-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Synthesis, identification and physicochemical properties of novel potential ultrashort-acting beta-adrenergic blockers
Syntéza, identifikácia a fyzikálno-chemické vlastnosti nových potenciálnych ultrakrátkopôsobiacich beta-adrenergných blokátorov
Práca sa venuje syntéze, overeniu štruktúry a štúdiu fyzikálno-chemických vlastností derivátov aryloxyaminopropanolu, 2-hydroxy-3-[4-(2-fluórfenyl)-piperazín-1-yl]-propyl-4-[(alkoxykarbonyl)amino] benzoátov, ich solí s kyselinou chlorovodíkovou, s jedným až štyrmi atómami uhlíka v alkoxy skupine karbamoylovej skupiny. Štruktúra zlúčenín bola potvrdená elementárnou analýzou, IČ, UV a hmotnostnými spektrami. V rámci štúdia bola stanovená teplota topenia, rozpustnosť, povrchová aktivita, disociačná konštanta, ako aj parametre lipofility, t.j. rozdeľovací koeficient P, hodnoty RM z reverznej chromatografie na tenkej vrstve a retenčný faktor k pomocou vysokoúčinnej kvapalinovej chromatografie.
Kľúčové slová:
ultrakrátkopôsobiace beta-blokátory – syntéza – fyzikálno-chemické vlastnosti
Authors: S. Kečkéšová; E. Sedlárová; J. Csöllei 1; P. Mokrý 1
Authors place of work: Comenius University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry, Bratislava ; University of Veterinary and Sciences Pharmaceutical, Faculty of Pharmacy Institute of Chemical Drugs, Brno 1
Published in the journal: Čes. slov. Farm., 2008; 57, 160-164
Category: Původní práce
Summary
The paper deals with the synthesis, structure confirmation, and study of physicochemical properties of some aryloxyaminopropanol derivatives, compounds of 2-hydroxy-3-[4-(2-fluorophenyl)-piperazine-1-yl]-propyl-4-[(alkoxycarbonyl)amino]benzoates, their salts with hydrochloric acid, with one to four carbon atoms in the alkoxy group of the carbamoyl group. Their structure was confirmed by elemental analysis, IR, UV and mass spectra. The melting point, solubility, surface activity, dissociation constant, and some lipophilicity parameters, i.e. partition coefficient P, RM values from reversed phase thin-layer chromatography, and retention factor k obtained from HPLC of the substances under study were determined.
Key words:
ultrashort-acting beta-blockers – synthesis – physicochemical propertiesIntroduction
Antagonists of adrenergic β-receptors are widely used in hypertension, arrhythmias, hypertrophic cardiomyopathy, and ischemic heart disease. Adverse effects of classic beta-blockers include hypotension, bradycardia, heart failure, bronchospasm or peripheral vasoconstrictions, which can last up to several hours after intravenous application 1). Therefore, ultrashort-acting β-blockers have been developed for emergent intravenous treatment of atrial fibrillation or when control of heart rate is desirable 2).
This paper focuses on the synthesis, identification, and determination of physicochemical properties of four new derivatives of ß-adrenergic receptor-blocking phenoxyaminopropanols. The structure of the pattern molecule was modified by the ester group inserted between the aromatic and amine moieties of the connecting chain.
EXPERIMENTAL PART
Synthesis
Synthesis of the novel derivatives of arylcarbonyloxyaminopropanols is shown in the Scheme and was carried out according to a general method first reported by Kam et al. 3).
The corresponding 4-carbalkoxyaminobenzoic acids 1a-d were prepared by the reaction of 4-aminobenzoic acid p.a. (Lachema, CR) with the appropriate alkylchloroformiates. To a solution of p-aminobenzoic acid (0.1 mol.l-1) and pyridine p.a. (LOBA Chemie, CR) (0.1 mol.l-1) in acetone p.a. (Kulich, CR) (100 ml), alkylchloroformiate (0.1 mol.l-1) was added dropwise. The mixture was refluxed for 1.5 hour, evaporated to dryness and washed with water (500 ml). The crude acid was recrystallized from ethanol (96%, Lachema, CR).
To a suspension of 4-carbalkoxyaminobenzoic acid (0.1 mol.l-1) in toluene p.a. (Lachema, CR) (120 ml), pure thionylchloride (Lachema, CR) (0.2 mol.l-1) was added. The resulting mixture was refluxed for 2 hours and evaporated to dryness. The crude 4 carbalkoxyaminobenzoyl chloride 2a-d recrystallized from toluene.
