-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA combination of grapefruit seed extract and concentrated cranberry juice as a potential antimicrobial preservative for the improvement of microbiological stability of hypromellose gel
Kombinace extraktu z grapefruitových semínek a koncentrované šťávy z klikvy velkoplodé jako potenciální protimikrobní konservans ke zvýšení mikrobiologické stability hypromelosového gelu
Vodné gely z hypromelosy nejsou mikrobiologicky stálé – během uchovávání se v nich mohou pomnožit mikroorganismy. Ke zvýšení doby použitelnosti gelů je nutná přísada protimikrobních konzervačních látek. Některé látky rostlinného původu jsou charakteristické svými antimikrobiálními vlastnostmi, a tak by mohly sloužit jako alternativa k syntetickým konzervantům. Proto cílem této práce bylo sledování mikrobiologické stability hypromelosového gelu a účinnosti přírodních látek – extraktu z grapefruitových semínek (GSE), koncentrované šťávy z klikvy velkoplodé a jejich kombinace – na antimikrobiální ochranu gelu. Hodnocení antimikrobiální aktivity GSE a klikvové šťávy ukázalo, že se míra jejich působení liší. Jak klikvová šťáva, tak GSE potlačovaly růst standardních gram-pozitivních a gram-negativních bakterií, avšak účinek GSE byl silnější. Candida albicans byla citlivá pouze k GSE. Z tohoto důvodu s cílem zasáhnout všechny zkoumané mikroorganismy, lze použít buď kombinaci 0,7 % GSE a 10 % klikvové šťávy nebo samotný GSE v 5% koncentraci. Kombinace GSE a klikvové šťávy byla účinná pouze v kyselém prostředí (pH 2,5–5), kdežto antimikrobiální efekt GSE nebyl závislý na hodnotě pH.
Klíčová slova:
extrakt z grapefruitových semínek • klikvová šťáva • hypromelosový gel • antimikrobiální ochrana
Authors: Jurga Bernatoniene; Rasa Keraitė; Ruta Masteiková; Alvydas Pavilonis; Arūnas Savickas
Authors place of work: Department of Drug Technology and Social Pharmacy, Academy of Medicine, Lithuanian University of Health Sciences, Kaunas, Lithuania ; Pharmacy of Lithuanian University of Health Sciences, Kaunas, Lithuania *
Published in the journal: Čes. slov. Farm., 2013; 62, 212-219
Category: Původní práce
Summary
Aqueous hypromellose gels are not microbiologically stable – they show signs of microorganism growth during storage. To extend the shelf-life of the gels, antimicrobial preservatives are needed. Some substances of plant origin are known for their antimicrobial properties, and thus they may be used as an alternative to synthetic preservatives. Therefore, the aim of this study was to evaluate the microbiological stability of aqueous hypromellose gel and the effectiveness of natural substances – grapefruit seed extract (GSE), concentrated cranberry juice, and a combination thereof – on the antimicrobial protection of the gel. The evaluation of the antimicrobial activity of GSE and cranberry juice showed that their antimicrobial effects differed. Both cranberry juice and GSE inhibited the growth of the standard gram-positive and gram-negative bacteria, but the effect of GSE was significantly stronger. Candida albicans was sensitive only to GSE. For this reason, in order to affect all the microorganisms studied, either a combination of 0.7% GSE and 10% cranberry juice, or 5% GSE alone may be used. The combination of GSE and cranberry juice was effective only in acidic medium (pH being 2.5–5), while the antimicrobial effect of GSE was not dependent on the pH value.
Keywords:
grapefruit seed extract • cranberry juice • hypromellose gel • antimicrobial protectionIntroduction
Hypromellose (hydroxypropyl methylcellulose, HPMC, Methocel) is a semisynthetic polymer (cellulose ether) widely used as excipient in pharmaceutical, cosmetic, and food industry1, 2). HPMC products give neutral-flavoured, odourless and colourless solutions2), and may be used in oral and topical (dermal, ophthalmic or nasal) liquid or semisolid pharmaceuticals as viscosifiers, gel-forming, thickening, and suspending agents3–5). The protective colloid action and emulsifying properties of hypromellose also benefit many liquid formulations1). HPMC in the powder form is resistant against microbial spoilage, but with addition of water (hydrophilic solutions or gels) it becomes susceptible to microbial growth. Such preparations must be protected by the addition of preservatives that prevent the alteration and degradation of the product. Preservatives are mainly effective in controlling mould, inhibiting yeast growth, and protecting against bacterial proliferation. Their antimicrobial and antifungal properties make them an integral part of the product formulation6). Commonly, synthetic preservatives such as some organic acids (benzoic and sorbic acid or parabens), alcohols, organic mercurials, or quaternary ammonium compounds are used7). Unfortunately, at present there is no perfect preservative, and all materials are a trade-off of a number of frequently contradicting properties7). It is not surprising that especially synthetic preservatives sometimes prove toxic to humans (irritation, hypersensitivity reactions, allergy, accumulation of organic mercurials in the body, etc.). The search for new antimicrobial agents has led to a tendency of avoiding synthetic substances and involving products of natural origin with less toxic effects as a possible safer alternative8). Naturally occurring antimicrobials include compounds that originate from microbial, plant (extracts, essential oils, or active ingredients isolated from plants), and animal sources9–11). Unfortunately, a negative feature of a number of products is a narrower spectrum of the antimicrobial effect and lower activity levels, compared to synthetic preservatives12). This drawback may partially be solved by suitable combinations of various substances12). In addition to that, antimicrobial protection of the preparation may also come from substances with a primarily therapeutic effect. Thus, creation of formulations requires an evaluation of the effective antimicrobial concentrations.
