-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
Francisella tularensis is a Gram-negative bacterium that infects macrophages and other cell types causing tularemia. F. tularensis is considered a potential bioterrorism agent and is a prime model intracellular bacterium to study the interaction of pathogens with the host immune system. The role of the proinflammatory cytokines IL-1β and IL-18 during lung infection with F. tularensis has not been characterized in detail. Here, using a mouse model of pneumonic tularemia, we show that both cytokines are protective, but through different mechanisms. Mice deficient in IL-18 quickly succumbed to the infection but administration of IFNγ rescued their survival. In contrast, mice lacking IL-1β appeared to control the infection in its early stages, but eventually succumbed and were not rescued by administration of IFNγ. Rather, IL-1β-deficient mice had significantly reduced serum level of IgM antibodies specific for F. tularensis LPS. These antibodies were generated in a IL-1β-, TLR2-, and ASC-dependent fashion, promoted bacteria agglutination and phagocytosis, and were protective in passive immunization experiments. B1a B cells produced the anti-F. tularensis IgM and were significantly decreased in the spleen and peritoneal cavity of infected IL-1β-deficient mice. Collectively, our results show that IL-1β and IL-18 activate non-redundant protective responses against tularemia and identify an essential role for IL-1β in the rapid generation of pathogen-specific IgM by B1a B cells.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004706
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004706Summary
Francisella tularensis is a Gram-negative bacterium that infects macrophages and other cell types causing tularemia. F. tularensis is considered a potential bioterrorism agent and is a prime model intracellular bacterium to study the interaction of pathogens with the host immune system. The role of the proinflammatory cytokines IL-1β and IL-18 during lung infection with F. tularensis has not been characterized in detail. Here, using a mouse model of pneumonic tularemia, we show that both cytokines are protective, but through different mechanisms. Mice deficient in IL-18 quickly succumbed to the infection but administration of IFNγ rescued their survival. In contrast, mice lacking IL-1β appeared to control the infection in its early stages, but eventually succumbed and were not rescued by administration of IFNγ. Rather, IL-1β-deficient mice had significantly reduced serum level of IgM antibodies specific for F. tularensis LPS. These antibodies were generated in a IL-1β-, TLR2-, and ASC-dependent fashion, promoted bacteria agglutination and phagocytosis, and were protective in passive immunization experiments. B1a B cells produced the anti-F. tularensis IgM and were significantly decreased in the spleen and peritoneal cavity of infected IL-1β-deficient mice. Collectively, our results show that IL-1β and IL-18 activate non-redundant protective responses against tularemia and identify an essential role for IL-1β in the rapid generation of pathogen-specific IgM by B1a B cells.
Introduction
Francisella tularensis (Ft) is a Gram-negative bacterium that infects macrophages and other cell types causing tularemia [1]. Ft is considered a potential bioterrorism agent and is used as a prime model intracellular bacterium to study the strategies adopted by microbes to evade and minimize innate immune detection.
Although the innate immune response to Ft infection has been examined in a great number of publications (reviewed in [2] [3]), much remains to be learned. Ft is known to evade various host defense mechanisms [4] and to produce an atypical LPS that does not stimulate TLR4 and does not possess proinflammatory activity [5] [6] [7,8] [9]. However, like others, we have shown that Ft stimulates a proinflammatory response primarily through TLR2 [10] [11] [12], which recognizes Ft lipoproteins [13]. The other innate immune pathway preferentially stimulated by Ft in mice is the inflammasome composed of AIM2-ASC-caspase-1 [14]. It is believed that genomic DNA released by lysing bacteria localized in the cytosol activates this inflammasome, leading to secretion of IL-1β and IL-18 and death of the infected cells by pyroptosis. This form of caspase-1-dependent cell death has been shown to effectively restrict intracellular replication of several bacteria, including Ft, by exposing them to extracellular microbicidal mechanisms [15]. Activation of the NLRP3 inflammasome in human macrophages has also been reported [16]. Whether this inflammasome is also activated in mice is unclear.
IL-1β and IL-18 are powerful proinflammatory cytokines that have been shown to be protective in a large number of experimental infection models. Both cytokines signal through the MyD88 pathway but elicit varied responses in different cell types [17]. Despite intensive research on Ft, the role of IL-1β and IL-18 during lung infection with this bacterium has not been characterized in detail. A confounding factor that affects the tularemia research field is that the majority of the studies are performed using either of two Francisella subspecies, F. novicida or Ft live vaccine strain (LVS), which are pathogenic in mice, but not humans, and differentially engage innate immune responses [1] [18]. An additional strain, the virulent Ft type A SchuS4 strain, displays an exaggerated virulence in mice, which has severely limited its use for the genetic analysis of the host immune response to this infection. A further complication in the analysis, comparison, and interpretation of the studies on tularemia, is that different routes of infection (i.p., i.d., i.n.) are used, which determine the severity of disease, and the relative contribution to protection of various immune pathways [19] [20]. For our studies we decided to use LVS because of its potential use as prophylactic vaccine and we adopted the intranasal infection route because it is the most lethal and the most relevant to biodefense.
Early evidence that IL-1β and IL-18 played protective roles during infection with Francisella species was provided by studies of Denise Monack’s group that showed that intraperitoneal injection of neutralizing antibodies against IL-18 and IL-1β increased bacterial burdens in mice infected intradermaly with F. novicida [21]. The same group showed that mice deficient in both IL-1β /IL-18 are more susceptible than C57BL/6J mice, yet not as much as mice deficient in ASC or caspase-1, suggesting that other caspase-1-dependent pathways, most likely pyroptosis, significantly contributed to protection from infection [14]. Collazo et al. analyzed Il-1r1-/- and Il-18-/- mice infected intradermally with Ft LVS, but did not find significant differences in the survival of these mice, compared to C57BL/6J mice [22]. Collectively, these studies indicated that IL-1β and IL-18 may contribute to protection from tularemia but it remains unclear to what extent, through which mechanism, and under which conditions. Despite the uncertainties about the role of IL-1β and IL-18 during Ft infection, several groups, including ours, have shown that Ft mutants that hyper-activate the inflammasome leading to increased IL-1β and IL-18 secretion are attenuated in vivo [23] [24] [25] [26,27], suggesting that these cytokines in fact play a protective role in tularemia.