Glycidyl esters 3a-d were prepared by the reaction of the acid chloride with glycidol. To a three-neck round-bottom flask, glycidol (96%, Aldrich, Germany) (0.25 mol.l-1) and tetrahydrofurane p.a. (Lachema, CR) (50 ml) were added. The solution was cooled to -5 °C and triethylamine (> 99%, Merck-Schuchardt, Germany) (0.25 mol.l-1) was added. To the resulting solution, a solution of 2a-d (0.2 mol.l-1) in tetrahydrofurane p.a. (Lachema, CR) (250 ml) was added dropwise at -5 to 5 °C. The mixture was stirred at room temperature for 3 hours, filtered and evaporated to dryness. The product, 2,3-epoxypropyl-4-carbalkoxyaminobenzoate – 3a-d, was recrystallized from propan-2-ol p.a. (Kulich, CR).
In the last step of the synthesis, a mixture of 3a-d (0.2 mol.l-1) and 1-(2-fluorophenyl)piperazine (97%, Aldrich, Germany) (0.25 mol.l-1) in isopropyl alcohol p.a. (Kulich, CR) (150 ml) was heated at 65 °C for 4 hours. The solvent was evaporated under reduced pressure and residing oil was dissolved in diethylether p.a. (Kulich, CR). The solution of the base 4a-d was converted to its chloride salt 5a-d by addition of ethereal hydrochloric acid p.a. (35%, Central System, SR). The amine salt was collected by filtration and recrystallized from an appropriate solvent to give white crystals.
Chemical structure confirmation
Elemental analysis
Elemental analysis was made by an Elemental Analyzer EA 1108 (Carlo Erba Instuments).
Ultraviolet spectra
Electronic spectra of the prepared compounds were measured in the UV region by a spectrophotometer (8425 A Diode Array, Vectra 286/12), the solvent was methanol, concentrations 1.0 × 10-6 mol.l-1 and quartz (1 cm).
Infrared spectra
IR spectra of the synthetized compounds were measured on an FTIR spectrophotometer Nicolet Model Impact 410 in the 400–4000 cm-1 range.
Mass spectra
Mass spectra were recorded by a mass spectrometer Agilent 1100, LC/MSD VL Trap with a linear mechanic pump (KD Scientific, USA), the solvent was methanol, concentrations were approximately 1 mg.l-1.
Thermic properties
Melting points were determined using a Kofler hotplate apparatus (HMK Franz Küstner, Germany).
Estimation of solubility and dissociation constants
Solubility
Solubility of prepared compounds was determined in distilled water, 96% ethanol (Lachema, CR), and in a chloroform medium p.a. (Lachema, CR) according to 4).
Dissociation constant
The dissociation constant of prepared substances was estimated by alkalimetric titration with potentiometric indication (pH-meter PO 811/1, Radelkis, Hungary) according to 5).
Surface activity determination
The relative surface activity was determined by stalagmometric method according to 5). Activity of the solution of the compounds (c = 1.0 × 10-3 mol.l-1) was measured where the solvent was a mixture of H2O methanol p.a. (SPOLCHIM, SR) (φr = 3 : 2). The reference solution was the same as the solvent.
Determination of lipophilic properties
The apparent partition coefficient P of prepared compounds was measured by the shake-flask method in octan-1-ol (Merck, Germany)/phosphate buffer medium at pH = 7.0 according to 6) using 0.5 ml of the organic solvent.
The chromatographic reversed-phase thin layer analysis was carried out according to 7) on the silica gel Silufol® UV254. RM values were obtained in the mobile phase consisting of 1 mol.l-1 HCl-acetone (φr = 4 : 2) after silica gel plates impregnation with 1% solution of silica oil in heptane (Lachema, CR). Chromatograms after developing and drying were detected by UV light at the wavelength 254 nm using a UV lamp TL-900 (Camag, Switzerland).
The chromatographic system for HPLC analysis for the retention factor k determination consisted of a pump Delta Chrom SDS (Watrex, Slovakia) with an injection valve and a UV detector Delta Chrom UVD 200 (Watrex, Slovakia). The analytical chromatography column was Sepharon SGX C18 (254 × 4 mm, particle size 7 μm). The mobile phase was a mixture of 94% methanol in an amount of 500 ml and 3.4 g of natrium acetate, the pH value was adjusted with acetic acid to pH = 6.0. The flow rate of the mobile phase was 0.6 ml.min-1, the injection volume was 1.10-2 ml, the chromatograms were scanned at the wavelength 274 nm. The solution of NaNO2 (c = 0.1 mol.l-1) was used to determine the dead time t, and the solution of the analyzed compound in a methanol medium (c = 0.4 × 10-3 mol.l-1) was used for determination of tR.