The existing literature does not provide an unequivocal opinion concerning the antimicrobial effectiveness of the grapefruit (Citrus paradisi Mact.) seed extract (GSE). Von Woedtke et al.13) stated that the antimicrobial effect was due to the presence of synthetic preservatives in commercially available extracts. Their self-produced extracts did not inhibit bacterial or fungal growth. Later studies in Croatia14) disproved these results: GSE produced in their laboratory (solvent: 70% ethanol) proved to be effective against all the studied gram-positive bacteria and fungi as well as against some gram-negative bacteria. However, the antimicrobial effect of GSE was weaker, compared to that of some commercially available extracts containing synthetic substances. According to the authors, these differences might not necessarily have been related to the presence of synthetic preservatives – the differences in phenolic compounds (especially flavonoids) content and composition might have had the greatest influence. Recent testimonials report grapefruit-seed extract (Citricidal) to be effective against more than 800 bacterial and viral strains, 100 strains of fungus, and a large number of single - and multi-celled parasites15). A stronger inhibitory effect is observed against gram-positive than against gram-negative bacteria16). In a study of Edwards-Jones et al.17), grapefruit seed extract (Citricidal) showed antibacterial effect even against MRSA – particularly in combination with essential oils. It has been presumed that GSE disrupts the bacterial membrane and liberates the cytoplasmic contents within 15 minutes after contact15). These processes are caused by a complex of biologically active substances found in grapefruit seed extract18). Grapefruit seeds are the major depository for limonoids as well as flavonoid glycosides, naringenin, quercetin, kaempherol, hesperidin, and apigenin being the most abundant among their aglycones; both saturated and unsaturated fatty acids are present as well15, 19–22). Some papers suggest that grapefruit seeds or bioactive compounds of GSE may have various therapeutic properties. Oyelami et al.23) described successful treatment of urinary tract infection with grapefruit seeds. Zayachkivska et al.24) demonstrated that GSE reduced acute gastric lesions induced by a corrosive concentration (100%) of ethanol, suggesting that it may be capable of protecting the gastric mucosa against necrotizing substances and possibly be useful in the treatment of acute and chronic gastric ulcerations. Dembinski et al.25) in their study found that GSE had a protective effect against ischemia/reperfusion-induced pancreatitis – probably due to the activation of anti-oxidative mechanisms in the pancreas and the improvement of pancreatic blood flow. Adeneye26) proved a hematopoietic effect of the methanol-based grapefruit seed extract.
Cranberries (Vaccinium macrocarpon Ait.) also have an antibacterial and antifungal effect27, 28). A number of studies have shown that cranberries are highly effective against bacteria that cause urinary tract infection – especially against E. coli29). This effect was thought to be due to the ability of the acids accumulated in cranberries to reduce urine pH, which in turn inhibits bacterial growth30), or due to the presence of polyphenols and tannins in cranberries31). However, recently, a group of proanthocyanidins (PACs) with A-type linkages were isolated from cranberries which exhibit bacterial anti-adhesion activity against both antibiotic-susceptible and resistant strains of uropathogenic P-fimbriated E. coli bacteria32). A study of Howell et al.32) performed on an in vivo Caenorhabditis elegans model showed that cranberries also act against bacterial virulence: E. coli strain presented a reduced ability to kill worms after cultivation in urine samples of patients who took cranberry capsules. Fructose is the second compound with bacterial anti-adhesion activity in cranberries; it inhibits the mannose-sensitive fimbrial adhesins30). The anti-adhesion activity of cranberries on non-urinary bacterial species such as Helicobacter pylori, Streptococcus mutans, Porphyromonas gingivalis, Enteroccocus faecalis, and Neisseria meningitidis has also been verified33, 34). Urinary tract infections, dental caries, periodontitis, and certain ulcers all occur when pathogenic bacteria adhere to the epithelial tissue in the body. GSE is also believed to possibly have a similar anti-adhesive effect19). However, cranberries do not only have anti-adhesive properties, but they also directly affect microorganisms35). Wu et al.36) hypothesized that cranberries may interact with the outer cell membrane – by disrupting it, cranberry compounds enter the cell and inhibit the transcription of genes; this may prevent the synthesis of proteins that are required for bacterial growth. Antimicrobial activity is due to phenolic compounds and anthocyanins found in the raw material27) in combination with other biologically active compounds37) – e.g. organic acids36). Cranberries are also characterized by certain antifungal activity38). In the case of Candida albicans, cranberry proanthocyanidins exert anti-biofilm (anti-adhesion) activities while having no effect on fungal growth39).
The literature focuses on the oral use of grapefruit seed extract and cranberries; in this case, determination of the minimal concentration of the active components that ensures microbiological stability of the preparation is of importance. The antimicrobial effectiveness of these biologically active substances may also be applied to ensure the microbiological safety of dermatological products. Thus, the aim of this study was to evaluate the microbiological stability of aqueous hypromellose gel and the effectiveness of biologically active substances – grapefruit seed extract (GSE), concentrated cranberry juice, and a combination of these substances – on the microbiological protection of the gel.
