-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Biocompatibility of the human mesenchymal stem cells with bovine bone tissue at the cellular level in vitro
Authors: Trebuňová Marianna 1,2; Gromošová Sylvia 2; Bačenková Darina 2,3; Rosocha Ján 2,3; Živčák Jozef 1
Authors place of work: Department of Biomedical Engineering and Measurement, Technical University, Košice, Slovakia 1; Associated Tissue Bank of University Hospital of L. Pasteur, Košice, Slovakia 2; Associated Tissue Bank, Faculty of Medicine, Pavol Jozef Šafárik University, Košice, Slovakia 3
Published in the journal: Lékař a technika - Clinician and Technology No. 2, 2018, 48, 59-65
Category: Original research
Summary
Abstract
The purpose of this study was to investigate biocompatibility of the human mesenchymal stem cells (hMSCs) with bovine bone tissue at the cellular level in vitro. Phenotypic analysis of cells was made by flow cytometry. Cells were grown on the bone for 12 days. Metabolic activity of cells was assessed with the MTS assay. The growth data were used to calculate the population doubling times. The scanning electron microscopy was used to verify the attachment of cells on the bone surface. The results were analyzed by using ANOVA test. Immunophenotypic characteristics were positive for CD105, CD90, CD73, and negative for CD34, CD45. The growth curves of stem cells of the 1st and the 2nd passages for both media, with and without, bovine bone were constructed. The increase of approximately 60% of the doubling time for mesenchymal cells co-cultivated with bovine bone tissue was observed for both passages in comparison with the control. Our study confirmed that human mesenchymal stem cells are able to adhere to the bovine bone, even not being modified with bone-targeting elements. The proliferation rate and metabolic activity of cells co-cultivated with bone decrease in comparison with the control. Better survival was observed for cells of the 1st passage.
Keywords:
human mesenchymal stem cells, bovine bone tissue, biocompatibility, flow cytometry, MTS assay
Introduction
The tissue engineering as a rapidly growing research discipline evolved from the biomaterial development. This interdisciplinary field of research applies the principles of engineering to life sciences. The tissue engineering covers a broad range of therapeutic and diagnostic applications. The regeneration or reparation of damaged tissue is the main challenge in this field of investigation. Understanding the mechanisms of tissue regeneration is inevitable in healing (repairing) or replacement of tissue. In general, tissue engineering is the regeneration of biological tissue using cells and supporting structures and/or biomolecules [1]. The tissue engineering implies the use of convenient connection to scaffolds, cells, biologically active molecules for the manufacture of a functional tissue. Another object of interest is to assemble a functional design that renews, maintains or improves the damaged tissue or the whole organ [2].
The successful tissue engineering relies on several specific criteria. One of them is the choice of cell type. The decision about the cell source plays a pivotal role in the design strategy of the tissue engineering for clinical applications. Key concern is to get the valid amount of the cells. The cells must be able to integrate into the matrix (e.g. bovine bone). Using growth factors, critical signaling molecules that instruct cells during development, cells will start to replicate and create new tissue. As a step toward engineering tissue by cell-based technologies is the use of native progenitor cells such as embryonic or adult stem cells. A specific subtype of multipotent stem cells, mesenchymal stem cells (MSCs), belongs to the most promising and frequent research in the field of biomedical engineering and regenerative medicine.
The permanent presence of stem cells in the tissues can help in regeneration of tissues in therapy. MSCs differentiate along a specific lineage pathway, thus replacing the damaged tissue, and/or induce tissue repair by endogenous cells through the paracrine release of trophic factors. In this way, MSCs offer the possibility of spontaneous reactions of the body and create conditions for a differentiation of cells of healthy tissue that replace damaged ones [3].
The bone marrow harbors the population of MSCs that possesses the potential to differentiate into bone, cartilage and fat [4]. When MSCs injected into cardiac muscle, they acquire the phenotype of cardiac myoblasts [5]. These characteristics indicate that MSCs can be used as powerful tools in reconstructive medicine [6]. MSCs transplanted into the bone and cartilage defects are able to differentiate into osteoblasts and cartilage and can repair damaged tissue by newly synthesized bone or hyaline cartilage, respectively [7–9]. To date, MSCs from various species have been studied. The bovine experimental model is being widely used in experiments in vivo and in vitro, but there is limited information about the regenerative effect of human mesenchymal stem cells on bovine bone tissue.
The tissue engineering is also looking for suitable material for the bone transplants, which in combination with MSCs can rapidly repair the bone damage. There is a large number of candidates for a transplant on one side, but insufficient supply of tissues and organs from human donors on the other. Therefore, the aim of this study was the preliminary evaluation of survival of human mesenchymal stem cells on bovine bone tissue to understand the mechanisms of bone remodeling and repair. This study was performed on the basis of the hypotheses that bovine bone elicit differential stem cell response and may be osteoinductive, and that stem cells isolated from human bone marrow can adhere to bovine bone when seeded on this type of scaffold. This investigation is aimed at analyzing the potential of bovine bone to function as hMSCs carriers and to induce their osteogenic differentiation.
Materials and methods
Human mesenchymal stem cells were obtained from bone marrow. Bone marrow cells were isolated from the knee of patient during orthopedic surgery (Department of Trauma Surgery, Louis Pasteur University Hospital in Košice, Slovakia). The cells were donated by 68-year-old man after informed consent. The written informed consent was obtained from the patient in accordance with the national ethical guidelines. Bone marrow was transported to the laboratory of Associated Tissue Bank of Louis Pasteur University Hospital in Košice on ice in a portable cooling container. Mononuclear cells were isolated by loading bone marrow sample into the lysing solution, which is important for lysis of erythrocytes. After centrifugation of lysate at 1500 RPM for 20 minutes at room temperature, the hMSCs layer was removed from the interphase and washed twice with Phosphate Buffered Saline (PBS, Invitrogen, USA). Then they were seeded into uncoated T75 flask (Sarstedt, Germany) at a cell concentration of 1×105 cells per square centimeter. Cells were cultured in basic growth medium (KKM) consisting of alpha-minimum essential medium (α-MEM) (Lonza, Switzer-land) with 100 U/ml penicillin, 0.1 mg/ml streptomycin (Lonza, Switzerland), 2 mmol/l L‑glutamine (Lonza, Switzerland), 0.025 mg/ml amphotericin B (Lonza, Switzerland) with 15% (vol/vol) of pre-screened Fetal Bovine Serum. Cultures were maintained in a humidi-fied atmosphere with 5% CO2 at 37°C. After incubation for 3 days, the medium was changed for the first time, later as needed. After reaching approximately from 60% to 80% confluence, the adherent cells were detached by treatment with 0.05% (vol/vol) trypsin/1 mmol/l EDTA solution (Gibco, USA). For our experiments, only cells from the first and the second passages (P1, P2) were used.
