-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
Sex reversal can occur in XY humans with only a single functional WT1 or SF1 allele or a duplication of the chromosome region containing WNT4. In contrast, XY mice with only a single functional Wt1, Sf1, or Wnt4 allele, or mice that over-express Wnt4 from a transgene, reportedly are not sex-reversed. Because genetic background plays a critical role in testis differentiation, particularly in C57BL/6J (B6) mice, we tested the hypothesis that Wt1, Sf1, and Wnt4 are dosage sensitive in B6 XY mice. We found that reduced Wt1 or Sf1 dosage in B6 XYB6 mice impaired testis differentiation, but no ovarian tissue developed. If, however, a YAKR chromosome replaced the YB6 chromosome, these otherwise genetically identical B6 XY mice developed ovarian tissue. In contrast, reduced Wnt4 dosage increased the amount of testicular tissue present in Sf1+/ − B6 XYAKR, Wt1+/ − B6 XYAKR, B6 XYPOS, and B6 XYAKR fetuses. We propose that Wt1B6 and Sf1B6 are hypomorphic alleles of testis-determining pathway genes and that Wnt4B6 is a hypermorphic allele of an ovary-determining pathway gene. The latter hypothesis is supported by the finding that expression of Wnt4 and four other genes in the ovary-determining pathway are elevated in normal B6 XX E12.5 ovaries. We propose that B6 mice are sensitive to XY sex reversal, at least in part, because they carry Wt1B6 and/or Sf1B6 alleles that compromise testis differentiation and a Wnt4B6 allele that promotes ovary differentiation and thereby antagonizes testis differentiation. Addition of a “weak” Sry allele, such as the one on the YPOS chromosome, to the sensitized B6 background results in inappropriate development of ovarian tissue. We conclude that Wt1, Sf1, and Wnt4 are dosage-sensitive in mice, this dosage-sensitivity is genetic background-dependant, and the mouse strains described here are good models for the investigation of human dosage-sensitive XY sex reversal.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002569
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002569Summary
Sex reversal can occur in XY humans with only a single functional WT1 or SF1 allele or a duplication of the chromosome region containing WNT4. In contrast, XY mice with only a single functional Wt1, Sf1, or Wnt4 allele, or mice that over-express Wnt4 from a transgene, reportedly are not sex-reversed. Because genetic background plays a critical role in testis differentiation, particularly in C57BL/6J (B6) mice, we tested the hypothesis that Wt1, Sf1, and Wnt4 are dosage sensitive in B6 XY mice. We found that reduced Wt1 or Sf1 dosage in B6 XYB6 mice impaired testis differentiation, but no ovarian tissue developed. If, however, a YAKR chromosome replaced the YB6 chromosome, these otherwise genetically identical B6 XY mice developed ovarian tissue. In contrast, reduced Wnt4 dosage increased the amount of testicular tissue present in Sf1+/ − B6 XYAKR, Wt1+/ − B6 XYAKR, B6 XYPOS, and B6 XYAKR fetuses. We propose that Wt1B6 and Sf1B6 are hypomorphic alleles of testis-determining pathway genes and that Wnt4B6 is a hypermorphic allele of an ovary-determining pathway gene. The latter hypothesis is supported by the finding that expression of Wnt4 and four other genes in the ovary-determining pathway are elevated in normal B6 XX E12.5 ovaries. We propose that B6 mice are sensitive to XY sex reversal, at least in part, because they carry Wt1B6 and/or Sf1B6 alleles that compromise testis differentiation and a Wnt4B6 allele that promotes ovary differentiation and thereby antagonizes testis differentiation. Addition of a “weak” Sry allele, such as the one on the YPOS chromosome, to the sensitized B6 background results in inappropriate development of ovarian tissue. We conclude that Wt1, Sf1, and Wnt4 are dosage-sensitive in mice, this dosage-sensitivity is genetic background-dependant, and the mouse strains described here are good models for the investigation of human dosage-sensitive XY sex reversal.
Introduction
Testis differentiation in humans is gene dosage-sensitive. For example, heterozygosity for WT1 or SF1 (NR5A1) mutations and duplications containing WNT4 has been associated with XY sex reversal (SR) [1]–[10]. Testis differentiation in humans also appears to be genetic background-sensitive because fathers who are heterozygous for SF1 mutations or hemizygous for SRY mutations can pass these mutations to their heterozygous/hemizygous XY daughters [4], [11], [12]. In contrast, initial studies in mice suggested testis differentiation is dosage-insensitive because heterozygosity for Wt1 or Sf1 null alleles or transgenic Wnt4 over-expression did not cause XY SR [13]–[17]. This apparent human/mouse species difference led to the hypothesis that some genetic events leading to testis development differed between these species [18]. However, given the evidence that in mice genetic background plays a critical role in testis differentiation [19], [20], we examined the possibility that Wt1, Sf1 and/or Wnt4 would be haploinsufficient for normal testis determination on specific genetic backgrounds.
Work by our group and others has shown that testis determination in C57BL/6J (B6) inbred mice is particularly sensitive to genetic perturbation and a number of inherited gonadal sex reversal conditions have been identified in which B6 XY mice develop ovarian tissue [19]–[23]. The founding member of this group, B6-YPOS sex reversal, occurs when the Y Chromosome (Chr) from Mus domesticus poschiavinus (YPOS) is transferred to the B6 genetic background, replacing the Mus musculus-derived YB6 Chr [19]. B6 XYPOS fetal gonads develop as either ovotestes (gonads that contain both ovarian and testicular tissue) or ovaries, but not normal testes. The YPOS Chr is not inherently defective, however, because it is testis-determining when transferred to most other inbred strain backgrounds, including DBA/2J (D2), BALB/cBy, C3H/HeJ and 129, or when present in F1 hybrids, such as in (D2×B6)F1 mice [24]–[27]. Furthermore, not all M. domesticus-derived Y Chrs (i.e., Sry alleles) behave like YPOS (SryPOS) when transferred to B6. For example, testis differentiation in B6 XY mice containing the BUB/BnJ Y Chr appears to be normal whereas testis differentiation in B6 XY mice containing the AKR/J Y Chr is transiently delayed [28]. B6 XYAKR mice do not develop permanent ovaries or ovotestes like B6 XYPOS mice. However, if B6 XYAKR mice are heterozygous for either the TOrl or Thp deletion on Chr 17 they develop bilateral ovaries. In contrast, B6 XYB6 or B6 XYBUB mice heterozygous for the TOrl deletion develop testes. Thus, the B6-YAKR consomic strain is more sensitive to XY sex reversal than the B6 strain. We previously showed that the transient delay in testicular cord differentiation is caused by the presence of particular SRY protein isoforms found in M. domesticus mice and that the reduced expression of some of these variants, such as the SryPOS allele, causes permanent sex reversal [29].
Further investigations revealed that B6-YPOS sex reversal occurs because the Sry testis-determining gene present on the YPOS Chr does not properly induce testis development if specific interacting genes, designated tda (testis determining autosomal) genes, are homozygous for B6 alleles. Two tda loci that differ between the B6 and D2 genomes and play a role in B6-YPOS sex reversal were mapped to Chrs 2 (tda2) and 4 (tda1) [24]. Intriguingly, the Wt1 (Wilms' tumor 1) and Sf1 (steroidogenic factor 1, officially Nr5a1; nuclear receptor subfamily 5, group A, member 1, also known as Ad4BP, adrenal 4-binding protein) map to the tda2 chromosomal region and Wnt4 (wingless-related MMTV integration site 4) to the tda1 region. Thus, we investigated Wt1 and Sf1 as candidates for tda2, and Wnt4 as a candidate for tda1.
The Wt1 and Sf1 genes share a number of characteristics in addition to those noted above, some of which result from the fact that Wt1 is a direct activator of Sf1 expression [30] including: 1) both encode zinc finger transcription factors [31]–[33]; 2) both are expressed in XX and XY mouse urogenital ridges from about embryonic day (E) 9.5 until E12.5, when expression becomes sexually dimorphic and cell-type restricted; and 3) the gonad progenitor (i.e., genital ridge) is initially present in fetal mice that are homozygous for a null allele of either gene, but differentiation of the genital ridge does not progress and it regresses via apoptosis [14]–[17]. The Wnt4 gene encodes a member of the WNT family of secreted signaling molecules that regulate cell-cell interactions during development. Wnt4 is initially expressed in XX and XY genital ridges; thereafter expression is down-regulated in XY gonads but maintained in XX gonads [34]. Homozygosity for a Wnt4 null mutation causes partial sex reversal of XX gonads resulting in the development of a testis-like coelomic vessel and the presence of cells expressing steroidogenic enzymes [34], [35]. In contrast, the gonadal phenotype in homozygous Wnt4 null XY mice is relatively less severe; the gonads develop as testes, but the cords are initially fewer in number and disorganized [36]. To our knowledge, Wnt4 heterozygotes have no reported gonad differentiation defects. In XY mice, transgenic Wnt4 over-expression also causes testis differentiation defects but not sex reversal [13], [35].
To examine the possibility that the Wt1, Sf1, and Wnt4 genes would be haploinsufficient on the B6 genetic background, we transferred null alleles to the B6 and D2 backgrounds and determined if reduced dosage influenced gonadal development in XYB6 and XYAKR mice. Gonad morphology and marker gene expression in mutant versus normal fetal mice was analyzed using immunohistochemistry and confocal microscopy, RNA in situ hybridization, and quantitative RT-PCR. The results of these experiments indicated that testis differentiation was compromised at a very early stage and that Sry expression was significantly reduced in Wt1+/ − or Sf1+/− B6 XYAKR but not in Wt1+/ − or Sf1+/− (D2×B6)F1 XYAKR fetal gonads. We also found that testis development was mostly rescued in Wt1+/ − and Sf1+/− B6 XYAKR fetuses that contained two Sry alleles. These results are consistent with the hypothesis that the Wt1B6 and Sf1B6 alleles behave as hypomorphic alleles of testis-determining pathway genes in B6 XY mice if other genes in the genome are homozygous for the B6 allele. In contrast, reduced Wnt4 dosage did not exacerbate the transient delay in testis differentiation in B6 XYAKR gonads but rather appeared to ameliorate the phenotype, which suggests that the B6-derived Wnt4 allele (Wnt4B6) is a hypermorphic allele of a gene in the ovary determination pathway. We also found that B6 Wt1+/ − XYAKR, B6 Sf1+/ − XYAKR, and B6 XYPOS fetuses with reduced Wnt4 dosage developed more testicular tissue than Wnt4+/+ fetuses. These data are consistent with the hypothesis that Wnt4B6 is an early functioning allele of an ovary-determining gene that antagonizes testis differentiation in XY mice. Expression of Wnt4 and four other ovary-biased or ovary-specific genes was found to be elevated in E12.5 B6 vs. D2 XX ovaries. Together, our results suggest that B6 mice are sensitive to XY sex reversal compared to D2 mice because genes in the ovary determination pathway function earlier in the B6 genome than they do in the D2 genome.
The results reported here show that the Wt1, Sf1, and Wnt4 genes are dosage-sensitive during mouse primary sex determination, as they are in humans, and that this effect is modulated by genetic background in the mouse, as is probably the case in humans. We propose that the dosage-sensitive C57BL/6 XY mouse strains described here are powerful models for further investigation of human dosage-sensitive XY sex reversals. In addition, our results support the candidacy of Wt1, Sf1, and Wnt4 as tda genes, and suggest the testable hypothesis that B6 XYPOS sex reversal is cause by the additive effects of: 1) hypomorphic Wt1B6 and Sf1B6 alleles, and reduced activation of the testis differentiation pathway, 2) a hypermorphic Wnt4B6 allele and hyperactivation of the ovary differentiation pathway, and 3) reduced function and expression of the SryPOS allele.
Results
Wt1+/ − and Sf1+/ − B6 XYAKR mice are sex-reversed
To examine the possibility that Wt1 and/or Sf1 would be haploinsufficient on the B6 genetic background and to investigate their candidacy as tda genes, we transferred null alleles to the B6 and D2 backgrounds and determined if reduced dosage influenced gonadal development in XYB6 and XYAKR mice. We reasoned that if the B6 alleles of Wt1 and/or Sf1 function as hypomorphs in the testis determination pathway, then further reducing their dosage in B6 XY mice should cause sex reversal, whereas reducing their dosage in D2 XY mice should not. Sex reversal was not evident in Wt1+/ − or Sf1+/ − B6 or D2 XY weanling or adult mice, and the mice were fertile. To increase the sensitivity of the genetic background to sex reversal, we mated Wt1 +/ − and Sf1+/ − B6 XX females to B6 XYAKR males. Based on the external sexual phenotype of 39 Wt1+/ − B6 XYAKR mice examined, 20 presented as male, 11 as hermaphrodite, and 8 as female (Table 1). In contrast, of the 20 Sf1+/ − B6 XYAKR offspring, none presented as male, 4 as hermaphrodite, and 16 as female. We noted a deficiency in the expected number of B6 Sf1+/ − animals recovered, regardless of chromosomal sex. To test if these XY sex reversals were dependent on genetic background like B6-YPOS sex reversal, offspring were analyzed from mating Wt1+/ − B6 XX females to AKR/J XYAKR males and Sf1+/ − D2 XX females to B6 XYAKR males. (D2 Wt1+/ − mice were not available when these analyses were performed but similar results were obtained in the analysis of gonad morphology in E14.5–16 fetuses when mating Wt1+/ − D2 XX females to B6 XYAKR mice, Table 2.) In F1 hybrids, the heterozygous XY offspring presented as normal males and the number of Sf1+/ − (D2×B6)F1 offspring recovered was Mendelian. We conclude that Wt1+/ − and Sf1+/ − B6 XYAKR mice are sex reversed, Sf1+/ − B6 mice have reduced viability, and both sex reversal and reduced viability are sensitive to genetic background. These results also indicate that the sex reversal phenotype is stronger in Sf1 mutants because more Sf1+/ − than Wt1+/ − B6 XYAKR mice presented as females and hermaphrodites.
