-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
Autism spectrum disorders (ASD) are a heterogeneous group of neurodevelopmental disorders with a complex inheritance pattern. While many rare variants in synaptic proteins have been identified in patients with ASD, little is known about their effects at the synapse and their interactions with other genetic variations. Here, following the discovery of two de novo SHANK2 deletions by the Autism Genome Project, we identified a novel 421 kb de novo SHANK2 deletion in a patient with autism. We then sequenced SHANK2 in 455 patients with ASD and 431 controls and integrated these results with those reported by Berkel et al. 2010 (n = 396 patients and n = 659 controls). We observed a significant enrichment of variants affecting conserved amino acids in 29 of 851 (3.4%) patients and in 16 of 1,090 (1.5%) controls (P = 0.004, OR = 2.37, 95% CI = 1.23–4.70). In neuronal cell cultures, the variants identified in patients were associated with a reduced synaptic density at dendrites compared to the variants only detected in controls (P = 0.0013). Interestingly, the three patients with de novo SHANK2 deletions also carried inherited CNVs at 15q11–q13 previously associated with neuropsychiatric disorders. In two cases, the nicotinic receptor CHRNA7 was duplicated and in one case the synaptic translation repressor CYFIP1 was deleted. These results strengthen the role of synaptic gene dysfunction in ASD but also highlight the presence of putative modifier genes, which is in keeping with the “multiple hit model” for ASD. A better knowledge of these genetic interactions will be necessary to understand the complex inheritance pattern of ASD.
Published in the journal: . PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002521
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002521Summary
Autism spectrum disorders (ASD) are a heterogeneous group of neurodevelopmental disorders with a complex inheritance pattern. While many rare variants in synaptic proteins have been identified in patients with ASD, little is known about their effects at the synapse and their interactions with other genetic variations. Here, following the discovery of two de novo SHANK2 deletions by the Autism Genome Project, we identified a novel 421 kb de novo SHANK2 deletion in a patient with autism. We then sequenced SHANK2 in 455 patients with ASD and 431 controls and integrated these results with those reported by Berkel et al. 2010 (n = 396 patients and n = 659 controls). We observed a significant enrichment of variants affecting conserved amino acids in 29 of 851 (3.4%) patients and in 16 of 1,090 (1.5%) controls (P = 0.004, OR = 2.37, 95% CI = 1.23–4.70). In neuronal cell cultures, the variants identified in patients were associated with a reduced synaptic density at dendrites compared to the variants only detected in controls (P = 0.0013). Interestingly, the three patients with de novo SHANK2 deletions also carried inherited CNVs at 15q11–q13 previously associated with neuropsychiatric disorders. In two cases, the nicotinic receptor CHRNA7 was duplicated and in one case the synaptic translation repressor CYFIP1 was deleted. These results strengthen the role of synaptic gene dysfunction in ASD but also highlight the presence of putative modifier genes, which is in keeping with the “multiple hit model” for ASD. A better knowledge of these genetic interactions will be necessary to understand the complex inheritance pattern of ASD.
Introduction
Autism spectrum disorders (ASD) are characterized by impairments in reciprocal social communication and stereotyped behaviors [1]. The prevalence of ASD is about 1/100, but closer to 1/300 for typical autism [2]. ASD are more common in males than females, with a 4∶1 ratio. Previously, twin and family studies have conclusively described ASD as the most “genetic” of neuropsychiatric disorders, with concordance rates of 82–92% in monozygotic twins versus 1–10% in dizygotic twins [3], but a recent study finds evidence for a more substantial environmental component [4]. In the absence of Mendelian inheritance patterns, ASD were first considered to be polygenic, i.e., a disorder caused by multiple genetic risk factors, each of weak effect. More recently, an alternative model was proposed that considered ASD as a group of disorders caused by heterogeneous genetic risk factors influencing common neuronal pathways [5], [6]. It was supported by the identification of apparently monogenic forms of ASD, each affecting a limited number of patients (1–2% for the most replicated genes) [7]–[14]. In this model, eventually a single highly penetrant mutation would be sufficient to produce ASD. However, the occurrence of two or more deleterious copy number variants (CNV) or mutations in a subset of patients also suggested that independent loci could act in concert to induce the development of ASD [9], [13]–[16]. In line with these findings, the recent observation that patients with a deletion at 16p12.1 were more likely to carry an additional large CNV agrees with a “two-hit model” for developmental disorders [17].
The genetic causes of ASD are diverse [18], but the main category of genes associated with the disorder is related to the development and function of neuronal circuits [6], [19]. Mutations of genes coding for synaptic cell adhesion molecules and scaffolding proteins, such as neuroligins (NLGN), neurexins (NRXN) and SHANK, have been recurrently reported in patients with ASD [7]–[10], [13], [14], [20]. These proteins play a crucial role in the formation and stabilization of synapses [21], as well as in synaptic homeostasis [22]. SHANK2 and SHANK3 code for scaffolding proteins located in the postsynaptic density (PSD) of glutamatergic synapses. Deletions of ProSAP2/SHANK3 at chromosome 22q13 are one of the major genetic abnormalities in neurodevelopmental disorders [20], and mutations of ProSAP2/SHANK3 have been identified in patients with ASD, intellectual disability (ID) and schizophrenia [7], [23]–[25]. Mutations of ProSAP1/SHANK2 have also recently been reported in both, ASD and ID [9], [26]. The difference in clinical outcome of mutation carriers has been attributed to the presence of still uncharacterized additional genetic, epigenetic and/or environmental factors [27].
In order to better understand the role of the NRXN-NLGN-SHANK pathway in ASD, we first aimed to describe SHANK2 isoform expression in different tissues of healthy individuals. To investigate the role of this pathway in ASD, we screened for SHANK2 CNVs and coding mutations in a large sample of patients with ASD and controls. We provide genetic and functional evidence that SHANK2 is associated with ASD, and that its mutations affect the number of synapses. Additionally, we report the co-occurrence of SHANK2 de novo deletions and inherited CNVs altering neuronal genes, suggesting that epistasis between specific loci in the genome could modulate the risk for ASD.
Results
SHANK2 isoforms are differentially expressed in human tissues
In order to characterize all isoforms of SHANK2, we scanned genomic databases for specific Expressed Sequence Tags (ESTs) and spliced isoforms. The human SHANK2 gene (NM_012309.3) spans 621.8 kb and contains 25 exons (Figure 1). The longest SHANK2 isoform (SHANK2E, AB208025) contains ankyrin (ANK) repeats at the N-terminus, followed by a Src homology 3 (SH3) domain, a PSD95/DLG/ZO1 (PDZ) domain, a proline-rich region and a sterile alpha motif (SAM) domain at its C-terminus region. All these domains are involved in protein-protein interactions that bridge glutamate receptors, scaffolding proteins and intracellular effectors to the actin cytoskeleton [28], [29]. Two additional isoforms, ProSAP1A (AB208026) and ProSAP1 (AB208027), originating from distinct promoters, were previously detected in the rat [30], [31]. Finally, the shortest isoform (AF141901), also originally described in the rat, results in premature termination of the transcription before the SAM domain due to an alternative 3′ end in exon 22 [32] (Figure 1A). To validate these SHANK2 isoforms in humans, we used specific RT-PCRs and sequencing (Figure 1B). Almost all tissues tested (brain, liver, placenta, kidney, lung, pancreas and lymphoblastoid cell lines) expressed SHANK2 mRNA, except heart and skeletal muscle, for which no expression was detected. We observed inter-individual differences in the relative amount of SHANK2 mRNA that were confirmed by using independent RT-PCRs and primers (not shown). Such differences have been previously reported for other synaptic genes such as NLGN1-4Y, PCHD11X/Y, and SHANK3 [7], [8], [33] and might be the consequence of polymorphisms located in specific regulatory sequences and/or activity dependent expression of this family of post-synaptic proteins [34]. Notably, exons 19, 20 and 23 were found to be expressed only in brain in all individuals tested (Figure 1C). Such brain specific splicing has been already observed for exon 18 in SHANK3 [7], which is similar to exon 19 and 20 in SHANK2. These ‘brain-specific exons’ code for a region in SHANK2/3 located between the PDZ and the proline rich domains. Finally, in contrast to previous results [26], we detected the longest SHANK2E isoform in all independent samples of human brain, with high expression in the cerebellum (Figure 1, Figure S1). This Shank2E isoform was also expressed in the cerebellum and in the liver of rat embryo at E19 (Figure S1).
