-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTHE CHANGE OF CARDIOSTIMULATION DEVICE PROGRAMMING DUE TO DETECTION OF ELECTROMAGNETIC INTERFERENCE
Authors: Jan Morava 1,2,3; Aleš Richter 1,3
Authors place of work: Faculty of Mechatronics, Informatics and Interdisciplinary Studies, Technical University of Liberec, Liberec, Czech Republic 1; Department of Cardiology, Regional Hospital Liberec, Liberec, Czech Republic 2; Faculty of Health Studies, Technical University of Liberec, Liberec, Czech Republic 3
Published in the journal: Lékař a technika - Clinician and Technology No. 2, 2020, 50, 65-68
Category: Původní práce
doi: https://doi.org/10.14311/CTJ.2020.2.04Summary
The study deals with the interdisciplinary topic of the electromagnetic compatibility of the cardiac implantable electronic devices that are used in patients with a defect of heart conduction system. We are focusing on the detection of disturbing signals on electrodes of cardiostimulation device and its interpretation. The detection of electromagnetic interference (EMI) is related to electrode choice, device placement, its configuration and programming. The aim of the study is the analysis of the pacemaker response in the presence of an external source of the disturbance fields. We point to possible risks of its interaction and discuss mechanisms that can influence the pacemaker sensitivity to EMI. Due to improper signal detection, the device programming changes can occur. We present an experiment of the exposure of the cardiostimulation system to a low-frequency harmonic interference signals and finally we analyse similar clinical episode and discuss proper functioning of the pacemaker.
Keywords:
Cardiostimulation implantable devices (CIED) – Electrophysiology – electromagnetic interference (EMI) – cardiology
Introduction
The wide spectrum of cardiac implantable electronic devices includes pacemakers (PCMs) for the bradyar-rhythmia treatment, cardioverter-defibrillators (ICDs) for the treatment of tachyarrhythmias, and devices for resynchronization therapy. All these devices have the possibility of cardiac pacing for bradyarrhythmia treat-ment. The cardiostimulation function of these devices is based on the same principle. The device senses and detects an atrial or a ventricular electrical signal from the distal poles of intracardiac leads located in the heart chambers. The stimulation of myocardial cells by an electric impulse of predefined parameters is the re-sponse of the device to sensed cardiac activity [1]. The detection of such signals that are not physiological activity of the heart can affect the proper function of the device and endanger the patient. Inhibition of cardiostimulation may occur and it can cause asystole in pacemaker dependent patients. In the case of ICDs, there is a risk of an inadequate antitachyarrhythmia high voltage therapy (defibrillation shock) [2]. The presence of signals of electromagnetic interference can also initiate temporary changes in the programming of CIEDs.
Millions of cardiac patients with an active implant are daily exposed to potentially dangerous external electromagnetic fields. Current CIEDs strive for maxi-mal electromagnetic susceptibility and try to minimize the risks associated with EMI. However, the potential danger cannot be completely eliminated [2].
The aim of this study is to point to possible risks of the interaction of CIED with the source of EMI. On the in vitro experiment and on the clinical episode we describe the CIED response in the presence of the interference field. We think it is important to discuss the possible risks of its interaction and to analyse the device's behaviour during improper signals detection.
Sensing and detection
Sensing is the ability of CIED to detect an electrical signal from atrial or ventricular heart chamber [3]. This is followed by signal interpretation. The device marks and classifies each electric heart event using various algorithms. CIED monitors signal properties such as amplitude, conduction intervals and signal regularity. In the case of ICDs, morphology and other discriminant algorithms may be applied. Signal amplitude is impor-tant for proper sensing. It depends on the electrode location and the position in the heart chamber (position relating to the conduction system).