Tab. 1. External sexual phenotype of Wt1+/− and Sf1+/− weaning age mice. 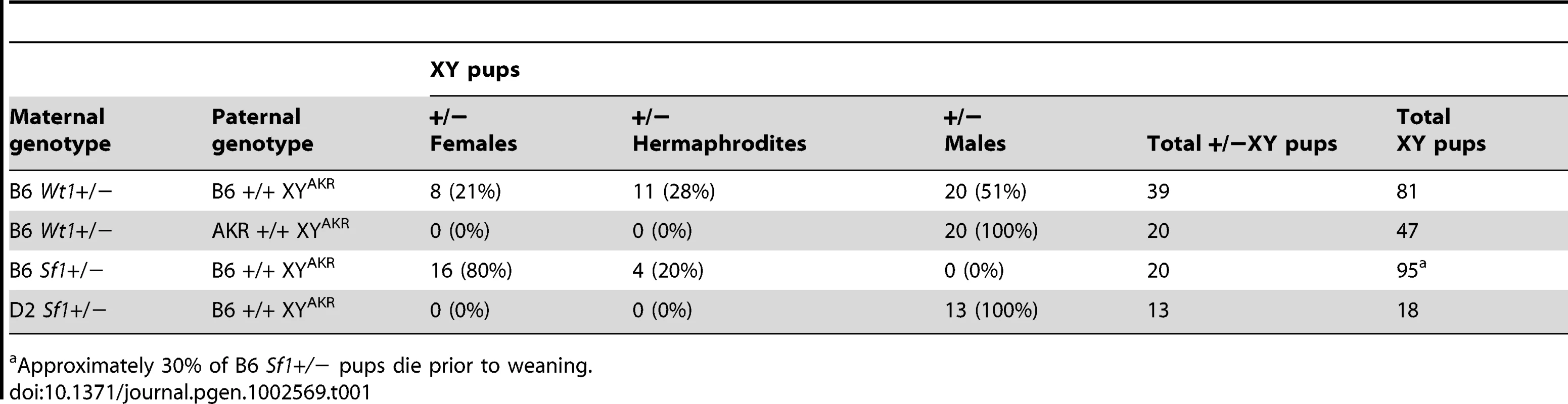
Approximately 30% of B6 Sf1+/− pups die prior to weaning. Tab. 2. Gonad phenotype in E14.5–16 Wt1+/− and Sf1+/− XY fetuses. 
Cord development is somewhat delayed at the anterior and/or posterior ends but the gonad is not an obvious ovotestis. Gonad morphology in E14.5–16 fetuses was examined because assessment of external sexual phenotype does not distinguish between primary and secondary sex reversal, and thus is only an estimate of the true sex reversal frequency. At this developmental stage small amounts of ovarian tissue are visually apparent in ovotestes and the initial delay in testicular cord development has resolved in B6 XYAKR fetuses, thus preventing the misclassification of the gonad as an ovotestis. All Wt1+/ − B6 XYB6, Wt1+/ − (B6×D2)F1 XYAKR and Wt1+/ − (D2×B6)F1 XYAKR gonads analyzed were testes (Figure S1 and Table 2). In contrast, approximately half of the 48 Wt1+/ − B6 XYAKR gonads analyzed were ovotestes (23) or ovaries (5), and half were normal (8) or abnormal testes (12). Similarly, all Sf1+/ − B6 XYB6 and Sf1+/ − (D2×B6)F1 XYAKR gonads analyzed were testes, whereas the 33 Sf1+/ − B6 XYAKR gonads analyzed were ovotestes (3) or ovaries (31). These results are consistent with the weanling analysis and show that sex reversal is the result of primary (gonadal) sex reversal.
Morphological and marker gene analysis of E13.5 Wt1+/ − and Sf1+/ − B6 XYAKR gonads
To gain further insight into the mechanism of sex reversal in Wt1+/ − and Sf1+/ − B6 XYAKR gonads, whole-mount immunohistochemistry (WIHC, Figure 1) and whole-mount RNA in situ hybridization (WISH, Figure 2) were used to analyze morphology and marker gene expression in E13.5 gonads. Overall, the morphological analyses were consistent with the E14.5–16 analyses: Wt1+/ − B6 XYAKR gonads were classified as ovaries, ovotestes and testes and Sf1+/ − B6 XYAKR gonads were classified as ovaries and ovotestes. The Wt1+/ − and Sf1+/ − B6 XYAKR gonads that developed as ovaries or ovotestes were phenotypically indistinguishable regardless of which gene was mutant. However, some Wt1+/ − B6 XYAKR gonads appeared to be very similar in morphology and marker gene expression pattern to those observed in control +/+ B6 XYAKR gonads, whereas none of the Sf1+/ − B6 XYAKR gonads were phenotypically similar to +/+ B6 XYAKR gonads.
Fig. 1. WIHC analysis of morphology and marker expression in Wt1+/− and Sf1+/− B6 XYAKR E13.5 gonads. 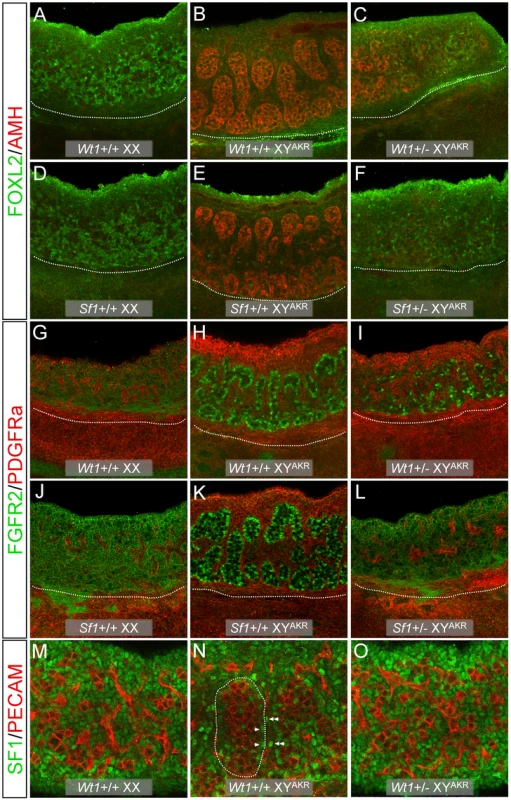
(A–L). Confocal images of gonad/mesonephros complexes with gonads shown above and mesonephroi below the white dotted line (20× magnification). Heterozygous XY gonads differentiated as ovotestes (C), ovaries (F, L, O), or gonads that were morphologically ovarian but expressed testicular markers (I). The Wt1+/− XY ovotestis in (C) expressed AMH, a Sertoli cell marker, in the central region containing testicular cords but not at poles that lacked cords. FOXL2, a granulosa cell marker, was expressed at the poles. FOXL2 also was expressed in isolated cells within regions containing testicular cords in B6 XYAKR gonads (E). Heterozygous XY ovaries (F, L, O) expressed ovarian but not testicular markers. The Wt1+/− XY ovary in (F) expressed FOXL2 but not AMH throughout. The Sf1+/− XY ovary in (L) expressed FGFR2 on the surface of somatic cells (ovarian pattern) but did not have cells with nuclear localization of FGFR2 (testicular pattern, note localization in Sertoli cells (H, K)). The Wt1 +/− XY gonad in (I) was ovarian morphologically but contained many cells with nuclear localization of FGFR2. These cells were not organized into testicular cords. PDGFRa was expressed at high levels in the coelomic epithelium of B6 XYAKR gonads (H, K) and at lower levels in interstitial cells of B6 XX (G, J) and XYAKR (H, K) gonads. Ovotestes, expressed PDGFRa at high levels in the coelomic epithelium of regions containing cords and at lower levels in regions without cords (data not shown). The heterozygous XY ovary in (L) expressed PDGFRa in a pattern similar to XX ovaries. The masculinized ovary in (I) expressed PDGFRa in a testis-like pattern. (M–O) Confocal images showing SF1 expression in gonads (40× magnification). At this stage, SF1 is expressed at fairly equal levels in all somatic cells in wild-type XX ovaries (M). In wild-type testes, expression is up-regulated in Sertoli cells (arrowheads) within testis cords (outlined with white dotted line) and in specific interstitial cells (double arrowheads), while it is down-regulated in most other interstitial cells (N). The expression of SF1 in Wt1+/− XY ovaries (O) resembled that of wild-type XX ovaries as expression was uniform in all somatic cells. Note that overall SF1 expression levels were similar in normal XX (M) vs. Wt1+/− XY (O) ovaries. Fig. 2. WISH analysis of the ovary somatic cell marker Wnt4 and the testis Leydig cell marker Ptch1 in E13.5 gonad/mesonephros complexes. 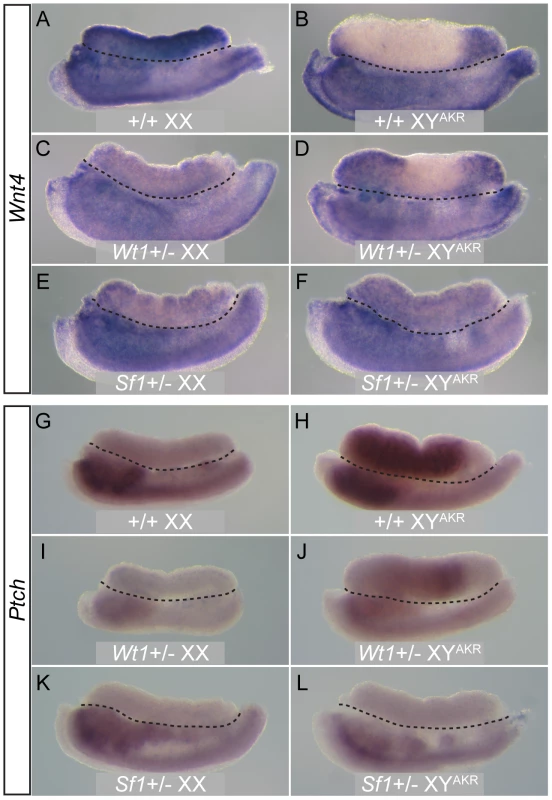
Wnt4 was expressed throughout +/+ XX ovaries, as expected (A), and at the anterior and/or posterior poles of +/+ B6 XYAKR gonads in the regions without cords, but not in the central region containing cords (B). Expression was detected at the anterior and/or posterior poles of Wt1+/−(D) and Sf1+/− (data not shown) B6 XYAKR ovotestes and throughout Wt1+/− (data not shown) and Sf1+/− B6 XYAKR ovaries (F). Wnt4 expression was reduced in B6 Wt1+/− XX (C) and Sf1+/− XX (E) ovaries compared to B6 Wt1+/+ XX (A) ovaries. Expression also was reduced in B6 Wt1+/− mesonephroi (C and D), but not in Sf1+/− mesonephroi (E and F). Ptch1 expression was restricted to central regions containing testicular cords in +/+ B6 XYAKR gonads (H) and was not detected in +/+ B6 XX ovaries (G), as expected. Expression was not detected in Wt1+/− and Sf1+/− B6 XX ovaries (I and K). Ptch1 was expressed in the central regions of Wt1+/− (J) and Sf1+/− (data not shown) B6 XYAKR ovotestes but at notably lower levels than in +/+ B6 XYAKR gonads, and it was not expressed in Wt1+/− (data not shown) and Sf1+/− (L) XYAKR ovaries. Ptch1 expression was reduced in the anterior region of B6 Wt1+/− mesonephroi (I and J), but not in Sf1+/− mesonephroi (K and L). The gonad/mesonephros complex in each panel is oriented with the gonad above the mesonephros and separated by a dotted black line with the anterior (cranial) pole to the left. All images are at 10× magnification. Generally, Wt1+/ − and Sf1+/ − B6 XYAKR gonads that developed as ovaries were morphologically indistinguishable from normal XX ovaries and strongly expressed ovary-specific markers, such as FOXL2 (Figure 1F), Wnt4 (Figure 2F), Fst (data not shown) and Bmp2 (data not shown) in a pattern similar to XX ovaries. Additionally, XY ovaries generally did not express testis-specific Sertoli cell markers, such as AMH (Figure 1F) or SOX9 (data not shown), or Leydig cell markers, such as Ptch1 (Figure 2L), or markers such as GATA4, WT1 (data not shown) or FGFR2 (Figure 1L) in a testis-like pattern. However, about 20% of XY mutant gonads that had ovarian morphology contained cells that expressed Sertoli cell-specific markers, such as AMH or SOX9 (data not shown), or expressed markers, such as PDGFRa and FGFR2 in a testis-like pattern (Figure 1I). However, these cells were not organized in a pattern that resembled testicular cords. We conclude that Sertoli cell differentiation was initiated in some XY gonads that were morphologically ovarian, but was insufficient to initiate or sustain cord formation.
In Wt1+/ − and Sf1+/ − B6 XYAKR ovotestes, testis markers generally were expressed in central regions containing cords whereas ovary markers were expressed at the anterior and posterior poles within regions lacking cords. For example, Figure 1C shows part of an ovotestis in which the central region contained cords and strongly expressed AMH, while the adjacent posterior region lacked cords and expressed FOXL2. Figure 2J shows an ovotestis that expressed Ptch1 in the central region that contained testicular cords, but not in the ovarian regions at the anterior and posterior poles. [Ptch1 expression in ovotestes was not as strong as that observed in B6 XYAKR control gonads (compare Figure 2H and 2J)]. Conversely, Figure 2D shows an ovotestis expressing Wnt4 in the anterior and posterior regions, but not in the central testicular region. Occasionally, testis - and ovary-specific markers were expressed in the same region (Figure 1E), where FOXL2 expression was detected in specific cells within a region containing well-formed testicular cords and strongly expressing the Sertoli cell marker AMH. We noted a few cells that appeared to express both AMH and FOXL2 (Figure S2A). However, because only whole-mount tissue was examined and because AMH is localized to the cell surface and FOXL2 to the nucleus, we were not able to exclude the possibility that the expression was in adjacent cells. Nevertheless, it is possible that some supporting cell progenitors can express both granulosa and Sertoli cell markers under certain conditions.
