-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mucinous carcinoma (non-intestinal type) arising in the ovarian mature cystic teratoma - a case report
Mucinózny adenokarcinóm (neintestinálneho typu) vzniknutý v zrelom cystickom teratóme vaječníka - kazuistika
Malígna somatická transformácia v zrelom cystickom teratóme je zriedkavý fenomén, pri ktorom vzniká zhubný nádor z diferencovaných tkanivových štruktúr niektorého zo zárodočných listov.
Autori prezentujú prípad 38 - ročnej ženy so zrelým cystickým teratómom oboch vaječníkov a mucinóznym adenokarcinómom vzniknutým z endodermálnej zárodočnej línie v pravom ováriu, ktorý imunohistochemicky vykazoval charakteristiky non-intestinálnej diferenciácie. V čase diagnostiky nádor metastázoval do panvových a retroperitoneálnych lymfatických uzlín.
Pacientka bola liečená troma líniami chemoterapie a po 15 mesiacoch od stanovenia diagnózy zomrela za príznakov progresie vo forme masívnej nádorovej propagácie do retroperitoneálnych, mediastinálnych a krčných lymfatických uzlín, infiltrácie retroperitonea a duodena a peritoneálneho rozsevu.
Malígna somatická transformácia v zrelom cystickom teratóme je spojená so zlou prognózou. Najdôležitejším prognostickým faktorom je štádium v čase stanovenia diagnózy.Kľúčové slová:
ovárium – zrelý cystický teratóm – malígna transformácia – adenokarcinóm – nádorový marker
Authors: Karol Kajo 1; Ladislav Masák 2; Dagmar Sorkovská 3; Miroslava Vallová 1; Michal Kajo 1; Katarína Macháleková 1; Lýdia Heľpianská 4
Authors place of work: Department of Pathology, St. Elisabeth Cancer Institute, Bratislava, Slovakia 1; Department of Gynaecological Oncology, St. Elisabeth Cancer Institute, Bratislava, Slovakia 2; Department of Clinical Oncology, St. Elisabeth Cancer Institute, Bratislava, Slovakia 3; 1st Department of Oncology, St. Elisabeth Cancer Institute, Bratislava, Slovakia 4
Published in the journal: Čes.-slov. Patol., 49, 2013, No. 4, p. 141-145
Category: Původní práce
Summary
Somatic malignant transformation in mature cystic teratoma is a rare phenomenon of a malignancy of differentiated tissue structures of any stem line.
The authors present a case of a 38-year-old female with mature cystic teratoma of both ovaries and with mucinous adenocarcinoma arising from endodermal germ line in the right ovary, showing immunohistochemical features of non-intestinal differentiation. At the time of diagnosis the tumour metastasized to the pelvic and retroperitoneal lymph nodes.
The patient was treated with three lines of chemotherapy and died after 15 months with signs of massive progression into the retroperitoneal, mediastinal and cervical lymph nodes, retroperitoneum, duodenal wall and peritoneal cavity.
Somatic malignant transformation in mature cystic teratoma is associated with poor prognosis. The most important prognostic factor is tumour stage at the time of diagnosis.Keywords:
ovary – mature cystic teratoma – malignant transformation – adenocarcinoma – tumour markerMature cystic teratoma (MCT) of the ovary is the most common tumour arising from germ cells and accounts for more than 20 % of all ovarian tumours (1). Rare complication of MCT is a malignant somatic transformation that occurs in 1 - 3 % of these tumours (1,2). The most common type of malignant tumour arising in the MCT is squamous cell carcinoma (2), which represents 75 - 85 % of all malignant transformations (1,3,4). Adenocarcinomas are much less frequent and occur in approximately 6.8 % of MCT with malignant change (1,3). Other rare malignancies reported in MCT were basal cell carcinoma, adenosquamous carcinoma, carcinoma of the thyroid, malignant melanoma, sarcoma, carcinosarcoma and neuroectodermal tumour (1,3). Also, there were described sporadic cases of MCT with malignant change of several tissue types in one tumour (5).