Experimental part
Materials
The following materials were used in the study: grapefruit seed extract (BAYERNWALD, Germany), concentrated cranberry juice (BAYERNWALD, Germany), hypromellose (Sigma-Aldrich Co.), purified water (Ph. Eur. quality).
Bacterial strains tested
Antimicrobial activity was evaluated by using standard microorganism cultures: bacteria Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumoniae ATCC 33499, Proteus mirabilis ATCC 12459, Bacillus subtilis ATCC 6633, and Bacillus cereus ATCC 8035, and fungi Candida albicans ATCC 60193.
Methods
Preparation of hypromellose gel and solutions with test substances
A 3% aqueous hypromellose gel was prepared by continuous stirring in a mixer until a transparent colourless mass was formed. In the case of the gel containing the active ingredients (GSE and cranberry juice), the amount of water used was reduced proportionately to the amount of the substance added into the product.
Solutions of the following concentrations were prepared by dissolving respective amounts of the test substances in purified water:
- grapefruit seed extract – 0.2%; 0.3%; 0.7%; 1.0%; 2.0%; 3.0%; 5.0%, and 10%;
- cranberry juice – 3.0%; 5.0%; 10.0%; 20.0%, and 30.0%;
Evaluation of microbial stability of hypromellose gels
Microbial stability of the gels was evaluated after storage in a thermostat (at 40 ± 1 °C and 20 ± 1 °C) and in a fridge (at 4 ± 1 °C). The gel that did not contain any active substances was stored for 1 month, and the gel with active ingredients for 3 months. Following that, disposable bacteriological loops were used to obtain samples of the gels under study; the samples were subsequently placed into separate sterile test-tubes containing liquid nutrient media – trypticase soy agar for bacteria, and Sabouraud agar for the cultivation of fungi. The prepared samples were stored for 24 hours in a thermostat at 35 ± 1 °C, and subsequently for 24 hours at room temperature (20 ± 2 °C). The appearance of the samples was evaluated visually – unaltered suspensions indicated that the preparations were stable (i.e. without microbial growth), while turbidity indicated microbial contamination, i.e. growth of microorganisms in the sample.
The test-tube contents that did not exhibit turbidity were placed on solid nutrient media and cultivated for 24 hours in a thermostat at 35 ± 1 °C, and subsequently for 24 hours at room temperature (20 ± 2 °C). The absence of bacterial or fungal growth on the surface of the media indicated bactericidal and fungicidal effect of the substances studied. The tests were performed in triplicate. Test-tube contents that showed turbidity were studied microscopically.
Microscopic evaluation of microorganisms
The morphology of the microorganisms (samples of the microbial culture that grew in the liquid nutrient medium) was evaluated under a biological microscope. The sample was dried at room temperature and fixed by applying the physical fixing technique. Gram staining was applied to differentiate between gram-positive and gram-negative bacteria. Samples were observed using a 100-times magnifying immersion lens. Gram-positive bacteria resulted in a purple-blue colour while gram-negative in pink-red.
Evaluation of antimicrobial activity
The study was conducted by applying the technique based on the diffusion of the tested substance into the nutrient medium: trypticase soy agar for bacterial growth, and Sabouraud agar for growing C. albicans. Agars were boiled, cooled to the temperature of 46 ± 1°C, and subsequently poured into sterile plastic Petri dishes, into which 1 ml of the suspension with each respective microorganism (1,5 ⋅× 108 of colony forming units in 1 ml) were introduced with a sterile medicine dropper. McFarland standards were used for the measurement of density of microorganisms in suspension. The resulting mixture was well stirred and left until the media containing the microorganisms cooled down and solidified. Then wells (8 mm in diameter) were formed on the surfaces, where 0.4 ml solutions of the respective test substances were introduced with a sterile medicine dropper. The Petri dishes were stored in a thermostat for 24 hours at 35 ± 1 °C. After that the diameters of inhibition zones were measured. In the control group, microbial cultures were mixed with the nutrient media in the Petri dishes, but no wells were formed, and no tested substances were introduced. The evaluation was performed in triplicate, and the arithmetical mean values and standard deviations were calculated.
Statistical analysis
The obtained results were processed using the statistical data software package IBM SPSS Statistics 5.5 for Windows, and Microsoft® Office Excel 2003 software.
Results and discussion
The investigation demonstrated that hypromellose-containing media became turbid under any storage conditions due to microbial growth. Microscopic examination with Gram staining revealed the presence of gram-positive bacteria (purple-blue), gram-negative bacteria (pink-red), and yeast, which indicate that hypromellose gels are not microbially stable. Thus their protection from microbial contamination requires an addition of substances with antimicrobial characteristics. For this reason antimicrobial properties of grapefruit seed extract (GSE) and concentrated cranberry juice were investigated.