In conventional dual-chamber devices, one electrode is located in the right atrium (right auricle) and the second one in the right ventricle (interventricular septum or apex). The atrial signal (P-waves) corre-sponds to myocardial depolarization in atria and the amplitude is between 0.5–5 mV. The ventricular signal is in the range of 2–30 mV and characterizes the depolarization of the ventricular myocardium (R‑waves). The signal amplitude is unstable and may vary during sinus rhythm and during arrhythmia, because the amplitude is usually lower during fibril-lation. The sensitivity level of each channel must be set lower than the value of the sensed signal and it is usually set to half because of the prevention of under-sensing. Due to the setting, the atrial channel is more sensitive to external interference than the ventricular one [2, 5].
Device configuration
The electrode leads from the device header to the heart chamber, where it is usually actively fixed to the endomyocardium. Passive fixation is used mainly in the case of left ventricular electrodes for resynchro-nization therapy. The electrode leads the electrical pulse to the myocardial cells and an electrical signal from the heart to the device [3]. Sense/pace poles are located at the distal end of the electrode at the fixation site. For bipolar electrodes, there are two poles at the distal end. The distal pole (tip) is a helix fixed to the myocardium and the proximal pole (ring) is located about 10 mm proximal above the tip. Such electrode allows bipolar (near field) sensing and pacing between the poles [4].
Previously implanted unipolar electrodes have only one pole (distal tip) and the pace/sense vector is between the distal pole (cathode) and the housing of the device (anode) as figured in Fig. 1. There are still many patients with the implanted unipolar electrode, because it is implanted for life. The unipolar configuration can also be set in bipolar leads if it is needed because of clinical effect or single pole defect. Sensing in the unipolar mode is more sensitive to interference detec-tion due to the size of the sensing vectors (induction loop) in the unipolar and bipolar configuration. The unipolar vector is several times larger (range of 10–20 cm) and depends on