We present a case of mucinous carcinoma arising in MCT, which is an interesting example of unusual type of malignant transformation of glandular epithelium and was presented at the time of diagnosis with metastases to the pelvic and interaortocaval lymph nodes.
CASE REPORT
In a 38-year-old woman, nulligravida and virgo intacta, menarche since 12 years of age, with no other remarkable gynaecological history, a large palpable tumour in her lower abdomen was found during a preventive gynaecological examination. On CT scan the tumour filled almost the whole pelvis and was presented as a lobed, sharply and smoothly bounded, solid-cystic formation attached to the right ovary, sized 105 x 105 x 128 mm. The tumour contained soft tissue structures, fluid, and coarse granular areas of calcification. In the left ovary there was a similar formation sized 49 x 39 x 47 mm. Several lymph nodes up to 12 mm in diameter were identified in the infrarenal retroperitoneum. The patient had elevated serum levels of tumour markers CA 19-9 (5054.9 U/ml; cut-off level 30 U/ml), CA 125 (550.4 U/ml; cut-off level 30 U/ml), CA 72-4 (7.9 U/ml; cut-off level 4.0 U/ml), CEA (32.9 ng/ml; cut-off level 4.0 ng/ml) and CA 15-3 (145.6 U/ml; cut-off level 28.0 U/ml).
The size of the right ovarian tumour, concurrent retroperitoneal lymphadenopathy, and elevated levels of tumour markers were suggestive of malignant behaviour of the tumour. Therefore abdominal hysterectomy and bilateral adnexotomy with frozen section of the right ovarian tumour were performed. After the result of frozen section confirming malignant somatic change in MCT, and presence of metastasis to the palpable paracaval lymph node, hysterectomy was followed by total omentectomy and removal of the pelvic and para-aortic infrarenal lymph nodes.
Macroscopically both ovaries were enlarged into solid–cystic tumour masses sized 12 x 13 x 10 cm in the right (Fig. 1) and 3 cm in diameter in the left ovary. Both tumours contained features of dermoid cysts, with the transitions into large compact areas of mucous and myxoid changes in the right ovarian tumour. Ovarian surface capsule was intact in both ovaries.
Figure 1. Macroscopic cut surface of the right ovarian teratoma with solid and cystic appearance with areas of mucinous deposits. 
Histological examination of the right ovarian mass proved the diagnosis of MCT with tissues of all three germ lines. Endodermal component was represented by urothelial and respiratory tissues, as well as non-intestinal type of epithelium resembling endocervical glandular epithelium. Exactly this type of epithelium showed malignant transformation into carcinoma with clearly defined irregular glands (Fig. 2), lakes of mucus forming pseudomyxoma ovarii, and large clusters and dissociated cellular elements of signet ring type (Fig. 3). Extensive endovascular spread of the tumour cells was present in the lymphatic vessels. Tumour cells accumulated in their cytoplasm PAS-positive mucous substances and showed immunohistochemical positivity of wide-spectrum cytokeratins (AE1/AE3), tissue-specific cytokeratin 7 (Fig. 4), p53, carcinoembryonic antigen (CEA) and oestrogen receptor (ER). Moreover, the neoplastic cells were negative for cytokeratin 20, CDX-2, TTF-1, p16, progesterone receptor, HER2 and chromogranin A (Table 1). FISH analysis did not prove amplification of HER2 gene (ZytoLight SPEC HER2/CEN 17 Dual Color Probe; ZytoLight FISH-Tissue Implementation Kit, Germany).
Figure 2. Mucinous carcinoma with characteristic glandular structures (haematoxylin - eosin, magnification x200). 
Figure 3. Differentiated (mature) glandular structure of MCT (the top of the image) close to the infiltration of disseminated signet ring cells of carcinoma (haematoxylin - eosin, magnification x200). 
Figure 4. Expression of cytokeratin 7 in tumour cells (magnification x200). 
