-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The gut microbiome in human immunodeficiency virus infection
HIV/AIDS causes severe dysfunction of the immune system through CD4+ T cell depletion, leading to dysregulation of both the adaptive and innate immune arms. A primary target for viral infection is the gastrointestinal tract, which is a reservoir of CD4+ T cells. In addition to being a major immune hub, the human gastrointestinal tract harbors trillions of commensal microorganisms, the microbiota, which have recently been shown to play critical roles in health. Alterations in the composition and function of microbiota have been implicated in a variety of ‘multi-factorial’ disorders, including infectious, autoimmune, metabolic, and neoplastic disorders. It is widely accepted that, in addition to its direct role in altering the gastrointestinal CD4+ T cell compartment, HIV infection is characterized by gut microbiota compositional and functional changes. Herein, we review such alterations and discuss their potential local and systemic effects on the HIV-positive host, as well as potential roles of novel microbiota-targeting treatments in modulating HIV progression and associated adverse systemic manifestations.
Keywords:
Microbiota, Dysbiosi,s Gastrointestinal tract, AIDS, HIV, Anti-retroviral therapy, CD4+ T cells
Authors: Gili Zilberman-Schapira† 1; Niv Zmora† 1; Shlomik Itav† 1; Stavros Bashiardes† 1; Hila Elinav 2*; Eran Elinav 1*
Authors place of work: Department of Immunology, Weizmann Institute of Science, 34 Herzl Street, Rehovot 76100, Israel 1; Hadassah AIDS Center, Department of Clinical Microbiology and Infectious Diseases, Hadassah-Hebrew University Medical Center, Jerusalem 91120, Israel 2
Published in the journal: BMC Medicine 2016, 14:83
Category: Minireview
doi: https://doi.org/10.1186/s12916-016-0625-3© 2016 Zilberman-Schapira et al. Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
The electronic version of this article is the complete one and can be found online at: https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-016-0625-3Summary
HIV/AIDS causes severe dysfunction of the immune system through CD4+ T cell depletion, leading to dysregulation of both the adaptive and innate immune arms. A primary target for viral infection is the gastrointestinal tract, which is a reservoir of CD4+ T cells. In addition to being a major immune hub, the human gastrointestinal tract harbors trillions of commensal microorganisms, the microbiota, which have recently been shown to play critical roles in health. Alterations in the composition and function of microbiota have been implicated in a variety of ‘multi-factorial’ disorders, including infectious, autoimmune, metabolic, and neoplastic disorders. It is widely accepted that, in addition to its direct role in altering the gastrointestinal CD4+ T cell compartment, HIV infection is characterized by gut microbiota compositional and functional changes. Herein, we review such alterations and discuss their potential local and systemic effects on the HIV-positive host, as well as potential roles of novel microbiota-targeting treatments in modulating HIV progression and associated adverse systemic manifestations.
Keywords:
Microbiota, Dysbiosi,s Gastrointestinal tract, AIDS, HIV, Anti-retroviral therapy, CD4+ T cellsBackground
Since the emergence of the acquired immunodeficiency syndrome (AIDS) epidemic in the early 1980s and the discovery of its causative agent, the human immunodeficiency virus (HIV), the deleterious effects of HIV infection on the human immune system, and mainly on the CD4+ T cell compartment, have been extensively studied. Upon the advent of highly active antiretroviral therapy (HAART), the burden of HIV-related morbidity and mortality dropped dramatically, although complete elimination of the disease has not yet been achieved. Additionally, effective antiretroviral therapy (ART) is accompanied by the emergence of long-term HIV - and treatment-related metabolic manifestations, suggesting that significant gaps remain in our comprehension of the mechanisms by which the virus exerts adverse effects on its host. Considering that 60 % of CD4+ T cells in the human body are estimated to reside in gut-associated lymphoid tissue, and since the reconstitution of these cell populations and the gut microbial composition is incomplete even under HAART, the human intestine has recently become the focus of attention in HIV research. Moreover, changes in gut microbial composition and function in HIV-positive individuals, aside from being secondary to HIV infection, may also play direct roles in mediating some disease manifestations. Potential mechanisms for such effects include local facilitation of viral infection, creation of viral sanctuary sites resistant to systemic ART treatment, and promotion of impaired gut mucosal barrier function, resulting in leakage of bacterial components into the main circulation. These mechanisms may potentially contribute to the long-term immune, infectious, metabolic, and neoplastic manifestations of HIV infection, collectively termed the ‘inflamm-aging’ of HIV. Herein, we focus on the intricate interactions between HIV, host gastrointestinal tract, and gut microbiota, and highlight how these interactions may contribute to immune and non-immune consequences on the host.
Immunological aspects of intestinal HIV infection
HIV infection is characterized by three, partially overlapping clinical stages – an acute phase, a chronic latency phase and a late advanced stage – during which clinical manifestations related to host immune deficiency emerge [1]. CD4+ T cell depletion characterizes all stages of the disease. The driving force behind the gradual CD4+ T cell depletion is chronic systemic immune activation, which increases co-receptor expression and allows viral cell entry into previously uninfected T cells [2], thus leading to cell death through a viral cytopathic effect and cytotoxic T cell attack of infected cells [3]. Latent HIV infection involves features of immune activation and dysregulation, which may be useful in complementing direct viral load measurements in assessing a patient's clinical status [4]. Late HIV stage is characterized by an increase in viral load and a decrease in circulating CD4+ T cell levels, typically to below 200 cell/mm3, as well as by the emergence of multiple opportunistic infections and immune deficiency-related neoplastic and neuro-degenerative disorders [5]. Over the past two decades, combined ART treatment has succeeded in reducing plasma viral loads to undetectable concentrations whilst restoring CD4+ T cell levels in peripheral blood, thus dramatically reducing – though not eliminating – the prevalence of an AIDS-related pathology in treated patients. A detailed description of the clinical course of HIV and AIDS is concisely described elsewhere [6].