Fig. 1. Genomic structure, isoforms, and expression of human SHANK2. 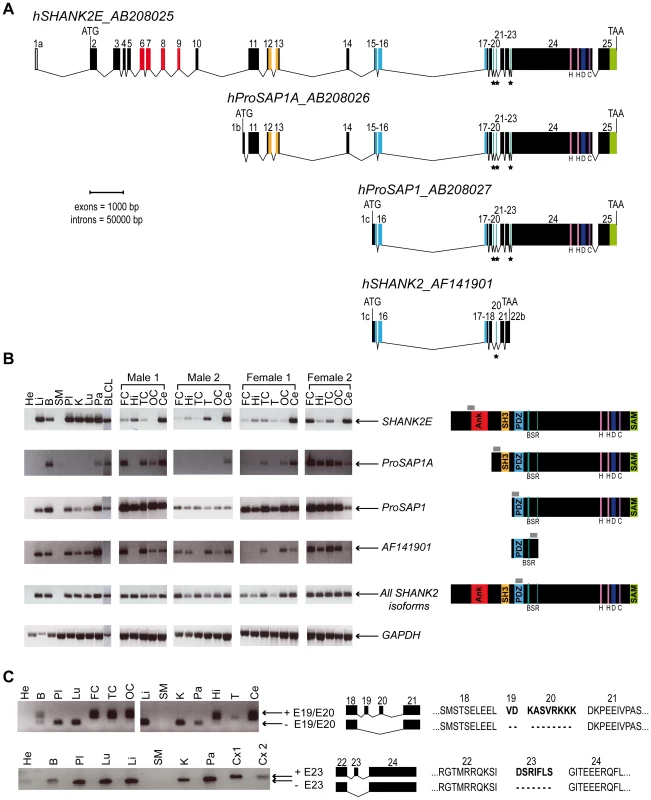
A. Genomic structure of the human SHANK2 gene. Transcription of SHANK2 produces four main mRNA from three distinct promoters: SHANK2E (AB208025), ProSAP1A (AB208026), ProSAP1 (AB208027) and AF141901. There are three translation starts: in exon 2 for SHANK2E, in exon1b for ProSAP1A, and in exon1c for ProSAP1 and AF141901; and two independent stop codons: in exon 22b for AF141901 and in exon 25 for SHANK2E, ProSAP1A and ProSAP1. Conserved domains of protein interaction or protein binding site are represented in color: ANK (red), SH3 (orange), PDZ (blue) and SAM (green), H (pink), D, (dark blue) and C (purple). Black stars identify the alternative spliced exons (‘brain-specific exons’ in turquoise: 19, 20 and 23). B. RT-PCRs of SHANK2 isoforms on RNA from different human control tissues (Clontech), and different brain regions of four controls (2 males and 2 females). The amplified regions specific to each isoform of SHANK2 are indicated by gray boxes. C. Alternative splicing of human SHANK2; exons 19, 20 and 23 are specific to the brain. ANK, ankyrin; SH3, Src homology 3; PDZ, PSD95/DLG/ZO1; SAM, sterile alpha motif; He, heart; Li, liver; B, brain; SM, skeletal muscle; Pl, placenta; K, kidney; Lu, lung; Pa, pancreas; FC, frontal cortex; Hi, hippocampus; TC, temporal cortex; T, thalamus; OC, occipital cortex; Ce, cerebellum; Cx, whole cortex; BLCL, B lymphoblastoid cell lines; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; BSR, brain specific region; H, homer binding site; D, dynamin binding site; C, cortactin binding site. The ages of the two males and the two females studied were 74, 42, 55, and 36 years with a post-mortem interval of 10, 21, 24, and 2 h, respectively. A de novo deletion of SHANK2 in a patient with ASD
Berkel et al. 2010 recently identified two independent de novo SHANK2 deletions in two patients, one with ID and another one with ASD [26]. In addition, whole genome analysis performed by the Autism Genome Project (AGP) using Illumina 1M single nucleotide polymorphism (SNP) arrays detected one additional de novo SHANK2 deletion in a patient (6319_3) with ASD [9] (the second patient described by the AGP, 5237_3, is patient SK0217-003 reported in Berkel et al. 2010 [26]). Recently, a 3.4 Mb de novo deletion including SHANK2 was observed in a female patient with speech and developmental delay [35]. To follow up on these results, we genotyped an independent sample of 260 patients with ASD using Illumina 1M Duo SNP arrays (Table S1). In this sample, we detected a 421.2 kb deletion within SHANK2 in patient AU038_3 with autism and moderate ID (see patient section in Materials and Methods, and Table S2). The deletion covered twelve exons (E5–E16) and altered all SHANK2 isoforms (Figure 2A). No other deleterious variants in the remaining copy of SHANK2 were detected by sequencing. The parents did not carry the deletion, indicating a de novo event. The deletion was validated by quantitative PCR analysis using DNA from an independent blood sample from all members of the family and SNP analysis indicated that the deletion originated on the maternal chromosome (Figure S2). SHANK2 deletions were absent in more than 5000 controls [9], [26] and not listed in the Database of Genomic Variants (DGV; http://projects.tcag.ca/variation/).
Fig. 2. SHANK2 mutations in patients with ASD. 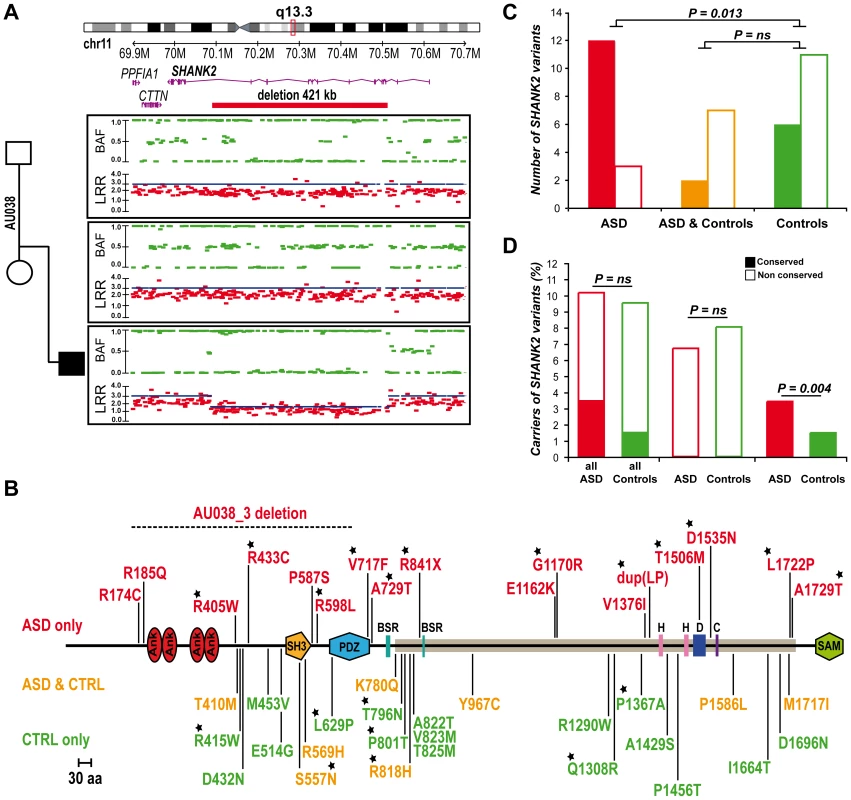
A. A heterozygous deletion of SHANK2 was identified with the Illumina Human 1M-Duo SNP array in a patient with autism (AU038_3). The deletion spans 421 kb on chromosome 11q13.3, covers twelve exons of the human SHANK2 and is not present in the parents. Each dot shows Log R Ratio (LRR; in red) and B allele frequency (BAF; in green). QuantiSNP score is represented with a blue line and indicates the deletion size. B. Location of the CNV and sequence variants (from this study and Berkel et al. 2010) along the SHANK2 protein: in red the variations specific to ASD, in orange the variations shared by ASD and controls and in green the variations specific to controls [26]. The breakpoints of the SHANK2 deletion in AU038_3 are represented with a dotted line on the protein. Stars indicate the variants affecting conserved amino acids. C. A total of 40 variants were identified and variants affecting conserved amino acids in other SHANK proteins are enriched in patients with ASD (nconserved = 12 and nnon-conserved = 3) compared with controls (nconserved = 6 and nnon-conserved = 11) (Fisher's exact test 1-sided, P = 0.013, OR = 6.83, 95% IC = 1.19–53.40). D. The percentage of carriers of SHANK2 variants in patients with ASD and Controls. Variants affecting a conserved amino acid among the SHANK proteins are enriched in patients with ASD (nconserved = 29 and nnon-conserved = 822) compared with controls (nconserved = 16 and nnon-conserved = 1074) (Fisher's exact test 1-sided, P = 0.004, OR = 2.37, 95% CI = 1.23–4.70). Open squares and filled squares represent the non-conserved and conserved amino acids, respectively. ANK, Ankyrin repeat domain; SH3, Src homology 3 domain; PDZ, postsynaptic density 95/Discs large/zona occludens-1 homology domain; SAM, sterile alpha motif domain; BSR, brain specific region; H, homer binding site; D, dynamin binding site; C, cortactin binding site. The proline-rich region is represented as a horizontal gray line. SHANK2 coding variants affecting conserved amino acids are enriched in patients with ASD
To probe for additional mutations, we first sequenced all exons of the longest SHANK2E isoform in 230 patients with ASD and 230 controls. We then sequenced an additional sample of 225 patients and 201 controls (Table S1) for the ProSAP1A isoform that corresponds to the major SHANK2 isoform in the brain. Since we screened all SHANK2 isoforms, we used a nomenclature including the SHANK2E isoform that differed from Berkel et al. 2010 [26]. Within the 9 coding exons specific to SHANK2E, we identified R174C (rs7926203) listed in dbSNP in 2 independent patients with ASD and R185Q in one patient with ASD. For this isoform, no variant was identified in the control sample. Within the ProsSAP1A isoform, we identified 24 non-synonymous variations. When these results are integrated with those obtained by Berkel et al. 2010, a total of 40 variants of ProsSAP1A including 3 already reported in dbSNP were identified (Figure 2B, Table 1, Figure S3). Only two variants (Y967C and R569H) with MAF>1% are detected and there is no enrichment of rare variants of SHANK2 (MAF<1%) in patients with ASD compared with controls. Because variants affecting conserved amino acids in the SHANK proteins are most likely to have a functional effect, we tested whether there was an enrichment of these variants in patients compared to controls. The alignment of the SHANK protein sequences and the conservation of the variants are indicated in the Table S5. In both mutation screening studies, the first performed by Berkel et al. 2010 and the second presented here, we observed an enrichment of variants affecting conserved amino acids in patients compared with controls (Figure 2C, Table S5 and Table S7). Overall, 12 of 15 (80%) of the variants identified only in the patient sample affected conserved amino acids compared with only 6 of 17 (35.3%) in controls (Fisher's exact test 1-sided, P = 0.013, OR = 6.83, 95% IC = 1.19–53.40). Because several independent patients carried these variants (Table 1), the enrichment is even more significant when the number of carriers was considered. The variants affecting conserved amino acids were observed in 29 of 851 (3.4%) patients and in 16 of 1090 (1.5%) controls (Fisher's exact test 1-sided, P = 0.004, OR = 2.37, 95% CI = 1.23–4.70). A total of 8 variants were identified in patients and controls. Among these 8 variants, 2 affected conserved amino acids (R818H and S557N). The variant S557N was observed in 9 of 851 (1.06%) independent families with ASD and in 3 of 1090 (0.28%) controls (Fisher's Exact Test one sided, P = 0.029, OR = 3.87; 95% CI = 0.96–22.29). It affects a conserved serine with a high probability of being phosphorylated and located in the SH3 domain of all SHANK proteins. This domain binds to GRIP and b-PIX, two proteins linking SHANK to glutamate AMPA receptors and actin skeleton, respectively [36]. In our initial mutation screen, R818H was observed in 5 of 230 patients with ASD and 0 of 230 controls. In order to determine if R818H was more frequent in the patients with ASD, we screened an additional sample of 3020 individuals with ASD, 1783 controls from European descent, and the Human Genome Diversity Panel (HGDP) control dataset (Table S3 and S4). R818H was virtually absent outside Europe and had the highest allelic frequency (2.37%) in Finland, but overall its frequency was not higher in patients with ASD compared with controls (ASD 32/3250 (1.0%); controls 27/2030 (1.33%); Fisher's exact test 2-sided, P = 0.28) (Table S3).