Tab. 1. List of primary antibodies 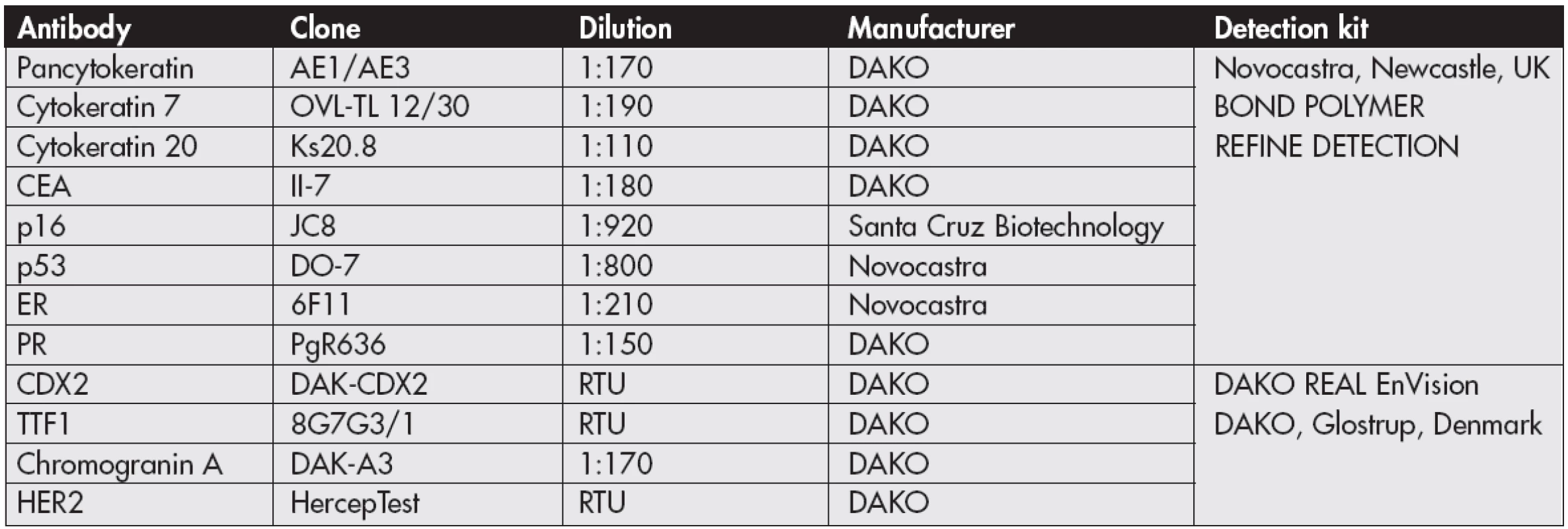
CEA – carcinoembryonal antigen, ER – oestrogen receptor, PR – progesterone receptor, RTU – ready to use The tumour in the left ovary was fully mature MCT with tissues of all three germ lines without any signs of malignant change. Focally regressive changes occurred in the form of giant cell reaction to hairy material.
The paracaval lymph node from frozen section contained a large metastasis of adenocarcinoma with predominant component of signet ring cells (Fig. 5). Of a total of 51 examined lymph nodes, metastases were observed in four lymph nodes from the right pelvic and interaortocaval area. The disease was evaluated as pathological stage pT1bpN1pMx and eventual gastrointestinal malignancy was completely excluded. Genetic testing performed from the tumour tissue did not reveal mutations in codons 12 and 13 of KRAS and codon V600E of BRAF.
Figure 5. Metastasis of mucinous carcinoma to the lymph node, composed of the tubular structures (left) and signet ring cells (right) (haematoxylin - eosin, magnification x100). 