The studies described here analyzed for the first time the response of IL-1 - or IL-18-deficient mice to intranasal infection with Ft LVS. Our results demonstrate that different protective mechanisms are activated by IL-18 and IL-1β and reveal a critical role for IL-1β in the production of anti-Ft LPS IgM by B1a B cells.
Results
Different susceptibility of Il-1r1-/- and Il-18-/- mice to Ft LVS lung infection
To increase our understanding of the role of IL-1 and IL-18 during lung infection with Ft LVS, we intranasally infected mice deficient in the IL-1 receptor, Il-1r1-/-, or mice deficient in IL-18 and measured their survival. As shown in Fig. 1A, both mouse strains were significantly more susceptible than the wild type C57BL/6J mice. Mice deficient in both IL-1 receptor and IL-18 (DKO) were also more susceptible. Interestingly, the mean time to death of Il-18-/- mice was much shorter than that of Il-1r1-/- mice, an observation that may suggest either that IL-18 plays a more critical role than IL-1 in this model of infection, or that each cytokine may be required at a different time point during infection (see below). While the bacterial burden in organs 72 hours post infection were not consistently increased in the knock-out mouse strains compared to C57BL/6J mice, 6 days p.i. Il-18-/- mice had significantly higher burdens than Il-1r1-/- mice (Fig. 1B) confirming the relative contribution of each cytokine. Compared to other gram-negative bacterial infection models, infection and innate immune response occur with relatively slower kinetics during tularemia and this may explain why the differences in bacterial burdens become more evident at later time points.
Fig. 1. IL-1RI and IL-18-deficient mice are more susceptible to lung infection with Ft LVS. 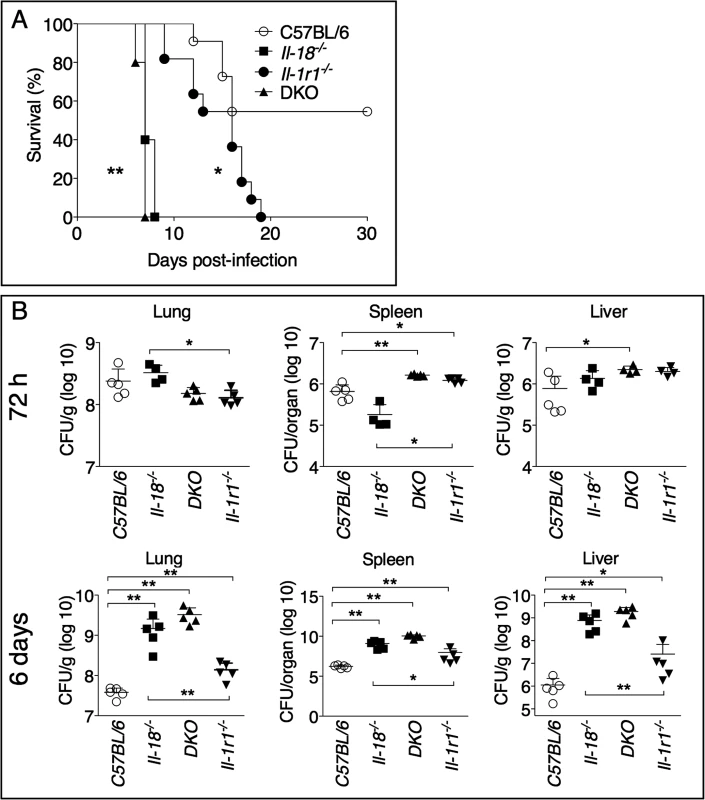
(A) C57BL/6J or Il-1r1-/- mice (n = 11), or Il-18-/- and Il-1r1-/-/Il-18-/- (DKO) mice (n = 5) were intranasally infected with 4x103 CFU Ft LVS and survival was monitored. (B) Mice infected as in A were euthanized 72 hours or 6 days p.i. and bacterial burden in organs was measured. One representative experiment of two is shown. Data are expressed as mean + S.D. *p<0.05, **p<0.01. (B) Mann-Whitney U test. Role of IL-18 during lung infection with Ft LVS
IL-1β and IL-18 levels were measured in the bronchoalveolar lavage fluid (BALF) or serum obtained from infected mice 72 hours or 6 days post-infection (Fig. 2A, B). IL-1β was not detectable in the sera of infected mice, but it was detected in higher amount at 72 hours than at 6 days p.i. in the BALF of C57BL/6J mice, a pattern that paralleled the reduction in bacterial burdens (see Fig. 1B). Production of IL-1β was drastically increased in Il-1r1-/- and Il-18-/- mice. Both IL-1 and IL-18 are known to induce Il-1b mRNA [28], which may explain why IL-1β level in BALF of DKO mice was severely reduced, compared to the other strains, despite high bacterial burden. IL-18 was detected in higher amounts in BALF, at 72 h, or in sera, at 6 days, in Il-1r1-/- mice, compared to C57BL/6J mice. The levels IFNγ, a cytokine that is known to be protective during several bacterial infections, including tularemia [29] [30] [31] [32] [33], were reduced in Il-18-/- mice, a finding consistent with the established function of IL-18 as an IFNγ-inducing cytokine [29], but were significantly increased in BALF or sera of Il-1r1-/- mice, a likely reflection of the high level of IL-18 in these mice. Collectively, these results suggest that the reduced resistance of Il-18-/- mice may be due to inability to produce sufficient IFNγ. This hypothesis was confirmed by the observation that administration of recombinant IFNβ to Il-18-/- mice during the first 6 days of infection completely rescued their survival (Fig. 2C). In contrast, IFNγ administration did not affect the survival of Il-1r1-/- mice, whose BALF already contained sustained level of IFNγ. These results show that distinct protective mechanisms are triggered by IL-18 and IL-1β during lung infection with Ft LVS. Interestingly, IFNγ administration to DKO mice significantly improved their survival but not to the extent of Il-1r1-/- mice, suggesting that IL-18 may have other protective functions not strictly related to induction of IFNγ.