Tab. 1. ProSAP1A/SHANK2 variations identified in 851 patients with ASD and 1,090 controls. 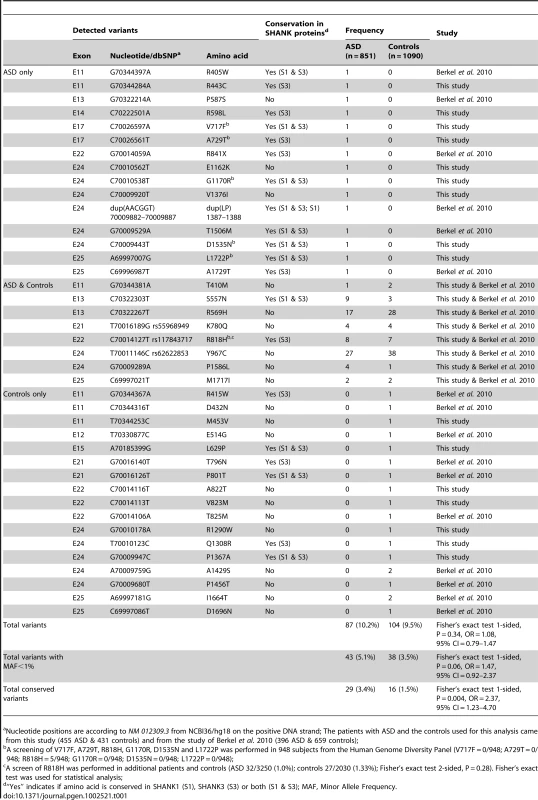
Nucleotide positions are according to NM 012309.3 from NCBI36/hg18 on the positive DNA strand; The patients with ASD and the controls used for this analysis came from this study (455 ASD & 431 controls) and from the study of Berkel et al. 2010 (396 ASD & 659 controls); Finally, and unexpectedly, during this additional mutation screening, we detected a variation (IVS22+1G>T) altering the consensus donor splice site of exon 22 in a Swedish control, SWE_Q56_508 (Figure 3A). This variant was predicted to disrupt all SHANK2 isoforms by deleting the proline rich and the SAM domain, except for the shortest isoform AF141901, where the mutation is located in the open reading frame (ORF) and should lead to a G263V change. This variant was not observed in 1786 patients or 1407 controls, and is not listed in dbSNP. This control female was part of a previous epidemiological study [37] and had been extensively examined for anthropometrics and cardiovascular risk factors such as blood pressure and levels of all major hormones. In addition, she was ascertained for axis I psychiatric disorders and personality traits using the Temperament and Character Inventory (TCI) [38] and the Karolinska Scales of Personality (KSP) [39]. Notably, despite the predicted deleterious effect of the mutation, this subject had no major somatic or psychiatric health problems. Regarding personality traits, none of her scores for TCI items were different from those found in the general population. KSP assessment showed that her scores for neuroticism (51.3), nonconformity/aggressiveness (56.7), and psychoticism (50.5) were not different from the general population (mean ± SD = 50±10). However, she displayed a high score (61.4) for the extraversion factor, and for one of its subscales, monotony avoidance [40].
Fig. 3. Genetic alterations identified in the control subject SWE_Q56_508. 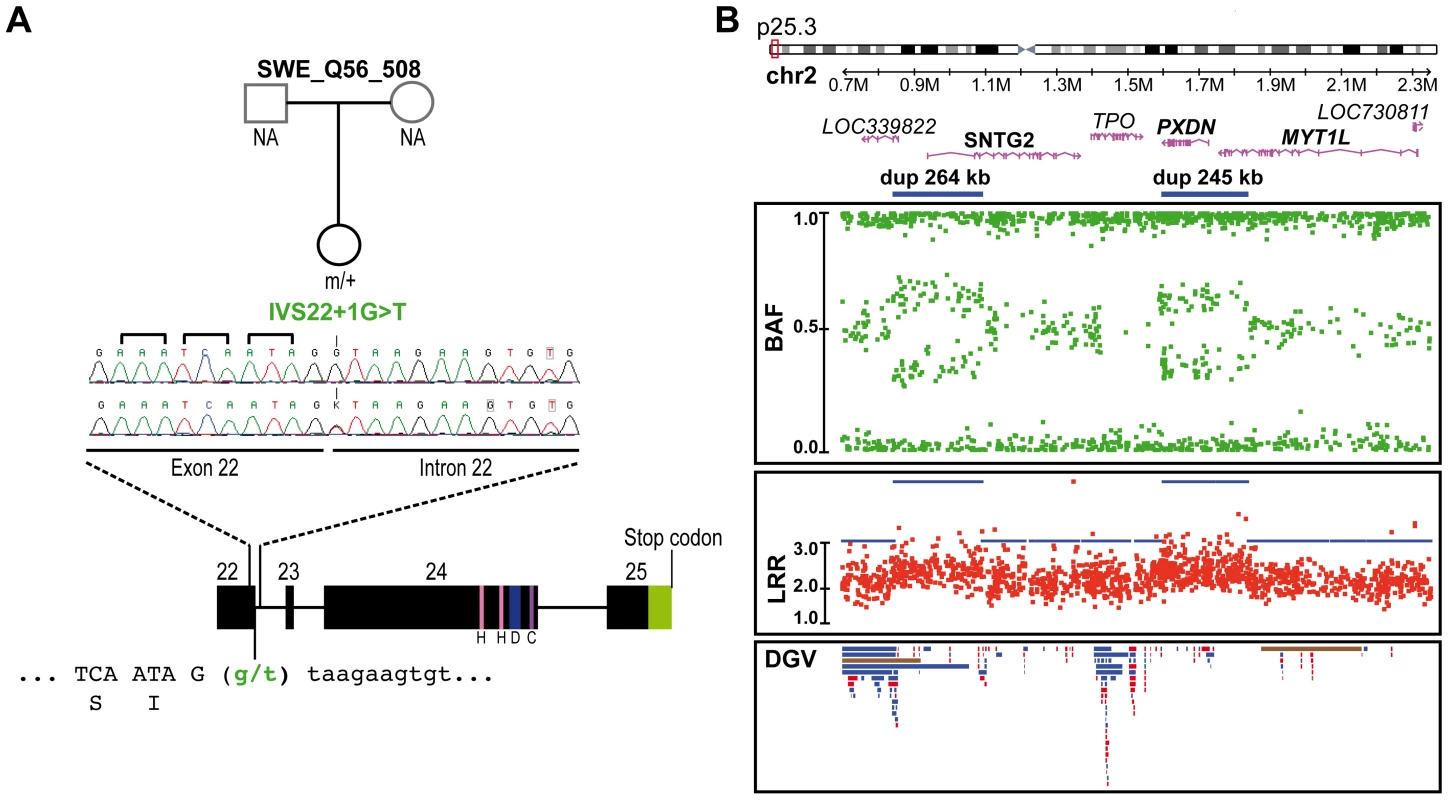
A. SHANK2 splice mutation (IVS22+1G>T) detected in a Swedish female control, SWE_Q56_508. The mutation altered the donor splicing site of exon 22 and led to a premature stop in all SHANK2 isoforms except for the AF1411901 isoform, where it altered the protein sequence (G263V). B. CNVs in the same individual altering LOC339822, SNTG2, PXDN and MYT1L. The two close duplications span 264 kb and 245 kb on chromosome 2 and altered LOC339822 and SNTG2, and PXDN and MYT1L, respectively. Dots show the B allele frequency (BAF; in green), Log R ratio (LRR; in red), and QuantiSNP score (in blue). Lower panel: all CNVs listed in the Database of Genomic Variants (DGV) are represented: loss (in red), gain (in blue), gain or loss (in brown). H, homer binding site; D, dynamin binding site; C, cortactin binding site. Several SHANK2 variants identified in patients alter synapse density in cultured neurons
In order to establish the functional impact of SHANK2 variations, we performed expression studies in primary neuronal cell cultures after over-expression of wild-type vs. mutant ProSAP1A/Shank2A cDNA (Figure 4). All the variants (n = 16) identified by our first screen of 230 patients and 230 controls were tested: 5 were identified only in patients (V717F, A729T, G1170R, D1535N and L1722P), 6 were detected in patients and controls (S557N, R569H, K780Q, R818H, Y967C and P1586L) and 5 were only found in controls (L629P, A822T, V823M, R1290W and Q1308R). All variants were predicted as damaging by Polyphen2 DIV except Q1308R identified only in controls and predicted as benign [41]. In the patient sample, 5/5 variants affected conserved amino acids in the SHANK proteins compared with only 2/6 in the group of variants identified in patients and controls, and 2/5 in the control group. All mutation sites were introduced into the rat ProSAP1A cDNA and confirmed by sequencing. The effect of the Shank2 variants was further investigated in cultured hippocampal neurons. Upon transfection, Western blot analysis revealed that the different GFP-Shank2 fusion proteins were expressed with the expected size (Figure S4). Results from quantification showed that none of the variants affected the cluster formation of Shank2 protein along the dendrites, the number or the general branching pattern of dendrites (Figure S4). In contrast, 8 variants identified in patients or in patients and control group reduced significantly the density of Shank2 positive synapses per 10 µm dendrite length compared with wild-type GFP-Shank2 (Figure 4). None of the variants identified in controls only were shown to have a significant effect. After Bonferonni correction for the 16 tests, 4 variants significantly affected synapse density. Among these variants, A729T, G1170R and L1722P were identified only in patients and S557N was observed more frequently in patients than in controls. As expected, the majority of the variants leading to reduced synaptic density altered conserved amino acids present in SHANK proteins (7/8), and the majority of variants not changing synaptic density affected amino acids present only in SHANK2 (7/8). The 4 strongly associated (after Bonferroni correction) variants affecting synaptic density modified conserved amino acids in other SHANK proteins. Because the significant threshold of 0.05 is arbitrary, we additionally tested for the quantitative effect of the variant on synaptic density as a continuous trait (Figure S4) and found that variants identified in patients were associated with a significant decrease of synapse density in vitro compared with those shared by patients and controls (Student's t test 2-sided P = 0.022) or those only detected in the controls (Student's t test 2-sided P = 0.0013). As expected, variants affecting conserved amino acids were associated with a higher reduction of synapse density in vitro (Student's t test 2-sided P = 0.014).