One month after surgery, the patient underwent six cycles of first line chemotherapy in combination of paclitaxel and carboplatin and half a year after surgery the patient was without signs of recurrence of the disease. A control CT scan and MRI disclosed only small multiple haemangiomas and benign cysts in the liver. Tumour markers were normal (CA 19-9 28.0 U/ml; CA 125 16.8 U/ml; CA 72-4 0.1 U/ml and CEA 2.5 ng/ml). However, during the next three months, the levels of tumour markers significantly increased (CA 19-9 39402.6 U/ml; CA 125 424.5 U/ml; CA 72-4 15.0 U/ml and CEA 69.2 ng/ml) and CT scan demonstrated tumour progression in the form of retroperitoneal lymphadenopathy and nodular dissemination on the ileum serous surface. Therefore, a second line chemotherapy was applied in the patient in combination of gemcitabine and cisplatin, the latter being subsequently replaced by carboplatin due to ototoxicity. Course of the disease was complicated by serious phlebothrombosis (left jugular vein, subclaviculary vein, axillary and cubital vein) that led to full anticoagulation therapy. In spite of the chemotherapy, the patient developed massive metastases of mucinous carcinoma into the retroperitoneal, mediastinal and cervical lymph nodes, with infiltration of retroperitoneum, duodenal wall (Fig. 6) and peritoneal surfaces which was followed by extreme increase of serum tumour markers (the last detected levels were: CA 19-9 40350.0 U/ml; CA 125 4427.7 U/ml; CA 72-4 > 440.0 U/ml and CEA 614.5 ng/ml). According to these findings the patient started a third line metronomic chemotherapy combined with immunomodulatory therapy. In addition, in the terminal stage of the disease the patient had to undergo urgent surgery due to ileus with antecolic gastroenteroanastomosis and gastrostomy, and died 15 months from initial diagnosis with the signs of generalized carcinoma. Autopsy was not performed.
Figure 6. Tumour infiltration and endovascular propagation of tumour cells in the duodenal mucosa (haematoxylin - eosin, magnification x200). 
DISCUSSION
Malignant transformation in MCT is a rare phenomenon defined as somatic malignancy of some tissue structures differentiated from embryonic layers of MCT. Squamous epithelium is the most often prone to undergo malignant change, which results from the predominance of ectodermal epidermal structures in dermoid cyst (4). Alternatively, squamous cell carcinoma may arise from metaplastic bronchial epithelial structures of MCT (6). Much more rarely adenocarcinomas can occur in MCT, representing malignant transformation of the tissues of the endodermal cell line. Of these, there prevail adenocarcinomas derived from the tissue structures of the lower gastrointestinal tract (7). Therefore, most of the previously described mucinous carcinomas in MCT showed characteristics of intestinal differentiation with immunohistochemical expression of cytokeratin 20 and CDX-2, and with negativity of cytokeratin 7 (8,9). In our case, we detected an opposite expression of these immunohistochemical markers, suggesting together with certain histological features for the origin of carcinoma from Müllerian or respiratory type of epithelium (4). Recently, there was described a case of MCT with signet ring cell carcinoma along with adenocarcinoma of lung type, in which tumour cells were positive for TTF-1 as one of the markers of respiratory differentiation (10). In our case, according to the negativity of TTF-1 and focal positivity of ER, we suggested that adenocarcinoma in MCT might arise from the Müllerian endocervical differentiation line. Hypothetically, this malignant transformation might have occurred less likely also in the endodermal structures of the upper gastrointestinal tract or pancreatobiliary tract (8), but these should be positive for CDX-2 with co-expression of CK20 (11).
The majority of MCT represents probably a result of the first meiotic division failure (8), but it is still not clear how this leads to malignant transformation. It is assumed that it is a long-term process (12) occurring in cases of MCT that were not removed in time (13).
MCT often involves both ovaries, but malignant transformation is usually restricted only to one ovary (2,6), forming a large tumour mass (14), as it was in our case. Moreover, unlike the majority of other ovarian tumours, malignant transformed MCT could be presented also with pain (1-3).