Studies focusing on CD4+ T cell depletion and its direct contribution to HIV-related immune deficiency mostly assess easily accessible peripheral blood samples [7, 8, 9]. However, the initial and most pronounced depletion of CD4+ T cells commonly occurs in the gastrointestinal mucosa during the acute phase of HIV infection [10], partially due to the high concentration of CCR5 co-receptor-expressing CD4+ T cells within the gut, allowing HIV entry and replication [9]. CD4+ T cell depletion in the acute phase of HIV infection is less pronounced in peripheral blood compared to the gastrointestinal tract, possibly stemming from the presence of CCR5-low CD4+ T cell populations within the peripheral blood compartment [9]. Infective simian immunodeficiency virus models have indicated that this rapid intestinal CD4+ T cell depletion occurs within as little as 7 days post-infection [11].
Chronic HIV infection within the gastrointestinal tract, as well as the closely associated secondary reduction in CD4+ T cells, significantly affect gut physiology. Whilst allowing the digestion and absorption of luminal nutrients into the host, epithelial cells in the mucosal epithelia of the gastrointestinal tract simultaneously prevent the activation of a systemic immune response triggered by commensal microorganisms [12]. HIV infection leads to a dysregulation of the gastrointestinal immune-epithelial network [12]; early in acute HIV infection, mucosal infiltration with activated memory CD4+ and CD8+ T cells leads to apoptosis and impaired barrier function [13], mediated by tight junction disruption through the secretion and activation of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-8 [14]. Further, gut HIV infection causes depletion and dysfunction of key resident immune populations, such as Th17 and CD103+ dendritic cells, in addition to CD4+ T cell depletion [12]. HIV infection may also lead to lower levels of IgA, possibly contributing to HIV-associated enhanced microbial translocation, which may lead, in turn, to a chronic state of immune activation as noted in many HIV carriers [15]. Enhanced gut microbial translocation is exemplified by an elevation in plasma systemic lipopolysaccharide (LPS) levels in HIV-positive patients [16] Elevated LPS levels have also been correlated with activated CD8+ T cell numbers [16] and have been suggested to drive chronic stimulation of monocytes.
During progression to AIDS, further immune dysfunction, at both the local gut and systemic levels, results in a marked propensity to develop infectious diarrhea in up to 90 % of patients [17]. AIDS-associated gastrointestinal symptoms are driven by both ‘regular’ pathogens, such as Escherichia coli, Salmonella, andShigella, and opportunistic ‘pathobionts’, such as Cryptosporidium, Cytoisospora belli, Microsporidium,Cytomegalovirus, and Micobacterium avium-intracellulare [18, 19], all of which lead to severe morbidity and mortality [20]. In addition, treatment for HIV infection or its complications is often associated with a variety of gastrointestinal symptoms [21]. Thus, HIV infection has a profound effect on gut immune function as characterized by CD4+ T cell depletion and immune modulation.
HIV and the gut microbiota
The human gastrointestinal tract harbors a complex microbial ecosystem within its epithelial cell lining, with microorganisms outnumbering host cells by a factor of 10–100 [22]. As a normal gut microbiota is essential for immune homeostasis; disruptions in intestinal immunity can precipitate gut dysbiosis, which may in turn induce chronic inflammation in the mucosa and periphery. The latter (summarized in Table 1) is commonly observed in HIV-positive individuals [23, 24].
Tab. 1. Summarizing Microbiota in HIV Table 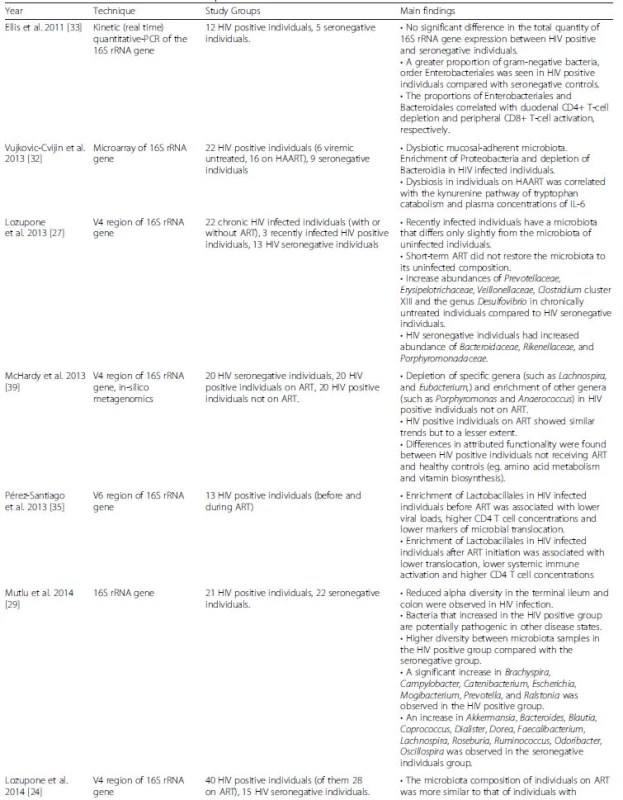

Sequencing of the highly conserved 16S rDNA gene, which features species-specific highly variable regions, enables the characterization of genus-, and at times, species-level relative microbiota compositions [25]. Emerging evidence from 16S rDNA sequencing of stool samples of HIV-negative, HIV-positive, and ART-treated infected individuals indicated that the gut microbiota differs between these populations [26, 27]. In the ART-treated population, while viral suppression was noted, the microbiota composition was not completely restored to its HIV negative state. Nevertheless, the exact microbiome configuration in HIV-positive individuals, ART-treated patients and healthy controls remain inconsistent between studies.