Fig. 2. IFNγ administration rescues survival of Il-18-/-, but not Il-1r1-/-, mice intranasally infected with Ft LVS. 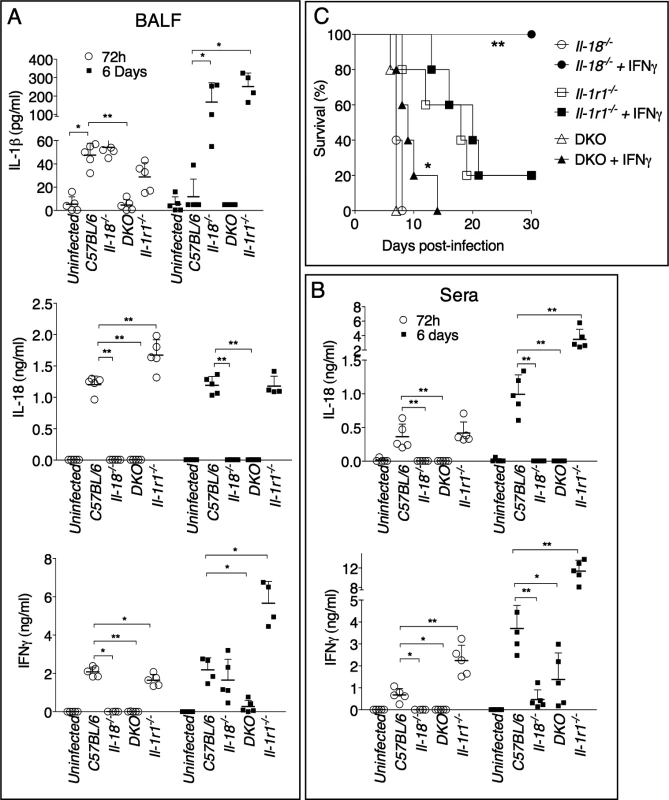
(A) Cytokine levels were measured 72 hours or 6 days p.i. in BALF (A) or serum (B) of mice intranasally infected with 4x103 CFU Ft LVS. (C) Mice (n = 5), of shown genotypes, were intranasally infected with 4x103 CFU Ft LVS and recombinant IFNγ (1 μg) was administered daily by i.p. injection for the first 6 days. Survival was monitored. One representative experiment of two is shown. Data are expressed as mean + S.D. *p<0.05, **p<0.01. Mann-Whitney U test. IL-1β, not IL-1α, mediates protection during Ft LVS infection and is important in the early phase of the infection
The IL-1RI mediates response to both IL-1α and IL-1β. Although both cytokines are produced during infection with Ft LVS (Fig. 3A), Il-1b-/-, but not Il-1a-/-, mice were found to be more susceptible than C57BL/6J mice to Ft LVS infection (Fig. 3B). In agreement with this result, bacteria burdens were significantly increased in organs of Il-1b-/-, but not Il-1a-/-, mice, compared to C57BL/6J mice (Fig. 3C), confirming the protective role of IL-1β. Interestingly, the combined absence of IL-1α and IL-1β appeared to result in a more drastic phenotype than the sole absence of IL-1β. Although the bacterial burden in organs was not significantly different between Il-1a-/-/Il-1b-/- and Il-1b-/- mice, absence of both cytokines significantly decreased mice survival compared to Il-1b-/- mice suggesting that, in absence of IL-1β, IL-1α may have a protective role. This effect is likely due to the fact that both cytokines are known to engage in compensatory mechanisms [34] [35] so that, in absence of IL-1β, the contribution of IL-1α, that is negligible in wild type mice, become somewhat more relevant.
Fig. 3. IL-1β, not IL-1α, is protective and acts in the early phase of Ft LVS intranasal infection. 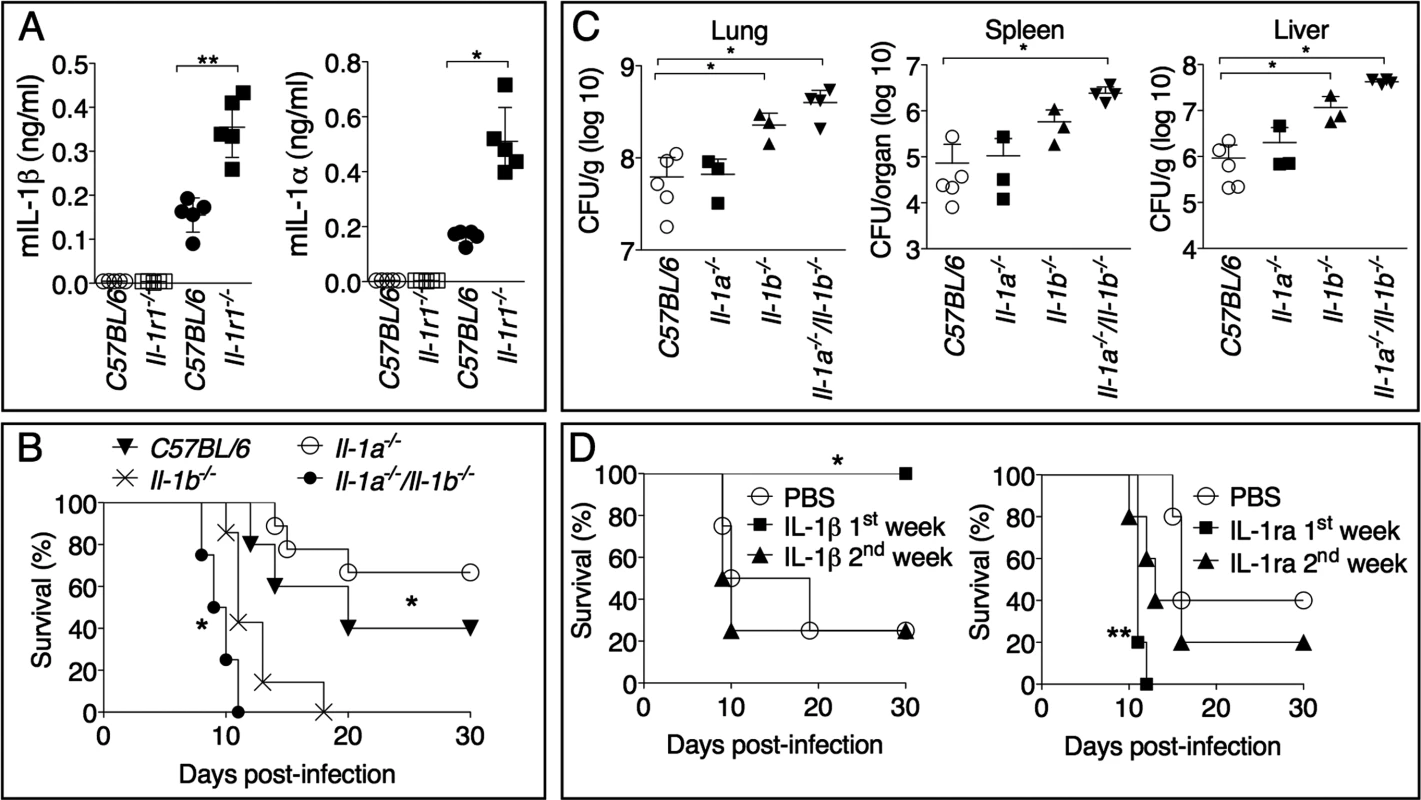
(A) IL-1α or IL-1β levels were measured 72 hours p.i. in BALF of mice intranasally infected with 4x103 CFU Ft LVS (solid symbols) or mock infected (empty symbols). (B) Mice (n = 5), of shown genotypes, were intranasally infected with 4x103 CFU Ft LVS and survival was monitored. * C57BL/6J or Il-1a-/-/Il-1b-/- compared to Il-1b-/-. (C) Organ bacterial burdens were measured 6 days p.i. in the indicated mouse strains infected as in B. (D) Il-1b-/- mice intranasally infected with 4x103 CFU Ft LVS received daily intraperitoneal injections of recombinant IL-1β (1 μg). In a similar fashion, C57BL/6J mice were treated with IL-1ra (4 mg) and infected. One representative experiment of two is shown. Data are expressed as mean + S.D. *p<0.05, **p<0.01, ***p<0.001. Mann-Whitney U test. As shown in Fig. 2A, production of IL-1β in C57BL/6J mice is higher at 72 h than at 6 days p.i., even though the morbidity and mortality caused by its absence becomes apparent several days later. To test the hypothesis that IL-1β is protective when produced in the early phase of the infection but not in the late phase, recombinant IL-1β was administered daily during the first week or the second week post infection to Il-1r1-/- mice. As shown in Fig. 3D, exogenous IL-1β was found to be protective only when administered during the first six days post infection. Conversely, blockage of IL-1, through administration of the IL-1 receptor antagonist IL-1ra to C57BL/6J mice, was deleterious during the first week but not during the second week post infection.