The diagnosis of malignant transformation in MCT is rarely determined preoperatively. Among the risk factors of malignant behaviour there are age, tumour size, some growth characteristics seen in imaging techniques and elevated tumour markers (6,9). Although malignant transformation in MCT was recorded in a broad range between 19 and 88 years of age (8), it is more common in postmenopausal women with the highest incidence in the 5th and 6th decades of life (1). Therefore, greater caution is recommended in patients over 45 years with dermoid cysts (6). Logically, the risk of malignant transformation increases with size of the tumour. In our case, suspicion of possible malignant behaviour was set preoperatively on the basis of tumour size, heterogeneous appearance in ultrasound imaging, finding of retroperitoneal lymphadenopathy and elevated tumour markers. Markers in MCT with malignant transformation may also be normal, e.g. if squamous cell carcinoma is present (2). The best screening markers of malignant squamous transformation are CEA and SCC (“Squamous Cell Carcinoma”) antigen, which along with the patients age over 45 and MCT size over 9.9 cm can provide good preoperative information and thus help differentiate pure MCT from MCT with squamous cell carcinoma (6,15). In adenocarcinomas there are usually found elevated serum levels of CEA and CA 125 (1,16). In our case, the markers used for diagnosis and monitoring of the disease progression were CA 19-9, CA 125, CA 72-4, CEA and CA 15-3, all of which showed increased levels. We also detected CEA in the cancer cells by immunohistochemical staining.
An interesting feature in our case was also the finding of metastasis to the regional lymph node. A similar phenomenon has been previously described in only one case of mucinous carcinoma arising in MCT with metastasis to paraaortic lymph node (1).
Essential in the differential diagnosis is to exclude a metastatic infiltration of ovaries from primary adenocarcinoma of gastrointestinal tract. This is particularly difficult to realize in adenocarcinomas arising in MCT from structures with intestinal endodermal type of differentiation, which often exhibit identical phenotypic characteristics as metastatic adenocarcinomas from gastrointestinal tract. It should be noted that presence of MCT alone in ovary is an important feature for confirming the origin of adenocarcinoma from the germ cells. In every doubtful case, it is necessary to perform particular gastrointestinal tract examination to rule out the possibility of secondary promotion to ovaries. Mucinous ovarian carcinomas of signet ring cell type are the most often metastases of primary carcinomas of gastrointestinal tract, e.g. from stomach, biliary tract, appendix, colon and rectum and they are referred as Krukenberg tumours. Primary ovarian signet ring cell carcinoma is extremely rare and only few cases have been yet described (17,18). In our case, we consider the signet ring cancer cells as a sign of dedifferentiation of originally differentiated mucinous carcinoma. Signs suggestive of metastatic disease are bilateral involvement of ovaries with tumours of moderate size (up to 10 cm), lymphovascular invasion, localization of mucus, a form of destructive invasion, involvement of ovarian surface, the presence of signet ring cells, and more advanced stage of the disease (5,19). Furthermore, it is important to distinguish from collision tumours, as two independent tumour processes arising in the same ovary, one of which is dermoid cyst, e.g. a serous ovarian carcinoma in association with dermoid cyst (20). In our case, immunohistochemical stains confirmed the ovarian origin of signet ring cells (positive expression of CK7 and negativity of CK20 and CDX-2) and also the patient in the postoperative period underwent further investigations, which definitely excluded the possibility of other primary tumours.
Mutation analysis of molecular alterations in mucinous neoplasms of the colon and ovary does not contribute to differential diagnosis, because KRAS mutations were observed in 43 % of ovarian mucinous carcinomas and in 30 % of colorectal carcinomas. Similarly, the incidence of p53 gene mutations is comparable in the tumours of both locations, 26 % and 33 %, respectively. Identification of mutations in the BRAF gene seems to be of some value in differential diagnosis, whereas these have not yet been demonstrated in ovarian tumours, but about one-fifth of mucinous tumours of the colon and rectum is associated with alterations of this gene. In contrast to this, the amplification of the HER2 gene was demonstrated in 18 % of ovarian mucinous carcinomas, but in less than 1 % of colorectal carcinomas (19). In our case we did not prove any mutations in KRAS and BRAF genes, or gene amplification of HER2, only expression of p53 was recorded immunohistochemically.
The treatment of choice for these tumours is surgery. Because the subsequent optimal therapeutic management of the patients with mucinous adenocarcinoma arising in the MCT has not yet been established (1), each case must be approached individually. It is essential to differentiate metastatic (intestinal) tumours from primary ovarian mucinous carcinomas, because the therapy of the latter is based on platinum and taxane drugs and the former are treated with fluoropyrimidine drugs (19).