Because Wt1 is functionally upstream of Sf1 and is necessary for the initiation of Sf1 expression [30], we examined SF1 expression in Wt1+/ − B6 XYAKR gonads using WIHC and found that expression levels appeared normal (Figure 1N–1O). Additionally, quantitative real-time RT-PCR (qRT-PCR) analysis did not detect a significant difference in Sf1 expression in E12 gonads from Wt1+/ − B6 XYAKR vs. +/+ B6 XYAKR fetuses (data not shown). On the other hand, Wnt4 expression appeared to be reduced in Wt1+/ − and Sf1+/ − B6 XX ovaries compared to +/+ B6 XX ovaries, and Ptch1 expression was strongly reduced in Wt1+/ − B6 XX and B6 XY mesonephroi (Figure 2). Overall, the morphological and marker gene expression analyses showed that the testis determination pathway is aberrant at an early stage, that the ovarian pathway is activated in Wt1+/ − and Sf1+/ − B6 XYAKR gonads, and that gene expression is affected in both XX and XY heterozygous gonad/mesonephros complexes.
A multigene qRT-PCR strategy was used to quantify gene expression changes in E12 +/ − vs. +/+ B6 XYAKR gonads, and E14 +/ − ovaries vs. +/+ B6 XYAKR testes (Table 3). These quantitative results were consistent with the morphological and marker gene analyses. For example, Adamts19, an ovary-specific gene, was significantly up-regulated in E14 Wt1+/ − and Sf1+/ − B6 XYAKR ovaries vs. +/+ B6 XYAKR control testes, and Cyp11a1 and Cyp17a1, both Leydig cell specific genes, were expressed at significantly lower levels in Wt1+/ − and Sf1+/ − B6 XYAKR ovaries.
Tab. 3. Quantitative RT–PCR analyses of E12 +/− versus +/+ B6 XYAKR gonads and E14 +/− B6 XYAKR ovaries (ovotestes excluded) versus +/+ B6 XYAKR testes. 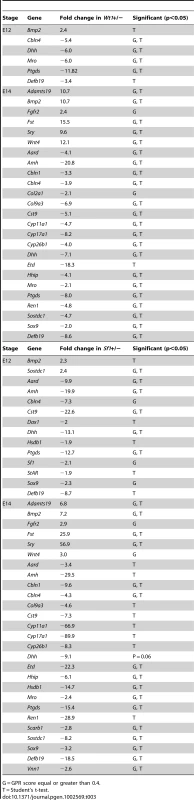
G = GPR score equal or greater than 0.4. Ovary-specific markers are expressed at the anterior and posterior of B6 XYAKR gonads
During analysis of marker gene expression in E13.5 Wt1+/ − and Sf1+/ − B6 XYAKR gonads using WIHC and WISH we observed the expression of ovary-specific somatic cell markers in gonads from +/+ B6 XYAKR fetuses. As expected, testis-specific markers, such as SOX9, AMH and Ptch1, were expressed in the central regions that contained testicular cords (Figure 1, Figure 2, and Figure S2). However, FOXL2, Wnt4, and Fst often were expressed in the anterior and posterior regions that lacked cords (Figure 1, Figure 2, Figure S2, and data not shown), indicating that the ovary differentiation pathway was activated in these regions. WISH analysis of the meiotic germ cell markers Stra8 and Rec8 [37] and the ovary-specific somatic cell marker Irx3 [38] was used to determine if activation of the ovary differentiation pathway extended to the germ cells and to quantify the occurrence of ovary-specific marker gene expression (Figure S2B). We found that 11 of 13 B6 XYAKR gonads clearly expressed either Stra8 or Rec8 at one or both poles and that 7 of 7 gonads expressed Irx3 at both poles. In the 9 gonads where Stra8 or Rec8 were expressed at both poles, expression was higher at the anterior pole than the posterior pole, suggesting that the anterior-to-posterior wave of meiotic germ cell gene expression is maintained despite the presence of intervening testicular tissue [37]. It is important to note that testicular cords are present throughout B6 XYAKR gonads after about E14.5. Therefore, these results are consistent with the idea that B6 XYAKR gonads develop as transient ovotestes [39], [40].
Testis and adrenal gland development is abnormal in B6 XYB6 fetuses that are double-heterozygous for Wt1 and Sf1
Because the Wt1+/ − and Sf1+/ − B6 XYAKR sex reversal phenotypes appear to be very similar and because Wt1 is necessary for activation of Sf1 expression in fetal gonads [30], we examined if B6 XYB6 mice were sex reversed when double heterozygous for Wt1 and Sf1 (Wt1+/−; Sf1+/−). We first attempted to ascertain the external sexual phenotype of the double heterozygotes at weaning. However, no Wt1+/−; Sf1+/ − mice were recovered among the 84 progeny from B6 Wt1+/−×B6 Sf1+/ − matings (Table 4). Because our previous results suggested that lethality could be sensitive to genetic background, progeny from B6 Wt1+/−×D2 Sf1+/ − matings were analyzed. Among the 23 offspring were 4 Wt1+/−; Sf1+/ − mice, indicating that lethality was rescued in (B6×D2)F1 hybrids. To determine at which developmental stage the lethality occurred, E14.5–15.5 fetuses from B6 Wt1+/−×B6 Sf1+/ − matings were analyzed. Among the 12 offspring were 3 Wt1+/−; Sf1+/ − mice (2 XY and 1 XX), indicating that lethality occurred between E15.5 and weaning. Histological examination of the three Wt1+/−; Sf1+/ − fetuses showed that each had bilateral adrenal agenesis, which likely would cause neonatal lethality (Figure 3A and 3B). Both of the Wt1+/−; Sf1+/ − XY fetuses had bilateral testes that appeared morphologically normal, but were smaller than those in wild-type XY littermates.
Fig. 3. Histological and WIHC analysis of Wt1+/−; Sf1+/− B6 XYB6 fetuses. 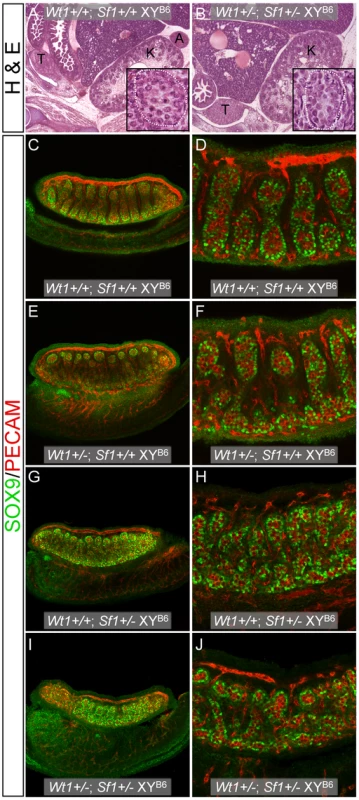
(A and B, 10× magnification) Hematoxylin and eosin staining of E14.5–15.5 fetal sagittal sections showed the absence of adrenal glands in Wt1+/−; Sf1+/− B6 XYB6 and XX (data not shown) fetuses. The inset panels in A and B are a magnified view showing that normal testicular cords were present in both Wt1+/+; Sf1+/+ and Wt1+/−; Sf1+/− B6 XYB6 fetuses (white dotted line outlines a section of a single cord). (C–J) WIHC analysis of E13.5 B6 XYB6 gonad/mesonephros complexes stained with SOX9 (Sertoli cells, green) and PECAM (germ cells and vascular endothelial cells, red). Panels C, E, G and I show 10× images taken with 0.7× zoom. Panels D, F, H, and J show 20× images. The gonads in all wild-type and single heterozygous E13.5 B6 XYB6 gonads were classified as testes, but testis cords in Wt1+/−; Sf1+/+ (E and F) and Wt1+/+; Sf1+/− (G and H), B6 XYB6 gonads were less developed and more disorganized than cords in Wt1+/+; Sf1+/+ (C and D) B6 XYB6 gonads. B6 Wt1+/−; Sf1+/+ gonads, however, were more similar to wild-type gonads than were Wt1+/+; Sf1+/− gonads. Testis differentiation was most affected in Wt1+/−; Sf1+/− B6 XYB6 gonads and these were classified as ovotestes: Sertoli cells in the anterior and posterior poles often expressed SOX9 but were not organized into cords (I and J). Tab. 4. Survival of <i>Wt1</i>+/−; <i>Sf1</i>+/− offspring. 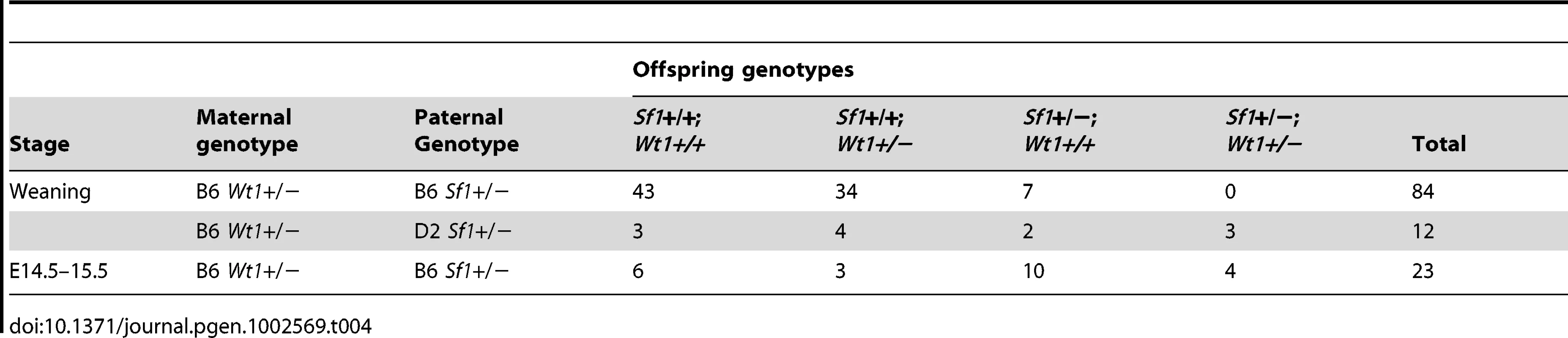
To extend these observations, gonad development was examined in E13.5 fetuses from B6 Wt1+/−×B6 Sf1+/ − matings using WIHC (Figure 3C–3J). Of the 15 fetuses examined, 7 were Wt1+/−; Sf1+/−, which is consistent with the E14.5–15.5 survival data. Of the 9 XYB6 fetuses, 4 were Wt1+/−; Sf1+/−, 4 were Wt1+/+; Sf1+/−, and 1 was Wt1+/+; Sf1+/+. All 8 Wt1+/−; Sf1+/ − XY gonads were classified as ovotestes, with large central testicular regions (Figure 3I and 3J). (Ovotestes in E13.5 double heterozygous fetuses appear to resolve into testes at later stages because the gonads in E14.5–15.5 double heterozygous fetuses do not contain ovarian tissue.) The 8 gonads from Wt1+/+; Sf1+/ − XY fetuses were classified as testes but their cords appeared to be less well developed and more disorganized than +/+ B6 XY gonads (Figure 3). None of the XY offspring from these intercrosses were Wt1+/−; Sf1+/+, so we examined gonad development in E13.5 Wt1+/ − B6 XYB6 fetuses generated from B6 Wt1+/−×B6 +/+ XYB6 crosses. All 6 gonads from Wt1+/ − B6 XYB6 fetuses were classified as testes. However, their cords appeared to be less well-developed and more disorganized than normal B6 XY gonads: 4 displayed disorganized anterior and posterior poles and 2 had disorganized posterior poles (Figure 3E and 3F). In comparison, of the 6 gonads from +/+ B6 XYB6 fetuses, 2 had testicular cords throughout and 4 had disorganized posterior poles. In summary, the degree of testicular cord development in the E13.5 B6 XYB6 gonads could be ranked from most complete to least complete as: Sf1+/+; Wt1+/+>Sf1+/+; Wt1+/−>Sf1+/−; Wt1+/+>Sf1+/−; Wt1+/−. These results indicate that testis development is compromised in Wt1+/ − and Sf1+/ − B6 XY fetuses, even in the absence of the YAKR Chr, and that testis development is further compromised in mice that are double heterozygous for Wt1 and Sf1 null alleles.
Sry expression is reduced in Wt1+/ − and Sf1+/ − B6 XYAKR gonads and sex reversal is partially rescued by increased Sry dosage
Because the morphological and marker gene expression analyses in E13.5 gonads suggested an early defect in the testis determination pathway, Sry expression in E11.5 Wt1+/− and Sf1+/− B6 XYAKR vs. +/+ B6 XYAKR gonad/mesonephros complexes was quantified. (Sry is not expressed in the mesonephros.) During peak Sry expression (E11.75), transcript levels in both mutants were less than 50% of the controls. We found that Sry transcript levels were reduced in Wt1+/− vs. +/+ samples at E11.25 (15–17 ts, t24 = 2.224 , p<0.0179), E11.5 (18–20 ts, t25 = 3.653, p<0.0012), and E11.75 (21–23 ts, t3 = 11.51, p<0.0014) but not significantly different at E12.0 (24–26 ts, Figure 4A). Sry transcript levels also were reduced in Sf1+/− vs. +/+ samples at E11.5 (18–20 ts, t29 = 2.125, p<0.0422) and E11.75 (21–23 ts, t5 = 6.142, p<0.0016), but were not significantly different at E11.25 (15–17 ts) or E12.0 (24–26 ts, Figure 4A). These results suggest that sex reversal in Wt1+/− and Sf1+/− B6 XYAKR gonads is at least partially due to a failure to sufficiently up-regulate Sry expression and properly initiate the testis determination pathway. However, because pairwise comparisons between Wt1+/− vs. Sf1+/− samples did not reveal significant differences in Sry expression at any of the embryonic stages analyzed, differences in the level of Sry expression do not explain the differences in the severity of the Wt1+/− and Sf1+/− phenotypes.