When considering bacterial composition as a whole, rather than focusing on specific taxa, a variety of studies identified different patterns of composition for each of the study groups (summarized in Fig. 1a). Lozupone et al. [27] demonstrated that HIV-negative and chronically infected HIV-positive individuals have distinguishable gut microbial compositions. One recurring finding is that the Prevotella genus is significantly enriched in HIV-positive gut microbiota compared with HIV-negative individuals; however, to date, the effects of this microbial expansion remain to be elucidated [27, 28, 29]. Nowak et al. [26] suggest that the distinct microbial compositions within HIV-positive individuals may stem from differences in HIV viral load. In a small cohort, they demonstrate that elite controllers, i.e., HIV-positive individuals with controlled viral load, have a microbial composition that is distinct from that of viremic patients, and overall more similar to healthy controls. Of note, some of the above studies utilized stool samples, which are known to be representative of the lumen microbiota composition. The mucosal microbiota may be more clinically relevant due to its close proximity to the host intestinal niche. Nevertheless, mucosal microbiota differs greatly from the luminal fraction, even within the same individual [30, 31]. It is therefore unsurprising that different patterns emerge when considering the mucosal microbiota of HIV-positive and HIV-negative individuals. Vujkovic-Cvijin et al. [32] investigated the changes in mucosal microbial composition in recto-sigmoid biopsy specimens from HIV-negative, HIV-positive, and ART-treated subjects using a bacterial 16S rRNA probe microarray; the microbiota composition of the mucosal-adherent bacteria was found to differ between HIV-positive and HIV-negative individuals. Exploring other regions of the gut, Mutlu et al. [29] found that the different compositional patterns between HIV-positive and HIV-negative individuals were also present in samples obtained from the ileum and colon. Specifically, the HIV-positive terminal ileum and the colon featured reduced species richness (i.e., alpha diversity), while the luminal microbiota featured less pronounced differences. Dillon et al. [28] also investigated alterations in the colonic mucosal microbiota between HIV-positive individuals and healthy controls. Within the ten most abundant bacterial families, they found a significant increase in the Prevotellaceae and Ruminococcaceae families and a significant decrease in the Becteroidaceae and Lachnospiraceae families in HIV-positive individuals versus healthy controls. Elliset al. [33] applied a slightly different approach of quantitative PCR of the 16S rRNA region. In this study the total quantity of universal 16S rRNA did not differ between the HIV positive and the control groups, however, a trend for a higher proportion of the gram-negative bacteria order Enterobacteriales in the HIV-positive group compared to control was demonstrated.
Fig. 1. Gut microbiota alterations during HIV infection and their potential effects on the host. a. In different studies, distinct gut microbiome compositions have been identified in HIV infected individuals with or without ART, as compared to healthy controls. Importantly, HIV-associated microbiome configurations vary between these studies. While ART dramatically lowers the viral load in infected individuals, gut microbiome composition is not fully restored to a healthy composition. ‘Elite controllers’ differ in their microbial composition from HIV- infected individuals and are more similar to healthy individuals. b. The characteristic HIV microbiota possibly contributes to some of the common HIV manifestations, including modification of the level if infectivity, occurrence of opportunistic infections, increased gut permeability and resultant bacteria and bacterial product translocation, increased immune activation and T cell polarization, metabolic complications and variability in the response to HIV treatment 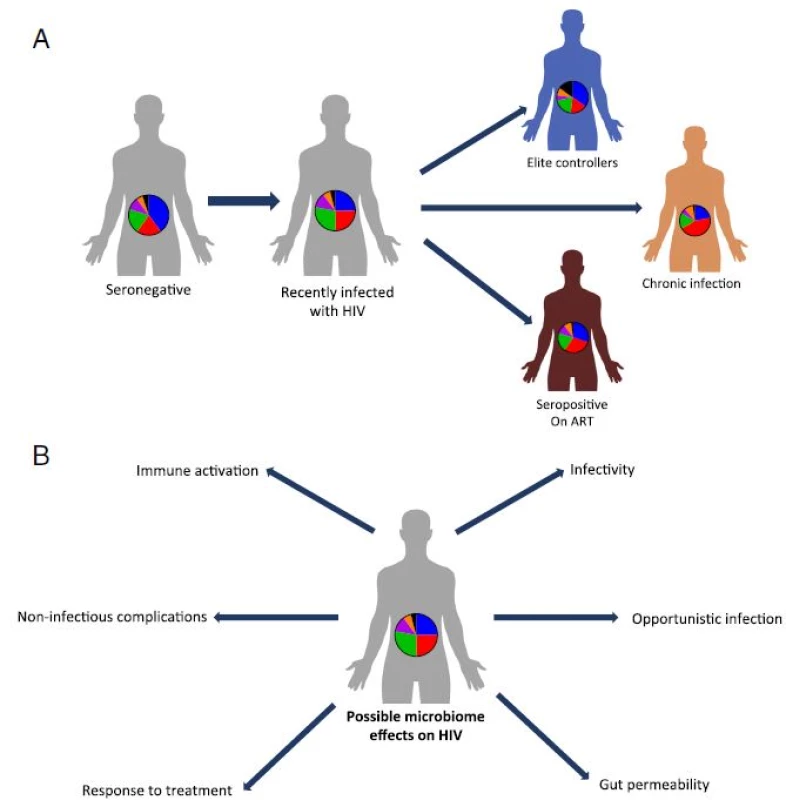