IL-1β is required for production of protective IgM
Our results so far indicate that IL-1β is produced and exerts its protective effect in the early phase of the infection, but morbidity and mortality starts to become apparent in infected Il-1r1-/- mice only in the second week post-infection when mice definitively loose the ability to contain the infection and bacteria burden dramatically increase. This is in clear contrast to Il-18-/- mice that shows signs of morbidity and mortality in the early days of infection (Fig. 1A). These results, together with the observation that IFNγ administration failed to rescue Il-1r1-/- mice (Fig. 2C), suggest that the innate immune response of Il-1r1-/- mice is sufficient to effectively control the infection and protect the mice for several days. Rather, the eventual death of Il-1r1-/- mice may be due to failure of immune responses that are dependent on the presence of IL-1β in the early phase of infection but are effectively deployed only in the second week of infection. These features are consistent with immune responses that bridge innate and adaptive immunity, such as the generation of IgM.
IgM is among the earliest immune effector mechanisms produced during infection and plays an important role during bacterial infections [36], including tularemia. As shown in Fig. 4A, Ft-specific IgM started to appear in the serum of infected mice 7 days post infection. Remarkably, the level of Ft-specific IgM was significantly reduced in Il-1b-/-, Il-1b-/--Il-1a-/-, or Il-1r1-/- mice compared to C57BL/6J or Il-1a-/- mice, suggesting that the susceptibility of IL-1-deficient mice may be due to insufficient production of pathogen-specific IgM and demonstrating for the first time that IL-1β is required for optimal production of this antibody isotype. The total serum IgM levels were similar among the different mouse strains (Fig. 4B) whereas the titers of Ft-specific IgG in serum or IgA in BALF of infected mice were undistinguishable from those of non-infected mice. (S1 Fig.). Importantly, anti-Ft IgM was also present in the BALF of infected mice (Fig. 4C). Supporting the hypothesis that reduced titer of Ft-specific IgM is responsible for the susceptibility of Il-1r1-/- mice, passive transfer of serum obtained from Ft LVS infected C57BL/6J mice seven days p.i. protected naïve C57BL/6J mice from infection with lethal doses of Ft LVS (Fig. 4D). Transfer of pre-immune serum did not confer protection, suggesting that innate antibodies were not mediating the observed protection. Remarkably, the serum obtained from Il-1b-/- mice was not protective, supporting the hypothesis that the observed protection was mainly mediated by Ft-specific IgM generated in an IL-1-dependent way. This was confirmed by the observation that serum of infected C57BL/6J mice depleted of IgM lost its protective activity. This result ruled out the possibility that other antimicrobial molecules produced during infection could play a role in the observed protection. Moreover, before passive transfer, sera were dialyzed using 100 kDa cut-off membranes to eliminate cytokines and other small inflammatory mediators.