Fig. 4. Sry expression is reduced in Wt1+/− and Sf1+/− B6 XYAKR gonads, but increased Sry dosage did not fully restore normal cord differentiation. 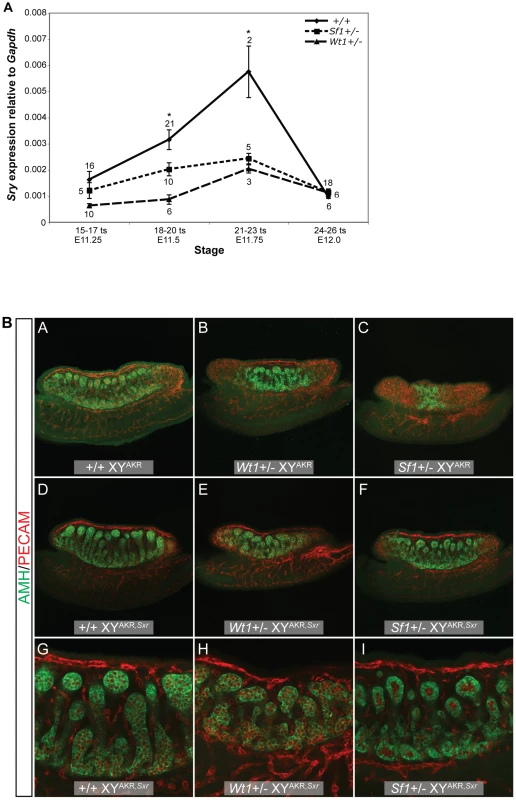
(A) Real-time qRT-PCR analysis of Sry expression relative to Gapdh (2−ΔCt) showing mean Sry transcript levels +/− standard error. The sample size for each group is shown adjacent to the corresponding data point. An asterisk denotes statistically significant effects of genotype (ANOVA, α = 0.05). There was a marginally significant effect of genotype at E11.25 (15–17 ts, ANOVA, F2,28 = 3.1670, p<0.0576) and a significant effect of genotype at E11.5 (18–20 ts, ANOVA, F2,34 = 6.999, p<0.0028) and E11.75 (21–23 ts, ANOVA, F2,7 = 15.41, p<0.0027). Pairwise comparisons revealed a significant reduction in Sry transcript levels in Wt1+/− vs. +/+ samples at E11.25 (15–17 ts, t24 = 2.224 , p<0.0179), E11.5 (18–20 ts, t25 = 3.653, p<0.0012), and in E11.75 (21–23 ts, t3 = 11.51, p<0.0014). Similarly, Sry expression was reduced in Sf1+/− vs. +/+ samples at E11.5 (18–20 ts, t29 = 2.125, p<0.0422) and E11.75 (21–23 ts, t5 = 6.142, p<0.0016). (B) The amount of tissue expressing the Sertoli cell marker AMH (green) was greater in Wt1+/− and Sf1+/− B6 XYAKR,Sxr gonads (E and F), which have two copies of the Sry gene, compared to Wt1+/− and Sf1+/− B6 XYAKR gonads (B and C), which have the normal single copy of the Sry gene (top two rows, 10× magnification with 0.7× zoom). However, while Wt1+/− and Sf1+/− B6 XYAKR,Sxr gonads developed more testicular tissue than Wt1+/− and Sf1+/− B6 XYAKR gonads, they consistently develop less testicular tissue than +/+ B6 XYAKR,Sxr controls. Additionally, cords were less differentiated and less organized in heterozygous mutants when compared to wild-type B6 XYAKR,Sxr gonads, even in the central regions of the gonads where cord differentiation was most complete (G–I, bottom row, 20× magnification). Germ and vascular endothelial cells are labeled by PECAM staining (red). To test the hypothesis that sex reversal in Wt1+/− and Sf1+/− B6 XYAKR gonads is due, at least in part, to reduced Sry expression, gonad morphology was examined by WIHC in gonads from E13.5 fetuses that had increased Sry dosage due to the presence of the YAKR,Sxr Chr. [Classification was done independently by two investigators (SMC and KHA)]. This rearranged Y Chr carries two Sry alleles, one from the AKR/J inbred strain (M. domesticus) and one from the RIII inbred strain (M. musculus). Gonads in Wt1+/− and Sf1+/− B6 XYAKR,Sxr fetuses consistently developed more testicular tissue than gonads in Wt1+/− and Sf1+/− B6 XYAKR fetuses; however, it appeared that cord differentiation in the Wt1+/− and Sf1+/−B6 XYAKR,Sxr gonads was not completely normal (Figure 4B, Table 5). Of the 8 Wt1+/− B6 XYAKR,Sxr gonads analyzed, 7 were classified as abnormal testes because the testicular cords appeared to be more disorganized and less well developed than those in +/+ B6 XYAKR,Sxr gonads. In fact, these cords appeared to be similar to the abnormal cords in Wt1+/− B6 XYB6 testes. One of the Wt1+/− B6 XYAKR,Sxr gonads was classified as an ovotestis because it lacked cords in a small region at the posterior pole and had disorganized testicular cords in the remainder of the gonad. Of the 8 Sf1+/− B6 XYAKR,Sxr gonads analyzed, 3 were classified as abnormal testes, with testicular cords throughout the gonad, and 5 were classified as ovotestes due to the absence of testicular cords at the posterior pole. The cords in these 8 gonads also were somewhat disorganized and less differentiated. In contrast, all 17 of the +/+ B6 XYAKR,Sxr gonads examined developed robust, well-organized testicular cords throughout the anterior, center and posterior regions. These data indicate that increasing Sry dosage in Wt1+/− and Sf1+/− B6 XYAKR gonads by replacing the YAKR Chr with the YAKR,Sxr Chr dramatically increased the amount of testicular tissue that develops, but did not fully rescue cord differentiation.
Fig. 5. Wt1 but not Sf1 transcript levels were significantly lower in B6 versus D2 gonad/mesonephros complexes. 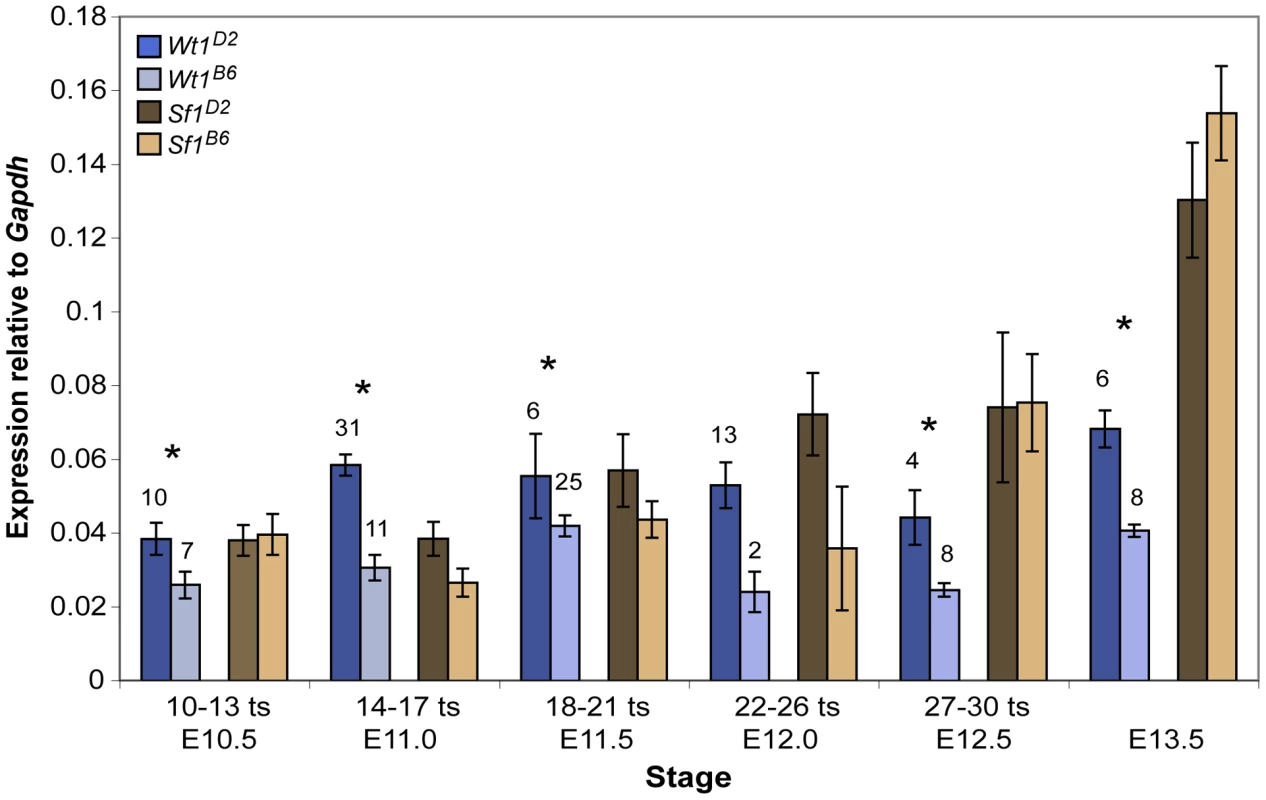
Real-time qRT-PCR analysis of Wt1 and Sf1 expression normalized to Gapdh (2−ΔCt) showing mean transcript levels +/− standard error. Wt1 and Sf1 expression were determined in the same B6 and D2 samples, therefore the samples sizes are only shown above the corresponding error bars for the Wt1B6 and Wt1D2 comparisons. An asterisk denotes statistically significant differences in pairwise comparisons (α = 0.05). Wt1 expression was significantly lower in B6 vs. D2 samples at E10.5 (10–13 ts, t15 = 2.063, p<0.0284), E11.0 (14–17 ts, t40 = 5.355, p<0.00002), E11.5 (18–21 ts, t29 = 1.699, p<0.0500), E12.5 (27–30 ts, t10 = 3.441, p<0.0032), and E13.5 (t12 = 5.985, p<0.0003). Wt1 expression also was lower in B6 than in D2 samples at E12.0 (27–30 ts) but the difference did not reach statistical significance (t13 = 1.741, p<0.0526). Sf1 expression was lower in B6 vs. D2 gonad/mesonephros complexes at E11.0 (t40 = 1.466, p<0.0753), E11.5 (t29 = 1.182, p<0.1235), and E12.0 (t13 = 1.208, p<0.1243), but these differences were not statistically significant. Tab. 5. Increasing <i>Sry</i> dosage partially rescues the sex reversal phenotypes of E13.5 B6 XY<sup>AKR</sup>, <i>Wt1</i>+/− B6 XY<sup>AKR</sup>, and <i>Sf1</i>+/− B6XY<sup>AKR</sup> gonads. 
The Wt1 and Sf1 B6 alleles require additional interacting genes to be homozygous for the B6 allele to function as hypomorphs
The results presented above suggest that the Wt1B6 and Sf1B6 alleles are hypomorphic alleles. One possibility is that the Wt1B6 and Sf1B6 alleles behave as intrinsic hypomorphs. An alternative possibility is that the Wt1B6 and Sf1B6 alleles function as hypomorphs only when one or more additional interacting gene(s) are homozygous for B6 alleles. To distinguish between these two alternatives, genetic crosses were designed that would generate hybrid (D2×B6)F1 XYAKR mice that are heterozygous for the null (knockout) allele of Wt1 or Sf1 and the Wt1B6 or Sf1B6 allele, respectively (e.g. Wt1KO/Wt1B6 or Sf1KO/Sf1B6 ) (Table 2). These F1 hybrid mice are genetically identical to Wt1+/ − and Sf1+/ − B6 XYAKR mice at the Wt1 and Sf1 loci; however, the remainder of the genome is different because it is (D2×B6)F1 hybrid (except for the regions immediately around the Sf1KO and Wt1KO alleles that are derived from the 129 strain). Because Wt1+/ − and Sf1+/ − (D2×B6)F1 XYAKR mice are not sex reversed, we conclude that the Wt1B6 and Sf1B6 alleles function as hypomorphs only when other genes (modifier genes) are homozygous for B6 alleles.
Molecular analysis of the difference between the B6 and D2 alleles of the Wt1 and Sf1 genes
The B6 and D2 alleles of the Wt1 and Sf1 genes could encode different protein variants and/or have different expression patterns. A PCR primer-walking strategy using cDNA and genomic DNA as template was employed to determine the DNA sequence of the Wt1 and Sf1 genes present in the D2 genome and these sequences were compared to the mouse B6 reference sequence (data not shown). For Wt1, the ORF, 5′ and 3′ UTRs, and about 1 kb of the upstream flanking region were sequenced. One synonymous difference was identified in the ORF: a T (B6) vs. C (D2) substitution at the third position of codon 339, which was confirmed by its presence in dbSNP Build 128 (NCBI, rs27444886). There were no differences in the 5′ UTR and upstream flanking region. Four differences were identified in the 3′ UTR, two of which were confirmed by their presence in dbSNP (rs33129887 and rs13467742). Two single base pair differences in homopolymer length were not confirmed in dbSNP. The functional significance, if any, of the 3′ UTR polymorphisms is not readily apparent.
For Sf1, the ORF, 5′ and 3′ UTRs, and ∼1.5 kb of the upstream and 500 bp of the downstream flanking regions were sequenced. One synonymous and one non-synonymous difference were identified in the ORF. The synonymous polymorphism was a T (B6) vs. C (D2) substitution at the third position of the third codon. This polymorphism is represented in dbSNP (rs3142930) for certain inbred strains, but not for DBA/2J. The non-synonymous polymorphism was a G (B6) vs. T (D2) substitution in the first position of codon 172 resulting in an alanine (B6) vs. serine (D2) amino acid substitution and was confirmed by its presence in dbSNP (rs3142929). This polymorphism was independently discovered by Frigeri and colleagues who first identified it in ACTH resistant derivatives of the mouse Y1 adrenocortical tumor cell line [41]. There were no differences in the 5′ and 3′ UTRs. The upstream and downstream regions each had one difference, both in CA repeat units: The upstream CAAA repeat had 6 units in B6 vs. 5 units in D2, while the downstream CA repeat had 17 units in B6 vs. 22 units in D2. Neither polymorphism is present in dbSNP and their functional significance is not readily apparent.