RESULTS AND DISCUSSION
The paper focused on the synthesis, spectral description, and study of physicochemical properties of the new compounds.
The derivatives of 2-hydroxy-3-[4-(2-fluorophenyl)-piperazine-1-yl]-propyl-4-[(alkoxycarbonyl)amino]benzoates with one to four carbon atoms in the alkoxy group of the carbamoyl group have been synthesized by a five-step reaction according to a similar procedure, which was described in the literature 2). The final compounds were isolated as the salts of hydrochloric acid (Scheme 1).
Fig. 1. Synthetic route of the compounds 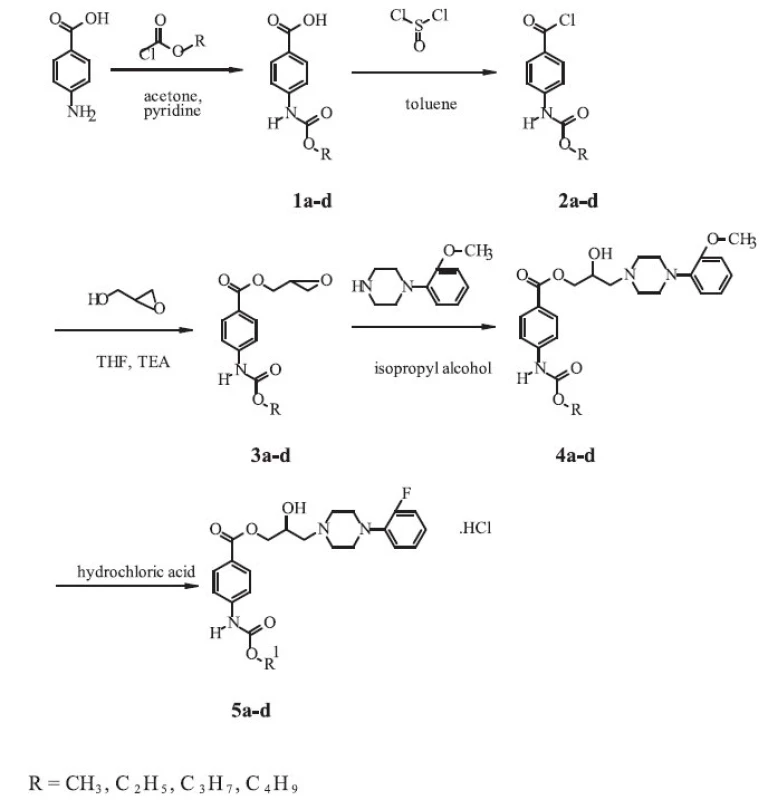
The methods of identification included elemental analysis, ultraviolet, infrared, and mass spectra. The prepared compounds were solid, colourless and their melting points, yields, and formulas are presented in Table 1.
The structure of the substances under study was confirmed by elemental analysis, the values were in good agreement with the respective structures for carbon, hydrogen and nitrogen. The differences between the calculated and experimentally obtained data were in the range of Ī 0.4 % and both data are given in Table 1.