Fig. 4. Rapid generation of protective anti-Ft IgM is dependent on IL-1β. 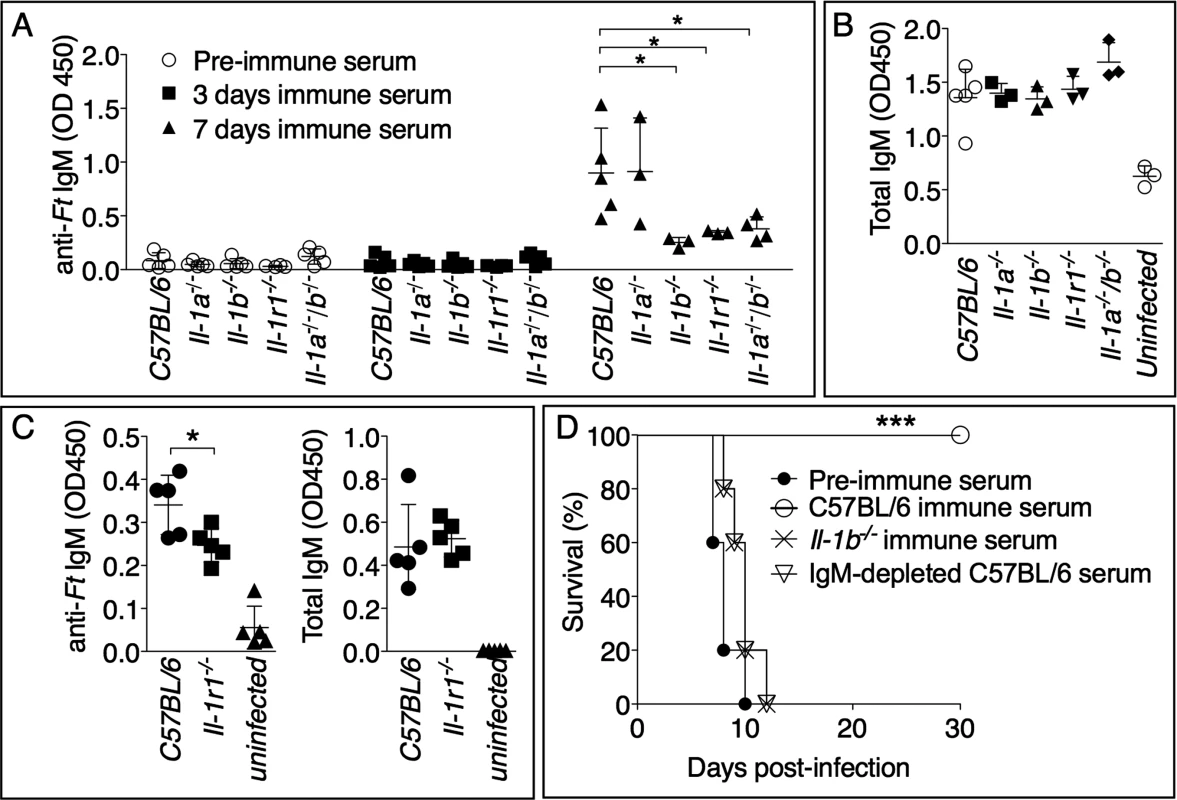
Mice, of shown genotypes, were intranasally infected with Ft LVS 103 CFU and bled at 0, 3, or 7 days p.i. Ft-specific (A) or total IgM titers (B) were measured in serum or BALF (C). (D) C57BL/6J mice (n = 5) were injected intraperitoneally with 300 μl of preimmune serum, or the indicated immune sera. 24 hours later, mice were intranasally infected with 8x103 CFU Ft LVS and survival was monitored. One representative experiment of three (A, B) or two (C, D) is shown. Data are expressed as mean + S.D. **p<0.01, ***p<0.001. Mann-Whitney U test. Anti-Ft IgM generation is dependent on TLR2 and ASC
As shown in Fig. 5A, and in agreement with previous works [12,21], production of mature IL-1β in response to Ft LVS infection is dependent on TLR2 and the inflammasome adaptor ASC. Interestingly, production of IL-18 was dependent on ASC but not TLR2 (Fig. 5B). Differently from IL-1β, IL-18 is known to be constitutively expressed, which may relieve the necessity of TLR-mediated priming steps. Reinforcing the notion that IL-1β is required for the production of anti-Ft IgM, the level of this antibody was significantly decreased in the serum of infected Tlr2-/- and Asc-/- mice (Fig. 5C). As previously shown [10] [11,21], these mice had increased bacterial burdens in the organs (Fig. 5D), another indication that anti-Ft IgM is protective.