Antimicrobial effect of 0.2–10% GSE solutions on gram-positive and gram-negative bacteria, and Candida albicans is shown in Fig. 1. The greatest sensitivity to GSE was observed in S. aureus, B. cereus and E. faecalis – their growth was already inhibited by a 0.2% concentration. A GSE 0.3% solution also affected E. coli and K. pneumoniae, while 5% and higher concentrations of GSE inhibited the growth of all standard microorganisms. Among the microorganisms studied, P. aeruginosa demonstrated the highest resistance – the growth of bacteria was unaffected by GSE concentrations below 5%. The fungus C. albicans was sensitive to relatively greatly diluted (i.e. 0.7%) solutions. Our findings correlate with those obtained by other researchers14, 16) indicating that the most sensitive to the effect of GSE are gram-positive bacteria.
Fig. 1. Antimicrobial activity of solutions of grapefruit seed extract 
Experiments with concentrated cranberry juice demonstrated inhibition of the growth of studied gram-positive and gram-negative bacteria too, but the inhibitory effect required higher concentrations of the substance, compared to GSE (Fig. 2). The most sensitive to cranberry juice were S. aureus, E. faecalis, B. cereus, and E. coli – their growth was already inhibited by a 3% concentration of cranberry juice. S. aureus was also one of the most sensitive microorganisms in a study of Margariños et al.35). 5% cranberry juice affected P. mirabilis, while 10% and greater concentrations influenced all the bacteria studied. The fungus C. albicans was not affected by cranberry juice at any concentration.
Fig. 2. Antimicrobial activity of solutions of concentrated cranberry juice 
The obtained results indicate that the most resistant microorganisms in the study were P. aeruginosa (concentration required for inhibition was 10% for cranberry juice and 5% for GSE) and C. albicans (was not affected by cranberry juice). S. aureus, B. cereus, and E. faecalis demonstrated the highest sensitivity to both substances under study. E. coli was less sensitive to GSE than S. aureus and E. faecalis bacteria were – the inhibition zones were, accordingly, by 1.3–1.6 times smaller.
The literature sources contain extensive descriptions of the effect of cranberry juice on E. coli29, 33, 35). Our findings confirmed that all the studied concentrations of cranberry juice inhibited the growth of this bacterial culture. Antimicrobial activity of cranberries has also been extensively studied at Kaunas University of Technology, where the ability of extracts from cranberry berries and pulp to inhibit bacterial growth was evaluated. The extracts were found to have a bacteriostatic effect on S. aureus, E. faecalis, and B. subtilis cultures40). These results are similar to those obtained in our study – only the diameters of the inhibition zones were somewhat different, which might have been due to differences in the species of cranberries, the raw material, the biologically active components contained, and/or the concentrations used in the study.
Thus, the obtained results showed that the growth of all the standard microorganisms under study was inhibited by 5% and higher concentrations of GSE. 10% solutions of cranberry juice inhibited the growth of all the bacteria studied but had no effect on C. albicans the growth of which was inhibited starting from a 0.7% concentration of GSE. In order to affect the growth of all microorganisms, a 5% solution of GSE or a combination of the substances under study can be used. For this reason, a mixture of 10% cranberry juice (inhibited all microorganisms except for C. albicans) and 0.7% GSE (the lowest concentration that inhibited C. albicans) was investigated. Figure 3 shows that all microorganisms were thus affected, but a greater inhibitory effect was observed with respect to the microorganisms that were also affected by individually used solutions of the substances under study. The effectiveness of this combination on the microbiological protection of hypromellose gel was also evaluated. Liquid nutrient media with the studied preparations remained clear throughout the monitoring period. This indicates that the selected substances inhibited microbial growth. For the verification of the absence of microorganisms in the preparations studied, their samples were inoculated into solid nutrient media. No microbial growth was observed in these media either. This suggests that the combination of the studied natural substances in gels had an antimicrobial effect in addition to the biological one.
Fig. 3. Comparison of the antimicrobial activity of the solutions of 0.7% grapefruit seed extract (GSE), 10% cranberry juice, and their combination 
Cranberry juice and grapefruit seed extract contain a number of organic acids that create the acidic environment of these substances19, 20). During the study, pH values of hypromellose gel and solutions of GSE and cranberry juice were measured, and an evaluation of how an addition of these substances changed the pH value of the gel was performed. The pH value of hypromellose gel was 5.9 ± 0.5, and 5% GSE only slightly reduced this value (pH 5.5 ± 0.5), while the combination of GSE and cranberry juice had the greatest influence – the gel became significantly acidic (pH 2.5 ± 0.5). For this reason, our next important task was to determine the influence of pH value on the antimicrobial activity of this combination. During our study, the formulation was alkalized, monitoring changes in the sensitivity of the microorganisms under study with respect to changes in the pH value. Since it was determined that the pH value of the mixture of 0.7% GSE and 10% cranberry juice did not differ from the pH value of hypromellose gel containing the same combination, a subsequent evaluation (in order to facilitate the work) was performed with the aqueous solutions of active substances (Table 1). The obtained results showed that the sensitivity of bacteria and fungi to the combination of 0.7% GSE and 10% cranberry juice decreased with increasing pH value (p < 0.05). With pH values increasing from 2.5 to 5, a decrease in the diameters of the inhibition zones was indicated; nevertheless all the standard microbial cultures remained still sensitive to the combination under study. Only when the pH value of the medium reached pH 6, the mixture of 0.7% GSE and 10% cranberry juice was no longer able to inhibit the growth of P. aeruginosa, K. pneumoniae, or P. mirabilis, and in a neutral medium (pH 7) E. coli also became resistant to the combination studied. Reduction of antimicrobial effect with increasing pH value was slower in the case of gram-positive bacteria or fungi, compared to gram-negative prokaryotes – even in a neutral medium the solution of the studied substances inhibited the growth of S. aureus, E. faecalis, B. cereus, or B. subtilis (gram-positive bacteria), and C. albicans.