Tab. 1. Chemical structure of studied compounds 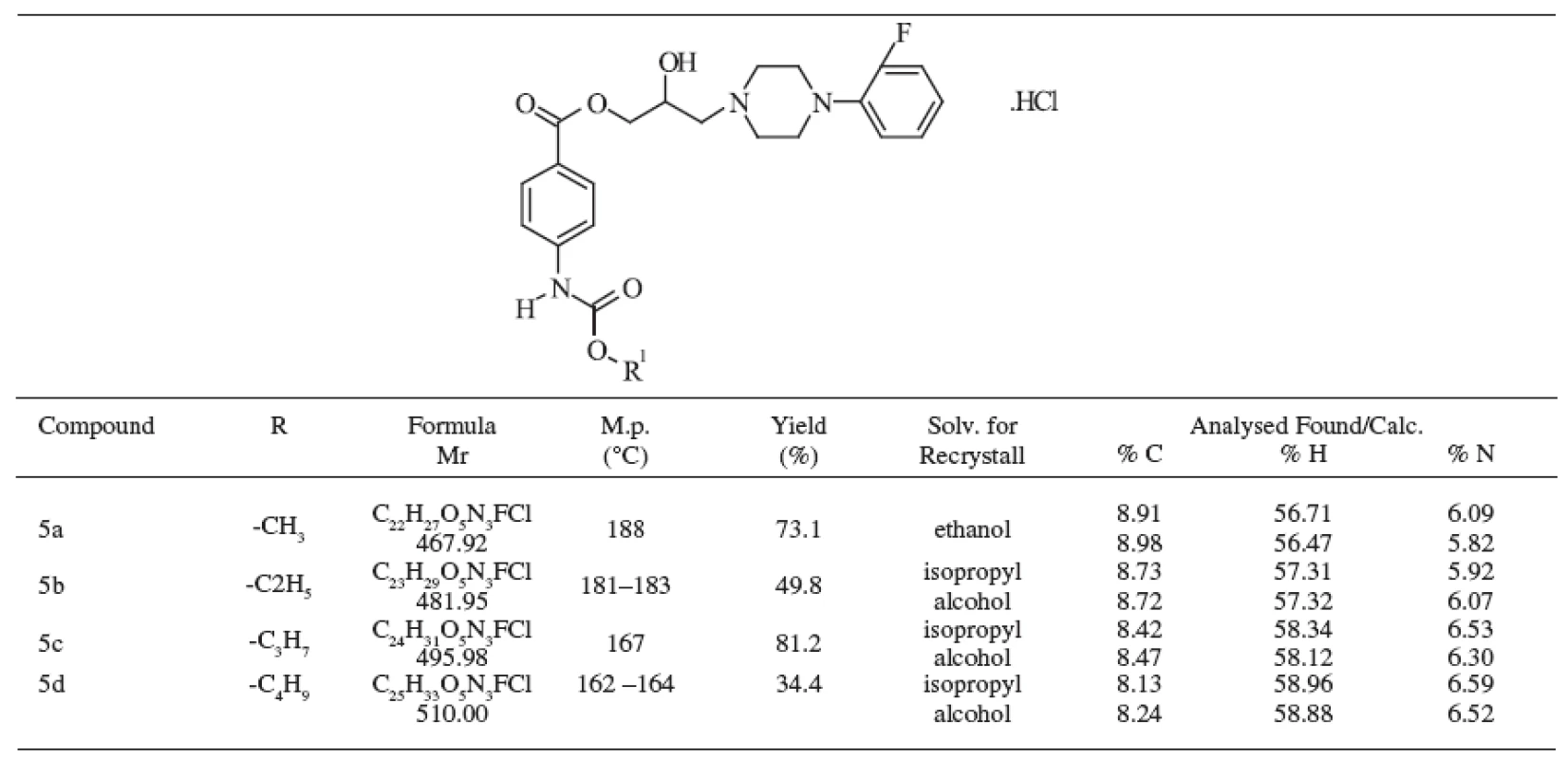
The mass spectra of prepared compounds revealed the presence of [M + H+] ions in all cases, the value m/z = 432.3 for the compound 5a2, m/z = 446.3 for 5b2, m/z = 460.3 for 5c2 and m/z = 474.3 for 5d2.
The spectrum in the ultraviolet region showed two absorption maxima at the wavelength γ = 202–203 nm and γ = 270–271 nm. The values of log ε of the analyzed substances are shown in Table 2.