Flow cytometry analysis
For evaluation of surface markers expression, cell suspension was incubated for 30 minutes with phycoerythrin-conjugated antibodies against following human antigens, CD105 clone 43A3 (BioLegend, USA), CD90-PE clone 5E10 (BD Biosciences, USA), CD73-PE clone AD2 (Miltenyi Biotec, Germany), which had to be positive, and also to the specific hematopoietic cell surface antigens, fluorescein isothiocyanate-conjugated antibodies against human antigens CD34 clone AC136 (Miltenyi Biotec, Germany), CD45-FITC clone 5B1 (Miltenyi Biotec, Germany), which had to be negative. Samples were analyzed with a FACS Calibur (BD Biosciences, USA) and the CellQuest software (BD Biosciences, USA).
Bovine bone tissue
Bovine bone tissue was obtained from University of Veterinary Medicine and Pharmacy in Košice. Bovine bone tissue was processed according to the standard essential laboratory procedures of Associated Tissue Bank of Louis Pasteur University Hospital in Košice. At first, bone was stripped of soft tissues and cartilage. The preparing samples for experimental purposes consisted in cutting the bone into matrices of 5×5×5 mm size. Matrices were partially deproteinized by 0.07 mol/l sodium phosphate and subsequently treated with the same solution for 16 hours at room temperature. After intensive washing with distilled water, the samples were being demineralized in 0.5 mol/l HCl for 2 hours at room temperature. After demineralization, the samples were equilibrated with distilled water and sterilized using chemical sterilization solution of 0.36 mol/l peracetic acid, 4.40 mol/l ethanol and distilled water. Chemical sterilization lasted 24 hours under vacuum (0.5–0.6 bar) at room temperature. After sterilization, the samples were equilibrated with sterile distilled water and saline under aseptic conditions.
Viable cell count and test
Proliferations of hMSCs were measured using standard colorimetric test. Briefly, cell suspensions containing 15×103 viable cells were cultivated in 96-well tissue culture plates (Sarstedt, Germany) in medium in a final volume of 200 μl in duplicates. The plate with bovine bone tissue was treated as the sample and the plate without bone tissue as the control. Bovine bone tissue with medium served as a negative control. The sample, the positive and the negative control were measured parallel. The first experiment was made approximately 24 hours after seeding. The optical density of each well was measured at 490 nm in a TriStar LB 941 device (Berthold Technologies, Germany). The obtained values were used to calculate a percentage of metabolic activity in comparison with controls considered to have 100% metabolic activity. Cells were stained with trypan blue (Sigma Aldrich, USA) with a 1 : 9 ratio of trypan blue to the cell suspension. Cells were counted in a Bürker counting chamber under light microscopy. Dark blue cells were evaluated as dead ones. The growth curves were constructed for both the sample and the control. Metabolic activity of the first passages (P1) as well as the second passages (P2) of hMSCs was evaluated. The whole experiment took 12 days. Proliferation was observed on the first, on the fifth, on the ninth and on the twelfth day of the cell growth.
Scanning electron microscopy
Scanning electron microscope was used to examine adhesion and proliferation of human mesenchymal stem cells on bovine bone blocks. After 14 days of co‑cultivation, samples were washed with PBS, fixed with 2.5% glutaraldehyde (Fluka-Biochemika, Switzerland) in 0.1 mol/l sodium cacodylate buffer (pH 7.2) (Merck-Darmstadt, Germany) at 4°C for 24‑hour periods and post fixed with 1% osmium tetraoxide (WC Heraeus GmbH, Germany) for 3 hours and washed with PBS. Then samples were immersed into isoamylacetate, dehydrated through an ethanol graded series and critical-point dried. Samples were attached to an aluminium stub and the sputter coated with gold prior to the observation. The surfaces of bovine bone tissue, with or without cells, were inspected by using the scanning electron microscope JSM 7000F (SEM Jeol, USA) at acceleration voltage of 20 kV.
Statistical analysis
The data are presented as the mean ± SEM (Standart Error of Mean). Significant differences between groups of means were analyzed by ANOVA. Statistical significance was assumed at the 95% confidence limit or greater (p<0.05).
Results
The growth curves present activity of hMSCs for both cells of the first and the second passage in comparison with the control. The cell number for all samples increased up to the 12th day of cultivation with the typical sigmoid population dynamics for the control. The pattern of the adherent cells in the sample is slightly different; the curves did not reach the stabilization or equilibrium stage during the period of cultivation. There is also an obvious decreased number of attached cells of the sample observed in all days (except for the first day) in relation to the control. Compared to hMSCs seeded directly onto tissue culture plastic, the scaffold condition itself significantly reduces the growth of cells.To distinguish hMSCs from other cells in the bone marrow compartment, negative and positive hMSCs markers proposed by the International Society for Cell Therapy were used. Immunophenotyping of bone marrow hMSCs revealed that they were positive for several markers common to bone marrow hMSCs. The cell-surface markers analyzed using FACS (Fluorescence-Activated Cell Sorting) showed that the bone marrow hMSCs had a positive expression (≥95%) of CD105, CD90, CD73 and negative expression (≤2%) of hematopoietic surface markers CD34, CD45 (Fig. 1).