A more mechanistic understanding of these compositional changes has been provided by recent studies focusing on the association between disease progression and disease markers with specific bacterial taxa [27, 28]. Nevertheless, to date, CD4+ T cell count remains the primary laboratory marker used in the follow-up of disease progression in HIV [34]. The bacterial order Lactobacillales was found to be positively correlated with CD4+ T cell count, a correlation that persisted even after initiation of ART [35], while the abundance of the Bacteroides genus trended towards a negative correlation with CD4+ T cell count [28]. Another study indicated that higher CD4+ T cell counts positively correlate with higher bacterial diversity within a sample [26]. Recently, Monaco et al. [36] reported an association between immunodeficiency in HIV-positive individuals and alterations in the gut bacterial and viral component of the microbiome [36]. Low peripheral T cell counts were associated with an overall reduced phylogenetic diversity and richness in the gut bacterial populations, while there was an increase in abundance of specific bacteria such as Enterobacteriaceae, which have been associated with inflammation and may be a contributing factor in AIDS-related enteropathy [36]. Of note, some studies did not identify any correlation between specific taxa and CD4+ T cell counts [29, 32]. Several bacterial genera were found in lower abundances in HIV-positive individuals and correlated positively with protective immune markers (e.g., low T cell activation) [28, 32]. In contrast, markers of disease progression (e.g., pro-inflammatory cytokine levels) were found to be correlated with bacteria genera enriched in HIV-positive individuals [32]. Other studies linked viral load, CD4+/CD8+ ratio, systemic biomarkers, cytokines, immune activation, bacterial translocation, and thymic function to gut bacterial abundance in different taxonomical levels [26, 28, 29, 35, 37].
Next generation sequencing-based characterization of the total microbial DNA content (whole genome shotgun sequencing) allows the reconstruction of genes, pathways, and modules of various microbiota configurations and enables the characterization of the functional basis of microbiota dysfunction noted in HIV-infected individuals. By applying this method, Vázquez-Castellanos et al. [37] began to unravel the metabolic pathway alterations taking place in the dysbiotic environment and their association with immune activation and inflammation as observed in HIV-infected patients. One interesting finding was the LPS biosynthesis pathway, which was found to be enriched in the HIV-positive group and to be a discriminative factor between the HIV-positive and HIV-negative groups [37]. Moreover, they demonstrated that an increased prevalence of LPS biosynthesis genes is associated with a decrease in strain diversity and an increase in the proportion of gram-negative bacterial presence. Interestingly, an increase in plasma LPS concentration is an established marker of HIV infection progression, and LPS is a known immune-stimulant, possibly contributing to systemic immune activation in infected patients [38]. McHardy et al. [39] addressed the issue of changes in intestinal mucosal bacterial composition occurring during HIV infection and subsequently following ART by combining 16S rRNA sequencing with in-sillico methods to infer the functionality of the bacterial populations. By applying PICRUSt [40] and HUMAnN [41] to the 16S rRNA sequencing data they were able to infer not only changes in composition, but also to reveal changes in metabolic functional potential in pathways involving, among others, amino acid metabolism and vitamin biosynthesis, suggesting alterations in nutrient availability between the study groups.
Further, in a principal coordinate analysis performed in Vázquez-Castellanos et al. [37], HIV-positive versus control individuals depicted clear and statistically significant differences, highlighting a typical and specific microbiota composition in the HIV-positive as compared to the non-infected control group; these differences stem mainly from enrichment in Prevotella and Succinivibrio in the HIV-positive group, and Bacteroides and Faecalibacterium in healthy controls. When performing a correlation analysis between these typical compositions (namely the first principal component) and immunological markers of disease progression, some statistically significant correlations were identified. Specifically, the first principal component positively correlated HIV microbiota changes with C-reactive protein (an inflammation marker) levels and with markers of T cell activation such as CD4 + HLA–DR + CD38+, CD4 + CD25+, CD8 + HLA–DR + CD38+, and CD8 + CD38+ T cell percentages. While causality was not demonstrated in these associations, they may potentially point towards involvement of microbiota dysbiosis in immune alteration in HIV [37]. Mechanistic determination of potential causality between microbiota commensal changes and these immune phenomena merit further study.
Collectively, these preliminary studies suggest that HIV-driven interference to the delicate balance of gut mucosal immunity may cause a disruption of gastrointestinal tolerance, which may in turn promote dysbiosis in HIV-positive individuals. Dysbiosis can result in activation of immune cells and promotion of bacterial translocation leading to systemic inflammation, a hallmark of chronic HIV infection (Fig. 1b).