To gain insight into whether the presence of the Sf1A172 (B6) vs. Sf1S172 (D2) allele was correlated with B6 XY sex reversal, and thus of potential functional significance, we took advantage of the fact that when females from different inbred strains are mated to B6 XYPOS hermaphrodites the F1 XY offspring are either sex reversed or normal, and thus the inbred strains can be classified as B6-like or D2-like, respectively (Table 6). Using this assay we identified two strains (C57BL/10J and NZB/BlNJ) that are B6-like and five strains (129S1/SvImJ, C3H/HeSnJ, SM/J C58/J and BALB/cBy) that are D2-like. A PCR-RFLP genotyping assay that was developed by Frigeri and colleagues [41] was used to determine the strain distribution pattern of the Sf1A172 and Sf1S172 alleles among these seven strains. As shown in Table 6, two strains were phenotypically and genotypically discordant. NZB/BlNJ was B6-like phenotypically but it has an Sf1S172 allele, and BALB/cBy was D2-like phenotypically but it has an Sf1A172 allele. We thus conclude that the presence of an Sf1A172 vs. Sf1S172 allele alone is not strictly correlated with B6 XY sex reversal.
Tab. 6. Strain distribution pattern of the Sf1A172 versus Sf1S172 alleles. 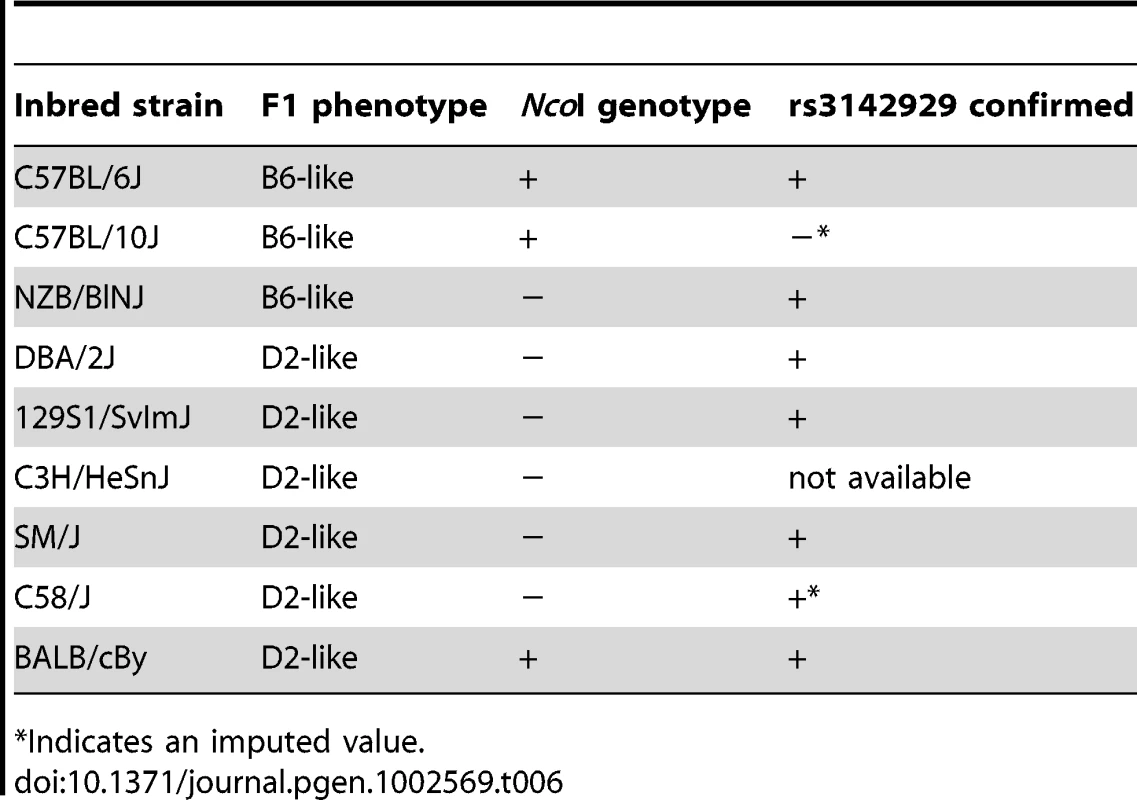
Indicates an imputed value. The Wt1 gene encodes multiple splice isoforms. Of particular interest here, alternative splicing of exon nine gives rise to two protein isoforms that differ by three amino acids and are referred to as the +KTS and −KTS isoforms. Both isoforms and their correct relative expression are necessary for normal gonad development [42]. For example, Frasier syndrome in humans is associated with XY sex reversal and caused by reduced expression of the Wt1 +KTS isoform [43]. Thus, it was possible that B6 XYAKR Wt1+/ − sex reversal could be caused by altered +KTS/−KTS isoform ratios and qRT-PCR using primers that span the exon nine splice junction was employed to examine if the ratio of +KTS/−KTS splice isoforms is different in E10.5–14.5 B6 vs. D2 XX and XY gonad/mesonephros complexes. We found that the relative expression of the Wt1 +KTS and −KTS isoforms did not differ between B6 and D2 gonad/mesonephros complexes (data not shown).
To test the hypothesis that the Wt1B6 and Sf1B6 alleles function as hypomorphs because they are expressed at lower levels or with different temporal profiles than the Wt1D2 and Sf1D2 alleles, respectively, transcript levels in B6 vs. D2 XY gonad/mesonephros complexes from E10.5–13.5 fetuses were quantified using qRT-PCR (Figure 5). Wt1 expression was significantly lower in B6 vs. D2 samples at all stages. Sf1 expression also was lower in B6 vs. D2 gonad/mesonephros complexes from E11.0–12.0 fetuses, but these differences were not statistically significant. These Wt1 and Sf1 expression results were corroborated in XX samples from similar stages, and the Sf1 expression results were further confirmed by using an independent SYBR green qRT-PCR assay to analyze a subset of the samples (data not shown). In summary, these molecular analyses suggest that Wt1B6 is a hypomorph due to reduced expression and that Sf1B6 is a hypomorph possibly due to altered protein structure and/or the presence of other modifier B6 loci.
Reducing Wnt4 dosage increases the amount of testis tissue that develops in multiple cases of B6 XY sex reversal
To examine the possibility that Wnt4 would be haploinsufficient on the B6 genetic background and to investigate its candidacy as a tda gene, we transferred a Wnt4 null allele onto the B6 genetic background and assessed if Wnt4 heterozygosity exacerbated XY sex reversal in B6 XYPOS, B6 XYAKR, Sf1+/ − B6 XYAKR, and Wt1+/ − B6 XYAKR fetuses. WIHC was used to examine gonad morphology and marker gene expression at E13.5. Each gonad/mesonephros complex was assessed at dissection for the presence of cords and male-specific vasculature, and classified as an ovary, ovotestis, or testis (Table 7). Classification was done prior to ascertainment of the fetal genotype and was done by a single investigator (SMC) for consistency. A subset of complexes of each genotype was processed for WIHC and examined by two investigators (SMC and KHA) to confirm the initial classification (Figure 6). In all four experimental groups, reducing Wnt4 dosage increased the area containing testicular cords and reduced the area without obvious structure in XY gonads. In B6 XYPOS fetuses, 5 of the 12 gonads analyzed from Wnt4+/+ fetuses were classified as ovaries and none were classified as testes, whereas 7 of the 28 gonads analyzed from Wnt4+/− fetuses were classified as ovaries and 4 were classified as testes. These data indicate that reducing Wnt4 dosage in B6 XYPOS fetuses decreased the percentage of ovaries from 42% to 25% and increased the percentage of testes from 0% to 14%. In B6 XYAKR fetuses, 12 of the 16 gonads analyzed from Wnt4+/+ fetuses were classified as ovotestes and 4 were classified as testes, whereas 6 of the 18 gonads analyzed from Wnt4+/−fetuses were classified as ovotestes and 12 were classified as testes. Thus, reducing Wnt4 dosage in B6 XYAKR mice decreased the percentage of ovotestes from 75% to 33% and increased the percentage of testes from 25% to 67%. In B6 Wt1+/−XYAKR fetuses, 3 of the 12 gonads analyzed from Wnt4+/+ fetuses were classified as ovaries, 5 as ovotestes, and 4 as testes, whereas none of the 6 gonads analyzed in Wnt4+/− fetuses were classified as ovaries, 1 was an ovotestis and 5 were testes. Thus, reducing Wnt4 dosage in Wt1+/ − B6 XYAKR mice decreased the percentage of ovaries from 25% to 0%, decreased the percentage of ovotestes from 42% to 17%, and increased the percentage of testes from 33% to 83%. In Sf1+/ − B6 XYAKR fetuses, all 8 gonads analyzed from Wnt4+/+ fetuses were classified as ovaries, whereas 2 of the 12 gonads analyzed from Wnt4+/− fetuses were classified as ovaries, 3 as ovotestes, and 7 as testes. Thus reducing Wnt4 dosage in B6 Sf1+/ − XYAKR mice decreased the percentage of ovaries from 100% to 17%, increased the percentage of ovotestes from 0% to 25%, and increased the percentage of testes from 0% to 58%. The WIHC analyses confirmed these results (Figure 6).
Fig. 6. Reduced Wnt4 dosage partially rescued cord differentiation in B6 XY genotypes that typically develop ovarian tissue. 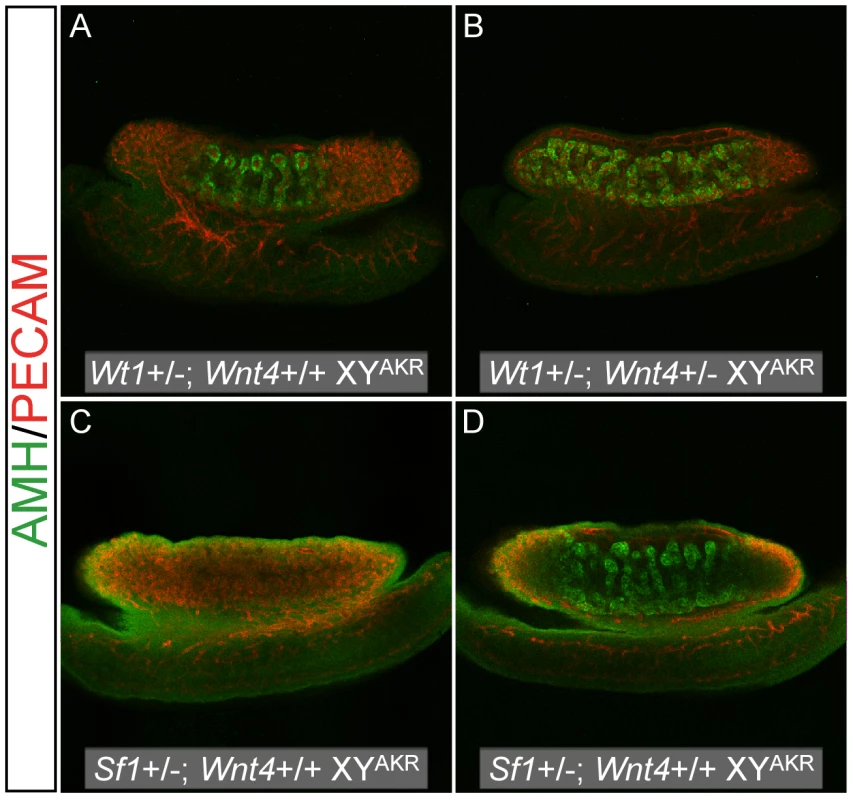
Representative images are shown for Wt1+/− (A and B) and Sf1+/− (C and D) B6 XYAKR E13.5 gonads that were either Wnt4+/+ (A and C) or Wnt4+/− (B and D). The amount of tissue expressing the Sertoli cell marker AMH (green) was consistently greater in Wnt4+/− vs. Wnt4+/+ gonads for all four sex reversals examined. Germ and vascular endothelial cells are labeled by PECAM staining (red). Tab. 7. Reducing the <i>Wnt4</i> dosage partially rescues the sex reversal phenotypes of E13.5 B6 XY<sup>POS</sup>, B6 XY<sup>AKR</sup>, B6 <i>Wt1</i>+/− XY<sup>AKR</sup>, and B6 <i>Sf1</i>+/− XY<sup>AKR</sup> gonads. 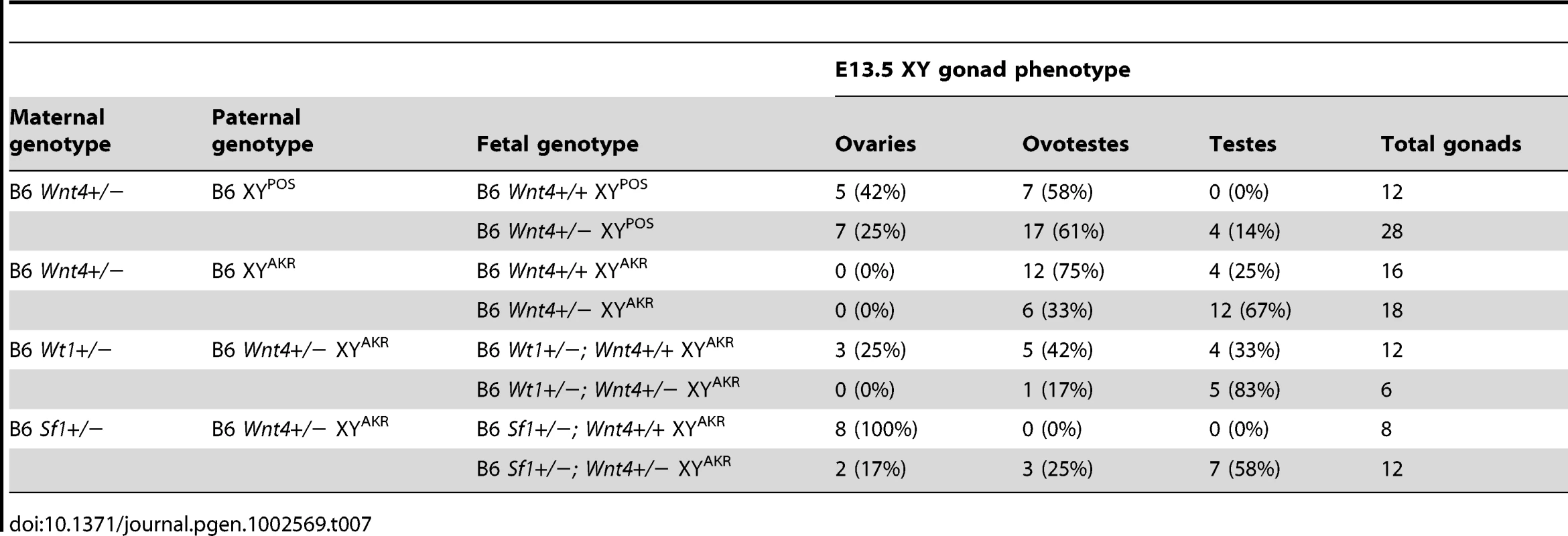
These data indicate that Wnt4B6 acts like an anti-testis gene. Using qRT-PCR we found that Sry expression in Wnt4+/+ vs. Wnt4+/ − B6 XYAKR was not significantly different during peak Sry expression in E11.75 (21–23 ts) gonad/mesonephros complexes (data not shown), suggesting that Wnt4B6 compromises the testis pathway downstream of Sry [36]. Together, these data suggest that Wnt4B6 behaves as a hypermorphic allele of a gene in the ovary determination pathway, rather than a hypomorphic allele of a gene in the testis determination pathway.