Fig. 1. Immunophenotypic bone marrow characteristics of hMSCs examined by flow cytometry: positive for CD105, CD90, CD73 and negative for CD34 and CD45. 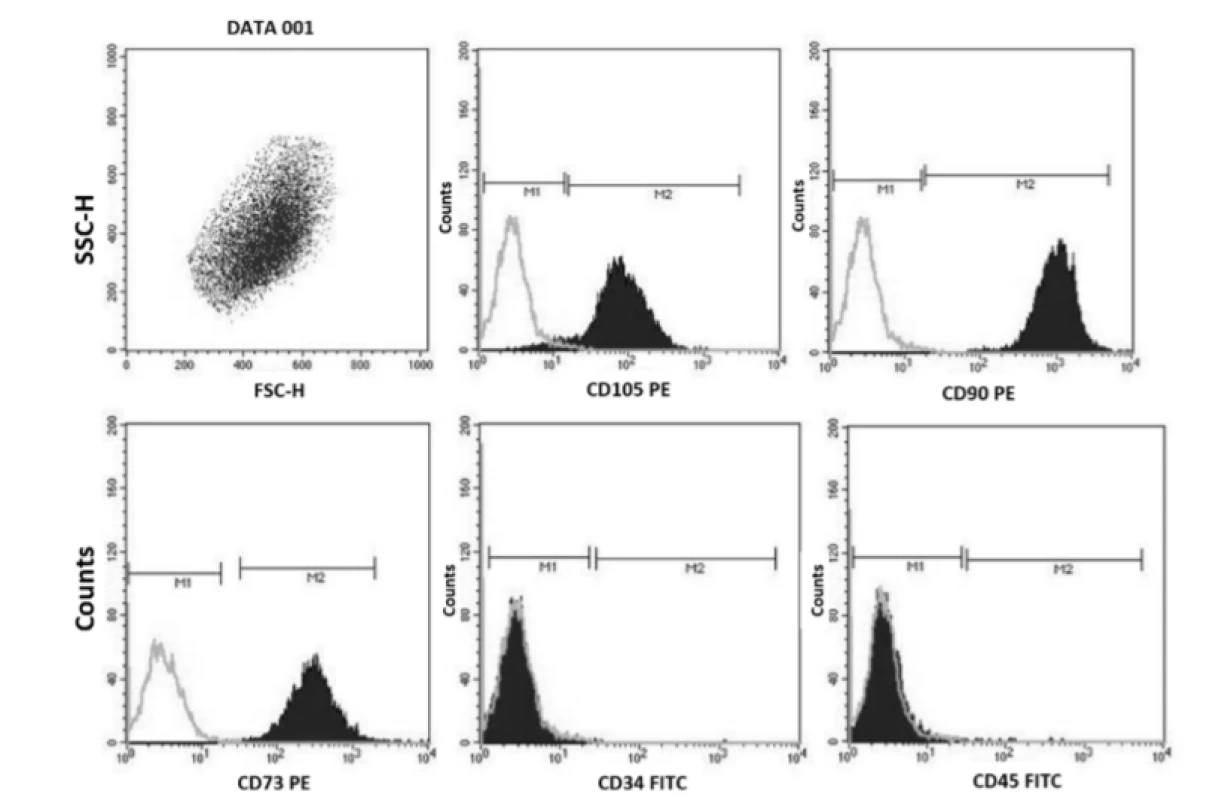
The metabolic activity was measured and compared after seeding. Fig. 3 depicts metabolic activity of the cell population seeded on the bovine bone tissue of the first and the second passage in comparison with the control. Results show that the human mesenchymal cells have comparable metabolic activity through day 1 and 5 after seeding for both passages. Metabolic activity was decreasing up to the 12th day of cultivation. The statistically significant difference between groups of P1 and P2 samples was assessed using ANOVA (*p<0.05). It can be seen from the graph that the cell growth on bovine bone tissue of the second passage cell population is significantly lower in comparison with the cell growth of the second passage on the 9th and on the 12th day of cultivation.
The results of cell adhesion and proliferation of hMSCs are shown in Fig. 2. Five independent experiments were performed with the control (hMSCs) and the sample (hMSCs co-cultivated with bovine bone tissue).Fig. 2. Growth curves of hMSCs. The data represent the mean ± SEM of five independent