the patient's anatomy, doctor skills and the position of CIED [4]. The implantation side is chosen in reverse according to the dominant side of the patient. Therefore, about 90% of systems are implanted on the left side. Defibrillation electrodes have discharge coil or coils that allow high-voltage therapy to be delivered and offer additional sensing vectors.
Fig. 1. The anteroposterior x-ray projection of the dual chamber pacemaker with figured pace/sense vectors. 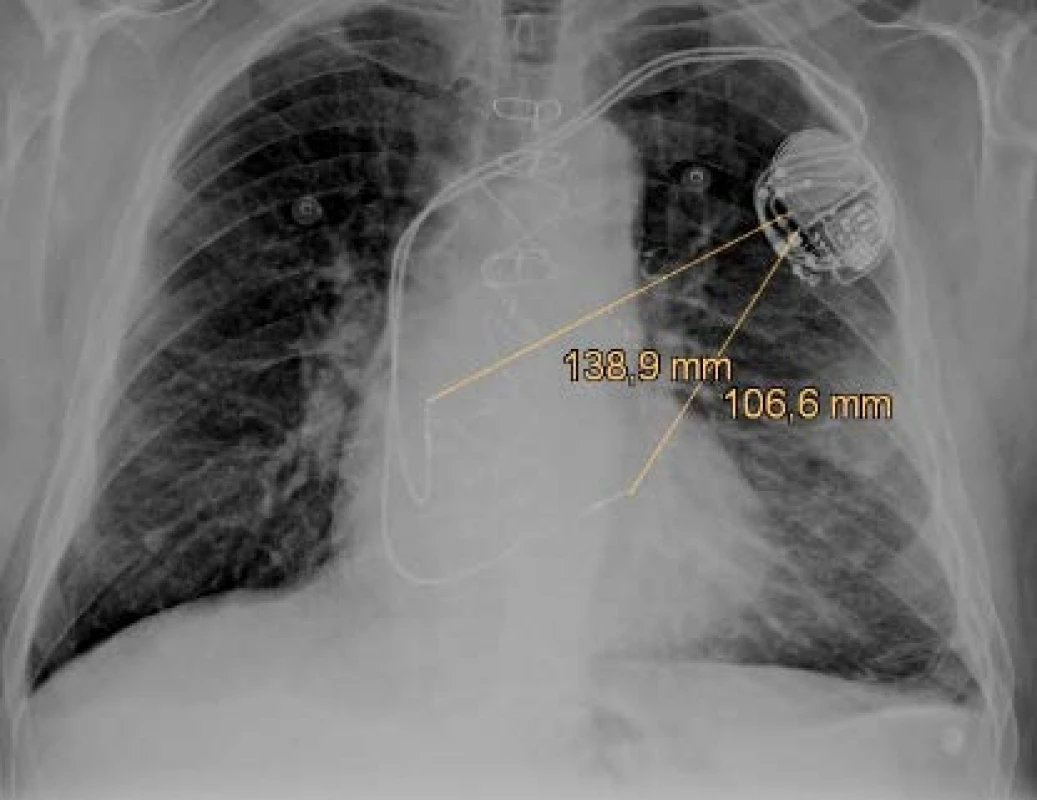
Electromagnetic interference
EMI detection and bad interpretation can have serious clinical or technical consequences. Cardio-stimulation may be inhibited because of the misinter-pretation of EMI as its own cardiac action. ICD can deliver an inadequate defibrillation shock, because the device interprets the signal as a malignant ventricular arrhythmia (ventricular fibrillation) and tries to treat it. There are also frequent changes in CIED programming due to the EMI presence. The most common case is the detection of disturbing signals on the atrial channel and misinterpretation as atrial fibrillation. Then a specific device response and change in pacing mode (triggered mode) occurs. It prevents the rapid triggered pacing in ventricles [5].
It can be confusing for CIED that the signal captured on the intracardiac electrodes may have similar charac-teristics and look like the heart action during running arrhythmias (as atrial fibrillation). When a strong elec-tromagnetic field is applied, the device can be reset to the factory settings (Power-On reset). But it is very rare. CIEDs are still vulnerable in this regard despite efforts to minimize the risks.
Methods
Simulated episode
We used a conventional dual chamber pacemaker and two common bipolar electrodes right atrial (RA) and right ventricular (RV) with active fixation. Pace-maker stimulation mode was DDD (dual chamber programmed stimulation) as presented in Tab. 1.
Tab. 1. Programming of the tested pacemaker. 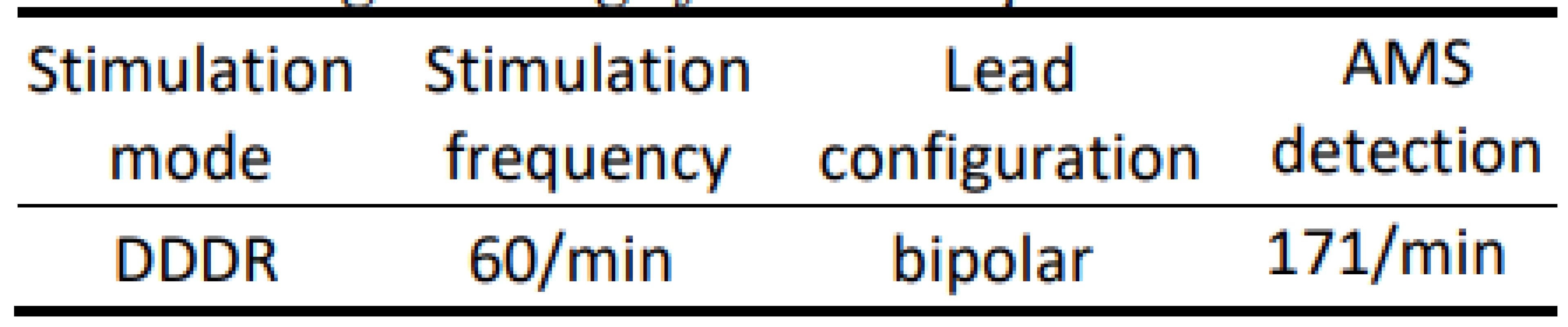
AMS - Automatic Mode Switching We placed the distal part of the atrial electrode into a container with saline. Then we simulated EMI by an external stimulator with a fixed frequency of 10 Hz with predefined pulse parameters (Tab. 2).