Mucosal dysbiosis as a risk factor for HIV acquisition
The mucosal barrier confers effective protection against sexual transmission of HIV; indeed, only 0.1 % of unprotected receptive vaginal intercourses and 1.4–1.7 % of unprotected receptive anal intercourses result in the acquisition of the virus [42]. However, the presence of mucosal inflammation and immune activation increases the risk for HIV transmission [42, 43]; it is believed that tissue-residing bacteria may affect human mucosal immune function, which may modulate this risk [44]. An example of the effects of the microbiota–immune interface on HIV infectivity resides in vaginal microbiota, where dysbiosis (termed bacterial vaginosis) is suggested to enhance HIV infectivity, as observed in the marked increase in susceptibility for HIV acquisition and transmission in bacterial vaginosis patients [44]. This enhanced infectivity is associated with elevated pro-inflammatory cytokine concentrations, especially IL-1β, and CCR5 + CD4+ cell activation and recruitment in the female genital tract mucosa [45, 46, 47]. In contrast, an increase in the relative abundance of Lactobacilli, an important member of the ‘healthy’ indigenous vaginal microbiota, induces an anti-inflammatory state contributing to the relative resistance to HIV infection [48] Indeed, a recent study by Anathar et al. [47] categorized vaginal microbiota profiles (cervicotypes) to four distinct bacterial community structures. Lactobacillus crispatus-rich vaginal microbiota were found to manifest the lowest inflammation, while highly diverse communities, namely Prevotella-containing and to a lesser extent Gardnerella-dominant microbiota, correlated with multiple pro-inflammatory mucosal cytokines even in the absence of overt sexually transmitted diseases. Notably, similar to what has been previously described for the gut, successful HAART with serum viral load suppression does not fully attenuate HIV-induced immune activation in the female genital tract [49]. Intriguingly, semen dysbiosis was found to be correlated with pro-inflammatory semen cytokines and elevated HIV viral load in the semen, which implies that male genital tract dysbiosis may also play a role in HIV sexual transmission [50]. These noted associations between male and female reproductive microbial changes in the mucosa and the risk for HIV acquisition remain to be further explored, and will constitute an exciting future avenue in HIV research. Potentially, understanding these microbial effects on HIV transmissibility may assist in developing novel microbiota-associated HIV prevention strategies.
Non-immune HIV manifestations and possible links to dysbiosis
The emergence of HAART rendered HIV infection a chronic disease. Nevertheless, despite viral load suppression and immune reconstitution, infected individuals still encounter a reduced life expectancy and increased morbidity and mortality. The chronicity of HIV introduced a new challenge to its follow-up and treatment, as chronically infected patients feature a variety of non-AIDS disorders associated with long-term viral infection, ART, immune dysregulation, and, potentially, microbiota dysbiosis [51]. Chronic HIV infection is characterized by enhanced atherosclerosis, leading to a propensity to develop cardiovascular diseases, including myocardial infarction, cerebrovascular disease [52], and peripheral vascular disease [53]. It has been demonstrated that the intestinal microbiota of HIV-negative subjects featuring increased metabolism of choline into trimethylamine (TMA), which is further oxidized to trimethylamine-N-oxide, may carry atherogenic potential [54]. Interestingly, a recent study showed that HIV-positive patients exhibit worsened calcified coronary plaque features compared to non-infected individuals, as visualized by cardiac computed tomography angiography [55] and that these radiologic features were correlated to serum TMA and LPS levels, potentially linking microbiota to these systemic disease risks [55]. Similarly, another recent study showed that serum trimethylamine-N-oxide levels were associated with silent ischemia in HIV-positive individuals [56]. HIV-positive patients exhibit immune activation leading to atherosclerosis even after the initiation of HAART [57]. It has been found that common carotid intima media thickness measured by ultrasonography correlated with sCD14 and IL-6 levels in serum. Additionally, it was implied that altered tryptophan catabolism could account for the increased cardiovascular disease risk, as the kynurenine-to-tryptophan ratio was elevated in patients who exhibited enhanced atherosclerosis. Tryptophan catabolism was shown to be associated with cardiovascular disease risk in other settings and to be indicative of poor immune reconstitution and of all-cause mortality in HIV-positive patients [32, 58], regardless of treatment status. Since this pathway is activated in response to interferons and bacterial products, dysbiosis may play a pivotal role in its alteration [59]. Collectively, these findings suggest that altered intestinal microbiota profiles may potentially contribute to cardiovascular disease in HIV-positive patients. This dysbiosis could be ascribed to enrichment in Prevotella [60], which possess TMA-production capabilities known to be increased in HIV-positive individuals [27], or depletion of species, such as Akkermansia, found to be protective against metabolic disorders [29, 61]. Additionally, a Proteobacteria-enriched and Bacteroidia-depleted mucosal-adherent colonic bacterial profile was proposed to stimulate kynurenine pathways of tryptophan catabolism and was associated with chronic inflammation and disease progression in HIV-infected individuals [32]. Furthermore, microbial translocation-induced systemic endotoxemia was found to trigger obesity, insulin resistance, diabetes mellitus, hypertension, dyslipidemia, atherosclerosis, and endothelial dysfunction, which may account for the increased cardiovascular risk seen in HIV-positive individuals [53].