experiments (respective SEM is less than the size of the symbol for each point). C – control (hMSCs without bovine bone tissue), P1 – the first passage of hMSCs, S – sample (hMSCs with bovine bone tissues), P2 – the second passage of hMSCs. 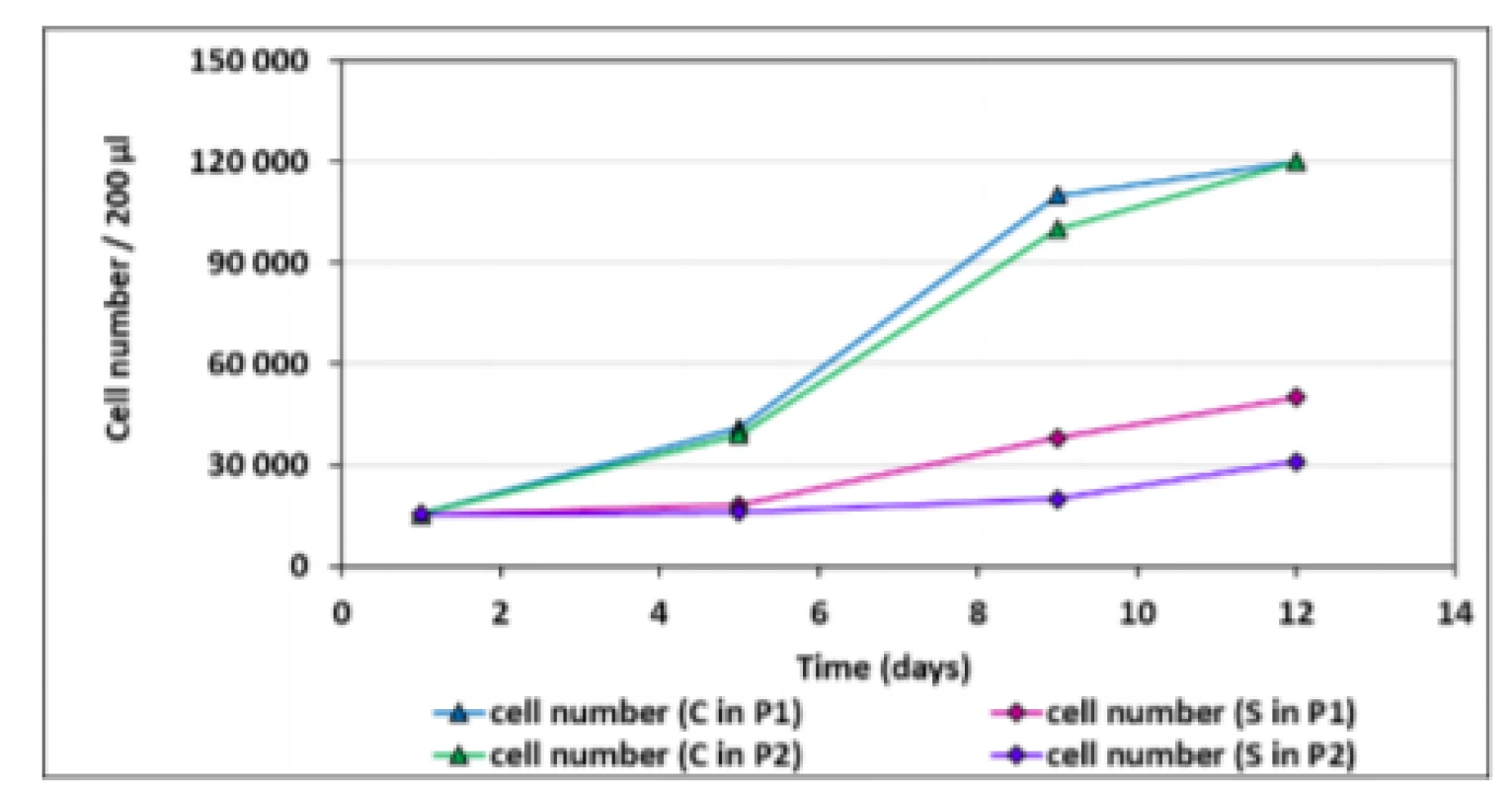
Fig. 3. Metabolic activity of hMSCs co-cultivated with bovine bone tissue of the first and the second passage. Bars represent the mean ± SEM of five independent experiments. Statistical significance was assessed by using ANOVA (*p<0.05). 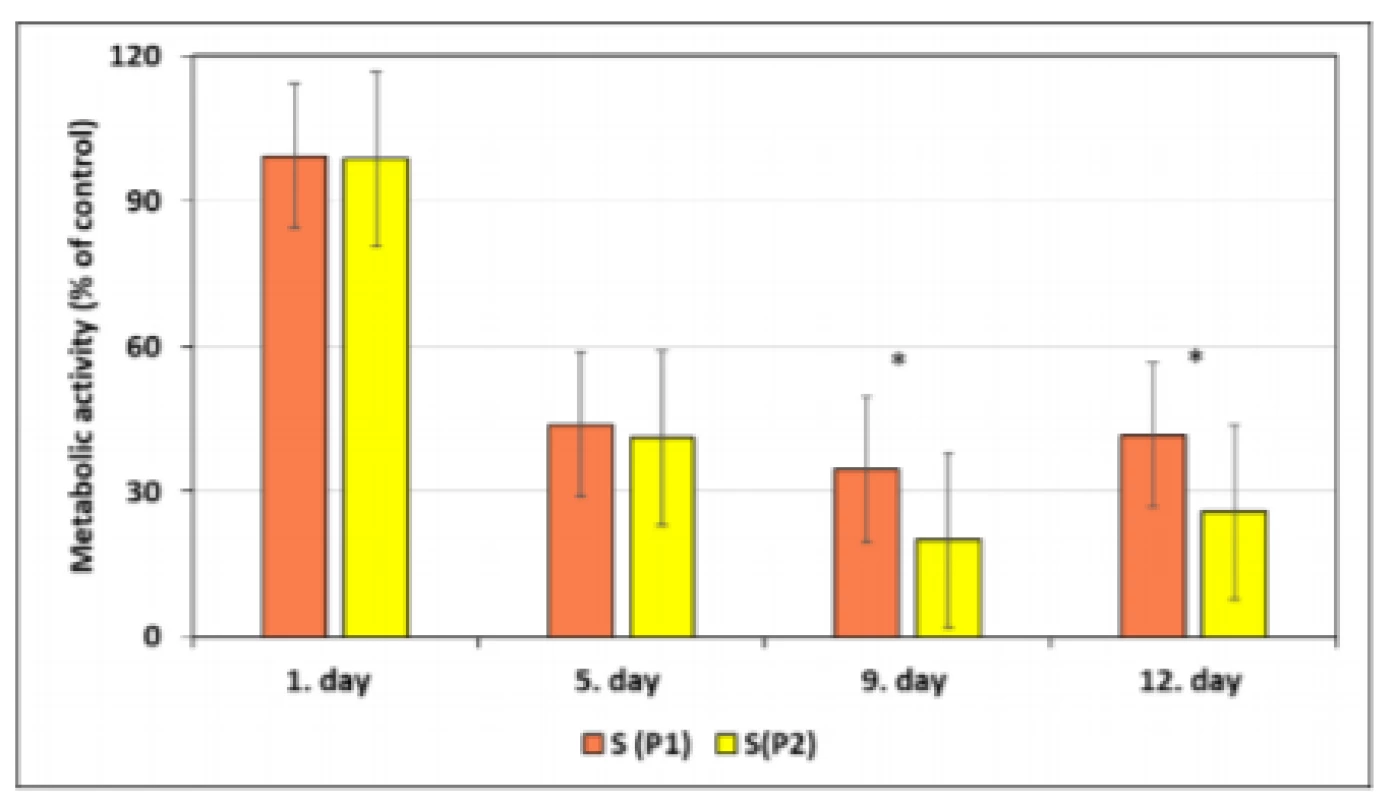
To measure cell growth kinetics, two-fold increase (doubling) in the total number of cells at exponential phase of growth of cell cultures was calculated. Population doubling times of hMSCs directly correlates with replicative senescence, which is linked to loss of potency, as well as with genomic instability. Fig. 4 illustrates population doubling times (PDTs) for hMSCs for both the first and the second passage of the control and the sample. PDTs show that cell cultures grow at different rates. A lower proliferation rate of sample in comparison with control was observed. An increase of approximately 60% of the doubling time for hMSCs co‑cultivated with bovine bone tissue was observed for both passages.