Fig. 5. Generation of protective anti-Ft LPS IgM is dependent on TLR2 and inflammasome. 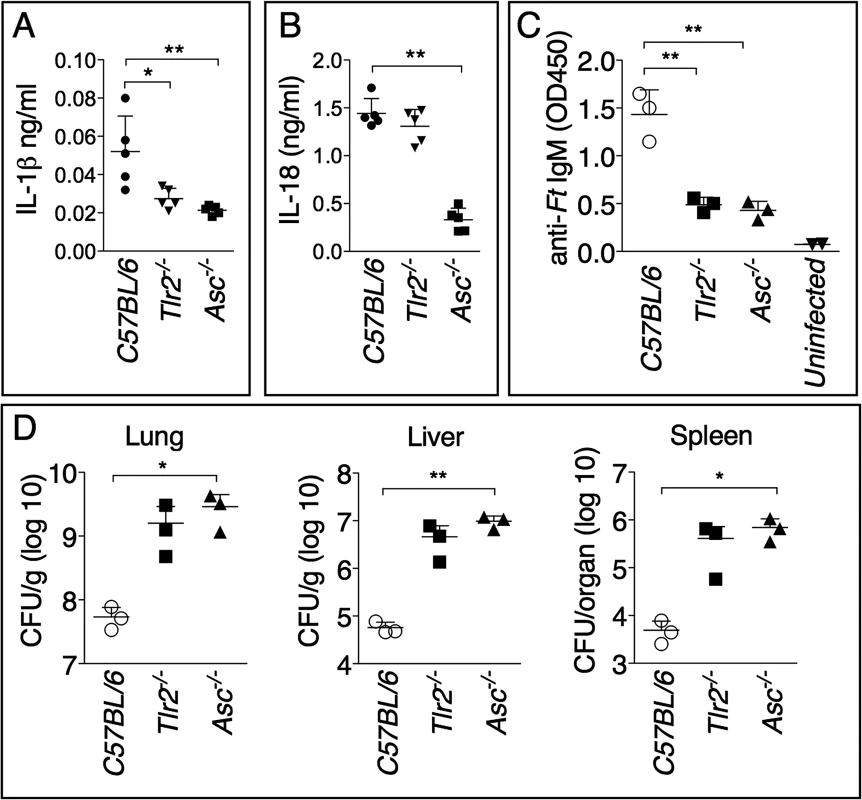
Cytokine levels were measured 6 days p.i. in BALF (A) or serum (B) of mice intranasally infected with 4x103 CFU Ft LVS. (C) Anti-Ft IgM were measured on day 7 p.i. in serum of mice infected as in A. (D) Organ bacterial burdens were measured 6 days p.i. in the indicated mouse strains infected as in A. Data are expressed as mean + S.D. *p<0.05, **p<0.01. Unpaired t-test. Protective IgM is specific for Ft LPS
Natural antibodies belonging to the IgM class are present in serum regardless of exposure to pathogens and tend to be poly-reactive and specific for T-independent antigens such as bacterial lipopolysaccharides [37]. As shown in Fig. 6A, pre-immune serum did not contain IgM reactive against a Ft LVS lysate. In contrast, the immune serum reacted with the Ft LVS lysate in a banding pattern characteristic of LPS. Competitive ELISA was used to show that the anti-Ft IgM was specifically directed against Ft LPS but did not react with E. coli LPS (Fig. 6B), reinforcing the notion that this is an Ft-specific response and that the measured anti-Ft LPS IgM is not part of the natural antibody repertoire.
Fig. 6. Protective anti-Ft IgM are specific for Ft LPS. 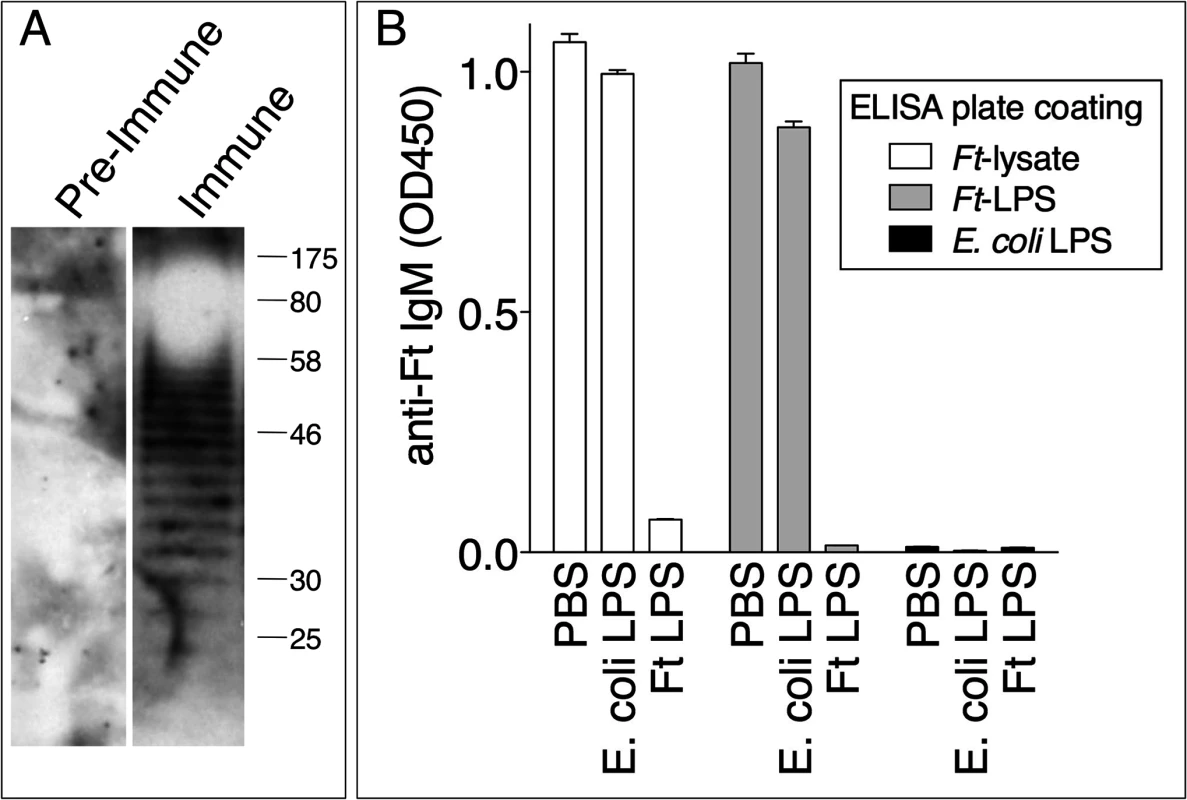
(A) Preimmune serum or day 7 Ft-immune serum were used to probe Ft lysate by immunoblot. (B) Ft immune serum was incubated with PBS, E. coli LPS, or Ft LPS and then used in a competitive ELISA assay on plates coated with Ft lysate, Ft LPS, or E. coli LPS. One representative experiment of two is shown. Data are expressed as mean + S.D. Anti-Ft IgM efficiently agglutinates Ft LVS and promotes phagocytosis
Complement fixation and agglutination are among the most effective antibacterial function of IgM. Ft LVS is known to be resistant to complement-mediated lysis [38] [39]. However, IgM-mediated C3 opsonization has been shown to enhance Ft LVS phagocytosis by PMN [40]. Day-7 immune serum from Ft LVS-infected C57BL/6 mice efficiently agglutinated Ft LVS in vitro, a phenomenon not observed with pre-immune serum and only partially with serum from infected Il-1b-/- mice (Fig. 7A). Pre-incubation of Ft LVS with immune serum significantly reduced infectivity and intracellular replication in bone marrow-derived macrophages (BMM) (Fig. 7B) or in C57BL6/J mice (Fig. 7C). Pre-agglutinated bacteria were also phagocytosed more efficiently by bone marrow-derived dendritic cells (BMDC) (Fig. 7D). When GFP-expressing Ft LVS was pre-incubated with pre-immune serum or serum from infected Il-1b-/- mice only 9.8% or 8.75% of BMDC phagocytosed bacteria and became GFP-positive, respectively. In contrast, agglutination of Ft LVS with serum from infected C57BL/6J mice dramatically increased phagocytosis of the bacteria (25.3% of cells were GFP-positive). Heat inactivation of this serum reduced the percentage of cells containing bacteria (16.5%) suggesting that the opsonic function of anti-Ft IgM is partially mediated by complement activation, a result consistent with the above-mentioned role of C3 in Ft LVS uptake. Pre-agglutination did not affect growth of bacteria in liquid cultures (S2 Fig.). Taken together, these results suggest that agglutination and C3-mediated opsonization are the main mechanisms responsible for the protective effect of anti-Ft IgM.