Elevated expression of ovary-biased genes in B6 versus D2 XX ovaries
The Wnt4 results presented above suggested the possibility that the ovary differentiation pathway is prematurely - or hyper-activated in B6 gonads, thereby suppressing the testis determination pathway. As with Wt1 and Sf1, the B6 and D2 Wnt4 alleles could encode different protein variants or have different expression patterns. We determined the DNA sequence of the Wnt4 ORF present in the D2 genome and found that it did not differ from that in B6, which was confirmed by data present in dbSNP Build 128 (data not shown). We used qRT-PCR to compare the expression of Wnt4 and seven other genes in the ovary differentiation pathway in E12.5 B6 vs. D2 XX ovaries to test the hypothesis that Wnt4B6 is an expression hypermorph and to investigate the possibility that the ovary differentiation pathway is prematurely - or hyper-activated in B6 gonads (Figure 7). The seven genes were chosen because their expression normally is ovary-biased at E12.5 (i.e., their expression is either ovary-specific or higher in ovaries vs. testes) [34], [38], [44]–[46]. We found that the expression of Wnt4 (p<0.0004), Adamts19 (p<0.026), Fst (p<0.0001), Igfbp2 (p<0.0001), and 1700106J16Rik (p<0.0001) was significantly higher in B6 vs. D2 ovaries; that expression of Irx3 (p<0.177) and Bmp2 (p<0.323) was not significantly different; and that expression of Rspo1 (p<0.0002) was significantly lower. These results demonstrate that Wnt4 and other genes known, or thought to be, in the ovary differentiation pathway are hyperactivated in E12.5 B6 vs. D2 XX gonads.
Fig. 7. Real-time qRT–PCR analysis of ovarian marker gene expression in B6 versus D2 E12.5 XX gonads. 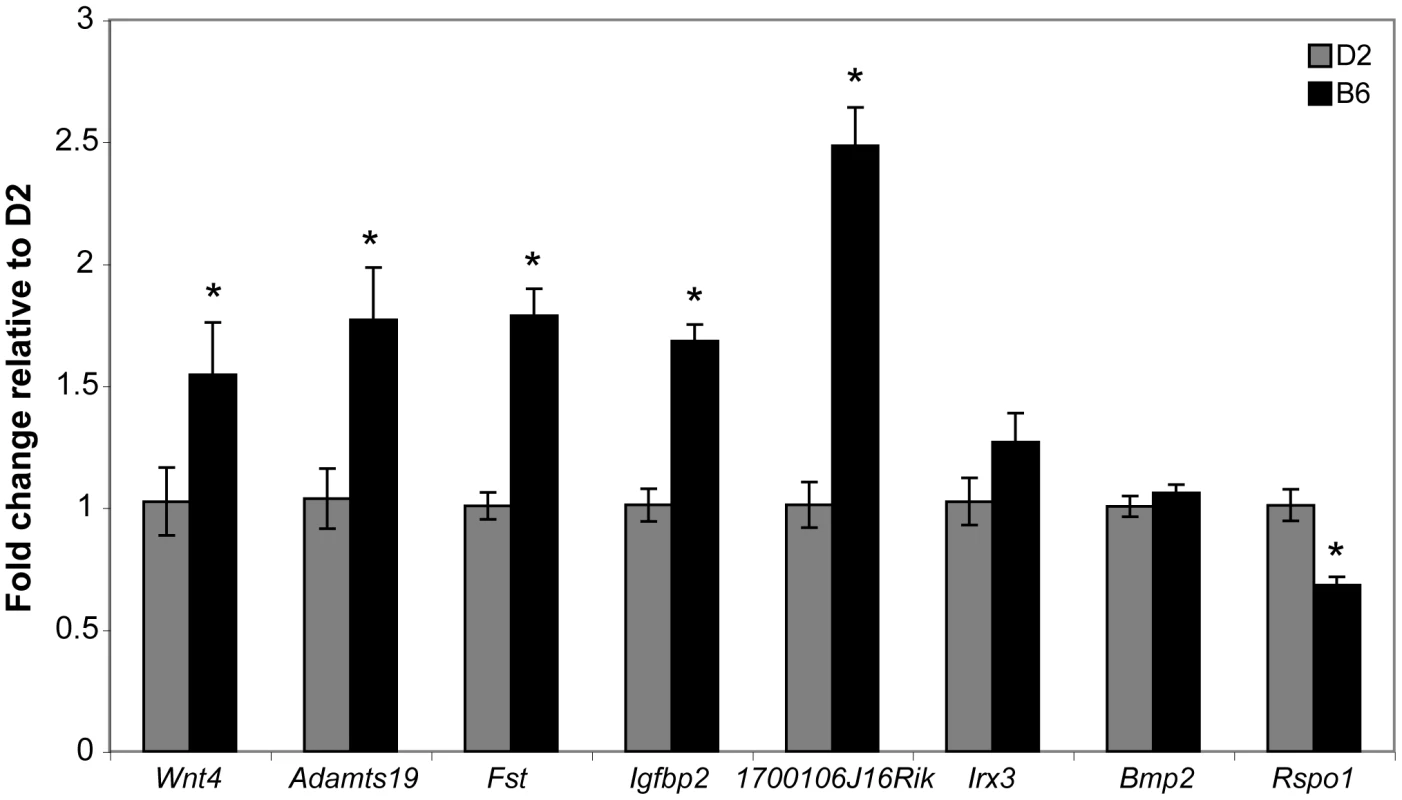
Expression of ovary-specific genes Wnt4, Adamts19, Fst, Ifgbp2, 1700106J16Rik, Irx3, Bmp2, and Rspo1 were measured in the same samples and normalized to Hprt (D2, n = 6 gonad/mesonephros complexes; B6, n = 10 gonad/mesonephros complexes). Fold change relative to mean D2 values was determined using the 2−ΔΔCt method and plotted as the average per sample group +/− standard error. An asterisk denotes statistically significant differences in pairwise comparisons (α = 0.05). The expression levels of Wnt4 (t14 = 1.938, p<0.0365), Adamts19 (t14 = 2.13, p<0.0257), Fst (t14 = 4.865, p<0.0001), Ifgbp2 (t14 = 4.987, p<0.0001), and 1700106J16Rik (t14 = 5.002, p<0.0001) were significantly higher in B6 vs. D2 XX ovaries. Expression of Irx3 and Bmp2 did not significantly differ between the two strains, and Rspo1 expression was significantly lower in B6 vs. D2 XX ovaries (t14 = 4.612, p<0.0002). Discussion
It has been suggested that some genetic events leading to testis determination differ between humans and mice because several genes that are haploinsufficient in humans appeared to be haplosufficient in mice [18]. However, based on our studies of B6-YPOS sex reversal we hypothesized that genetic background would have a significant influence and that some of these genes would be haploinsufficient on the B6 genetic background. We chose to analyze the Wt1, Sf1, and Wnt4 genes for two main reasons: 1) they are dosage-sensitive in humans but initial studies suggested they were dosage-insensitive in mice, and 2) they are good candidates for tda genes based on genetic location and because gonad differentiation is abnormal in homozygous knockout mice. Our analyses show that these three genes can be dosage-sensitive in mice, depending on the genetic background, and provide insights into the mechanisms of B6 XY sex reversal.
The Wt1 and Sf1 genes are dosage-sensitive on the B6 genetic background
Our results show that the Wt1 and Sf1 B6 alleles are haploinsufficient for normal testis differentiation when present on the B6 genetic background. We found that Wt1+/ − and Sf1+/−B6 XYB6 mice developed as males; however, testis differentiation was transiently delayed and was further compromised in double heterozygous Wt1+/−; Sf1+/ − B6 XYB6 fetuses. These data are consistent with evidence that Wt1 activates Sf1 transcription [30]. When the genetic background was further sensitized by substituting the YB6 Chr with the YAKR Chr, testis differentiation was more severely affected and Wt1+/ − and Sf1+/ − B6 XYAKR mice developed ovotestes and ovaries. In contrast, all Wt1+/ − and Sf1+/ − (D2×B6)F1 XYAKR mice developed testes. Taken together, these results show that the Wt1 and Sf1 B6 alleles are hypomorphic relative to the D2 alleles. Analysis of morphology and marker gene expression in E13.5 Wt1+/ − and Sf1+/ − B6 XYAKR gonads showed that some developed as ovaries and were indistinguishable from control XX ovaries in that they expressed ovary-specific markers, and did not express Sertoli cell or Leydig cell-specific markers. These data indicate that the testis-determining pathway is interrupted at a very early stage in these XY gonads.
In addition to defects in testis differentiation, we found that double heterozygous Wt1+/−; Sf1+/ − fetuses lacked adrenal glands and that neonates die shortly after birth, presumably from adrenal insufficiency. Because the gonads and adrenal cortex share a common origin [47], because the morphological defects we observed occur early in the development of these organs, and because the lethality and sex reversal phenotypes were sensitive to genetic background in a similar way, we hypothesize that the initial defect occurs during the adrenogonadal progenitor stage and that one or more tda gene(s) plays a role in the differentiation of this progenitor into the adrenal cortex and gonads. While it has been known that Wt1, Sf1 and Wnt4 are necessary for adrenal cortex differentiation [14]–[17], [34], [35], [48], our results show that Wt1 and Sf1 interact genetically during adrenal development. These results are consistent with results reported for fetuses that are double heterozygous for Sf1 and Cited2, a transcription cofactor that interacts with Wt1 [49]. Given that Wt1 is thought to directly activate Sf1 transcription in the gonadal progenitor, our results suggest that this genetic pathway is important for the differentiation of the adrenogonadal progenitor. It will be interesting to determine how Wnt4 fits into this genetic pathway in the adrenogonadal progenitor.
Wt1 and Sf1 regulate Sry expression, but reduced Sry transcript levels are not solely responsible for sex reversal in Wt1+/− or Sf1+/− B6 XY mice
Consistent with these indications of an early defect in testis differentiation, we found that Sry expression was markedly reduced in heterozygous mutant gonads. Our unpublished data indicate that reduced expression results from both a direct effect on Sry expression and an indirect effect on the differentiation of pre-Sertoli cells (manuscript in preparation). Interestingly, increasing Sry dosage by replacing the YAKR Chr in Wt1+/ − or Sf1+/ − B6 XYAKR fetuses with the YAKR,Sxr Chr dramatically increased the amount of testis tissue that developed but cord differentiation remained somewhat abnormal. Our previous experiments showed that the SryAKR and SrySxr alleles are expressed with similar profiles and at similar levels in gonads from B6 XYAKR,Sxr fetuses [29], suggesting that Sry expression is essentially doubled in these gonads. Thus, testicular cord differentiation was compromised in Wt1 and Sf1 heterozygotes, regardless of Sry dosage. These results suggest that reduced Sry transcript levels are not the sole cause of sex reversal in Wt1+/ − and Sf1+/ − B6 XYAKR mice and that the expression of critical genes other than Sry is likely to be affected. We consistently found that the Sf1 heterozygous phenotype was more severe than the Wt1 heterozygous phenotype. However, the reduction in Sry expression was not statistically different between the two heterozygotes, which supports our hypothesis that genes and cellular processes other than those controlled by SRY contribute to the phenotypic differences. Our result agree with previous reports suggesting that Sry expression levels alone do not predict whether a given Y Chr causes testis differentiation defects on the B6 genetic background [50] and that sex reversal is caused by both the presence of particular SRY protein isoforms found in M. domesticus mice and reduced Sry expression [29].
Analysis of Wt1 and Sf1 heterozygous gonads identifies potential downstream targets
The analysis of marker gene expression using WISH provided insights into potential Wt1 and Sf1 target genes. For example, Wnt4 expression was reduced in Wt1+/ − and Sf1+/ − B6 XX ovaries compared to +/+ B6 XX ovaries indicating that Wt1 and Sf1 might regulate Wnt4 expression in ovaries (Figure 2). In contrast, Wnt4 expression was elevated in Wt1+/ − and Sf1+/ − B6 XYAKR gonads compared to +/+ gonads, but we suspect that this increase is due to the presence of more ovarian tissue in these gonads. Expression of Wnt4 also was reduced in Wt1+/ − XX and XY mesonephroi. Because Sf1 is not expressed in mesonephroi, reduced Wnt4 expression indicates in this tissue WT1 regulates Wnt4 expression independently of SF1. Ptch1 expression also was reduced in Wt1+/ − XX and XY mesonephroi. Interestingly, Ptch1 is strongly expressed in the region where the mesonephric tubules form and the number of tubules is reduced in Wt1−/ − mesonephroi [14]. This result suggests that Wt1 regulates Ptch1 expression and that Ptch1 could have a role in the formation of mesonephric tubules. However, these WISH results, though quite intriguing, require confirmation with a more quantitative method such as qRT-PCR.