Fig. 4. Characterization of the functional impact of SHANK2 mutations in cultured neuronal cells. 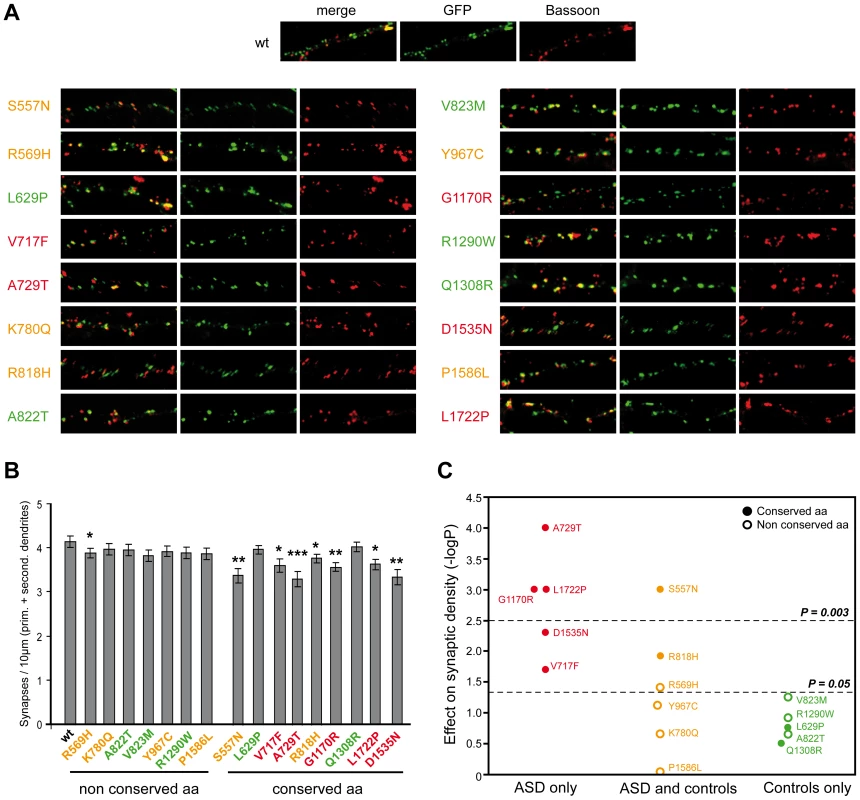
A. The colocalization of ProSAP1A/SHANK2-EGFP (postsynaptic marker) and Bassoon (presynaptic marker) indicated that the mutations did not disturb the formation of SHANK2 clusters at excitatory synapses along the dendrites. B. The quantification of synapse density was performed on 20 transfected hippocampal neurons per construct from at least three independent experiments. The majority of the ProSAP1A variants affecting a conserved amino acid among SHANK proteins reduced significantly the synaptic density compared with the variants that affect amino acid non conserved among SHANK proteins (Mann-Whitney U-test: nWT = 20, nmut = 20; US557N = 82.5, pS557N = 0.001; UR569H = 124, pR569H = 0.04; UL629P = 149, pL629P = 0.17; UV717F = 114, pV717F = 0.02; UA729T = 73, pA729T = 0.000; UK780Q = 154, pK780Q = 0.221; UR818H = 108, pR818H = 0.012; UA822T = 154.5, pA822T = 0.224; UV823M = 129, pV823M = 0.056; UY967C = 134, pY967C = 0.076; UG1170R = 78, pG1170R = 0.001; UR1290W = 142, pR1290W = 0.121; UQ1308R = 162, pQ1308R = 0.314; UD1535N = 97, pD1535N = 0.005; UP1586L = 137, pP1586L = 0.910; UL1722P = 79, pL1722P = 0.001, *p<0.05, **p<0.01, ***p<0.001). C. Effect of the variants on synaptic density. The y-axis represents −log P compared to WT (P obtained with Mann-Whitney test). After Bonferroni correction for 16 tests, only P values<0.003 were considered as significant. Variants represented in red were specific to ASD, in orange were shared by ASD and controls, and in green were specific to the controls. Open circles and filled circles represent non conserved and conserved amino acids, respectively. Prim, primary; second, secondary. Additional CNVs affect neuronal genes in patients with de novo SHANK2 deletions and in the control carrying the SHANK2 splice mutation
To test if additional CNVs may modulate the impact of SHANK2 mutations in the development of ASD, we analyzed the CNVs of patient AU038_3 and the two patients (5237_3 and 6319_3) carrying SHANK2 de novo deletions previously identified by Pinto et al. [9] (Figure 5 and Table S6). In addition to our CNV study group of 260 patients with ASD and 290 controls, we used the CNV dataset from the AGP, which includes 996 patients with ASD and 1287 controls genotyped with the Illumina 1M SNP array [9]. Remarkably, all three patients with SHANK2 de novo deletions also carried rare inherited genetic imbalances at chromosome 15q11–q13 (Figure 6), a region associated with Angelman syndrome, Prader-Willi syndrome and other neuropsychiatric disorders, including ASD [42]–[61]. This region is characterized by recurrent deletions/duplications with breakpoints generally located within five segmental duplications named BP1 to BP5, which act as hotspots of non-allelic homologous recombination. In the BP5 region, patients AU038_3 and 5237_3 carried the same 496 kb duplication of the nicotinic receptor CHRNA7 gene (29.8–30.3 Mb, hg 18; maternally inherited in patient AU038_3 and paternally inherited in patient 5237_3). This small CHRNA7 duplication was present in 13 of 1257 patients with ASD (1.03%) compared with 9 of 1577 controls (0.57%) (Fisher's exact test, 2-sided P = 0.19).
Fig. 5. Characterization of CNVs in three patients carrying a de novo deletion of SHANK2. 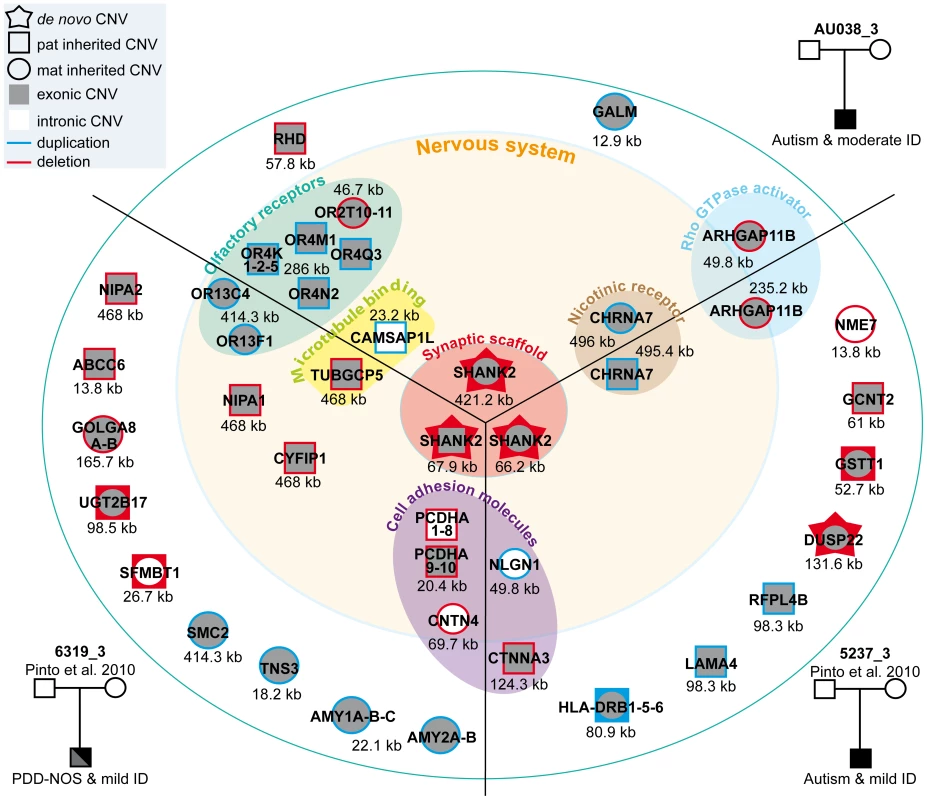
Paternally or maternally inherited CNVs are indicated by squares and circles, respectively. De novo CNVs are indicated by stars. Deletions and duplications are indicated in red and blue, respectively. CNVs hitting exons or only introns are filled with grey and white, respectively. Squares and circles within star represent de novo CNV of paternal or maternal origin; circles within squares represent CNV inherited by father or mother. ABCC6, ATP-binding cassette, sub-family C, member 6 pseudogene 2; ADAM, ADAM metallopeptidase; AMY1, amylase (salivary); AMY2A, amylase (pancreatic); ARHGAP11B, Rho GTPase activating protein 11B; CAMSAP1L1, calmodulin regulated spectrin-associated protein 1-like 1; CHRNA7, cholinergic receptor, nicotinic, alpha 7; CNTN4, contactin 4; CTNNA3, catenin (cadherin-associated protein), alpha 3; CYFIP1, cytoplasmic FMR1 interacting protein 1; DUSP22, dual specificity phosphatase 22; GALM, galactose mutarotase; GCNT2, glucosaminyl (N-acetyl) transferase 2; GOLGA, golgi autoantigen, golgin subfamily a; GSTT1, glutathione S-transferase theta 1; HLA-DRB, major histocompatibility complex, class II, DR beta; LAMA4, laminin, alpha 4; NIPA, non imprinted in Prader-Willi/Angelman syndrome; NLGN1, neuroligin 1; NME7, non-metastatic cells 7; OR, olfactory receptor; PCDHA, protocadherin alpha; RFPL4B, ret finger protein-like 4B; RHD, Rh blood group, D antigen; SFMBT1, Scm-like with four mbt domains 1; SHANK2, SH3 and multiple ankyrin repeat domains 2; SMC2, structural maintenance of chromosomes 2; TNS3, tensin 3; TUBGCP5, tubulin, gamma complex associated protein 5; UGT2B17, UDP glucuronosyltransferase 2 family, polypeptide B17. Fig. 6. Inherited 15q11–q13 CNVs identified in three ASD patients carrier of a de novo SHANK2 deletion. 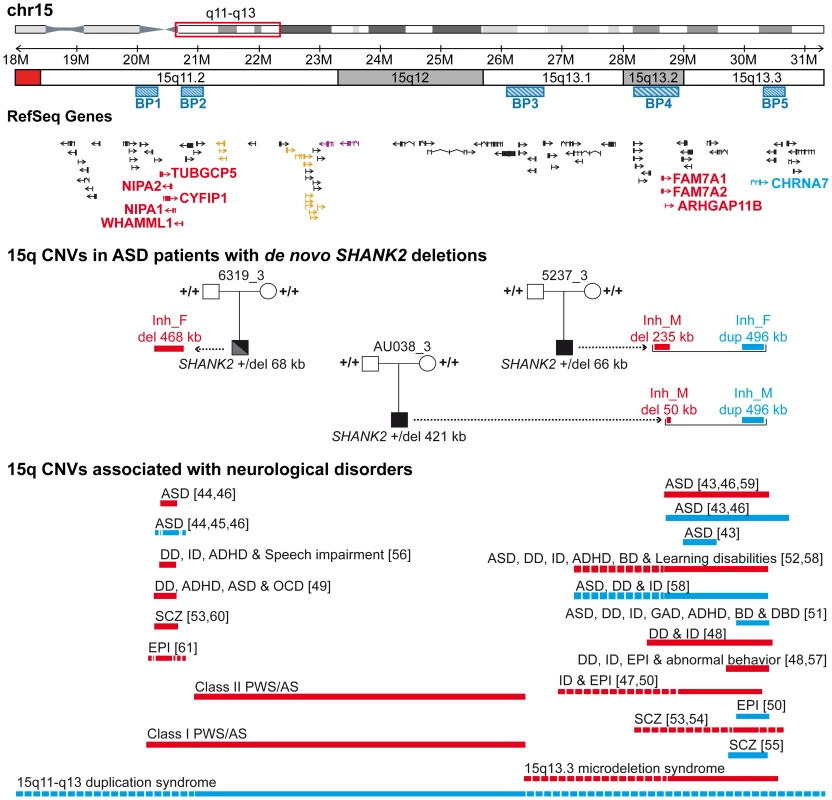
Deletions (del) and duplications (dup) are indicated in red and blue, respectively. Paternally and maternally imprinted genes are indicated in yellow and pink, respectively. Genes altered by the CNVs are indicated in blue or red. The bottom part of the figure indicates the location of the deletions/duplications previously associated with neuropsychiatric disorders [43]–[61]. BP, breakpoint; Inh_M, inherited by mother; Inh_F, inherited by father; AS, Angelman syndrome; ASD, Autism spectrum disorders; ADHD, attention deficit-hyperactivity disorder; BP, bipolar disorder; DD: developmental delay; DBD, disruptive behavior disorder; EPI, epilepsy; GAD, generalized anxiety disorder; OCD, obsessive-compulsive disorder; ID, intellectual disability; PWS, Prader-Willi syndrome; SCZ, schizophrenia. These duplications are considered of uncertain clinical significance since they were previously detected at similar frequencies in patients with epilepsy (6 of 647, 0.93%), in controls (19 of 3699, 0.51%) [50], and in subjects referred for chromosomal microarray analysis (55 of 8832, 0.62%) [51]. In contrast, larger 15q13.3 deletions (∼1.5 Mb) between BP4 and BP5, encompassing the CHRNA7 locus have been associated with disorders such as ID, epilepsy, schizophrenia, and ASD [43], [46]–[48], [50], [52]–[54], [57]–[59]. In the BP4 region, the same two patients AU038_3 and 5237_3 also carried two independent deletions of the rhoGAP ARHGAP11B gene. Loss of ARHGAP11B was detected in 8 of 1257 patients with ASD (0.64%) and in 4 of 1577 controls (0.25%) (Fisher's exact test, 2-sided P = 0.15). Patient 5237_3 carried a large deletion (235.2 kb) of the full gene, transmitted by the mother. Patient AU038_3 carried a smaller deletion of 49.8 kb of the first two exons, transmitted by the mother. Both deletions overlap the segmental duplications of BP4 and have been reported to accompany the majority of microduplications involving CHRNA7 [51]. However, in patient 5237_3, the two CNVs are present on distinct parental chromosomes since the CHRNA7 duplication and the ARHGAP11B deletion are paternally and maternally inherited, respectively. Finally, the third patient, 6319_3, carried a paternally-inherited BP1-BP2 deletion of 468 kb, removing NIPA1, NIPA2, CYFIP1, and TUBGCP5. This deletion was observed in 4 of 1257 patients with ASD (0.32%) and in 4 of 1577 controls (0.25%) (Fisher's exact test, 2-sided P = 0.74). The BP1-BP2 deletion is associated with phenotypic variability and has been reported in individuals with neurodevelopmental disorders [20], schizophrenia [53], [60], ASD [44]–[46], [49], and epilepsy [61]. In a recent screen for large CNVs (>400 kb) performed on 15,767 children with ID and various congenital defects, and 8,329 unaffected adult controls [20], deletions affecting CYFIP1, NIPA1, NIPA2 and TUBGCP5 were associated with neurodevelopmental disorder (P = 4.73×10−6), epilepsy (P = 1.48×10−3) and autism (P = 1.99×10−2).