Fig. 4. Population doubling times (PDTs) of hMSCs for the first (P1) and the second passage (P2) for the control (C) and the sample (S). Values represent mean ± SEM for five experiments. 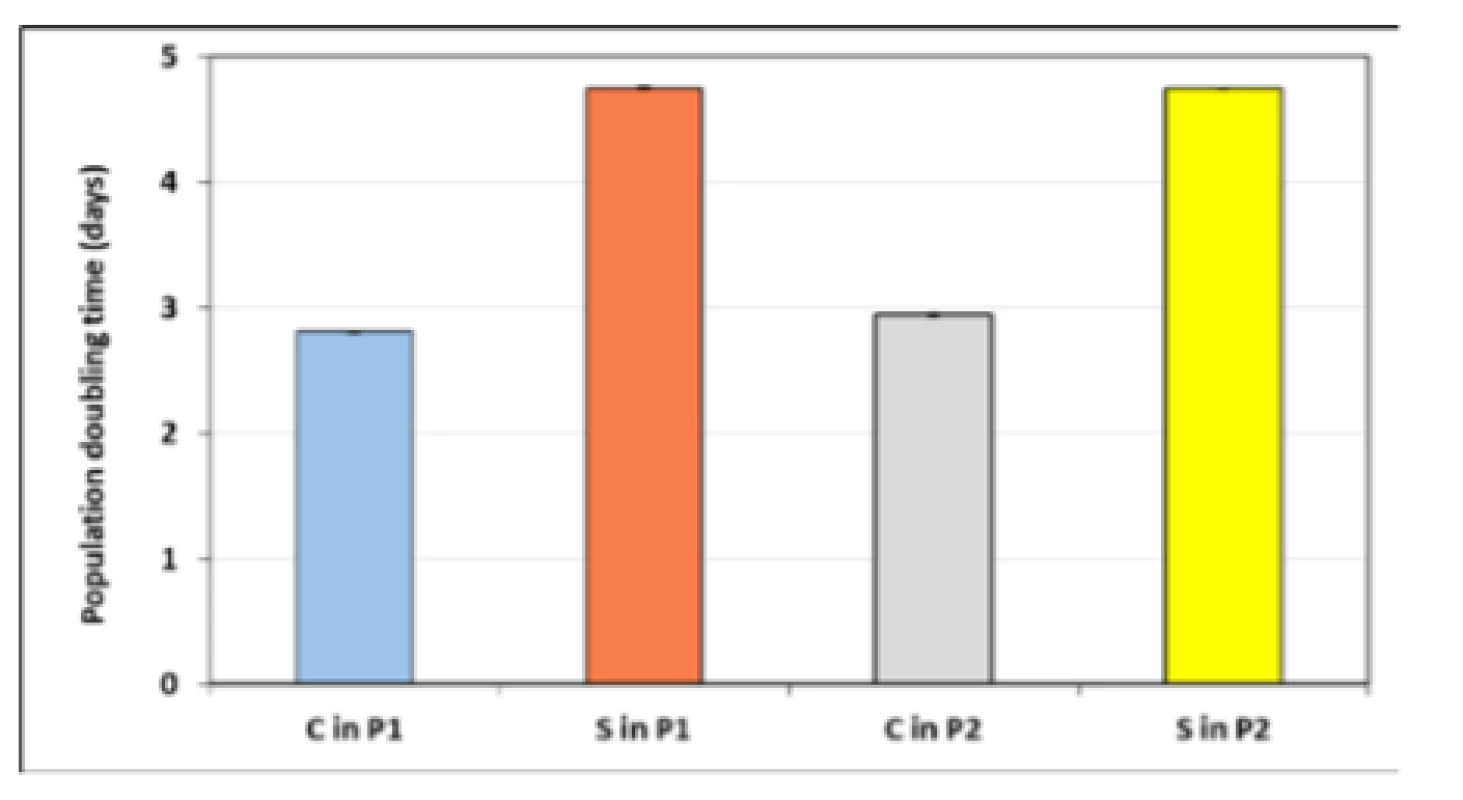
According to the statistical analysis, the change in the growth rate of hMSCs co-cultivated with bovine bone tissue between the first and second passage cell population (Tab. 1) was significant when p<0.005 and p<0.05. No significant difference was observed only between the cell cultures grow rates of the sample for both passages.
Tab. 1. Statistical analysis of growth of hMSCs co-cultivated with bovine bone tissue after the first and the second passage. 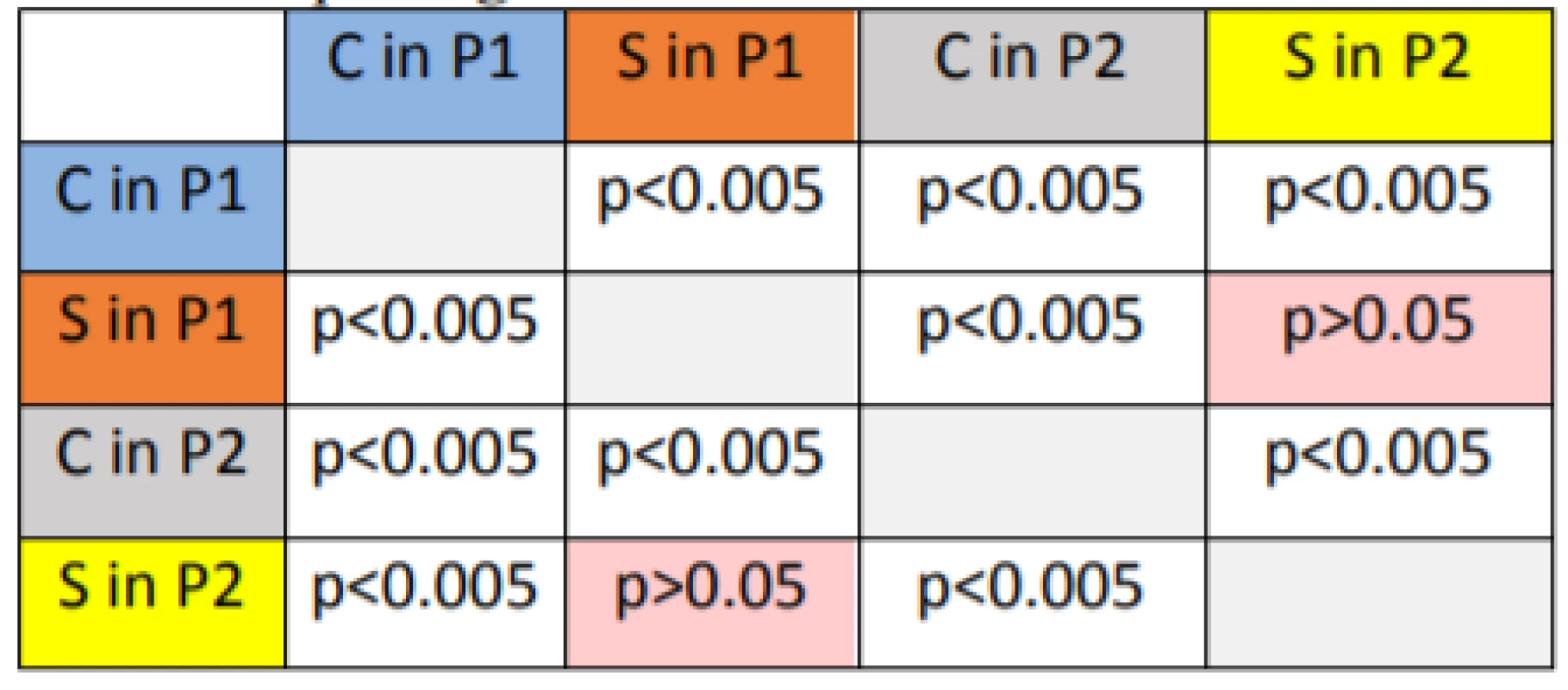
Finally, we evaluated the attachment of hMSCs to bovine bone tissue using scanning electron microscopy (Fig. 5, Fig. 6). SEM (Scanning Electron Microscope) micrograph shows the pore architecture of the bovine bone tissue without cells (Fig. 5) SEM micrograph bovine bone tissue with seeded cells demonstrates effective adhesion, spreading and intercellular contact of human mesenchymal stem cells within the pores bovine bone tissue during 14 days cultivation period (Fig. 6).
Fig. 5. The surfaces of bovine bone tissue without hMSCs. 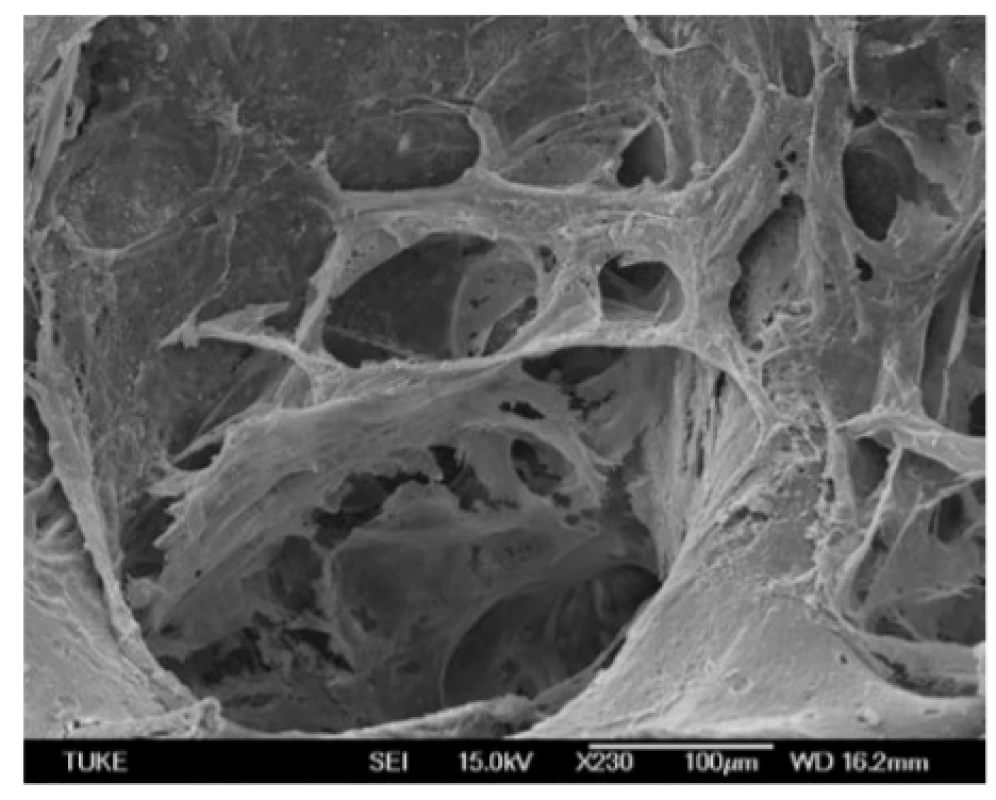
Fig. 6. The surfaces of bovine bone tissue with attached hMSCs 
Discussion
Human MSCs have the potential of self-renewal and differentiation into multiple lineages including cartilage, adipose and bone tissue. Generally, MSCs are characterized by their ability to adhere under standard cell culture conditions, expressing CD44, CD73, CD90, CD105, but not CD45, CD34, and CD14 [10]. We used flow cytometry using a panel of monoclonal antibodies to verify the identity of MSCs extracted from human bone marrow. Immunophenotypic bone marrow characteristics of hMSCs examined by flow cytometry were positive, as expected, for CD105, CD90, and CD73, and negative for CD34 and CD45.