Fig. 7. Anti-Ft IgM efficiently agglutinates Ft and promotes phagocytosis. 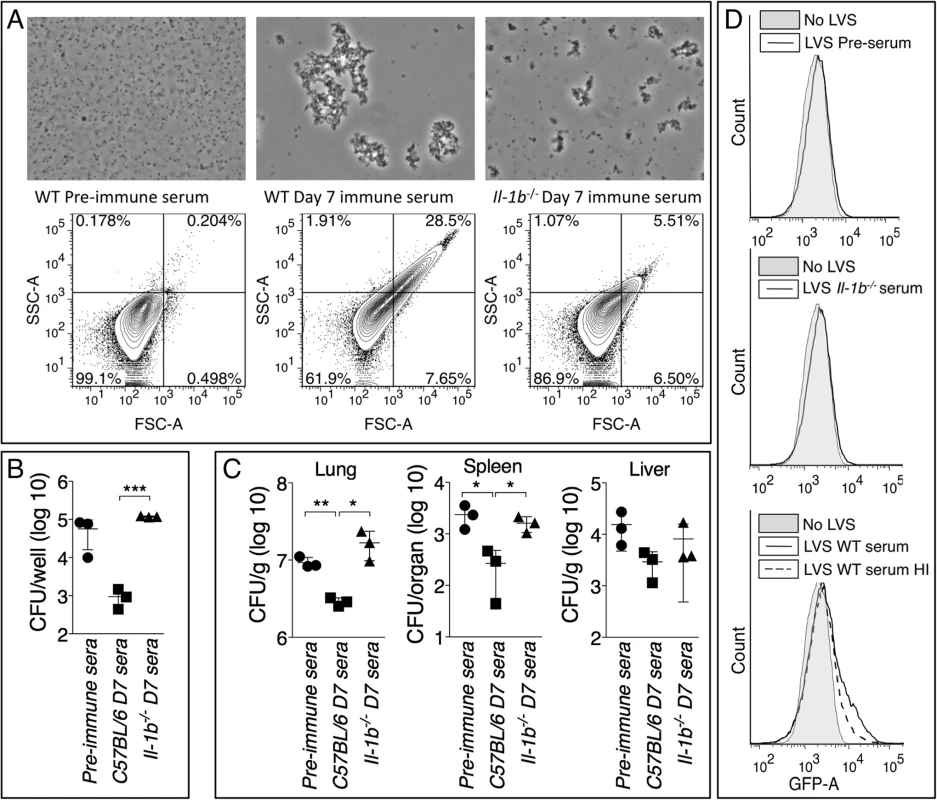
(A) Ft LVS agglutinated with the indicated sera was examined by microscopy or flow cytometry. (B) Agglutinated Ft LVS was used to infect BMM and intracellular bacteria replication was measured 18 hours later. (C) Agglutinated Ft LVS (6.5x103 CFU) was used to infect intranasally C57BL/6J mice and bacteria burdens were measured 48 hours later. (D) GFP-expressing Ft LVS was agglutinated with the indicated sera and used to infect BMDC. Bacteria uptake was measured by flow cytometry. HI, heat inactivated. Data are expressed as mean + S.D. *p<0.05, **p<0.01, ***p<0.001. Unpaired t-test. Role of B1a B cells in production of anti-Ft LPS IgM
Both B1 and B2 B cells subsets are known to contribute to rapid production of IgM during infection and to a different extent depending on the pathogen. B1 B cells and marginal zone B cells (MZ B cells) are a major source of natural antibodies and IgM specific for T-independent antigens such as LPS and self-antigens [41] [42]. Previous work has shown that immunization with purified Ft LPS caused expansion of B1a B cells [43]. Analysis of various B cells subsets in mice infected for 7 days with Ft LVS revealed that C57BL/6J and Il-1r1-/- mice or Il-1b-/- mice (S3 Fig.) had a similar percentage and total number of B cells, MZ B cells, and follicular B cells in different anatomical locations. In contrast, B1a B cells, but not B1b B cells, were detected in lower proportions in the peritoneal cavity and spleen of Il-1b-/- mice, compared to C57BL/6J mice (Fig. 8B). B1a B cells were present in equal amount in uninfected C57BL/6J or Il-1b-/- mice suggesting that absence of IL-1β affects the infection-induced expansion of these cells, not their homeostasis. Spleen cells from infected C57BL/6J and Il-1b-/- mice were cultured for 18 hours in presence of PMA and anti-Ft IgM was measured in the cultured supernatants. As shown in Fig. 8C, cells derived from Il-1b-/- mice released significantly lower amount of anti-Ft IgM compared to C57BL/6J cells. Given that B1a cells were the only B cell subset differentially represented in the spleen of C57BL/6J and Il-1b-/- mice (Fig. 8B and S3 Fig.), these data suggests that the anti-Ft LPS IgM is produced by B1a B cells. To demonstrate this point conclusively, B1a B cells were purified by flow cytometry from the spleen of C57BL/6J or Il-1b-/- infected mice and cultivated for 24 hours. Anti-Ft LPS IgM were present in the conditioned supernatants of purified B1a B cell cultures (Fig. 8D) and, more importantly, in higher amount in C57BL/6J than in Il-1b-/- cells. Together with the in vivo results, these data demonstrate that B1a B cells are the source of the Ft-specific IgM and that expansion of these cells depends on IL-1β.