Tab. 1. Sensitivity of the standard microorganisms to the combination of 0.7% GSE and 10% cranberry juice with respect to the pH value of the medium 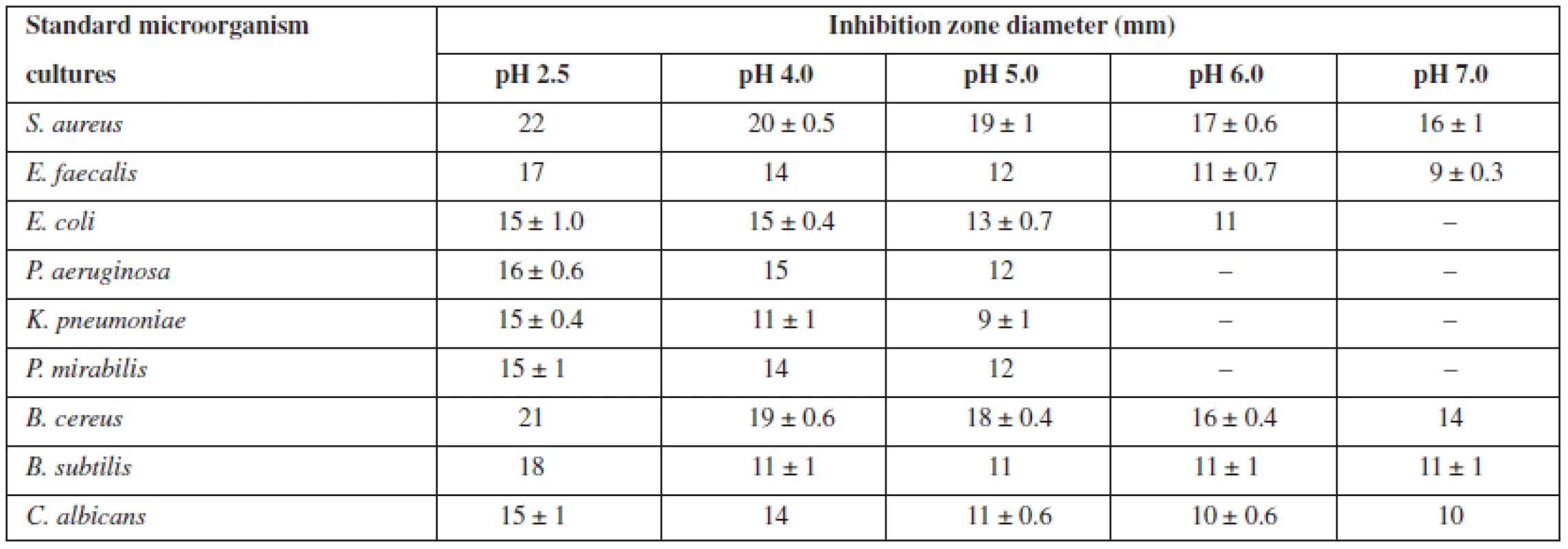
The fact that with pH 7 of the medium the combination of 0.7% GSE and 10% cranberry juice did not inhibit the growth of only gram-negative bacteria (Table 1) suggests that the relation between antimicrobial activity and acidity of the medium may be due to one component of the combination. Since previous studies have shown that the growth of C. albicans was inhibited only by GSE, and the present evaluation has demonstrated that the inhibitory effect on fungi is preserved in the neutral medium, the effect of GSE on microorganisms should not be dependent upon changes in pH value. This hypothesis was verified by separately evaluating antimicrobial activity of a 10% solution of cranberry juice and a 0.7% or 5% solution of GSE (Table 2). The evaluation demonstrated that in a neutral medium (pH 7) a 10% cranberry juice had no inhibitory effect on microbial growth, while the antimicrobial activity of a 0.7% aqueous solution of GSE did not change, i.e. there were de facto no changes in inhibitory zone diameters. This suggests that the relation between antimicrobial activity and pH value of the medium exists only in cranberry juice, i.e. the juice can only inhibit microbial growth in an acid environment. The obtained data confirmed the findings of numerous researchers. Wen et al.41) found that phenolic acids (cranberry contains some of them) could have an antimicrobial effect against Listeria monocytogenes, and the effect was pH-dependent. According to the study by Wu et al.39), a low pH of the cranberry concentrate (mainly contributed by organic acids) plays an important role in inhibiting pathogens. However, their pH effect analysis also indicated that at the same pH level, the cranberry concentrate showed greater antibacterial effects than the simple acidic solution did, implying that other bioactive compounds such as phenolics in the cranberry concentrate may also contribute to the antimicrobial effect36). A 5% aqueous GSE solution inhibited the growth of all the microorganisms studied regardless of the pH value of the medium: no statistical significance was found in the inhibition zone diameter between the acidic (pH 5.5) and neutral (pH 7) environment (Table 2). The obtained findings showed that GSE as an antimicrobial substance may be used independently of the pH value of the formulation.