Tab. 2. UV spectra of prepared compounds 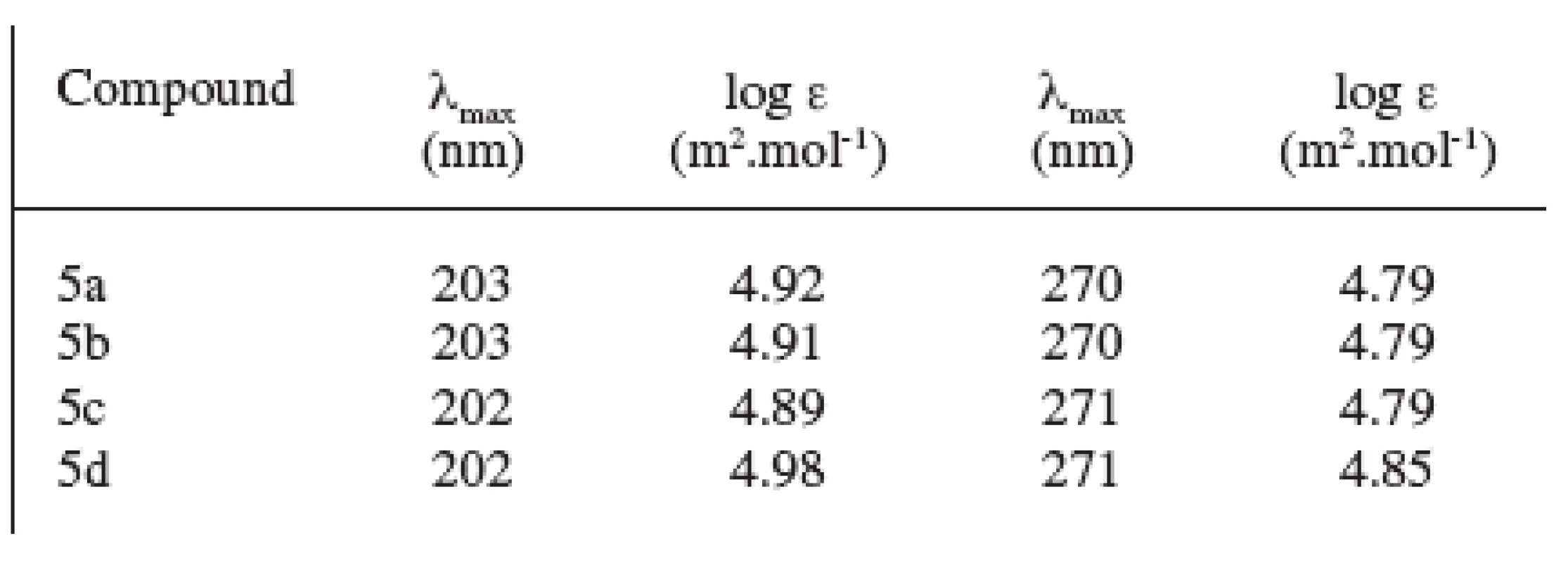
The results of the spectral parameters of the IR region are summarized in Table 3.
Tab. 3. Parameters of IR spectra (cm-1) of prepared compounds 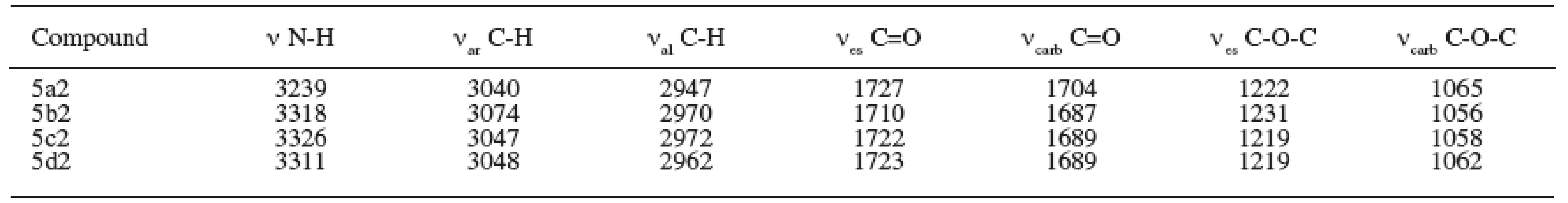
The solubility of liquids and solids in water is no doubt one of the most important molecular properties that affect their biological activity. Solubility of solid compounds depends on the free energy changes involved in changing the solid state to the liquid state, in addition to the interactions in the liquid phase 8).
Despite the fact that the compounds were salts with hydrochloric acid, their solubility was practically indissoluble in water, very limited in ethanol and practically indissoluble in chloroform.
The dissociation constant presents the rate of the dissociated and undissociated forms of the drug 9). The value of dissociation constant was obtained as the average value from three measurements and was determined according to the Henderson-Hasselbalch equation. The values of pKa are shown in Table 4.
Tab. 4. Physicochemical parameters of substances under study 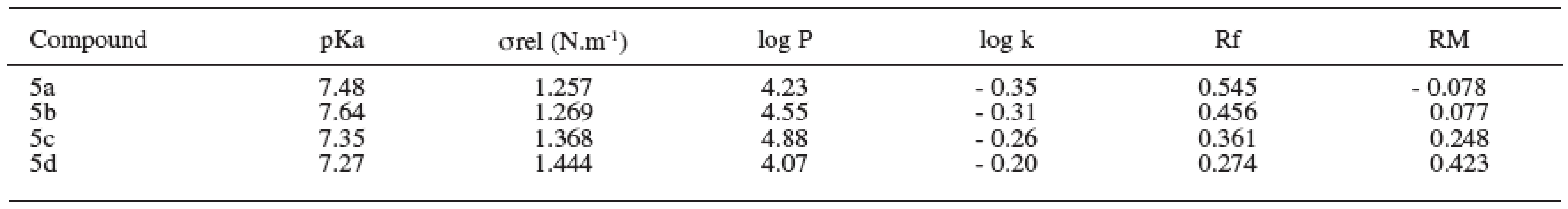
The relative surface activity values of the compounds increased with the alkoxy chain elongation. The values of relative surface tension were obtained as the average value from six measurements and are summarized in Table 4.