Several additional CNVs also altered compelling candidate genes for susceptibility to ASD. In patient AU038_3 we detected a previously unreported paternally inherited intronic duplication of CAMSAP1L on chromosome 1q32.1, coding for a calmodulin regulated spectrin-associated protein highly expressed in the brain. Patient 5237_3 carried a de novo deletion altering the coding sequence of the tyrosine phosphatase DUSP22 on chromosome 6p25.3 and a maternally inherited intronic duplication of NLGN1 on chromosome 3q26.3 [9]. These CNVs were observed at similar frequencies in patients with ASD compared with controls. DUSP22 deletions were observed in 8 of 1257 patients with ASD (0.64%) and in 14 of 1577 controls (0.89%), while NLGN1 intronic duplications were observed in 60 of 1257 patients with ASD (4.77%) and in 62 of 1577 controls (3.93%). Finally, patient 6319_3 carried an unreported maternally inherited intronic deletion of contactin CNTN4, a gene on chromosome 3p26.3 associated with ASD [62], as well as a paternally inherited deletion within the protocadherin PCDHA1-10 gene cluster on chromosome 5q31.3. Interestingly, this deletion removes the first exon of both PCDH8 and PCDH9 and was significantly less frequent in patients with ASD compared with controls (ASD: 62 of 1257; controls: 132 of 1577; Fisher's exact test, 2-sided P = 0.0003; OR = 0.57; 95% CI = 0.41–0.78).
We also analyzed the genome of the Swedish control SWE_Q56_508 carrying the SHANK2 splice mutation using the Human Omni2.5 BeadChip array from Illumina (Figure 3B). Two close duplications on 2p25.3 were detected, altering four genes, LOC391343, SNTG2, PXDN and MYT1L. The inheritance of these two duplications could not be investigated, because DNA samples from the parents were not available. However, 2 of 1577 controls also carried of the same close duplications, suggesting that these CNVs are located on the same chromosome. Among the affected genes, syntrophin-γ2 (SNTG2) and myelin transcription factor 1-like (MYT1L) are expressed in the brain. Alterations of SNTG2 and MYT1L have been previously reported in patients with ASD [20], [63], [64] and schizophrenia [65], respectively. SNTG2 is a scaffolding protein interacting with the NLGN3/4X proteins [66] and a component of the dystrophin glycoprotein complex [67]. MYT1L is a myelin transcription factor required to convert mouse embryonic and postnatal fibroblasts into functional neurons [68].
Discussion
Deleterious SHANK2 variations are enriched in patients with ASD, but also observed in controls
The identification of mutations in synaptic proteins such as NRXN1, NLGN3/4X and SHANK2/3 has demonstrated that a synaptic defect might be at the origin of ASD [5], [6]. Here we confirm the presence of SHANK2 de novo deletions in individuals with ASD, with a prevalence of 0.38% (1/260) in our cohort of ASD patients analyzed with the Illumina 1M SNP array. This frequency is similar to the one reported previously by the AGP in a larger sample of 996 patients with ASD (0.2%) [9]. SHANK2 deletions altering exons were not detected in controls, in agreement with previous findings [9], [26]. As reported for SHANK3 [7], no other coding variations were detected in the remaining SHANK2 allele of the deletion carriers, suggesting that, in some individuals, a de novo deletion of a single allele of SHANK2 might be sufficient to increase the risk for ASD. In one case, a patient carried two rare SHANK2 variants predicted as deleterious and inherited from different parents, indicating that they were separate alleles.
For the remaining SHANK2 variants, patients were heterozygous for non-synonymous rare variations inherited from one of their parents (Figure S3). Since parents were apparently asymptomatic, the causative role of these variants in ASD remains difficult to ascertain. However, we observed a significant enrichment of SHANK2 variants affecting conserved amino acids in patients with ASD compared with controls. This was also the case in the previous mutation screening by Berkel et al. 2010 [26]. The majority of the variants affecting conserved residues and identified in the patients were shown to alter the ability of SHANK2 to increase the number of synapses in vitro. Importantly, the assays performed in this study show that the variants can potentially impact on the function of the protein, but they do not confirm that they have deleterious effects on neuronal function in vivo in people that carry them. However, these results are consistent with previous findings showing that inherited variants of SHANK2 and SHANK3 cause synaptic defects in vitro [7], [69], [70]. Recently, Berkel et al. 2011 showed that two inherited (L1008_P1009dup, T1127M) and one de novo (R462X) SHANK2 mutations identified in patients with ASD affect spine volume and reduced Shank2 cluster sizes [70]. This deleterious effect was also observed in vivo since mice expressing rAAV-transduced Shank2-R462X present a specific long-lasting reduction in miniature postsynaptic AMPA receptor currents [70].
In patients, the only feature associated with carriers of SHANK2 mutations compared with other patients was a trend for low IQ (P = 0.025, OR = 3.75, 95% CI = 1.1–20.0) (Table S8). But, as observed for SHANK3 mutations, this correlation could differ from one individual to another (i.e. the patient with a SHANK2 de novo stop mutation reported by Berkel et al. 2010 presented with high-functioning autism [26]).
Our result also showed that potentially deleterious SHANK2 variants were detected in a heterozygous state in parents and in the general population without causing severe phenotypic consequences. Indeed, we showed that almost 5% of the Finnish population is heterozygous for the SHANK2 R818H variation, which modifies a conserved amino acid and is associated with lower synaptic density in vitro. Furthermore, we identified a SHANK2 splice site mutation in a control female without any apparent psychiatric disorders. Similarly, two frame-shift mutations and one splice site mutation of SHANK2 are listed in dbSNP and in the 1000 genomes project [71]. These nonsense variations should be interpreted with caution since none of them has been validated by Sanger sequence technology. Taken together, variants affecting conserved amino acids of SHANK2 might act as susceptibility variants for ASD, but, in some cases, additional genetic, epigenetic or environmental factors seem to be necessary for the emergence of the disorder.