Tab. 2. Sensitivity of standard microorganisms to the studied substances with respect to the pH value of the medium 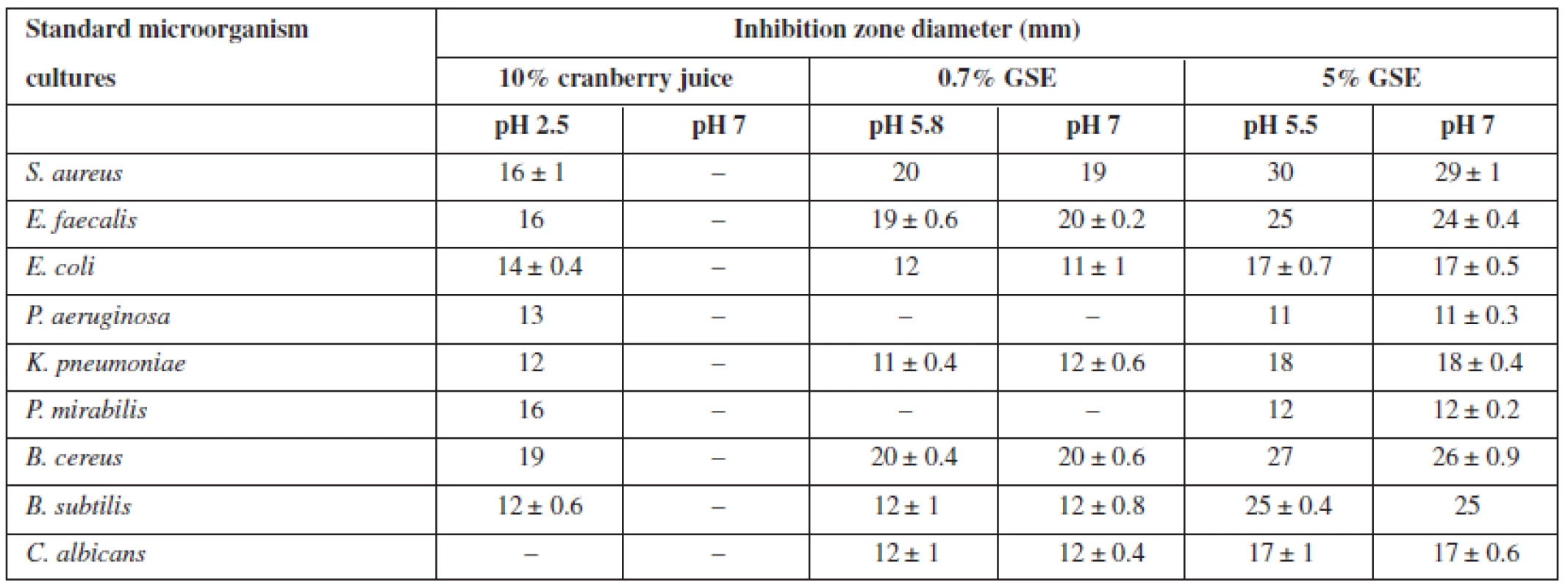
In conclusion, GSE, cranberry juice or their combination not only have a biological effect, but also demonstrate a wide spectrum of antimicrobial activity, and thus they may protect a preparation against contamination with microorganisms, and serve as preservatives in medicines for internal and external use. Both GSE and cranberry juice inhibited the growth of gram-positive and gram-negative bacteria; however the effect of GSE is significantly stronger – C. albicans was sensitive only to GSE. Therefore either a combination of 0.7% GSE and 10% cranberry juice, or 5% GSE alone may be used for microbial growth inhibition. GSE effect is pH independent, while the combination of GSE and cranberry juice is useful only in acidic medium.
Conflicts of interest: none.
Received 7 August 2013
Accepted 10 September 2013
Jurga Bernatonienė* • Rasa Keraitė • Arūnas Savickas
Department of Drug Technology and Social Pharmacy, Academy of Medicine, Lithuanian University of Health Sciences, Kaunas, Lithuania
*Pharmacy of Lithuanian University of Health Sciences, Kaunas, Lithuania
Assoc. Prof. Ruta Masteiková, Ph.D.
University of Veterinary and Pharmaceutical Sciences Brno
Department of Pharmaceutics, Faculty of Pharmacy
Palackého tř. 1/3, 612 42 Brno
e-mail: masteikovar@vfu.cz
Alvydas Pavilonis
Department of Microbiology, Lithuanian University of Health Sciences, Kaunas, Lithuania
Zdroje
1. Rawe R. C., Sheskey P. J., Owen S. C. (eds.) Handbook of Pharmaceutical Excipients. 5th ed. London: Pharmaceutical Press 2006; 948 p.
2. Murray J. C. F. Cellulosics. In: Phillips G.O., Williams P. A. eds. Handbook of Hydrocolloids. Boca Raton: CRC Press 2000.
3. Helin-Tanninen M., Naaranlahti T., Kontra K., Wallenius K. Enteral suspension of nifedipine for neonates. Part 1. Formulation of nifedipine suspension for hospital use. Clin. Pharmacol. Ther. 2001; 26, 49–57.