Human mesenchymal stem cells have generated a great deal of interest because of their potential use in regenerative medicine and tissue engineering. Numerous preclinical and clinical studies have been performed to support the safety of using MSCs for cell-based therapies. However, the ability of MSCs to produce intended result in vivo is still limited due to poor survival, retention, and engraftment of the cells. The treatment of musculoskeletal disorders has improved during the last 20 years due to the enormous progress in the understanding of basic biological and biomechanical principles. Based on this knowledge, new methods, modern rehabilitation programs and innovative approaches were developed and successfully applied for treatment [11]. Plenty of clinical and pre‑clinical studies illustrate their therapeutic value in cell therapy [12], in dental medicine for rebuilding lost periodontal tissue [13] or in soft tissue-engineering [14] and bone tissue regeneration [15, 16], studying cell therapeutics for use in endotoxic shock, suggested that bone marrow-derived mesenchymal stromal cells may be more effective in shock therapy and adipose-derived stromal/stem cells may be positioned for continued exploration of immunomodulatory diseases. New generation of dermal equivalents for dermal tissue engineering was presented in the study of Schneider et al. [17]. They demonstrated the promotion of epithelial cell proliferation and extracellular matrix remodeling of collagen-embedded MSCs on the human fibroblast‑derived dermal matrix.