Co-expression of ovarian and testicular markers suggests reduced antagonism of the ovary and testis differentiation pathways in B6 XY gonads
Analysis of E13.5 B6 XYAKR gonads showed that the anterior and posterior regions that lacked testicular cords expressed multiple ovarian somatic cell markers and that the germ cells within these domains had initiated meiosis. Therefore, these regions were not simply undifferentiated, but rather had initiated the ovary differentiation program in both somatic and germ cells. By E15.5, testicular cords are present throughout B6 XYAKR gonads and it is unclear what happens to the ovarian regions between these two stages. Two possibilities are that these ovarian cells might transdifferentiate into the analogous testicular cell types, or regress via apoptosis. A recent analysis showed that the ovarian regions in Sox9 transgenic XX ovotestes regress via apoptosis [51] and our preliminary analyses suggest that there is an increased number of apoptotic cells within the ovarian regions in B6 XYAKR ovotestes (data not shown). In these regions it was clear that most cells expressed either ovarian (FOXL2) or testicular (AMH) markers and that these cells could be interspersed. We propose that co-expression of ovarian and testicular markers in E13.5 B6 XY ovotestes suggests that antagonism between these opposing differentiation pathways is reduced in these XY gonads. Contrary to results reported by Bradford and colleagues [52], we did observe a few cells at the boundaries between testicular and ovarian domains that appeared to express both granulosa and Sertoli cell markers. We could not unequivocally demonstrate co-expression using the markers and techniques presented here, however, the possibility that cells expressing both female and male supporting cell progenitor markers might be present during fetal gonad differentiation warrants further investigation.
Why are the Wt1 and Sf1 B6 alleles haploinsufficient?
To determine why the Wt1B6 and Sf1B6 alleles are haploinsufficient and function as hypomorphs, we compared the coding sequences and expression levels of the B6 and D2 alleles. The results indicated that the Wt1B6 and Wt1D2 allele encode the same protein and that the ±KTS Wt1 isoform expression ratio was the same in B6 and D2 gonad/mesonephros complexes. However, we found that Wt1 expression throughout the initial stage of testis differentiation was lower in complexes from B6 fetuses, suggesting that in the B6 genetic background the Wt1B6 allele is an expression hypomorph. In contrast, we were unable to detect significantly lower Sf1 expression in complexes from B6 fetuses but we did identify one coding polymorphism in Sf1, suggesting that the Sf1B6 allele is haploinsufficient not because its expression is reduced but possibly because the SF1B6 protein variant has reduced function. It appears that two SF1 variants are present among the common inbred mouse strains: SF1A172 (present in B6) and SF1S172 (present in D2). However, we found that homozygosity for the Sf1A172 allele in F1 hybrids was not sufficient to cause sex reversal in the presence of the YPOS Chr [as in (NZB/BlNJ×B6 XYPOS) F1s] and that trans-heterozygosity for the Sf1A172 and Sf1S172 alleles was not sufficient to prevent sex reversal in the presence of the YPOS Chr [as in (BALB/cBy×B6 XYPOS) F1s]. These data show that the SF1A172 polymorphism alone does not account for sex reversal in the presence of the YPOS Chr. Therefore, if the SF1A172 protein variant does indeed have reduced function during testis determination, then homozygosity for B6 alleles at other loci likely is necessary to cause Sf1B6 to function as a hypomorph. Frigeri and colleagues [41] demonstrated that the SF1A172 and SF1S172 protein variants did not consistently differ in their ability to transactivate reporter genes containing tandem SF1 binding sites, in vitro. However, further experiments are needed to determine if the SF1A172 protein variant has reduced function during gonad differentiation in vivo.
The Wnt4 B6 allele is dosage-sensitive in the B6 genetic background and may contribute to hyperactivation of the ovary differentiation pathway
Our genetic results suggest that the Wnt4B6 allele functions as a hypermorph in the ovary determination pathway. To ascertain if the ovary determination pathway was hyperactive in B6 vs. D2 gonads we quantified the expression of eight ovary-biased or ovary-specific genes in E12.5 B6 vs. D2 XX ovaries. We chose this developmental stage because existing data suggested that the ovary pathway would be active and that the chosen markers would be expressed at detectable levels. We chose to analyze XX and not XY gonads because an increase in ovary gene expression in XY gonads could be caused by failure to activate the testis pathway and subsequent failure to suppress the ovary pathway. We found that the expression of Wnt4, and four other genes was higher in B6 ovaries, indicating that the ovary determination pathway is activated earlier and/or to a greater degree in B6 vs. D2 ovaries. These results are consistent with those reported in an eQTL study of B6 vs. 129 gonads [53]. The B6 vs. D2 expression differences do not appear to be caused by gross differences in development between these two strains because we did not detect differences in limb morphology or in the size of the gonads or mesonephroi at the 27–28 ts stage used for these analyses (data not shown). However, not all eight genes were up-regulated in B6 ovaries: two were not significantly different, and one was down-regulated. We find it intriguing that Rspo1, which maps close to Wnt4 within the tda1 region, had reduced expression in B6 ovaries. However, because the Rspo1 and Wnt4 knockout phenotypes are very similar in that ovary and not testis differentiation is primarily affected, and because Wnt4 expression is greatly reduced in Rspo1−/ − XX gonads [54], [55], we think it is unlikely that Rspo1 is tda1 because we would expect that a strain with reduced Rspo1 expression like B6 would be less sensitive to XY sex reversal, whereas B6 is, in fact, more sensitive. We note that for Rspo1 no SNPs that differ between the B6 and D2 strains are present in dbSNP (Build 128), indicating that the RSPO1 protein does not differ between these strains. Furthermore, we suggest that the interaction between Rspo1 and Wnt4 may be indirect because Rspo1 expression in decreased in B6 mice while Wnt4 expression is increased. Nevertheless, these data do not formally exclude Rspo1 as a tda1 candidate.
Are Wt1, Sf1, and Wnt4 tda genes?
More than twenty-five years ago, two of the authors hypothesized that the B6-derived tda genes could function later than normal in the testis determination pathway and/or, because the testis and ovary determination pathways antagonize each other, could function earlier than normal in the ovary determination pathway [25], [56]. Thus, if a tda gene functions in the testis-determining pathway, then reducing its dosage in B6 XY mice should cause sex reversal, whereas reducing its dosage in D2 XY mice should not. Similarly, if a tda gene functions in the ovary-determining pathway and antagonizes testis differentiation, then reducing its dosage should ameliorate B6 XY sex reversal. The Wt1 and Sf1 genes fulfill the criteria as late functioning testis-determining genes and Wnt4 fulfills the criteria as an early function ovary-determining gene that antagonizes testis differentiation. Furthermore, our results show that B6 XYPOS, Wt1+/ − B6 XYAKR, and Sf1+/ − B6 XYAKR sex reversal share similar gonadal phenotypes and that these three sex reversals are sensitive to genetic background in a similar way. Additionally, we have recently determined that a major modifier of Sf1+/ − B6 XYAKR sex reversal that differs between the B6 and D2 genomes, maps to the distal region of Chr 4, which suggests that the tda1 modifier gene plays a prominent role in both Sf1+/ − B6 XYAKR and B6 XYPOS sex reversal (manuscript in preparation). However, while our data are consistent with the hypothesis that tda2 is comprised of Wt1 and Sf1 and that tda1 is Wnt4, the genetic intervals defined by the linkage analyses are fairly broad, and further experiments are needed to directly test this hypothesis and to exclude the possibility of other candidate genes in the tda1 and tda2 intervals.
Undoubtedly, tda genes in addition to tda1 and tda2 contribute to B6 XYPOS sex reversal because, as shown in the original linkage analysis, some XYPOS fetuses that were homozygous for B6 alleles at the tda1 and tda2 loci contained normal appearing testes [24]. Nevertheless, it appears that tda1 and tda2 play an important role in many cases of B6 XY sex reversal because we have mapped a major modifier of Dax1 −/Y sex reversal that differs between the B6 and D2 genomes to the same chromosome region as tda1 [22], and have recently determined that major modifiers of Gata4 +/ − B6 XYAKR sex reversal [23] map to the same chromosome regions as tda1 and tda2 (manuscript in preparation).
An additive model to explain B6 XY sex reversal
Overall, our data suggest that the B6 inbred strain is sensitive to XY sex reversal, at least in part, due to hypomorphic alleles of Wt1 and/or Sf1 that compromise the testis determination pathway, as well as a hypermorphic allele of Wnt4 that antagonizes testis development. We suggest that in XY gonads of most inbred strains, such as DBA/2J, the testis determination pathway reaches a critical threshold that is sufficient to inactivate the ovary determination pathway prior to the developmental stage when the ovary determination pathway reaches a threshold that is sufficient to inactivate the testis determination pathway. In contrast, in a few inbred strains such as B6, the testis determination pathway is compromised and the ovary determination pathway is hyperactivated, thus the interval between the developmental stages at which these two thresholds are reached is greatly reduced. We propose that this interval is even further reduced or eliminated in B6 mice carrying certain M. domesticus-derived Sry alleles that do not efficiently activate the testis determination pathway, thus allowing ovotestes to develop transiently in B6 XYAKR mice and allowing stable ovotestes and ovaries to develop in B6 XYPOS mice, for example. In summary, we propose that sex reversal in B6 XYPOS gonads is the result of the additive effects of 1) hypomorphic Wt1B6 and/or Sf1B6 alleles, 2) a hypermorphic Wnt4B6 allele and hyperactivation of the ovary pathway, 3) reduced function of M. domesticus-derived SRY proteins, 4) reduced expression of the SryPOS allele, and 5) the presence of other unidentified tda genes.
Mouse models of human dosage-sensitive sex reversal
These studies demonstrate that Wt1, Sf1, and Wnt4 are dosage-sensitive in mice, as they are in humans, and show that this effect is modulated by genetic background, as we suspect it is in humans. They also demonstrate that the Wt1+/− and Sf1+/− B6-YAKR mice are good models for the corresponding human XY sex reversals involving heterozygous mutations in these genes. We suggest that these heterozygous mice will be very useful for the elucidation of Wt1 and Sf1 functions during gonad differentiation because the heterozygous phenotype is far less severe than the homozygous phenotype, which is a complete abrogation of gonad differentiation and subsequent apoptosis at the genital ridge stage. Thus, we think it is likely that these strains will be particularly useful in providing further insights into the roles of Wt1 and Sf1 in organogenesis and human disease.
Materials and Methods
Animals and genotyping
Null (knockout) alleles of Wt1 (Wt1tm1Jae) [14], Sf1 (Nr5a1tm1Klp) [16], and Wnt4 (Wnt4tm1Amc) [34] were transferred to the B6 and D2 inbred strain backgrounds using standard backcrossing. No sex ratio disturbances were noted in heterozygous progeny during construction of these congenic strains. Experimental results reported here were conducted after backcross generation N10 or greater was achieved. The B6-YPOS, B6-YAKR, B6-YAKR,Sxr and D2-YAKR consomic strains were previously described [19], [20], [23], [29], [57]. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Jackson Laboratory and Boston University Laboratory Animal Science Center are AALAS accredited and all animal procedures were approved by The Jackson Laboratory Animal Care and Use Committee (protocols LAH:93-26, LAH:95-01, LAH:95-02) or the Boston University Animal Care and Use Committee (protocol AN-13935).
The presence of a Wt1, Sf1, or Wnt4 knockout allele was determined by multiplex PCR using primers to detect the neomycin cassette and the myogenin gene as a control [23]. In crosses involving multiple knockout alleles, gene-specific PCR assays were used to amplify both the normal and the targeted alleles [46], [58]. The genetic sex of fetuses was determined by PCR using Y Chr specific primers and myogenin control primers [59]. The B6-YAKR,Sxr strain carries a rearranged Y Chr containing two Sry alleles: the M. domesticus-derived SryAKR allele and the M. musculus-derived SrySxr allele. The presence of the M. domesticus and/or M. musculus Sry alleles was determined by PCR followed by digestion with NlaIV restriction enzyme using primers that flank a polymorphism within the Sry gene [29].
Embryo isolation and staging, and determination of sexual phenotype
Timed matings were used for all embryonic experiments. Noon on the day of vaginal plug detection was designated as E0.5. Embryos between E10.5 and 12.5 were precisely staged by counting the number of tail somites (E10.5∼8 ts, E11.5∼18 ts, E12.5∼30 ts) [60] and those between E12.5 and E16 were staged by limb morphology and the extent of cranial vessel formation [61].
To classify the gonads of individual E14.5–16 fetuses, the gonads were removed from the body cavity and immediately examined with the aid of an inverted microscope using transmitted light, and then photographed [62]. Testes were identified as having distinctive cords and vasculature, and an incipient tunica albuginea. Ovaries were identified as having a reticular appearance and lacking the structures noted above in the testes. Ovotestes contained a region with a reticular appearance at one or both ends of the gonad with the remainder containing cords. The sexual phenotype of weaning age (∼3 weeks postpartum) mice was determined by examining the external genitalia, noting the anal-genital distance, and observing whether mammary gland-associated yellow-pigmented hairs were present [63]. Gonads from E13.5 fetuses were classified at the time of dissection, when the observer was blind to the fetal genotype. Gonads without any testicular cords were classified as ovaries, those with testicular cords only in the central region of the gonad were classified as ovotestes, and those with testicular cords throughout were classified as testes. Those with disorganized testicular cords throughout the gonad were classified as abnormal testes.
Whole-mount immunohistochemistry and RNA in situ hybridization
Gonad/mesonephros complexes were fixed overnight in 4% paraformaldehyde in PBS at 4°C. Whole-mount indirect fluorescent immunohistochemistry (WIHC) was performed essentially as previously described [64], except that a blocking buffer containing 3% BSA, 10% donkey serum, 0.1% Triton-X, 0.02% sodium azide in PBS was used when the anti-PDGFRa or anti-MIS (AMH) antibodies were employed. Primary antibodies and dilutions are listed in Table S1. Secondary antibodies were donkey anti-IgG conjugated with Cy3 or Cy5 (Jackson ImmunoResearch, 1∶500) or AF488 (Molecular Probes, 1∶750). Samples were imaged using a Zeiss LSM510 confocal microscope.