The prognosis of patients with malignant transformation in MCT is generally poor (8) and the majority of women die within a year from diagnosis (1). Only sporadic cases can survive for exceptional long time (14). Poor prognostic factors are tumour dissemination, infiltration of the cyst wall, ascites, spontaneous or accidental rupture, adhesions, and a malignancy other than squamous cell carcinoma (3,7). Our case was unique due to the fact that there has not yet been described metastasis of malignant transformed MCT into the duodenal wall, although metastases from other neoplasms were recorded in this area (21,22).
Our case represents MCT with malignant transformation to mucinous non-intestinal adenocarcinoma with extensive endolymphatic spread. This malignant transformation in MCT is indeed an extremely rare but possible complication that has to be considered in differential diagnosis especially in large ovarian tumours.
AKNOWLEDGEMENT
The authors wish to thank the League against Cancer, Slovak Division, for their invaluable support of the Projects of Tumour tissue biobanking and FISH diagnostics at the Department of Pathology, St. Elisabeth Cancer Institute, Bratislava.
Correspondence address:
Karol Kajo, Assoc. Prof., MD., PhD.
Department of Pathology, St. Elisabeth Cancer Institute
Heydukova 10, 812 50 Bratislava, Slovakia
tel.: +421 2 3224 9536
e-mail: kkajo@ousa.sk
Zdroje
1. Park JH, Whang SO, Song ES, Choi SJ, Lee WY. An ovarian mucinous cystadenocarcinoma arising from mature cystic teratoma with para-aortic lymph node metastasis: a case report. J Gynecol Oncol 2008; 19(4): 275-278.
2. Nor Azlin MI, Isa MR, Zaleha AM. Bilateral ovarian teratoma with squamous cell malignancy in a young woman: a diagnostic and management challenge. Int Med J 2005; 4(2): 51 - 53.
3. Arora DS, Haldane S. Carcinosarcoma arising in a dermoid cyst of the ovary. J Clin Pathol 1996; 49(6): 519-521.
4. Cobellis L, Shürfeld K, Ignacchiti E, Santopietro R, Petraglia F. An ovarian mucinous adenocarcinoma arising from mature cystic teratoma associated with respiratory type tissue: a case report. Tumori 2004; 90(5): 521-524.
5. Hinshaw HD, Smith AL, Desouki MM, Olawaiye AB. Malignant transformation of a mature cystic ovarian teratoma into thyroid carcinoma, mucinous adenocarcinoma, and strumal carcinoid: A case report and literature review. Case Report in Obstetrics and Gynecology 2012; Article ID269489. Doi: 10.1155/2012/269489.
6. Suranagi VV, Malur PR. Squamous cell carcinoma arising from mature cystic teratoma of ovary. JK Science 2009; 11(3): 154-156.
7. Chang JL, Liu KY, Kuo YL, Lee WH. Adenocarcinoma arising from mature cystic teratoma of the ovary. J Med Sci 2002; 22(5): 239-244.
8. Talerman A, Vang R. Germ cell tumors of the ovary. In: Kurman RJ, Ellenson LH, Ronnett BM, eds. Blausteinęs pathology of the female genital tract (6th ed). New York: Springer; 2011 : 847-908.
9. Takai M1, Kanemura M, Kawaguchi H, et al. Mucinous adenocarcinoma of the intestinal type arising from mature cystic teratoma of the ovary: a rare case report and review of the literature. J Ovarian Res 2012, 5(1): 41, http://www.ovarianresearch.com/content/5/1/41.
10. Boyd C, Patel K, O’Sullivan B, Taniere P, McCluggage WG. Pulmonary-type adenocarcinoma and signet ring mucinous adenocarcinoma arising in an ovarian dermoid cyst: report of unique case. Hum Pathol 2012; 43(11): 2088-2092.