Bone tissue engineering is still a new research area. The subject of the study was to promote development of clinical applications to replace or restore the lost bone in case of orthopedic defects, stabilization of spinal segments, and restoration of bone defects in case of tumors and bone neoplasia and treatment of pseudo-arthrosis. Bone tissue engineering has already been used in craniofacial, maxillofacial, orthopedic reconstructive surgery, head surgery and trauma and neck surgery [18]. It may provide solutions for generating a new bone tissue with good mechanical and functional qualities. Reduction of the risks and expenses is possible by using allografts, autografts, metals and ceramics, and so on. According to the study of Rodrigues et al. [19], utilization of allografts in bone tissue engineering has several disadvantages consisting in potential immune response, transmission of diseases and induction of the loss of osteogenesis. Autografts have limitations due to the necessity of an additional surgery, anatomical and structural problems, limited donor bone supply and inadequate resorption rate during healing. Different biomaterials have been used as for example scaffolds or implants for bone tissue engineering [20–23] or artificial intervertebral discs [24] or part of endoprothesis [25]. Metals, alone or coated with bioactive and bioinert ceramics, have been used for load-bearing orthopedic applications, but problems due to metals corrosion, dense fibrous tissue formation on the bone-implant or ceramics-metal interface may occur [19]. For improving osteoinduction and osteoconduction, association of extracellular matrix scaffolds with osteogenic cells and differentiation and growth factors may be required [26].
The large number of candidates for a transplant on the one side and the insufficient supply of donated organs from human donors on the other, necessitate in alternative options. Such an alternative could also be the mechanisms of bone remodeling and repair with the aid of combinatorial approach including human mesenchymal stem cells and bovine bone tissue. Lately, a novel option for the delivery of human mesenchymal cells appeared there that employs bovine bone matrix as a scaffold [27]. Although MSCs differentiate into osteoblasts, naturally they do not migrate to bone. According to the studies examining the tropisms of MSCs to bone, MSCs can only be targeted to bone for bone regeneration by enhancing the transmigration of MSCs to the bone via the selectin-mediated cell adhesion pathway [28, 29]. Also, MSCs modified with bone-targeting polymer can be used to augment osteogenesis [30–32].
In our study, we tried to evaluate the survival of human mesenchymal stem cells delivered on bovine bone matrix as a scaffold. We examined the tropism of hMSC to bovine bone patches of unmodified hMSCs. The results show that bovine bone scaffolds can be used to harbour hMSCs and that bone can modulate human stem cell behaviour. hMSCs are able to adhere to the bovine bone even not being modified with bone-targeting elements. However, it must be said, that the proliferation rate and metabolic activity for hMSCs co‑cultivated with bovine bone decrease in comparison with the control. There are also observable differences in the behaviour of the first and the second passages of the cells, proliferation of the second passage is not as rapid as the proliferation of the first one.
Conclusion
This preliminary study is only the first step on the long way of examination of compatibility and validation of combinatorial approach using bones from other species and human MSCs for tissue engineering for transplantation. Therefore, future experimental studies need to focus on inducing multitudes of biochemical and physical cues to affect the microenvironment of the MSCs, which may enhance the desired efficacy.
Acknowledgement
This study was supported by the grant KEGA 069TUKE-4/2017 of the Ministry of Education, Science, Research and Sport of the Slovak Republic. This work was done in collaboration with the ATB UNLP in Košice.
Marianna Trebuňová, Ph.D.
Department of Biomedical Engineering and Measurement
Faculty of Mechanical Engineering
Technical University in Košice
Letná 9
042 00 Košice
Slovak RepublicPhone: +421 556 022 380
Zdroje
- Hüsing, B., Bührlen, B., Gaisser, S.: Human Tissue Engineered Products – Today´s Markets and Future Prospects, Final Report for Work Package. Fraunhofer Institute for System and Innovation Research, Karlsruhe 2003.
- Castells-Sala, C., Alemany-Ribes, M., Fernández-Muiños, T., Recha-Sancho, L., López-Chicón, P., Aloy-Reverté, C., Caballero-Camino, J., Márquez-Gil, A., Semino, C. E.: Current applications of tissue engineering in biomedicine. J Biochips Tissue Chip 2013, vol. S2, 1–14.
- Filip, S., Mokrý, J., Hruška, I.: Kmeňové Bunky. Galén, Praha 2006.
- Azouna, N. B., Jenhani, F., Regaya, Z., Berraeis, L., Othman, T. B., Ducrocq, E., Domenech, J.: Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow, comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res Ther 2012, vol. 3, 1–14.
- Orli, D., Kajstura, J., Chimenti, S., Jakoniuk, I., Anderson, S. M., Li, B., Pickel, J., McKay, R., Nadal-Ginard, B., Bodine, D. M., Leri, A., Anversa, P.: Bone marrow cells regenerate infarcted myocardium. Nature 2001, vol. 410, 701–705.
- Bosnakovski, D., Mizuno, M., Kim, G., Takagi, S., Okumura, M., Fujinaga, T.: Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res 2005, vol. 319, 243–253.
- Bruder, S. P., Kurth, A. A., Shea, M., Hayes, W. C., Jaiswal, N., Kadiyala, S.: Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res 1998, vol. 16, 155–162.
- Liu, Y., Chen, F., Liu, W., Cui, L., Shang, Q., Xia, W., Wang, J., Cui, Y., Yang, G., Liu, D., Wu, J.: Repairing large porcine full-thickness defects of articular cartilage using autologous chondrocyte-engineered cartilage. Tissue Eng 2002, vol. 8, 709–721.
- Wakitani, S., Imoto, K., Yamamoto, T., Saito, M., Murata, N., Yoneda, M.: Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartilage 2002, vol. 10, 199–206.
- Natunen, S., Lampinen, M., Suila, H., Ritamo, I., Pitkänen, V., Nairn, A. V., Räbinä, J., Laitinen, S., Moremen, K. W., Reutter, W., Valmu, L.: Metabolic glycoengineering of mesenchymal stromal cells with N-propanoylmannosamine. Glycobiology 2013, vol. 23, 1004–1012.