Standard protocols for whole-mount in situ hybridization (WISH) were used with minor changes [46], [65]. Gene-specific probes are listed in Table S1. The Rec8 probe was synthesized from a full-length cDNA clone (IMAGE ID:6335959, GenBank: BC052155.1). Antisense and sense probes were tested in parallel, and only signal specific to the antisense probes was reported as gene-specific expression. Each result reported was replicated in a minimum of four samples.
RNA extraction, reverse transcription, and quantitative RT–PCR
Total RNA was isolated using the RNeasy mini kit (Qiagen). Samples were treated with Turbo DNase (Ambion) and subsequently treated with DNase inactivation reagent using the DNA-free protocol (Ambion). RNA Samples were determined to be free of DNA by PCR using primers specific for the Myog or Lhx1 genes [29]. cDNA was synthesized using oligo-dT primers and reverse transcriptase (RT). The RT reaction was tested for the presence of intact cDNA by PCR amplification with primers specific for the Hprt transcript [46].
Relative Sry expression in mutant vs. normal gonad/mesonephros complexes was determined using TaqMan Universal PCR Master Mix, and Sry (Mm00441712_s1) and Gapdh (Mm99999915_g1) Assays-on-Demand following the manufacturer's standard protocol (Applied Biosystems). Relative Wt1 and Sf1 expression in B6 vs. D2 gonad/mesonephros complexes was determined using Wt1 (Mm00460570_m1), Sf1(Mm00446826_m1) and Gapdh TaqMan Assays-on-Demand. Relative expression of ovary pathway genes in B6 vs. D2 ovaries was determined using Power SYBR Green PCR Master Mix (Applied Biosystems) and the following primers: Adamts19 (5′-CCAGATGCCTCCTGCTTTTA and 5′-GGTGCGGGTGACCTATGAT: 165 bp product), Wnt4 (5′-AGGATGCTCGGACAACATCG and 5′-CGCATGTGTGTCAAGATGGC: 149 bp product), Igfbp2 and 1700106J16Rik as in [46], Fst, Bmp2, and Hprt as in [44], and Irx3 as in [38].
Quantitative RT-PCR (qRT-PCR) assays were performed on an ABI PRISM 7900HT Sequence Detection System using SDS 2.3 software to determine the average cycle threshold (Ct) value from three technical replicates for each sample. Average Ct values for Hprt or Gapdh were subtracted from the average Ct values for each gene for each sample, to determine the normalized average Ct value (ΔCt). When expression was profiled across developmental stages, the results are represented as expression relative to the endogenous control gene (2−ΔCt), when expression was compared within a single developmental stage, the results were determined by subtracting the average ΔCt for D2 ovaries according to the 2−ΔΔCt method [66]. In pairwise comparisons of gene expression levels with a priori predictions about the direction of the effect, such as detecting lower expression of Wt1 and Sf1 or higher expression of Wnt4 and other ovarian genes in B6 samples, statistically significant differences were identified by one-tailed unpaired Student's t-tests (α = 0.05) using Microsoft Excel. For statistical analysis of Sry expression, one-way ANOVA was used to test for a significant effect of genotype in multiple comparisons and two-tailed unpaired Student's t-tests (α = 0.05) in pairwise comparisons within a developmental stage using JMP version 8 software (SAS Institute Inc.).
Multi-gene quantitative real time RT-PCR was conducted using RNA extracted from E12 gonad-mesonephros complexes and E14 gonads as described in [44]. Only E14 XYAKR mutant gonads lacking any testicular cords (classified as ovaries) were used for this analysis.
Genotyping assay for the presence of Sf1A172 versus Sf1S172 alleles
The presence of the Sf1A172 and Sf1S172 alleles was determined using a previously published genotyping assay [41]. Briefly, a region of Sf1 exon 3 including codon 172 was amplified from genomic DNA using 5′-TGCGTGCTGATCGAATGC and 5′-CCAGTCGACAATGGAGATAAAGG as primers and the PCR product digested using NcoI. Genomic DNA containing the Sf1A172 allele contains a NcoI recognition site resulting in two fragments of 331 and 262 bp, while the Sf1S172 allele lacks the NcoI recognition site resulting in an undigested 593 bp fragment. Genomic DNA was obtained from mice in the Eicher Laboratory mouse colony or purchased from the JAX Mice DNA Resource [C58/J (000669), NZB/BlNJ (000684), C3H/HeSnJ (000661), BALB/cBY (000650), SM/J (000687)].
Supporting Information
Zdroje
1. AchermannJCItoMHindmarshPCJamesonJL 1999 A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22 125 126
2. CorreaRVDomeniceSBinghamNCBillerbeckAERaineyWE 2004 A microdeletion in the ligand binding domain of human steroidogenic factor 1 causes XY sex reversal without adrenal insufficiency. J Clin Endocrinol Metab 89 1767 1772
3. MalletDBretonesPMichel-CalemardLDijoudFDavidM 2004 Gonadal dysgenesis without adrenal insufficiency in a 46, XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. J Clin Endocrinol Metab 89 4829 4832
4. LourencoDBraunerRLinLDe PerdigoAWeryhaG 2009 Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med 360 1200 1210
5. PhilibertPZenatyDLinLSoskinSAudranF 2007 Mutational analysis of steroidogenic factor 1 (NR5a1) in 24 boys with bilateral anorchia: a French collaborative study. Hum Reprod 22 3255 3261
6. LinLPhilibertPFerraz-de-SouzaBKelbermanDHomfrayT 2007 Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab 92 991 999
7. PelletierJBrueningWKashtanCEMauerSMManivelJC 1991 Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 67 437 447
8. PelletierJBrueningWLiFPHaberDAGlaserT 1991 WT1 mutations contribute to abnormal genital system development and hereditary Wilms' tumour. Nature 353 431 434
9. PatekCELittleMHFlemingSMilesCCharlieuJP 1999 A zinc finger truncation of murine WT1 results in the characteristic urogenital abnormalities of Denys-Drash syndrome. Proc Natl Acad Sci U S A 96 2931 2936
10. JordanBKMohammedMChingSTDelotEChenXN 2001 Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet 68 1102 1109
11. JagerRJHarleyVRPfeifferRAGoodfellowPNSchererG 1992 A familial mutation in the testis-determining gene SRY shared by both sexes. Hum Genet 90 350 355
12. VilainEMcElreaveyKJaubertFRaymondJPRichaudF 1992 Familial case with sequence variant in the testis-determining region associated with two sex phenotypes. Am J Hum Genet 50 1008 1011
13. JordanBKShenJHOlasoRIngrahamHAVilainE 2003 Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/beta-catenin synergy. Proc Natl Acad Sci U S A 100 10866 10871
14. KreidbergJASariolaHLoringJMMaedaMPelletierJ 1993 WT-1 is required for early kidney development. Cell 74 679 691
15. ShinodaKLeiHYoshiiHNomuraMNaganoM 1995 Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204 22 29
16. LuoXIkedaYParkerKL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77 481 490
17. SadovskyYCrawfordPAWoodsonKGPolishJAClementsMA 1995 Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci U S A 92 10939 10943
18. VeitiaRASalas-CortesLOttolenghiCPailhouxECotinotC 2001 Testis determination in mammals: more questions than answers. Mol Cell Endocrinol 179 3 16
19. EicherEMWashburnLLWhitneyJBIIIMorrowKE 1982 Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science 217 535 537
20. WashburnLLEicherEM 1983 Sex reversal in XY mice caused by dominant mutation on chromosome 17. Nature 303 338 340
21. NagamineCMTaketoTKooGC 1987 Studies on the genetics of tda-1 XY sex reversal in the mouse. Differentiation 33 223 231
22. BoumaGJAlbrechtKHWashburnLLRecknagelAKChurchillGA 2005 Gonadal sex reversal in mutant Dax1 XY mice: a failure to upregulate Sox9 in pre-Sertoli cells. Development 132 3045 3054
23. BoumaGJWashburnLLAlbrechtKHEicherEM 2007 Correct dosage of Fog2 and Gata4 transcription factors is critical for fetal testis development in mice. Proc Natl Acad Sci U S A 104 14994 14999
24. EicherEMWashburnLLSchorkNJLeeBKShownEP 1996 Sex-determining genes on mouse autosomes identified by linkage analysis of C57BL/6J-YPOS sex reversal. Nat Genet 14 206 209
25. EicherEMWashburnLL 1986 Genetic control of primary sex determination in mice. Annu Rev Genet 20 327 360
26. EicherEM 1994 Sex and trinucleotide repeats. Nat Genet 6 221 223
27. WhitneyJBMillsTMLewisRWWartellRAbneyTO 2000 A single genetic determinant that prevents sex reversal in C57BL-YPOS congenic mice. Biochem Genet 38 119 137
28. WashburnLLAlbrechtKHEicherEM 2001 C57BL/6J-T-associated sex reversal in mice is caused by reduced expression of a Mus domesticus Sry allele. Genetics 158 1675 1681
29. AlbrechtKHYoungMWashburnLLEicherEM 2003 Sry expression level and protein isoform differences play a role in abnormal testis development in C57BL/6J mice carrying certain Sry alleles. Genetics 164 277 288
30. WilhelmDEnglertC 2002 The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev 16 1839 1851
31. IkedaYLalaDSLuoXKimEMoisanM-P 1993 Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol 7 852 860
32. LittleMHolmesGWalshP 1999 WT1: what has the last decade told us? Bioessays 21 191 202
33. ReddyJCLichtJD 1996 The WT1 Wilms' tumor suppressor gene: How much do we really know? Biochim Biophys Acta 1287 1 28
34. VainioSHeikkilaMKispertAChinNMcMahonAP 1999 Female development in mammals is regulated by Wnt-4 signalling. Nature 397 405 409
35. Jeays-WardKHoyleCBrennanJDandonneauMAlldusG 2003 Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130 3663 3670
36. Jeays-WardKDandonneauMSwainA 2004 Wnt4 is required for proper male as well as female sexual development. Dev Biol 276 431 440
37. MenkeDBKoubovaJPageDC 2003 Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 262 303 312
38. JorgensenJSGaoL 2005 Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr Patterns 5 756 762
39. CarlisleCWinkingHWeichenhanDNagamineCM 1996 Absence of correlation between Sry polymorphisms and XY sex reversal caused by the M. m. domesticus Y chromosome. Genomics 33 32 45
40. NagamineCMCarlisleC 1996 The dominant white spotting oncogene allele KitW-42J exacerbates XYDOM sex reversal. Development 122 3597 3605
41. FrigeriCTsaoJCordovaMSchimmerBP 2002 A polymorphic form of steroidogenic factor-1 is associated with adrenocorticotropin resistance in y1 mouse adrenocortical tumor cell mutants. Endocrinology 143 4031 4037
42. HammesAGuoJ-KLutschGLehesteJ-RLandrockD 2001 Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106 319 329
43. KlamtBKoziellAPoulatFWieackerPScamblerP 1998 Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/−KTS splice isoforms. Hum Mol Genet 7 709 714
44. BoumaGJHartGTWashburnLLRecknagelAKEicherEM 2004 Using real time RT-PCR analysis to determine multiple gene expression patterns during XX and XY mouse fetal gonad development. Gene Expr Patterns 5 141 149
45. NefSSchaadOStallingsNRCederrothCRPitettiJL 2005 Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol 287 361 377
46. LeeHJPazinDEKahlonRSCorreaSMAlbrechtKH 2009 Novel markers of early ovarian pre-granulosa cells are expressed in an Sry-like pattern. Dev Dyn 238 812 825
47. HatanoOTakakusuANomuraMMorohashiK 1996 Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1 663 671
48. HeikkilaMPeltoketoHLeppaluotoJIlvesMVuolteenahoO 2002 Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology 143 4358 4365
49. ValPMartinez-BarberaJPSwainA 2007 Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development 134 2349 2358
50. LeeCHTaketoT 2001 Low levels of Sry transcripts cannot be the sole cause of B6-Y(TIR) sex reversal. Genesis 30 7 11
51. GregoireEPLaveryRChassotAAAkiyamaHTreierM Transient development of ovotestes in XX Sox9 transgenic mice. Dev Biol 349 65 77
52. BradfordSTWilhelmDBandieraRVidalVSchedlA 2009 A cell-autonomous role for WT1 in regulating Sry in vivo. Hum Mol Genet 18 3429 3438
53. MungerSCAylorDLSyedHAMagwenePMThreadgillDW 2009 Elucidation of the transcription network governing mammalian sex determination by exploiting strain-specific susceptibility to sex reversal. Genes Dev 23 2521 2536
54. ChassotAARancFGregoireEPRoepers-GajadienHLTaketoMM 2008 Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet 17 1264 1277
55. TomizukaKHorikoshiKKitadaRSugawaraYIbaY 2008 R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet 17 1278 1291
56. EicherEMWashburnLL 1983 Inherited sex reversal in mice: Identification of a new primary sex-determining gene. J Exp Zool 228 297 304
57. WashburnLLEicherEM 1989 Normal testis determination in the mouse depends on genetic interaction of a locus on chromosome 17 and the Y chromosome. Genetics 123 173 179
58. StarkKVainioSVassilevaGMcMahonAP 1994 Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372 679 683
59. CapelBAlbrechtKHWashburnLLEicherEM 1999 Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev 84 127 131
60. HackerACapelBGoodfellowPLovell-BadgeR 1995 Expression of Sry, the mouse sex determining gene. Development 121 1603 1614
61. KaufmanM 2003 The Atlas of Mouse Development; KaufmanM London Academic Press
62. EicherEMBeamerWGWashburnLLWhittenWK 1980 A cytogenetic investigation of inherited true hermaphroditism in BALB/cWt mice. Cytogenet Cell Genet 28 104 115
63. EicherEMWashburnLL 2001 Does one gene determine whether a C57BL/6J-YPOS mouse will develop as a female or as an hermaphrodite? J Exp Zool 290 322 326
64. AlbrechtKHEicherEM 2001 Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol 240 92 107
65. WilkinsonDG 1992 Whole mount in situ hybridization of vertebrate embryos. WilkinsonDG In situ hybridization: A Practical Approach. 2nd ed Oxford IRL Press 75 83
66. LivakKJSchmittgenTD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25 402 408
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