Tab. 2. Parameters of external stimulation. 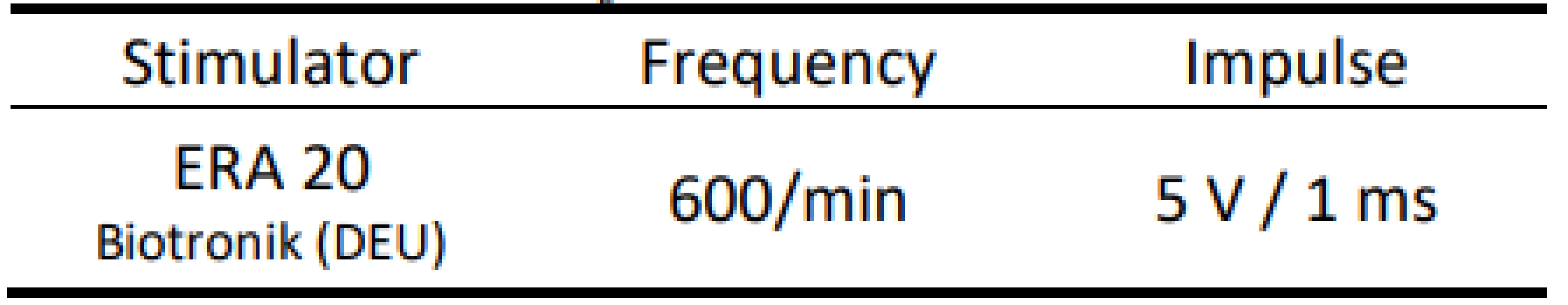
On the atrial recording in Fig. 2 there is figured that the device detects a fast atrial action. The signal fulfils the detection criteria for activating AMS mode. The behaviour of CIED corresponds to the description of the algorithm by the manufacturer.The goal was to use external stimulation to cause false detection by the device and to evoke the change in the CIED program and switch to AMS mode. Auto-matic mode switching (AMS) is an algorithm used to detect an atrial fibrillation and to prevent a rapid paced response in ventricles. After the atrial fibrillation is detected, the device switches to an inhibited DDI (inhibited stimulation mode in dual-chamber) or VVI (ventricular inhibited mode in single-chamber) pacing mode with a higher stimulation rate. In this case, we set the response in AMS mode according to the parameters in Tab. 3. This AMS mode setting is not standard and was selected for experiment purposes. By nominal set-ting the AMS has a pacing rate of 80/min for 10 min since the time the arrhythmia is detected.