- Maffulli, N., Renstrom, P., Leadbetter, W. B.: Tendon Injuries. Basic Science and Clinical Medicine. Springer, London 2005.
- Moll, G., Le Blanc, K.: Engineering more efficient multipotent mesenchymal stromal (stem) cells for systematic delivery as cellular therapy. ISBT Science Series 2015, vol. 10, 357–365.
- Wu, S. M., Chiu, H. C., Chin, Y. T., Lin, H. Y., Chiang, C. Y., Tu, H. P., Fu, M. M., Fu, E.: Effects of enamel matrix derivative on the proliferation and osteogenic differentiation of human gingival mesenchymal stem cells. Stem Cell Res Ther 2014, vol. 5, 1–11.
- Okabe, K., Yamada. Y., Ito, K., Kohgo, T., Yoshimi, R., Ueda, M.: Injectable soft-tissue augmentation by tissue engineering and regenerative medicine with human mesenchymal stromal cells, platelet-rich plasma and hyaluronic acid scaffolds. Cytotherapy 2009, vol. 11, 307–316.
- Knight, M. N., Hankenson, K. D.: Mesenchymal stem cells in bone regeneration. Adv Wound Care 2013, vol. 2, 306–316.
- Elman, J. S., Li, M., Wang, F., Gimble, J. M., Parekkadan, B.: A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm 2014, vol. 11, 1–8.
- Schneider, R. K., Anraths. J., Kramann, R., Bornemann, J., Bovi, M., Knüchel, R., Neuss, S.: The role of biomaterials in direction of mesenchymal stem cell properties and extracellular matrix remodelling in dermal tissue engineering. Biomaterials 2010, vol. 31, 7948–7959.
- Lawson, A. C., Czernuszka, J. T.: Collagen-calcium phosphate composites. Proc Instr Mech Eng 1998, vol. 212, 413–425.
- Rodrigues, C. V., Serricella, P., Linhares, A. B., Guerdes, R. M., Borojevic, R, Rossi, M. A., Duarte, M. E., Farina, M.: Characterization of a bovine collagen-hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials 2003, vol. 24, 4987–4997.
- Donzelli, E., Salvadè, A., Mimo, P., Viganò, M., Morrone, M., Papagna, R., Carini, F., Zaopo, A., Miloso, M., Baldoni, M., Tredici, G.: Mesenchymal stem cells cultured on a collagen scaffold, In vitro osteogenic differentiation. Arch Oral Biol 2007, vol. 52, 64–73.
- Hutmacher, D. W.: Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, vol. 21, 2529–2543.
- Kiel-Jamrozik, M., Szewczenko, J, Basiaga, M., Nowińska, K.: Technological capabilities of surface layers formation on implant made of Ti-6Al-4V ELI alloy. Acta Bioeng Biomec. 2015, vol. 17, 31–37.
- Rumian, L., Reczyńska, K., Wrona, M., Tiainen, H., Haugen, H. J., Pamuła, E.: The influence of sintering conditions on microstructure and mechanical properties of titanium dioxide scaffolds for the treatment of bone tissue defects. Acta Bioeng Biomech 2015, vol. 17, 3–9.
- Migacz, K., Chłopek, J., Morawska-Chochół, A., Ambroziak, M.: Gradient composite materials for artificial intervertebral discs. Acta Bioeng Biomech 2014, vol. 16, 3–12.
- Mróz, A., Skalski, K., Walczyk, W.: New lumbar disc endoprosthesis applied to the patient’s anatomic features. Acta Bioeng Biomech 2015, vol. 17, 25–34.
- Bruder, S. P., Fox, B. S.: Tissue engineering of bone. Clin Orthop Rel Res 1999, vol. 367S, S68–S83.
- Rodríguez-Fuentes, N., Reynoso-Ducoing, O., Rodríguez-Hernández, A., Ambrosio-Hernández, J. R., Piña-Barba, M. C., Zepeda-Rodríguez, A., Cerbón-Cervantes, M. A., Tapia-Ramírez, J., Alcantara-Quintana, L. E.: Isolation of human mesenchymal stem cells and their cultivation on the porous bone matrix. J Vis Exp 2015, vol. 96, 1–7.
- Park, J. S., Suryaprakash, S., Lao, Y.-H., Leong, K. W.: Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, vol. 84, 3–16.
- Sackstein, R., Merzaban, J. S., Cain, D. W., Dagia, N. M., Spencer, J. A., Lin, C. P., Wohlgemuth, R.: Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 2008, vol. 14, 181–187.
- D’Souza, S., Murata, H., Jose, M. V., Askarova, S., Yantsen, Y., Andersen, J. D., Edington, C. D., Clafshenkel, W. P., Koepsel, R. R., Russell, A. J.: Engineering of cell membranes with a bisphosphonate-containing polymer using ATRP synthesis for bone targeting. Biomaterials 2014, vol. 35, 9447–9458.
- Guan, M., Yao, W., Liu, R., Lam, K. S., Nolta, J., Jia, J., Panganiban, B., Meng, L., Zhou, P., Shahnazari, M., Ritchie, R. O.: Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med 2012, vol. 18, 456–462.
- Yao, W., Lane, N. E.: Targeted delivery of mesenchymal stem cells to the bone. Bone 2015, vol. 70, 62–65.
Štítky
Biomedicína
Článek vyšel v časopiseLékař a technika

2018 Číslo 2-
Všechny články tohoto čísla
- Response by an automated inspired oxygen control system to hypoxemic episodes: assessment of damping
- Workflow for bioprinting of cell-ladem bioink
- Application of sibgle wireless holter to simultaneous EMG, MMG and eim measurement of human muscles activity
- Biocompatibility of the human mesenchymal stem cells with bovine bone tissue at the cellular level in vitro
- Machine learning using speech utterances for parkinson disease detection
- Lékař a technika
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Application of sibgle wireless holter to simultaneous EMG, MMG and eim measurement of human muscles activity
- Response by an automated inspired oxygen control system to hypoxemic episodes: assessment of damping
- Workflow for bioprinting of cell-ladem bioink
- Machine learning using speech utterances for parkinson disease detection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