11. Goldstein NS, Bosler DS. Immunohistochemistry of the gastrointestinal tract, pancreas, bile ducts, gallbladder and liver. In: Babbs D, ed. Diagnostic immunohistochemistry (2nd ed). Philadelphia, PA: Churchill Livingstone Elsevier; 2006 : 442-508.
12. Rim SY, Kim SM, Choi HS. Malignant transformation of ovarian nature cystic teratoma. Int J Gynecol Cancer 2006; 16(1): 140-144.
13. Bal A, Mohan H, Singh SB, Sehgal A. Malignant transformation in mature cystic teratoma of the ovary: report of five cases and review of the literature. Arch Gynecol Obstetr 2007; 275(3): 179-182.
14. Ueda G, Fujita M, Ogawa H, Sawada M, Inoue M, Tanizawa O. Adenocarcinoma in a benign cystic teratoma of the ovary: report of a case with a long survival period. Gynecol Oncol. 1993 Feb; 48(2): 259-263.
15. Kikkawa F, Nawa A, Tamakoshi K, et al. Diagnosis of squamous cell carcinoma arising from mature cystic teratoma of the ovary. Cancer 1998; 82(11): 2249-2255.
16. Stewart CJ, Tsukamoto T, Cooke B, Leung YC, Hammond IG. Ovarian mucinous tumor arising in mature cystic teratoma and associated with pseudomyxoma peritonei: Report of two cases and comparison with ovarian involvement by low-grade appendical mucinous tumour. Pathology 2006; 38(6): 534-538.
17. McCluggage WG, Young RH. Primary ovarian mucinous tumors with signet ring cells: report of 3 cases with discussion of so-called primary Krukenberg tumor. Am J Surg Pathol 2008; 32(9): 1373-1379.
18. El-Safadi S, Stahl U, Tinneberg HR, Hackethal A, Muenstedt K. Primary signet ring cell mucinous ovarian carcinoma: a case report and literature review. Case Rep Oncol 2010; 3(3): 451-457.
19. Kelemen LE, Köbel M. Mucinous carcinomas of the ovary and colorectum: different organ, same dilemma. Lancet Oncol 2011; 12(11): 1071-1080.
20. Kajo K, Macháleková K. Collision of invasive serous adenocarcinoma and mature cystic teratoma in the ovary. Letter to the editor. APMIS 2007; 115(6): 769-771.
21. Benedeto-Stojanov DA, Nagomi AV, Živkovič VV, Milanovič JR, Stojanov DA. Metastatic melanoma of the stomach and the duodenum. Arch Oncol 2006; 14 (1-2): 60-61.
22. Kanthan R, Senger JL, Diudea D, Kanthan S. A review of duodenal metastases from squamous cell carcinoma of the cervix presenting as an upper gastrointestinal bleed. WJSO 2011; 9 : 113. http://www.wjso. com/content/9/1/113.
Štítky
Patologie Soudní lékařství Toxikologie
Článek vyšel v časopiseČesko-slovenská patologie

2013 Číslo 4-
Všechny články tohoto čísla
- Metodika molekulárně patologické diagnostiky - díl druhý
- Diagnostika neurodegenerací je detektivní práce
- MONITOR aneb nemělo by vám uniknout, že...
- Fluorescence in situ hybridization on histologic sections
- Laser capture microdissection and its practical applications
- Immunophenotypization by means of flow cytometry in pathology
- Minimal residual disease – detection possibilities in haematological and non-haematological malignancies
- Mucinous carcinoma (non-intestinal type) arising in the ovarian mature cystic teratoma - a case report
- Eosinophilic dysplasia of the cervix associated with HPV 6 infection – case report and review of the literature
- How to improve the histopathological diagnosis of hepatocellular benign affections (adenoma versus focal nodular hyperplasia) in daily practice?
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- How to improve the histopathological diagnosis of hepatocellular benign affections (adenoma versus focal nodular hyperplasia) in daily practice?
- Immunophenotypization by means of flow cytometry in pathology
- Minimal residual disease – detection possibilities in haematological and non-haematological malignancies
- Fluorescence in situ hybridization on histologic sections
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