Tab. 3. Programming in AMS mode. 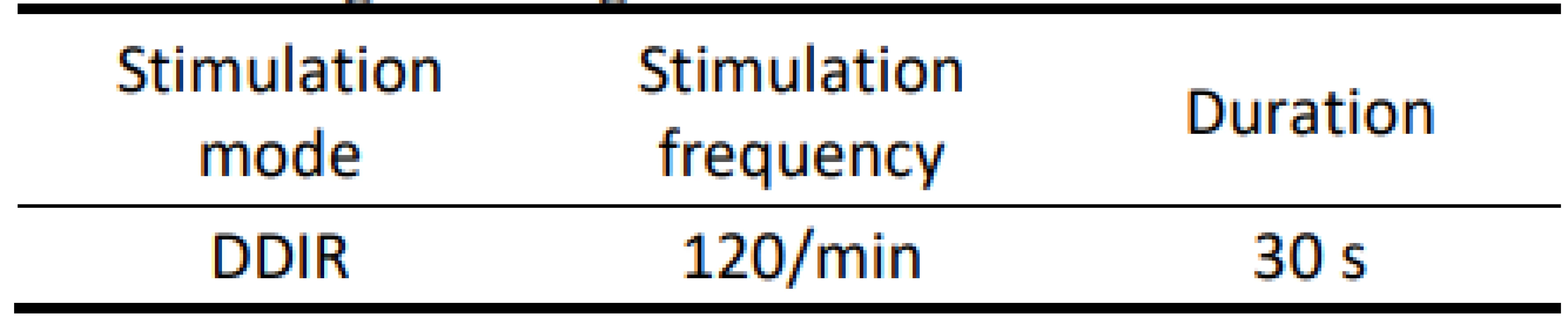
AMS - Automatic Mode Switching. Fig. 2. The recording of near-field channel of bipolar atrial lead during EMI simulation detected as atrial fibrillation. N.B.: Marker AP = Atrial Pace, AR/AB = Atrial Refractory/Blanking, VP = Ventricular Pace, MS = Mode Switching. 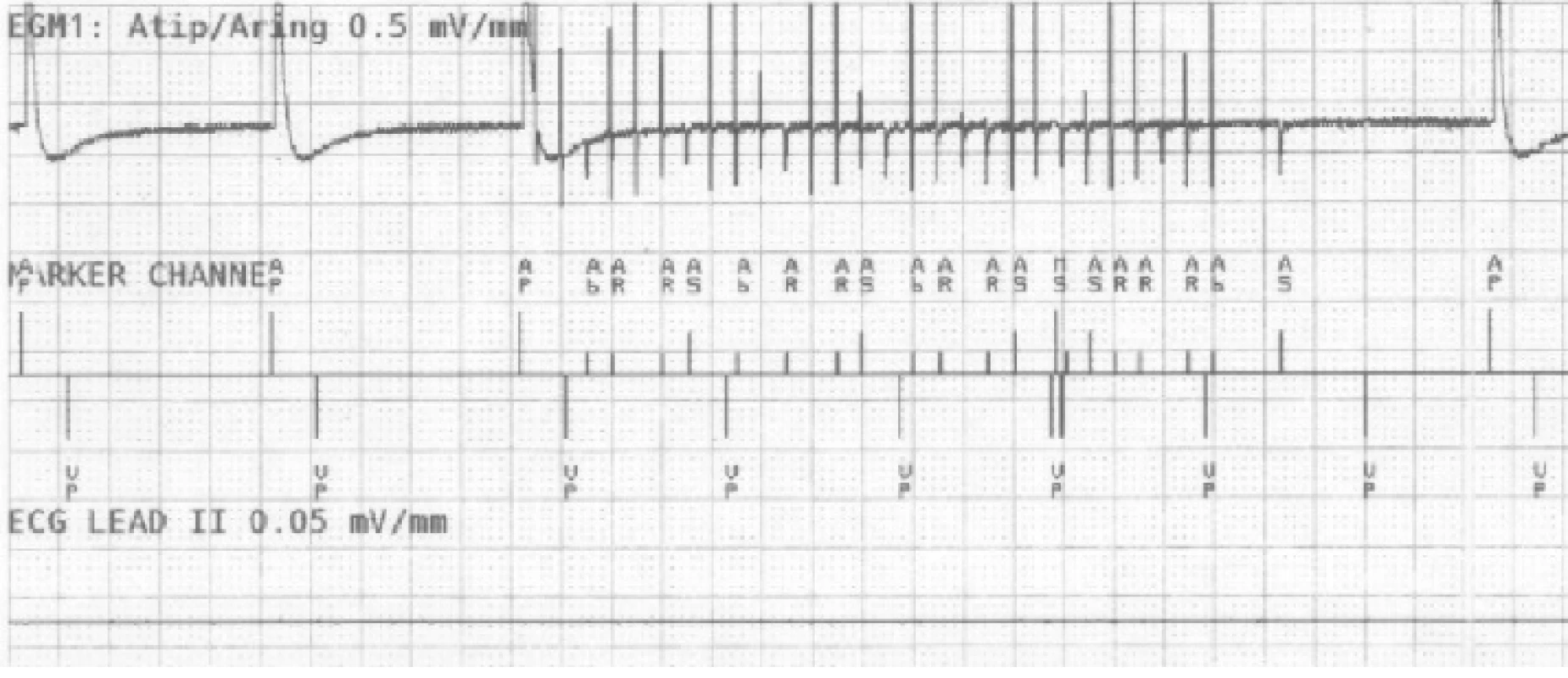
A sudden increase of frequency detected on the RA channel causes a fast triggered response in ventricles (VP markers), in this example up to 610 ms. The device compares the frequency of both (RA, RV) channels in the window of the last three VPs after activating the sudden onset (increase of the atrial frequency). This is followed by AMS activation and the change of the pacing program to DDIR mode (dual chamber inhibited mode with rate adaptive stimulation). After 30 s since the last fast action, as we set in this case, the device returns to the default mode.