4. Ji A. J., Ingham E., Wang J. Y. Effect of EDTA and methionine on preventing loss of viscosity of cellulose-based topical gel. AAPS PharmSciTech. 2009; 10, 678–683.
5. Koroloff N., Boots R., Lipman J. Thomas P., Rickard C., Coyer F. J. A randomised controlled study of the efficacy of hypromellose and Lacri-Lube combination versus polyethylene/Cling wrap to prevent corneal epithelial breakdown in the semiconscious intensive care patient. Int. Care Med. 2004; 30, 1122–1126.
6. Boukarim C., Jaoudé S. A., Bahnam R., Barada R., Kyriacos S. Preservatives in liquid pharmaceutical preparations. J. Appl. Res. 2009; 9, 14–17.
7. Gilbert P., Allison D. G. Preservation of pharmaceutical products. In: Swarbrick J., Boylan J. C. eds. Encyclopedia of Pharmaceutical Technology, 2nd ed. New York: Marcel Dekker 2002.
8. Ostrosky E. A., Marcondes E.M. C., Nishikawa S. O., Lopes P. S., Varca G. H. C., Pinto T. J. A., Consiglieri T. V. O., Baby A. R., Velasco M. V. R., Kaneko T. M. Rubus rosaefolius extract as a natural preservative candidate in topical formulations. AAPS PharmSciTech. 2011; 12, 732–737.
9. Davidson P. M., Harrison M. A. Resistance and adaptation to food antimicrobials, sanitizers, and other process controls. Food Technol. 2002; 56, 69–78.
10. Serra A. T., Matias A. A., Nunes A. V. M., Leitão M. C., Brito D., Bronze R., Silva S., Pires A., Crespo M. T., San Romão M. V., Duarte C. M. In vitro evaluation of olive - and grape-based extracts as potential preservatives for food. Innovative Food Sci. Emerg. Technol. 2008; 9, 311–319.
11. Soković M., Glamočlija J., Marin P. D., Brkić D., van Griensven L. J. L. D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010; 15, 7532–7546.
12. Lemay M. J., Choquette J., Delaquis P. J., Gariépy C., Rodrigue N., Saucier L. Antimicrobial effect of natural preservatives in a cooked and acidified chicken meat model. Int. J. Food Microbiol. 2002; 78, 217–226.
13. Von Woedtke T., Schlüter B., Pflegel P., Lindequist U., Jülich W.D. Aspects of the antimicrobial efficacy of grapefruit seed extract and its relation to preservative substances contained. Pharmazie 1999; 54, 452–456.
14. Cvetnić Z., Vladimir-Knežević S. Antimicrobial activity of grapefruit seed and pulp ethanolic extract. Acta Pharm. 2004; 54, 243–250.
15. Heggers J. P., Cottingham J., Gusman J., Reagor L., McCoy L., Carino E., Cox R., Zhao J. G. The effectiveness of processed grapefruit-seed extract as an antibacterial agent: II. Mechanism of action and in vitro toxicity. J. Altern. Complement. Med. 2002; 8, 333–340.
16. Bevilacqua A., Ficelo S., Corbo M. R., Sinigaglia M. J. Bioactivity of grapefruit seed extract against Pseudomonas spp. Food Process Preserv. 2010; 34, 495–507.
17. Edwards-Jones V., Buck R., Shawcross S. G., Dawson M. M., Dunn K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns 2004; 30, 772–777.
18. Xu W., Qu W., Huang K., Guo F., Yang J., Zhao H., Luo Y. Antibacterial effect of grapefruit seed extract on food-borne pathogens and its application in the preservation of minimally processed vegetables. Postharvest Biol. Technol. 2007; 45, 126–133.
19. Faleye F. J., Ogundaini A. O., Olugbade A. T. Antibacterial and antioxidant activities of Citrus paradisi (grapefruit seed) extracts. JPSI 2012; 1, 63–66.
20. Reagor L., Gusman J., McCoy L., Carino E., Heggers J. P. The effectiveness of processed grapefruit-seed extract as an antibacterial agent: I. An in vitro agar assay. J. Altern. Complement. Med. 2002; 8, 325–332.
21. Tirillini B. Grapefruit: the last decade acquisitions. Fitoterapia 2000; 71, S29–S37.
22. Yu J., Dandekar D. V., Toledo R. T., Singh R. K., Patil B. S. Supercritical fluid extraction of limonoids and naringin from grapefruit (Citrus paradisi Macf.) seeds. Food Chem. 2007; 105, 1026–1031.
23. Oyelami O. A., Agbakwuru E. A., Adeyemi L. A., Adedeji G. B. The effectiveness of grapefruit (Citrus paradisi) seeds in treating urinary tract infections. J. Altern. Complement. Med. 2005; 11, 369–371.
24. Zayachkivska O. S., Konturek S. J., Drozdowicz D., Konturek P. C., Brzozowski T., Ghegotsky M. R. Gastroprotective effects of flavonoids in plant extracts. J. Physiol. Pharmacol. 2005; 56, 219–231.