Additional CNVs in subjects with SHANK2 mutations may modulate the risk for ASD
In order to detect risk and protective genetic factors, we analyzed the CNV burden of the individuals carrying deleterious variations of SHANK2. Notably, the three ASD patients with de novo SHANK2 deletions also carried CNVs on chromosome 15q11–q13, a region associated with ASD [43], [47], [48], [50]–[52], [72]. In contrast, the patient reported by Berkel et al. 2010, who did not meet all the diagnostic criteria for ASD, seemed to have no CNV at chromosome 15q [26]. Although the probability to observe the co-occurrence of a de novo SHANK2 deletion and a duplication of CHRNA7 at 15q is very low, two of the three patients carrying a de novo SHANK2 deletion also carried the CHRNA7 duplication. While the numbers are small, this finding could suggest epistasis between these two loci. The role of CHRNA7 in ASD was recently supported by the observation of low levels of CHRNA7 mRNA in the post-mortem brain from patients with ASD [73]. Interestingly, it was also found that, in contrast to the gene copy number, the transcript levels CHRNA7 were reduced in neuronal cells [74] or brain samples with maternal 15q duplication [75]. Finally, functional studies have shown that NLGN and NRXN, which belong to the same synaptic pathway, are key organizers of the clustering of nicotinic receptors at the synapse [76]–[78]. Therefore the co-occurrence of a deletion of SHANK2 and a duplication of the nicotinic receptor CHRNA7 could act together within the same pathway to increase the risk of ASD in patients AU038_3 and 5237_3. In patient 6319_3 carrying the BP1–BP2 deletion, several genes might also play a role in the susceptibility to ASD. Among them, NIPA1 and TUBGCP5 encoding a magnesium transporter and a tubulin gamma associated protein, respectively, are highly expressed in the brain. However, the most compelling candidate in the deleted region is CYFIP1 [45], [53], which codes for a binding partner of FMRP, the protein responsible for fragile X syndrome. Both CYFIP1 and FMRP are involved in the repression of synaptic translation [79], one of the major biological mechanisms associated with ASD [80]. Therefore, the co-occurrence of a loss of one copy of SHANK2 and CYFIP1 might increase the risk of abnormal synaptic function in patient 6319_3.
If some individuals have a higher risk to develop ASD when a deleterious SHANK2 variant is present, others individuals may experience a protective effect by additional genetic factors. For example, control SWE_Q56_508 carried a SHANK2 splice mutation, but clinical examination revealed no major disorders. In addition, this control individual also carried a partial duplication of SNTG2 and MYT1L. Based on a single control subject, it is not possible to formally prove that these additional hits at SNTG2 and/or MYT1L acted as suppressor mutations, counteracting the phenotypic effects of the SHANK2 splice mutation. However, the encoded proteins may interact with the NRXN-NLGN-SHANK pathway. Both SNTG2 and SHANK2 are scaffolding proteins localized in actin rich structures [81]–[83] and bind directly to neuroligins [66]. Furthermore, mutations of NLGN3/4X identified in patients with ASD decrease their protein binding to SNTG2 [66]. In addition, MYT1L is a myelin transcription factor that is sufficient, with only two other transcription factors, ASCL1 and BRN2, to convert mouse embryonic and postnatal fibroblasts into functional neurons in vitro [67]. Therefore, alterations of SNTG2 and/or MYTL1 might modulate synapse physiology and counteract the effect of the SHANK2 splice site mutation. We recently highlighted the key role of synaptic gene dosage in ASD and the possibility that a protein imbalance at the synapse could alter synaptic homeostasis [6]. In the future, animal models should be developed to test whether the effect of a primary mutation in a synaptic protein complex (e.g. Shank2) can be reduced or suppressed by a second mutation (e.g. Sntg2 or Myt1l). A similar suppressor effect has been demonstrated by the decrease of abnormal behavior of the Fmr1 mutant mice carrying a heterozygous mutation of the metabotropic glutamate receptor mGluR5 [84].
Conclusions and perspectives
In summary, we confirmed that de novo SHANK2 deletions are present in patients with ASD and showed that several SHANK2 variants reduce the number of synapses in vitro. The genomic profile of the patients carrying deleterious de novo SHANK2 deletions also points to a possible genetic epistasis between the NRXN-NLGN-SHANK pathway and 15q11–q13 CNVs. CHRNA7 and CYFIP1 were already proposed as susceptibility genes for neuropsychiatric disorders [43], [45], [49], [51], and our study provides additional support for this association. Therefore, as previously observed for ID [85], our results suggest that the co-occurrence of de novo mutations, together with inherited variations might play a role in the genetic susceptibility to ASD. Finally, our analyses suggest the interesting possibility that deleterious mutations of neuronal genes (e.g. SNTG2 and MYT1L) could potentially counteract the effect of synaptic deleterious mutations (e.g. SHANK2). The identification of risk and protective alleles within the same subject is one of the main challenges for understanding the inheritance of ASD. Initial results from the 1000 genomes project has estimated that, on average, each person carries approximately 250 to 300 loss-of-function variants in annotated genes and 50 to 100 variants previously implicated in inherited disorders [71]. To date, it is not clear how many loci can regulate synaptic homeostasis and how these variants interact with each other to modulate the risk for ASD [6]. A better knowledge of these genetic interactions will be necessary to understand the complex inheritance pattern of ASD.
Materials and Methods
Ethics statement
This study was approved by the local Institutional Review Board (IRB) and written inform consents were obtained from all participants of the study. The local IRB are the “Comité de Protection des Personnes” (Île-de-France Hôpital Pitié-Salpêtrière Paris) for France; the Sahlgrenska Academy Ethics committee, University of Gothenburg for Sweden; the local IRB of the medical faculty of JW Goethe University Frankfurt/Main for Germany; the Committee #3 of the Helsinki University Hospital, Finland; the “Comitato Etico IRCCS Fondazione Stella Maris” at Stella Maris Institute, Calambrone (Pisa), Italy; the “Comitato Etico Azienda Ospedaliera-Universitaria Policlinico-Vittorio Emanuele”, Catania, Italy.
Patients
Patients with ASD and analyzed for CNV analysis and/or mutation screening are presented in Table S1. Patients were recruited by the PARIS (Paris Autism Research International Sibpair) study at specialized clinical centers disposed in France, Sweden, Germany, Finland, UK. The Autism Diagnostic Interview-Revised (ADI-R) and Autism Diagnostic Observation Schedule (ADOS) were used for clinical evaluation and diagnosis. In Sweden, in some cases, the Diagnostic Interview for Social and Communication Disorders (DISCO-10) was applied instead of the ADI-R. Patients were included after a clinical and medical check-up with psychiatric and neuropsychological examination, standard karyotyping, fragile-X testing and brain imaging and EEG whenever possible. All patients were from Caucasian ancestry.
The patient AU038_3 with a de novo SHANK2 deletion is an 11.05 year-old boy diagnosed with autism and moderate ID (Table S2). He was the only child of non-consanguineous parents from European descent. His parents had no relevant personal and familial history of psychiatric or medical illness. He was born at 40 weeks of gestation, after normal pregnancy and delivery. Birth weight, length and occipitofrontal head circumference were 2500 g (5th percentile), 48 cm (22nd percentile) and 31 cm (2nd percentile), respectively. Apgar scores were 7 and 10 at 1 and 5 minutes, respectively. In the first year of life, the pediatrician reports did not mention signs of hypotonia. At 2 months, he was operated for an inguinal hernia. Motor acquisition was apparently normal (sitting at 6 months), but with a late acquisition of walking, at 18 months. Speech was severely delayed, without any apparent regressive phase. Only a few words and sentences appeared when he was 4 y and 6.5 y, respectively. His expressive language remained limited to restrictive sentences, mainly dyssyntaxic. A formal diagnostic assessment for autism was performed when he was 11 years old. The scores of the Autism Diagnostic Interview-Revised (ADI-R) domains were: social 24, communication 23, and behavior 6 (cut-offs for autism diagnosis are 10, 8 (verbal autism) and 3, respectively); the age at first symptoms was before 36 months. Cognitive evaluation with the Kaufman Assessment Battery for Children (K-ABC) showed moderate intellectual deficit (composite score 40). He required assistance with basic activities such as eating and dressing. At examination, he had a normal facial appearance, with a prominent chin. General and neurological examinations were normal, except for hypermetropia and astigmatism. High-resolution karyotype, fragile X testing, MLPA analysis of telomeres and microdeletion/microduplication syndromes, and metabolic screening for inherited disorders of metabolism (urine amino acids, mucopolysaccharides and organic acids, uric acid in blood and urine) were all normal. No significant epileptic event was reported on the electroencephalogram.
The two male patients with de novo SHANK2 deletions reported by Pinto et al. 2010 [9] (5237_3 and 6319_3) shared several clinical features with patient AU038_3. Patient 5237_3 is a Canadian subject diagnosed with autism (based on ADI-R and ADOS) associated with below average non verbal IQ (<1st percentile) and language (<1st percentile). He had minor dysmorphic features including 5th finger clinodactyly and several curled toes, and no history of epilepsy. Patient 6319_3 was recruited in the same geographic area as patient AU038_3 (Grenoble, France) and was clinically diagnosed with PDD-NOS. The ADI-R scores were: social 14, communication 8, behaviors 2 (cut-off for autism: 3); with an age at first symptoms <36 months). He had mild ID as evaluated with the WISC-III (full scale IQ 60, performance IQ 60, verbal IQ 67). His language was delayed (first words 24 m, first sentences 48 m), but functional. He had no history of regression or epilepsy. The physical exam was normal, except for large and prominent ears and flat feet; the neurological exam was also normal. Similarly to patient AU038_3, he had hypermetropia.
The control female carrying the splice site mutation (IVS22+1G>T) was part of a cohort of 172 females recruited for a study on obesity, anthropometrics, and cardiovascular risk factors [37]. In addition, these women were assessed for axis I psychiatric disorders and for personality traits using the Temperament and Character Inventory (TCI) [38] and the Karolinska Scales of Personality (KSP) [39]. This subject had no psychiatric disorders and her TCI and KSP scores were similar to those found in the general population.
Genomic structure and transcripts analysis of SHANK2
To define the genomic structure of the human SHANK2 gene, we used the two reference sequence genes from UCSC (NM_012309 and NM_133266), one human mRNA from GenBank (DQ152234) and three Rattus reference sequence genes from UCSC (NM_201350, NM_133441 and NM_133440). SHANK2 is transcribed in four isoforms described in GenBank (AB208025, AB208026, AB208027 and AF141901) and is composed of 25 exons. Transcript analysis of SHANK2 was performed in human brain regions from four independent controls (two females and two males) and in human tissues (heart, brain, placenta, lung, liver, skeletal muscle, kidney, pancreas and B lymphoblastoid cell lines) using the Clontech Multiple Tissue cDNA panel (Clontech). Total RNA was isolated from control human brain tissues by the acid guanidinium thiocyanate phenol chloroform method and reverse transcribed by oligodT priming using SuperScript II Reverse Transcriptase (Invitrogen). The PCR was performed with HotStar Taq polymerase (Qiagen) and the protocol used was 95°C for 15 min, followed by 40 cycles at 95°C for 30 sec, 55 to 58°C for 30 sec, 72°C for 30 sec to 1 min, with a final cycle at 72°C for 10 min. PCR primers were designed to detect the ANK domain, the SH3 domain, the PDZ domain, and the SAM domain in order to distinguish the four SHANK2 isoforms and are indicated in Table S11. All RT-PCR products were directly sequenced. The expression of SHANK2E isoform was also studied by SYBR-Green real-time PCR approach. The fluorescence was read with the Applied Biosystems 7500 Real-Time PCR System. Each assay was conducted in three replicates. GAPDH was used for the ΔCt calculation and total brain was used as the reference for relative quantification calculation (RQ). The relative RQ of transcripts was calculated as 2−ΔΔCT with the magnitude of upper error as 2−(ΔΔCT−SEM)-2−ΔΔCT and the magnitude of lower error as 2−ΔΔCT-2−(ΔΔCT+SEM). The primers specific to SHANK2E isoform are indicated in Table S11. In situ hybridization was performed essentially as described previously [28]. Transcripts encoding the different ProSAP1/Shank2 cDNAs (ProSAP1/Shank2 starting with the PDZ domain, ProSAP1A, starting with the SH3 domain and ProSAP1E/Shank2E, starting with the ankyrin repeats) were detected with isoform specific S35 labeled cDNA antisense oligonucleotides purchased from MWG-Biotech (Ebersberg, Germany) directed against the ATG regions of the different mRNAs. All variants were evaluated for potential pathogenicity using the HumDIV method for rare alleles of PolyPhen2 [41].