Clinical episode
Similar clinical episodes of false atrial tachycardia detection are relatively common. Next, we analysed one anonymized episode (Fig. 3) captured by a remote monitoring system. This device is from other manu-facturer and it is a type of biventricular ICD. However, the algorithms for atrial tachycardia detection and AMS activation are in principle similar across indi-vidual devices.
Fig. 3. The intracardiac recording of improper detection on near-field channel (1st line is atrial, 3rd ventricular) and far-field RV Coil–Can channel (2nd line). N.B.: Marker AS = Atrial Sense, BP = Biventricular Pace, VS = Ventricular Sense, AMS = Automatic Mode Switching 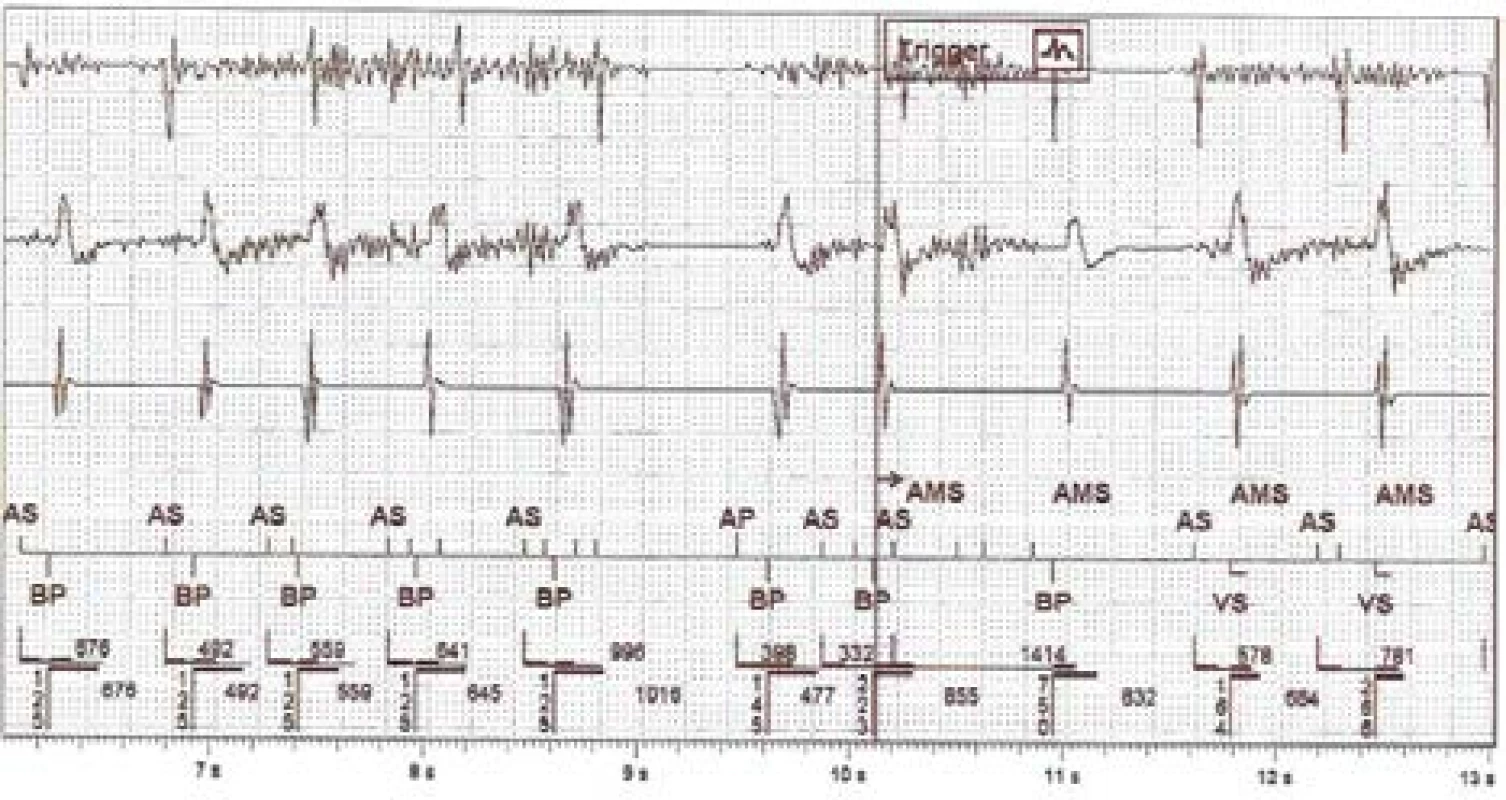
The captured disturbing signal, susp. EMI, has an amplitude floating around the level of sensitivity set-ting on the RA channel. It is also evident on the dis-crimination far-field channel. The signal is visible on the bipolar RV sensing vector due to the different signal gain. The fast atrial action evokes rapid biven-tricular pacing in the ventricles and subsequently acti-vates the AMS mode and changes the pacing program to DDIR 70/min. The atrial tachycardia detection limit is set to 180/min for this device.
Results
In this paper we demonstrated the easily induced erroneous response of different CIED based on the false detection of EMI on the atrial channel as an atrial fibrillation. We presented an EMI simulation and ob-served the change of the device behaviour and program changes to AMS mode. We subsequently analysed a similar clinical episode captured by the home moni-toring system. Changing of CIED programming by activating the AMS mode is not significant for the patient. It depends on the preset of the device and may not be perceived by the patient.