25. Dembinski A., Warzecha Z., Konturek S. J., Ceranowicz P., Dembinski M., Pawlik W. W., Kusnierz-Cabala B., Naskalski J. W. Extract of grapefruit-seed reduces acute pancreatitis induces by ischemia/reperfusion in rats; possible implication of tissue antioxidants. J. Physiol. Pharmacol. 2004; 55, 811–821.
26. Adeneye A.A. Haematopoetic effect of methanol seed extract of Citrus paradisi Macfad (grape fruit) in Wistar rats. Biomed. Res. 2008; 19, 23–26.
27. Côté J., Caillet S., Doyon G., Dussault D., Sylvain J. F., Lacroix M. Antimicrobial effect of cranberry juice and extracts. Food Control 2004; 22, 1413–1418.
28. Caillet S., Côté J., Sylvain J. F., Lacroix M. Antimicrobial effects of fractions from cranberry products on the growth of seven pathogenic bacteria. Food Control 2012; 23, 419–428.
29. Rahbar M., Diba K. In vitro activity of cranberry extract against etiological agents of urinary tract infections. Afr. J. Pharm. Pharmacol. 2010; 4, 286–288.
30. Raz R., Chazan B., Dan M. Cranberry juice and urinary tract infection. Clin. Infect. Dis. 2004; 38, 1413–1419.
31. Cimolai N., Cimolai T. The cranberry and the urinary tract. Eur. J. Clin. Microbiol. Infect. Dis. 2007; 26, 767–776.
32. Howell A. B., Botto H., Combescure C., Blanc-Potard A.-B., Gausa L., Matsumoto T., Tenke P., Sotto A., Lavigne J. P. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect. Dis. 2010; 10, 94. http://www.biomedcentral.com/1471-2334/10/94.
33. Pinzón-Arango P. A., Holguin K., Camesano T. A. Impact of cranberry juice and proanthocyanidins on the ability of Escherichia coli to form biofilms. Food Sci. Biotechnol. 2011; 20, 1315–1321.
34. Bodet C., Grenier D., Chandad F., Ofek I., Steinberg D., Weiss E. I. Potential oral health benefits of cranberry. Crit. Rev. Food Sci. Nutr. 2008; 48, 672–680.
35. Margariños H. L. E., Sahr C., Selaive S. D. C., Costa M. E., Figuerola F. E., Pizarro O. A. In vitro inhibitory effect of cranberry (Vaccinium macrocarpum Ait.) juice on pathogenic microorganisms. Appl. Biochem. Microbiol. 2008; 44, 300–304.
36. Wu V. C. H., Qui X., Bushway A., Harper L. Antibacterial effect of American cranberries (Vaccinium macrocarpon) on foodborne pathogens. LWT-Food Sci. Technol. 2008; 41, 1834–1841.
37. Lacombe A., Wu V. C. H., Tyler S., Edwards K. Antimicrobial action of the American cranberry constituents: phenolics, anthocyanins and organic acids against Escherichia coli O157:H7. Int. J. Food Microbiol. 2010; 139, 102–107.
38. Patel K. D., Scarano F. J., Kondo M., Hurta R. A., Neto C. C. Proanthocyanidin-rich extracts from cranberry fruit (Vaccinium macrocarpon Ait.) selectively inhibit the growth of human pathogenic fungi Candida spp. and Cryptococcus neoformans. J. Agric. Food Chem. 2011; 59, 12864–12873.
39. Feldman M., Tanabe S., Howell A., Grenier D. Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement. Altern. Med. 2012; 12, 6.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3273432/pdf/1472-6882-12-6.pdf.
40. Viskelis P., Rubinskienė M., Jasutienė I., Šarkinas A., Daubaras R., Česonienė L. Anthocyanins, antioxidative and antimicrobial properties of american cranberry (Vaccinium macrocarpon Ait.) and their press cakes. J. Food Sci. 2009; 74, C157–C161.
41. Wen A., Pasacal D., Stanich K., Toivonen P. Antilisterial activity of selected phenolic acids. Food Microbiol. 2003; 20, 305–311.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2013 Číslo 5- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
-
Standard prescriptions for extemporaneously produced medicinal preparations in pharmacies
VI. Collection Neues Rezeptur-Formularium - A combination of grapefruit seed extract and concentrated cranberry juice as a potential antimicrobial preservative for the improvement of microbiological stability of hypromellose gel
- Evaluation of liberation of caffeine from dermal semisolids drugs
- Influence of temperature and concentration of a surfactant on pharmaceutical availability
- Perception of body weight by pharmacists and pharmaceutical laboratory assistants in Slovakia I
- Perception of body weight by pharmacists and pharmaceutical laboratory assistants in Slovakia II
- Pracovní den u příležitosti životního jubilea prof. Jana Solicha a doc. Václava Ruska
- Prof. Ing. Milan Remko, DrSc. CChem FRSC – 65 ročný
-
Standard prescriptions for extemporaneously produced medicinal preparations in pharmacies
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle-
Standard prescriptions for extemporaneously produced medicinal preparations in pharmacies
VI. Collection Neues Rezeptur-Formularium - A combination of grapefruit seed extract and concentrated cranberry juice as a potential antimicrobial preservative for the improvement of microbiological stability of hypromellose gel
- Influence of temperature and concentration of a surfactant on pharmaceutical availability
- Prof. Ing. Milan Remko, DrSc. CChem FRSC – 65 ročný
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