CNV detection and validation
DNA was extracted from blood leukocytes or B lymphoblastoid cell lines. The SHANK2 CNV was detected with the Illumina Human 1M-Duo BeadChip, which interrogates 1 million SNPs distributed over the human genome. For the Swedish control SWE_Q56_508 carrying the SHANK2 splice mutation we used the Illumina Human Omni2.5 BeadChip array. The genotyping was performed at the Centre National de Génotypage (CNG) and the Institut Pasteur. Only samples that met stringent quality control (QC) criteria were included: call rate ≥99%; high confidence score log Bayes factor ≥15; standard deviation of the log R ratio (LRR) ≤0.35 and of the B allele frequency (BAF)≤0.13; number of consecutive probes for CNV detection ≥5; CNV size ≥1 kb. When the QC criteria were met, we used two CNV calling algorithms, QuantiSNP [86] and PennCNV [87], and the CNV viewer, SnipPeep (http://snippeep.sourceforge.net/). To obtain high-confidence calls, the CNVs identified by QuantiSNP were validated by visual inspection of the LRR and BAF values. PennCNV was used to confirm inheritance status of the resulting CNV calls. CNVs were validated by quantitative PCR analysis using the Universal Probe Library (UPL) system from Roche. UPL probes were labeled with FAM and the fluorescence was read with the Applied Biosystems 7500 Real-Time PCR System. Each assay was conducted in four replicates for target region probe-set and control region probe-set. Relative levels of region dosage were determined using the comparative CT method assuming that there were two copies of DNA in the control region. The relative copy number for each target region was calculated as 2−ΔΔCT with the magnitude of upper error as 2−(ΔΔCT−SEM)-2−ΔΔCT and the magnitude of lower error as 2−ΔΔCT-2−(ΔΔCT+SEM). UPL probes and primers are indicated in Table S12. For comparisons between patients and controls, statistical significance for each CNV was assessed using a 2-sided Fisher's exact test.
Mutation screening
The 24 coding exons of SHANK2 were amplified and sequenced for mutation screening. The PCR was performed on 20–40 ng of genomic DNA template with HotStar Taq polymerase from Qiagen for all exons the protocol used was 95°C for 15 min, followed by 35–40 cycles at 95–97°C for 30 sec, 55–62°C for 30 sec, 72°C for 30 sec to 90 sec, with a final cycle at 72°C for 10 min. Sequence analysis was performed by direct sequencing of the PCR products using a 373A automated DNA sequencer (Applied Biosystems). Genotyping of R185Q, V717F, A729T, R818H, G1170R, D1535N and L1722P was performed by direct sequencing or Taqman SNP Genotyping Assays system from Applied Biosystems designed with Custom TaqMan Assay Design Tool. All primers are indicated in Table S9. Enrichment of SHANK2 variations in the ASD sample compared with controls was assessed using a 1-sided Fisher's exact test (hypothesizing that cases will show an excess of SHANK2 variants compared to controls).
In vitro mutagenesis and transfection studies in hippocampal neurons
Rat GFP-ProSAP1A (Shank2A) cDNA was mutated according to the human mutations using the site directed mutagenesis kit (Stratagene). The mutagenesis primers were listed in Table S10. We have tested all the variants (n = 16) identified in our first screen of 230 patients with ASD and 230 controls: 5 were detected only in patients (V717F, A729T, G1170R, D1535N and L1722P), 6 were detected in patients and controls (S557N, R569H, K780Q, R818H, Y967C and P1586L) and 5 were only found in controls (L629P, A822T, V823M, R1290W and Q1308R). All mutated amino acids were conserved among human, rat and mouse ProSAP1/Shank2. All cDNAs were sequenced and subsequently tested for expression by Western blot analysis. After expression of the constructs in Cos7 cells, the cell homogenate was separated on a gel, transferred to a nitrocellulose membrane and subsequently protein bands were detected using a rabbit anti-GFP antibody. Thereafter, the cDNAs were transfected into primary hippocampal neurons. Cell culture experiments of rat hippocampal primary neurons (embryonic day 18–21: E18-21) were performed as described previously [88]. In brief, after preparation, hippocampal neurons were seeded on poly-l-lysine (0.1 mg/ml; Sigma-Aldrich, Steinheim, Germany) coated coverslips at a density of 4×104 cells/well (transfection experiments) or 2×104 cells/well (immunological staining). Cells were grown in Neurobasal medium (Invitrogen, Karlsruhe, Germany), complemented with B27 supplement (Invitrogen), 0.5 mM L-glutamine (Invitrogen), and 100 U/ml penicillin/streptomycin (Invitrogen) and maintained at 37°C in 5% CO2. Hippocampal cells were transfected using Lipofectamine 2000, according to the manufacturer's recommendation (Invitrogen). Fluorescence images were obtained using a camera attached to a fluorescence microscope. For immunofluorescence, the primary cultures were fixed with ice cold 4% paraformaldehyde/1.5% sucrose/PBS for 20 min at 4°C and processed for immunohistochemistry. After washing three times with 1× PBS for 5 min at room temperature the cells were permeabilized for 3 min on ice in a buffer containing 0.1% Triton X-100/0.1% Na-Citrate/PBS and washed again three times with 1× PBS. Blocking was performed with 10% fetal calf serum/PBS for 1 h at room temperature followed by incubation with the primary antibody (mouse anti-Bassoon) overnight at room temperature. After a further washing-step the cells were incubated with the secondary antibody coupled to Alexa555 (red) (Molecular Probes, Invitrogen) for 90 min at room temperature, washed first with 1×PBS and then with aqua bidest for 5 min and mounted in Mowiol (with or without DAPI for staining of the nucleus). All animal experiments were performed in compliance with the guidelines for the welfare of experimental animals issued by the Federal Government of Germany, the National Institutes of Health and the Max Planck Society.
Image acquisition and quantification
In morphological studies, dendrites were considered primary when processes extended directly from the cell body, and secondary when processes branched off primary dendrites. Twenty transfected neurons were chosen randomly for quantification from at least three independent experiments for each construct. Morphometric measurements were performed using Axiovision Zeiss microscope and Axiovision software with a 40× magnification. For the quantification of excitatory synapse number, cells were counterstained with anti-Bassoon antibodies. From randomly chosen transfected neurons, Bassoon-positive spots from primary dendrites were counted and the length of dendrites was measured. The total number of spines was expressed as density per 10 µm length of dendrite. Measured data were exported to Excel software (Microsoft), and the data of each variant were compared by using the Mann-Whitney U test. The comparisons of synaptic density for each phenotypic or conservation categories were performed using the Student's t test.