Lipodystrophy, a metabolic disorder visually characterized by impaired distribution of body fat, is commonly seen in HIV-positive individuals. Increases in Prevotella and decreases in Bacteroides species in these patients were suggested to contribute to metabolic diseases, especially when consuming a high-fat diet [24, 27]. Other non-immune pathologies associated with HIV infection and which may be related to dysbiosis include hepatic damage due to the translocation of microbial products passing through the portal circulation [62]; HIV-associated ‘wasting syndrome’, characterized by malabsorption, low weight gain, and steatorrhea, and suggested to result from an abnormal interplay between the intestinal microbiota, an impaired immune response, and a dysfunctional gut epithelium leading to decreased metabolic activity [63]; and oral candidiasis, a common manifestation in advanced stages of HIV, resulting from dysregulation in quorum-sensing between oral bacterial and fungal microbiota [64]. Additionally, it has been suggested that the gut microbiota may modulate HIV latency and promote progression of AIDS by regulating the epigenetic status of integrated proviral DNA, an effect that could be mediated by butyric acid-producing bacteria [65]. All of these HIV manifestations, potentially modulated by microbiota changes, merit further mechanistic studies. As microbiota research progresses, the impact of gut bacteria on other extra-intestinal phenomena may be also unraveled, and missing links in the interplay between the host immune system and the virus may be found to be orchestrated or mediated by bacterial functions.
Probiotics and future treatments
The abovementioned interactions between the HIV virus, the host gastrointestinal tract epithelium, and the microbiota have led to the development of treatments targeting microbiota-related manifestations or modifying the gut microbiota, focusing on oral or vaginal probiotic supplementation and aiming to improve disease status. Probiotic supplementation is expected to have an effect on CD4+ count, HIV infection manifestations such as diarrhea, weight loss, and cardiovascular diseases, or in the reversal of failure to thrive (a common HIV manifestation in resource-poor countries) in HIV-positive children, which remains a major concern in the ART era [66]. Trois et al. [67] assessed CD4+ count in 77 HIV1-infected children supplemented with either a Bifidobacterium bifidum - and Streptococcus thermophilus-enriched formula or standard formula over a 2-month period, and observed that, following the supplementation period, the mean CD4+ T cell count increased by 118 cells/mm3 to 791 cells/mm3 in the probiotic group, while it decreased by 42 cells/mm3 to 538 cells/mm3 in the control group. Similarly, Cunningham-Rundles et al. [66] assessed the progress of 14 children of them 12 diagnosed with failure to thrive prior to and following ART treatment for at least 1 month with subsequent Lactobacillus plantarum 299v (Lp299v) administration compared to controls, and demonstrated that improvement in weight was significant when comparing the pre - and post-treatment periods. However, these results may also stem from the recurrent weight loss observed in the non-responder group.
While ART treatment has increased the life expectancy of HIV-positive individuals, chronic inflammation remains central in HIV pathogenesis and morbidity. A recent study [68] followed 20 HIV-positive combined ART-treated individuals that consumed a probiotic supplement consisting of Streptococcus, Bifidobacteria, and Lactobacillus twice daily for 48 weeks. A variety of immune activation markers (e.g., CD4+, CD8+, CD38+, and HLA-DR+ lymphocyte counts and high-sensitivity C-reactive protein) were evaluated before and after the probiotic consumption period and were compared to those of healthy controls that did not consume any probiotics. After 48 weeks of probiotics consumption, many of the immune activation markers were significantly reduced to a level that was comparable to the marker level in the control group, including a moderate, non-significant increase in the CD4+ T cell count and a significant reduction in CD4 + CD38 + HLA-DR+ and CD8 + CD38 + HLA-DR+ T cell percentages. While IL-6 and C-reactive protein levels did not differ before and after probiotic treatment, the high-sensitivity C-reactive protein was found to significantly decrease post-treatment values compared with those prior to treatment. These findings suggest that long-term probiotics consumption may reduce some of the HIV inflammatory markers, potentially improving the chronic inflammation associated with chronic disease and ART treatment in HIV-positive individuals.
Conclusions
Since the introduction of HAART two decades ago, the characteristics of the HIV epidemic have changed considerably, with a dramatic reduction noted in both morbidity and mortality coupled with the emergence of new chronic clinical manifestations and treatment-associated complications. While the control of HIV infection and its related morbidity remains a major challenge in many regions of the world, treatment of HIV-positive individuals in developed regions is shifting towards the long-term control of multiple co-morbidities associated with chronic HIV infection and treatment. Newly emerging chronic manifestations of HIV include a number of metabolic disorders such as cardiovascular disease, chronic hepatic and renal abnormalities, non-HIV defining cancers, osteoporosis, and growth abnormalities in young children. To date, it is well accepted that inflammation and immune dysfunction are the main inducers of these non-AIDS-associated age-related clinical manifestations [51, 62]. Nevertheless, much remains to be explored on the roles that microbiota play in HIV infectivity, persistence, and drug responsiveness. Equally interesting are the effects of microbiota in the chronic manifestations of HIV. An understanding of these roles and effects, as well as their molecular mechanisms, may offer new insights into HIV biology and potentially introduce new microbiota-associated therapeutic targets to HIV infection and its associated co-morbidities.