In these examples we demonstrate, that CIEDs are prone to misinterpretation of electrical signals and that EMI can easily affect the proper function of the device. However, other more serious complications may occur due to the effects of EMI. It may already have a direct impact on the patient's actual health. This is caused, because the device does not monitor the shape of the sensed signal, according to which it could discriminate interference and arrhythmia, but mainly monitors the amplitude and frequency of oscillations. The risk of false detection is real and the consequences and manner of influencing the device depend on many other factors.
Discussion and Conclusion
The relevance of this issue is increasing with the rapidly growing number of cardiac patients and with increasing electronization. These patients are daily exposed to potentially dangerous sources of disturbing fields. Modern systems try to use software and hard-ware mechanisms to minimize the risks associated with EMI, but it still has limits. Therefore, it is necessary to bring more attention to this issue.
This issue must be approached comprehensively. When assessing the risks of CIEDs interaction with EMI, the CIED factor and the factor of interfering electromagnetic field need to be considered. For further research we consider that EMI detection is not influ-enced only by the programming of the device and the configuration of the electrodes, but also by the position of the system and the location (orientation) of the electrodes in patient's chest. The individual distribution of tissues around the implant can also have an effect. We also consider and focus on the possible influence of the coupling mechanism of transmission (capacitive, inductive or galvanic) of interfering signals. In further research we deal with the sources of low-frequency electromagnetic fields, because it is frequency-similar to the physiological activity of the heart muscle. Because of this, it is a more serious potential risk for the patient. The assessment of the EMI interaction with CIED is individual and requires a comprehensive view of the issue.