Supporting Information
Zdroje
1. American Psychiatric Association 1994 Diagnostic and Statistical Manual of Mental Disorders, 4th Ed American Psychiatric Press, Washington D.C
2. FernellEGillbergC 2010 Autism spectrum disorder diagnoses in Stockholm preschoolers. Res Dev Disabil 31 680 685
3. FreitagCM 2007 The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry 12 2 22
4. HallmayerJClevelandSTorresAPhillipsJCohenB 2011 Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Arch Gen Psychiatry
5. BourgeronT 2009 A synaptic trek to autism. Curr Opin Neurobiol 19 231 234
6. ToroRKonyukhMDelormeRLeblondCChasteP 2010 Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet in press
7. DurandCMBetancurCBoeckersTMBockmannJChasteP 2007 Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39 25 27
8. JamainSQuachHBetancurCRastamMColineauxC 2003 Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34 27 29
9. PintoDPagnamentaATKleiLAnneyRMericoD 2010 Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466 368 372
10. SzatmariPPatersonADZwaigenbaumLRobertsWBrianJ 2007 Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 39 319 328
11. ReddyKS 2005 Cytogenetic abnormalities and fragile-X syndrome in Autism Spectrum Disorder. BMC Med Genet 6 3
12. O'RoakBJDeriziotisPLeeCVivesLSchwartzJJ 2011 Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet 43 585 589
13. SandersSJErcan-SencicekAGHusVLuoRMurthaMT 2011 Multiple Recurrent De Novo CNVs, Including Duplications of the 7q11.23 Williams Syndrome Region, Are Strongly Associated with Autism. Neuron 70 863 885
14. LevyDRonemusMYamromBLeeYHLeottaA 2011 Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron 70 886 897
15. PootMBeyerVSchwaabIDamatovaNVan't SlotR 2010 Disruption of CNTNAP2 and additional structural genome changes in a boy with speech delay and autism spectrum disorder. Neurogenetics 11 81 89
16. SchaafCPSaboASakaiYCrosbyJMuznyD 2011 Oligogenic heterozygosity in individuals with high-functioning autism spectrum disorders. Hum Mol Genet 20 3366 3375
17. GirirajanSRosenfeldJACooperGMAntonacciFSiswaraP 2010 A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet 42 203 209
18. BetancurC 2011 Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res 1380 42 77
19. GilmanSRIossifovILevyDRonemusMWiglerM 2011 Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron 70 898 907
20. CooperGMCoeBPGirirajanSRosenfeldJAVuTH 2011 A copy number variation morbidity map of developmental delay. Nat Genet 43 838 846
21. SudhofTC 2008 Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455 903 911
22. YuLMGodaY 2009 Dendritic signalling and homeostatic adaptation. Curr Opin Neurobiol 19 327 335
23. MoessnerRMarshallCRSutcliffeJSSkaugJPintoD 2007 Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet 81 1289 1297
24. GauthierJChampagneNLafreniereRGXiongLSpiegelmanD 2010 De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci U S A 107 7863 7868
25. HamdanFFGauthierJArakiYLinDTYoshizawaY 2011 Excess of de novo deleterious mutations in genes associated with glutamatergic systems in nonsyndromic intellectual disability. Am J Hum Genet 88 306 316
26. BerkelSMarshallCRWeissBHoweJRoethR 2010 Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet 42 489 491
27. PersicoAMBourgeronT 2006 Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci 29 349 358
28. BoeckersTMKreutzMRWinterCZuschratterWSmallaKH 1999 Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci 19 6506 6518
29. ShengMKimE 2000 The Shank family of scaffold proteins. J Cell Sci 113 Pt 11 1851 1856
30. McWilliamsRRGideyEFouassierLWeedSADoctorRB 2004 Characterization of an ankyrin repeat-containing Shank2 isoform (Shank2E) in liver epithelial cells. Biochem J 380 181 191
31. BoeckersTMWinterCSmallaKHKreutzMRBockmannJ 1999 Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family. Biochem Biophys Res Commun 264 247 252
32. LimSNaisbittSYoonJHwangJISuhPG 1999 Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development. J Biol Chem 274 29510 29518
33. DurandCMKappelerCBetancurCDelormeRQuachH 2006 Expression and genetic variability of PCDH11Y, a gene specific to Homo sapiens and candidate for susceptibility to psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet 141 67 70
34. GutierrezRCFlynnRHungJKerteszACSullivanA 2009 Activity-driven mobilization of post-synaptic proteins. Eur J Neurosci 30 2042 2052
35. WischmeijerAMaginiPGiordaRGnoliMCicconeR 2010 Olfactory Receptor-Related Duplicons Mediate a Microdeletion at 11q13.2q13.4 Associated with a Syndromic Phenotype. Mol Syndromol 1 176 184
36. BrandstatterJHDickOBoeckersTM 2004 The postsynaptic scaffold proteins ProSAP1/Shank2 and Homer1 are associated with glutamate receptor complexes at rat retinal synapses. J Comp Neurol 475 551 563
37. RosmondRBjorntorpP 1998 Psychiatric ill-health of women and its relationship to obesity and body fat distribution. Obes Res 6 338 345
38. CloningerCRSvrakicDMPrzybeckTR 1993 A psychobiological model of temperament and character. Arch Gen Psychiatry 50 975 990
39. SchallingDAsbergMEdmanGOrelandL 1987 Markers for vulnerability to psychopathology: temperament traits associated with platelet MAO activity. Acta Psychiatr Scand 76 172 182
40. MelkeJWestbergLNilssonSLandenMSoderstromH 2003 A polymorphism in the serotonin receptor 3A (HTR3A) gene and its association with harm avoidance in women. Arch Gen Psychiatry 60 1017 1023
41. AdzhubeiIASchmidtSPeshkinLRamenskyVEGerasimovaA 2010 A method and server for predicting damaging missense mutations. Nat Methods 7 248 249
42. MarshallCRNoorAVincentJBLionelACFeukL 2008 Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82 477 488
43. MillerDTShenYWeissLAKornJAnselmI 2009 Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J Med Genet 46 242 248
44. DepienneCMoreno-De-LucaDHeronDBouteillerDGennetierA 2009 Screening for genomic rearrangements and methylation abnormalities of the 15q11–q13 region in autism spectrum disorders. Biol Psychiatry 66 349 359
45. van der ZwaagBStaalWGHochstenbachRPootMSpierenburgHA 2010 A co-segregating microduplication of chromosome 15q11.2 pinpoints two risk genes for autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet 153B 960 966
46. ShenYDiesKAHolmIABridgemohanCSobeihMM 2010 Clinical genetic testing for patients with autism spectrum disorders. Pediatrics 125 e727 735
47. SharpAJMeffordHCLiKBakerCSkinnerC 2008 A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet 40 322 328
48. Masurel-PauletAAndrieuxJCallierPCuissetJMLe CaignecC 2010 Delineation of 15q13.3 microdeletions. Clin Genet 78 149 161
49. DoornbosMSikkema-RaddatzBRuijvenkampCADijkhuizenTBijlsmaEK 2009 Nine patients with a microdeletion 15q11.2 between breakpoints 1 and 2 of the Prader-Willi critical region, possibly associated with behavioural disturbances. Eur J Med Genet 52 108 115
50. HelbigIMeffordHCSharpAJGuipponiMFicheraM 2009 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet 41 160 162
51. SzafranskiPSchaafCPPersonREGibsonIBXiaZ 2010 Structures and molecular mechanisms for common 15q13.3 microduplications involving CHRNA7: benign or pathological? Hum Mutat 31 840 850
52. Ben-ShacharSLanpherBGermanJRQasaymehMPotockiL 2009 Microdeletion 15q13.3: a locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. J Med Genet 46 382 388
53. StefanssonHRujescuDCichonSPietilainenOPIngasonA 2008 Large recurrent microdeletions associated with schizophrenia. Nature 455 232 236
54. International Schizophrenia Consortium 2008 Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 455 237 241
55. TamGWvan de LagemaatLNRedonRStrathdeeKECroningMD 2010 Confirmed rare copy number variants implicate novel genes in schizophrenia. Biochem Soc Trans 38 445 451
56. MurthySKNygrenAOEl ShakankiryHMSchoutenJPAl KhayatAI 2007 Detection of a novel familial deletion of four genes between BP1 and BP2 of the Prader-Willi/Angelman syndrome critical region by oligo-array CGH in a child with neurological disorder and speech impairment. Cytogenet Genome Res 116 135 140
57. ShinawiMSchaafCPBhattSSXiaZPatelA 2009 A small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nat Genet 41 1269 1271
58. van BonBWMeffordHCMentenBKoolenDASharpAJ 2009 Further delineation of the 15q13 microdeletion and duplication syndromes: a clinical spectrum varying from non-pathogenic to a severe outcome. J Med Genet 46 511 523
59. PagnamentaATWingKAkhaESKnightSJBolteS 2009 A 15q13.3 microdeletion segregating with autism. Eur J Hum Genet 17 687 692
60. KirovGGrozevaDNortonNIvanovDMantripragadaKK 2009 Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet 18 1497 1503
61. de KovelCGTrucksHHelbigIMeffordHCBakerC 2010 Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain 133 23 32
62. CottrellCEBirNVargaEAlvarezCEBouyainS 2010 Contactin 4 as an autism susceptibility locus. Autism Res
63. RosenfeldJABallifBCTorchiaBSSahooTRavnanJB 2010 Copy number variations associated with autism spectrum disorders contribute to a spectrum of neurodevelopmental disorders. Genet Med 12 694 702
64. ChristianSLBruneCWSudiJKumarRALiuS 2008 Novel submicroscopic chromosomal abnormalities detected in autism spectrum disorder. Biol Psychiatry 63 1111 1117
65. VrijenhoekTBuizer-VoskampJEvan der SteltIStrengmanESabattiC 2008 Recurrent CNVs disrupt three candidate genes in schizophrenia patients. Am J Hum Genet 83 504 510
66. YamakawaHOyamaSMitsuhashiHSasagawaNUchinoS 2007 Neuroligins 3 and 4X interact with syntrophin-gamma2, and the interactions are affected by autism-related mutations. Biochem Biophys Res Commun 355 41 46
67. AlessiABraggADPercivalJMYooJAlbrechtDE 2006 gamma-Syntrophin scaffolding is spatially and functionally distinct from that of the alpha/beta syntrophins. Exp Cell Res 312 3084 3095
68. VierbuchenTOstermeierAPangZPKokubuYSudhofTC 2010 Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463 1035 1041
69. DurandCMPerroyJLollFPerraisDFagniL 2011 SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol Psychiatry
70. BerkelSTangWTrevinoMVogtMObenhausHA 2011 Inherited and de novo SHANK2 variants associated with autism spectrum disorder impair neuronal morphogenesis and physiology. Hum Mol Genet
71. DurbinRMAbecasisGRAltshulerDLAutonABrooksLD 2010 A map of human genome variation from population-scale sequencing. Nature 467 1061 1073
72. The international schizophrenia consortium 2008 Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature 455 237 241
73. YasuiDHScolesHAHorikeSIMeguro-HorikeMDunawayKW 2011 15q11.2–13.3 chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum Mol Genet
74. Meguro-HorikeMYasuiDHPowellWSchroederDIOshimuraM 2011 Neuron-specific impairment of inter-chromosomal pairing and transcription in a novel model of human 15q-duplication syndrome. Hum Mol Genet 20 3798 3810
75. HogartALeungKNWangNJWuDJDriscollJ 2009 Chromosome 15q11–13 duplication syndrome brain reveals epigenetic alterations in gene expression not predicted from copy number. J Med Genet 46 86 93
76. Triana-BaltzerGBLiuZGounkoNVBergDK 2008 Multiple cell adhesion molecules shaping a complex nicotinic synapse on neurons. Mol Cell Neurosci 39 74 82
77. ChengSBAmiciSARenXQMcKaySBTreuilMW 2009 Presynaptic targeting of alpha4beta 2 nicotinic acetylcholine receptors is regulated by neurexin-1beta. J Biol Chem 284 23251 23259
78. NeffRA3rdGomez-VarelaDFernandesCCBergDK 2009 Postsynaptic scaffolds for nicotinic receptors on neurons. Acta Pharmacol Sin 30 694 701
79. SchenckABardoniBLangmannCHardenNMandelJL 2003 CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron 38 887 898
80. KelleherRJ3rdBearMF 2008 The autistic neuron: troubled translation? Cell 135 401 406
81. BoeckersTMBockmannJKreutzMRGundelfingerED 2002 ProSAP/Shank proteins - a family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J Neurochem 81 903 910
82. SugiyamaYKawabataISobueKOkabeS 2005 Determination of absolute protein numbers in single synapses by a GFP-based calibration technique. Nat Methods 2 677 684
83. NagaiRHashimotoRTanakaYTaguchiOSatoM 2010 Syntrophin-2 is required for eye development in Drosophila. Exp Cell Res 316 272 285
84. DolenGOsterweilERaoBSSmithGBAuerbachBD 2007 Correction of fragile X syndrome in mice. Neuron 56 955 962
85. VissersLEde LigtJGilissenCJanssenISteehouwerM 2010 A de novo paradigm for mental retardation. Nat Genet 42 1109 1112
86. ColellaSYauCTaylorJMMirzaGButlerH 2007 QuantiSNP: an Objective Bayes Hidden-Markov Model to detect and accurately map copy number variation using SNP genotyping data. Nucleic Acids Res 35 2013 2025
87. WangKLiMHadleyDLiuRGlessnerJ 2007 PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res 17 1665 1674
88. BoeckersTMLiedtkeTSpilkerCDresbachTBockmannJ 2005 C-terminal synaptic targeting elements for postsynaptic density proteins ProSAP1/Shank2 and ProSAP2/Shank3. J Neurochem 92 519 524
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 2- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Příjem alkoholu a menstruační cyklus
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
-
Všechny články tohoto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