Authors’ contributions
GZS, NZ, SI, and SB reviewed the existing literature and drafted the manuscript. HE and EE edited the manuscript and supervised the review process. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Received: 4 December 2015
Accepted: 10 May 2016
Published: 3 June 2016* Correspondence:
Hila Elinav
2Hadassah AIDS Center
Department of Clinical Microbiology and Infectious Diseases
Hadassah-Hebrew University Medical Center
Jerusalem 91120, Israelhilaelinav@gmail.com
Eran Elinav
1Department of Immunology
Weizmann Institute of Science
234 Herzl Street
Rehovot 76100, Israeleran.elinav@weizmann.ac.il
Zdroje
1. Fauci AS. HIV and AIDS: 20 years of science. Nat Med. 2003;9(7):839–43.
2. Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254(1):78–101.
3. Gadhamsetty S, Beltman JBB, de Boer RJJ. What do mathematical models tell us about killing rates during HIV-1 infection? Immunol Lett. 2015;168(1):1–6.
4. Ipp H, Zemlin AE, Erasmus RT, Glashoff RH. Role of inflammation in HIV-1 disease progression and prognosis. Crit Rev Clin Lab Sci. 2014;51(2):98–111.
5. Hernandez-Vargas EA, Middleton RH. Modeling the three stages in HIV infection. J Theor Biol. 2013;320 : 33–40.
6. Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet Elsevier Ltd. 2014;384(9939):258–71.
7. Cooper A, García M, Petrovas C, Yamamoto T, Koup RA, Nabel GJ. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature. 2013;498(7454):376–9.
8. Doitsh G, Galloway NLK, Geng X, Yang Z, Monroe KM, Zepeda O, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2013;505(7484):509–14.
9. Parmentier M. CCR5 and HIV Infection, a View from Brussels. Front Immunol. 2015;6 : 295.
10. Brenchley JM. CD4+ T Cell Depletion during all Stages of HIV Disease Occurs Predominantly in the Gastrointestinal Tract. J Exp Med. 2004;200(6):749–59.
11. Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26(1):2–18.
12. Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21(1):6–13.
13. Epple H, Allers K, Tröger H, Kühl A, Erben U, Fromm M, et al. Acute HIV Infection Induces Mucosal Infiltration With CD4+ and CD8+ T Cells, Epithelial Apoptosis, and a Mucosal Barrier Defect. Gastroenterology. 2010; 139(4):1289–300.e2. Elsevier Inc.
14. Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(4):e1000852.
15. Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9(4):235–45.
16. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71.
17. Bhaijee F, Subramony C, Tang S-J, Pepper DJ. Human immunodeficiency virus-associated gastrointestinal disease: common endoscopic biopsy diagnoses. Patholog Res Int. 2011;2011 : 247923.
18. Cabada MM, White AC. Treatment of cryptosporidiosis: do we know what we think we know? Curr Opin Infect Dis. 2010;23(5):494–9.
19. Sun H-Y, Chen M-Y, Wu M-S, Hsieh S-M, Fang C-T, Hung C-C, et al. Endoscopic appearance of GI mycobacteriosis caused by the Mycobacterium avium complex in a patient with AIDS: case report and review. Gastrointest Endosc. 2005;61(6):775–9.
20. Sharpstone D, Gazzard B. Gastrointestinal manifestations of HIV infection. Lancet (London, England). 1996;348(9024):379–83.
21. Montessori V, Press N, Harris M, Akagi L, Montaner JSG. Adverse effects of antiretroviral therapy for HIV infection. Can Med Assoc J. 2004;170(2):229–38.
22. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7.
23. Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, et al. The Intestinal Microbiota, Microbial Translocation and Systemic Inflammation in Chronic HIV Infection. J Infect Dis. 2014;211 : 19–27.
24. Lozupone CA, Rhodes ME, Neff CP, Fontenot AP, Campbell TB, Palmer BE. HIV-induced alteration in gut microbiota: driving factors, consequences, and effects of antiretroviral therapy. Gut Microbes. 2014;5(4):562–70.
25. Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008; 57(11):1605–15.
26. Nowak P, Troseid M, Avershina E, Barqasho B, Neogi U, Holm K, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. Aids. 2015;29 : 1.
27. Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14(3):329–39.
28. Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014; 7(4):983–94.
29. Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2):e1003829.
30. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8.
31. Nava GM, Friedrichsen HJ, Stappenbeck TS. Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J. 2011;5(4):627–38.
32. Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5(193):193ra91.
33. Ellis CL, Ma Z-M, Mann SK, Li C-S, Wu J, Knight TH, et al. Molecular characterization of stool microbiota in HIV-infected subjects by panbacterial and order-level 16S ribosomal DNA (rDNA) quantification and correlations with immune activation. J Acquir Immune Defic Syndr. 2011;57(5):363–70.
34. Olubajo B, Mitchell-Fearon K, Ogunmoroti O. A Comparative Systematic Review of the Optimal CD4 Cell Count Threshold for HIV Treatment Initiation. Interdiscip Perspect Infect Dis. 2014;2014 : 625670.
35. Pérez-Santiago J, Gianella S, Massanella M, Spina CA, Karris MY, Var SR, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27(12):1921–31.
36. Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, et al. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell Host Microbe. 2016;19(3):311–22.
37. Vázquez-Castellanos JF, Serrano-Villar S, Latorre A, Artacho A, Ferrús ML, Madrid N, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2014;8(4):760–72.
38. Takeda K, Kaisho T, Akira S. Toll-Like Receptors. Annu Rev Immunol. 2003; 21(1):335–76.
39. McHardy IH, Li X, Tong M, Ruegger P, Jacobs J, Borneman J, et al. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 2013;1(1):26.
40. Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–21. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.
41. Abubucker S, Segata N, Goll J, Schubert AM, Izard J, Cantarel BL, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput Biol. 2012;8(6):e1002358.
42. Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol Elsevier Ltd. 2015;36 : 22–30.
43. Hirbod T, Kong X, Kigozi G, Ndyanabo A, Serwadda D, Prodger JL, et al. HIV acquisition is associated with increased antimicrobial peptides and reduced HIV neutralizing IgA in the foreskin prepuce of uncircumcised men. PLoS Pathog. 2014;10(10):e1004416.
44. Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PLoS Med. 2012;9(6):e1001251.
45. Marconi C, Santos-Greatti MMV, Parada CMGL, Pontes A, Pontes AG, Giraldo PC, et al. Cervicovaginal levels of proinflammatory cytokines are increased during chlamydial infection in bacterial vaginosis but not in lactobacillidominated flora. J Low Genit Tract Dis. 2014;18(3):261–5.
46. Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a crosssectional study. Sex Transm Infect. 2014;90(8):580–7.
47. Anahtar MNN, Byrne EHH, Doherty KEE, Bowman BAA, Yamamoto HSS, Soumillon M, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5): 965–76. Elsevier Inc.
48. Nunn KL, Wang Y, Harit D, Humphrys MS, Ma B, Cone R, et al. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus -Dominant Microbiota. MBio. 2015;6(5):1–9.
49. Kyongo JK, Crucitti T, Menten J, Hardy L, Cools P, Michiels J, et al. Cross-Sectional Analysis of Selected Genital Tract Immunological Markers and
Molecular Vaginal Microbiota in Sub-Saharan African Women, with Relevance to HIV Risk and Prevention. Clin Vaccine Immunol. 2015;22(5):526–38.
50. Liu CM, Osborne BJW, Hungate B a, Shahabi K, Huibner S, Lester R, et al. The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLoS Pathog. 2014;10(7):e1004262.
51. Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–33.
52. Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–8.
53. Trøseid M, Manner IW, Pedersen KK, Haissman JM, Kvale D, Nielsen SD. Microbial translocation and cardiometabolic risk factors in HIV infection. AIDS Res Hum Retroviruses. 2014;30(6):514–22.
54. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63.
55. Srinivasa S, Fitch KV, Lo J, Kadar H, Knight R, Wong K, et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiotagenerated trimethylamine. AIDS. 2015;29 : 443–52.
56. Haissman JM, Knudsen A, Hoel H. KjÆr A. Berge RK, et al. Microbiotadependent marker TMAO is elevated in silent ischemia but is not associated with first-time myocardial infarction in HIV infection. J Acquir Immune Defic Syndr: Kristoffersen US; 2015.
57. Siedner MJ, Kim J-H, Nakku RS, Bibangambah P, Hemphill L, Triant VA, et al. Persistent Immune Activation and Carotid Atherosclerosis in HIV-Infected Ugandans Receiving Antiretroviral Therapy. J Infect Dis. 2016;213(3):370–8.
58. Byakwaga H, Boum Y, Huang Y, Muzoora C, Kembabazi A, Weiser SD, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;210(3):383–91.
59. Mehraj V, Routy J-P. Tryptophan Catabolism in Chronic Viral Infections: Handling Uninvited Guests. Int J Tryptophan Res. 2015;8 : 41–8.
60. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85.
61. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Crosstalk
between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71.
62. Deeks SG, Tracy R, Douek DC. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity. 2013;39(4):633–45. Elsevier Inc.
63. Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17(12):1585–93.
64. Brown RE, Ghannoum MA, Mukherjee PK, Gillevet PM, Sikaroodi M. Quorum-Sensing Dysbiotic Shifts in the HIV-Infected Oral Metabiome. PLoS One. 2015;10(4):e0123880.
65. Victoriano AFB, Imai K, Okamoto T. Interaction between endogenous bacterial flora and latent HIV infection. Clin Vaccine Immunol. 2013;20(6):773–9.
66. Cunningham-Rundles S, Ahrné S, Johann-Liang R, Abuav R, Dunn-Navarra A-M, Grassey C, et al. Effect of Probiotic Bacteria on Microbial Host Defense, Growth, and Immune Function in Human Immunodeficiency Virus Type-1 Infection. Nutrients. 2011;3(12):1042–70.
67. Trois L, Cardoso EM, Miura E. Use of probiotics in HIV-infected children: a randomized double-blind controlled study. J Trop Pediatr. 2008;54(1):19–24.
68. d’Ettorre G, Ceccarelli G, Giustini N, Serafino S, Calantone N, De Girolamo G, et al. Probiotics Reduce Inflammation in Antiretroviral Treated, HIV-Infected Individuals: Results of the “Probio-HIV” Clinical Trial. PLoS One. 2015;10(9):e0137200.
Článek vyšel v časopiseBMC Medicine
Nejčtenější tento týden
2016 Číslo 83- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Alergie na antibiotika u žen s infekcemi močových cest − poznatky z průřezové studie z USA
- AUDIO: (Jak) je možné prodloužit si život?
- Koordinátoři onkologické péče zkrátí pacientům cestu systémem. Jak to bude fungovat v praxi?
- INFOGRAFIKA: Světový den boje proti rakovině... aneb jaké výzvy stojí před českou onkologií?
Nejčtenější v tomto čísle
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