We want to contribute the better knowledge of doc-tors and patients by the partial outputs of our research. The recommendations given by the manufacturers are currently not robust enough and sometimes too strict to patients.
Acknowledgement
This work was supported by Student Grant Compe-tition (SGS) of Technical University of Liberec in 2019.
This article is a result of the cooperation between the Technical University of Liberec, the Institute of Mechatronics and Computer Engineering, and the Regional Hospital Liberec, the Cardiology Department.
Ing. Jan Morava
Faculty of Mechatronics
Technical University of Liberec
Studentská 1402/2
461 17 Liberec 1
E-mail: jan.morava@tul.cz
Phone: +420 736 410 545
Prof. Ing. Aleš Richter, CSc.
Faculty of Health Studies
Technical University of Liberec
Studentská 1402/2
461 17 Liberec 1
E-mail: ales.richter@tul.cz
Phone: +420 602 189 739
Zdroje
- Napp A, Stunder D, Maytin M, Kraus T, Marx N, Driessen S. Are patients with cardiac implants protected against electro-magnetic interference in daily life and occupational environ-ment?. European heart journal. 2015 Jul 21;36(28):1798–804. DOI: 10.1093/eurheartj/ehv135
- Morava J, Richter A, Eichler J. Detection of Electromagnetic Interference on Electrodes of Cardiac Implantable Electronic Devices. In: 2019 12th International Conference on Measure-ment. IEEE; 2019 May 27. pp. 182–5. DOI: 10.23919/MEASUREMENT47340.2019.8780035
- Ellenbogen KA, Wilkoff BL, Kay GN, Lau CP, Auricchio A. Clinical Cardiac Pacing, Defibrillation and Resynchronization Therapy. Elsevier, 2017. ISBN 978-0 - 323-37804-8.
- Gercek C, Kourtiche D, Nadi M, Magne I, Schmitt P, Souques M. Computation of pacemakers immunity to 50 Hz electric field: Induced voltages 10 times greater in unipolar than in bipolar detection mode. Bioengineering. 2017 Mar;4(1). DOI: 10.3390/bioengineering4010019
- Morava J, Richter A, Kučera P. Electromagnetic Compatibility of Cardiostimulation Technology in Relation to Human Body—The Introductory Study. In: World Congress on Medical Physics and Biomedical Engineering 2018. Springer Singapore; 2019. pp. 755–60. DOI: 10.1007/978-981-10-9035-6_139
Štítky
Biomedicína
Článek vyšel v časopiseLékař a technika

2020 Číslo 2-
Všechny články tohoto čísla
- 3D PRINTED HYDROGEL GLUCOSE SENSOR ON ARGON PLASMA ACTIVATED POLYSTYRENE
- PREMATURE INFANT BLOOD VESSEL SEGMENTATION OF RETINAL IMAGES BASED ON HYBRID METHOD FOR THE DETERMINATION OF TORTUOSITY
- THE EFFECT OF FOOTWEAR TO THE POSTURE
- THE CHANGE OF CARDIOSTIMULATION DEVICE PROGRAMMING DUE TO DETECTION OF ELECTROMAGNETIC INTERFERENCE
- INAPPROPRIATE S-ICD PATIENT RECEIVES FALSE POSITIVE SHOCKS
- Lékař a technika
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- THE EFFECT OF FOOTWEAR TO THE POSTURE
- THE CHANGE OF CARDIOSTIMULATION DEVICE PROGRAMMING DUE TO DETECTION OF ELECTROMAGNETIC INTERFERENCE
- PREMATURE INFANT BLOOD VESSEL SEGMENTATION OF RETINAL IMAGES BASED ON HYBRID METHOD FOR THE DETERMINATION OF TORTUOSITY
- INAPPROPRIATE S-ICD PATIENT RECEIVES FALSE POSITIVE SHOCKS
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