Lipophilicity is the key parameter used in quantitative structure-activity relationships (QSAR), quantitative structure-property relationships (QSPR), or quantitative structure-retention relationships (QSRR) studies, for modelling the biological and physicochemical properties of large classes of chemical compounds. In many cases, this molecular parameter strongly correlates with the biological activity of chemicals, as well as with other physicochemical properties 10).
The logarithm of partition coefficient between octan-1-ol and water (log P) is the most widely used parameter in chemistry, pharmaceutical chemistry, and many other practical fields 10).
Partition coefficient was determined by the shake-flask method in octan-1-ol/phosphate medium. The log P values of the compounds increased with rising molecular weight of the substances. Degression was found in the substance with the working label 5d2. The values of log P are given in Table 4.
Logarithm of the retention factor k, like the lipophilicity parameter, was obtained by HPLC method. The results show that the log k values of evaluated compounds increase with the increasing number of carbon atoms in the alkoxy chain of the carbamoyl group. The values of log k are summarized in Table 4.
The RM values obtained from reversed-phase thin layer chromatography increased with the side alkyl chain elongation (Table 4).
This study was supported by the Comenius University grant No. UK/2/2008.
Received 2 Juny / Accepted 26 Juny 2008
Address for correspondence:
PharmDr. Simona Kečkéšová
Comenius University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry
Odbojárov 10, 832 32 Bratislava, SR
e-mail: skeckesova@yahoo.co.uk
Zdroje
1. Bartošová, L. et al.: Pharmazie, 2003; 58, 841-842.
2. Mokrý, P. et al.: Pharmazie, 2003; 58, 18-21.
3. Kam, S.T. et al.: J. Med. Chem., 1984; 27, 1007-1016.
4. Pharmacopea Slovaca PhS 1, 1st Edition (in Slovak). Bratislava, Herba 1999, p. 22.
5. Malík, I. et al.: Čes. Slov. Farm., 2005; 54, 235–239.
6. Sedlárová, E. et al.: Farm. Obzor, 2007; 76, 86-89.
7. Malík, I. et al.: Farm. Obzor , 2005; 74, 211-215.
8. Malík, I. et al.: Chem. Pap., 2006; 60, 42-47.
9. Sedlárová, E. et al.: Čes. a Slov. Farm., 2000; 49, 306-312.
10. SČrbu, C. et al.: Talanta, 2008; 75, 651-657.
Štítky
Farmacie Farmakologie
Článek UPOZORNĚNÍ
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2008 Číslo 4- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Zpráva o činnosti České farmaceutické společnosti ČLS J. E. Purkyně za období VII. 2004 až IV. 2008(přednesena 17. května 2008 na 10. sjezdu České farmaceutické společnosti v Olomouci)
- Actual acidity of the environment and efficacy of auxiliary substances used for antimicrobial stabilization of medicinal preparations prepared in pharmacies
- Synthesis, identification and physicochemical properties of novel potential ultrashort-acting beta-adrenergic blockers
- Characterization of microcrystalline celluloses by means of the parameters of a three-exponential compression equation
- Rada vlády ČR pro výzkum a vývoj
- A study of the properties of tablets from two types of directly compressible xylitol
- Nový způsob aplikace (IONSYS) Fentanylu transdermálním systémem
- Antiradical activity of substances with a potential effect on the cardiovascular system
- Monitoring of pharmacotherapy in seniors of rest homes in Brno region
- Z činnosti farmaceutických společností
- Prof. MUDr. Jaroslav Dršata, CSc. – 65 let
- UPOZORNĚNÍ
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Prof. MUDr. Jaroslav Dršata, CSc. – 65 let
- Actual acidity of the environment and efficacy of auxiliary substances used for antimicrobial stabilization of medicinal preparations prepared in pharmacies
- Monitoring of pharmacotherapy in seniors of rest homes in Brno region
- Z činnosti farmaceutických společností
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