Fig. 8. Reduced number of B1a B cells in Il-1b-/- mice infected with Ft LVS. 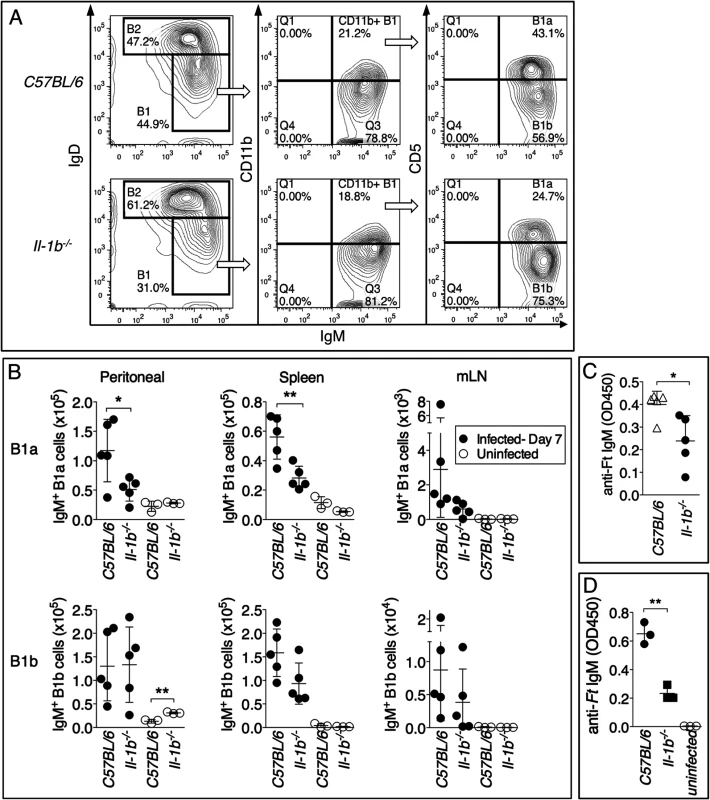
(A) Representative FACS profile and gating strategy to identify B1a and B1b B cells. (B) The number of B1a and B1b B cells was measured in peritoneal lavage, spleen, and mediastinal lymph nodes of indicated mouse strains intranasally infected with Ft LVS 103 CFU 6 days p.i. (C) Total spleen cells (5x106 cells/ml) or (D) purified B1a B cells (x106 cells/ml) from mice infected as in A were cultured for 24 hours. IgM was measured in culture supernatants. One representative experiment of three is shown. Data are expressed as mean + S.D. *p<0.05, **p<0.01. Unpaired t-test. Discussion
A number of studies over the past few years have characterized the innate immune response against Ft LVS infection and in particular the protective role played by activation of the AIM2 inflammasome [3,44]. However, by comparison, the role of IL-1β and IL-18 in this infection has been somewhat neglected and remains unclear.
Our study is the first to show that during lung infection with Ft LVS, IL-1β and IL-18 are protective, but through different mechanisms. IL-18-deficient mice quickly succumbed to infection with high organ bacterial burdens and low levels of IFNγ, a cytokine that has been shown to be critical for protection from tularemia [30] [31] [32] [33]. Administration of IFNγ increased the survival of Il-18-/- mice, suggesting that the lower resistance to tularemia is due to an inability to produce IFNγ. In contrast, IFNγ administration had no effect on Il-1r1-/- mice survival. Thus, different mechanisms determine the increased susceptibility of Il-1r1-/- and Il-18-/- mice to tularemia. This conclusion was also supported by the observation that Il-1r1-/- or Il-1b-/- mice appear to be able to control the infection, at least in its early stages, but eventually succumbed. This raised the possibility that responses at the boundary of innate and adaptive immunity were defective in absence of IL-1β. In fact, our results show that Il-1r1-/- mice produced Ft LPS-specific IgM in a significantly reduced amount compared to C57BL/6J mice. Passive immunization experiments showed that anti-Ft IgM were protective, suggesting that the higher susceptibility of IL-1-deficient mice is partly due to reduced production of anti-Ft-LPS IgM.
The antibody response to Ft infection has been characterized in detail in mice and humans and is mainly directed against LPS and composed of IgM and IgG [45]. Several studies have previously shown that Ft LVS infection, or immunization with Ft LPS, result in a protective humoral response that can be passively transferred [46] [47] [48] [49]. In all these studies, the passive immunization relied on immune sera obtained several weeks post infection/immunization, implying a role for Ft-specific IgG, rather than IgM. One study in fact showed that in order to be protective, serum should be collected at least 15 days p.i. [46]. In that study mice were infected intradermaly as opposed to the intranasal route we used. One novelty of our study is that it shows that during intranasal infection with Ft LVS the protective humoral response is deployed more rapidly than previously thought and that IgM is a critical component of this response. The fact that Ft LVS has been shown to have a significant extracellular phase in infected mice [50] explains why the humoral response can be so effective against infection with this facultative intracellular bacterium. Importantly, our results show that anti-Ft IgM is present in the BALF of infected mice suggesting a role for this Ig isotype at mucosal surfaces. It is likely that infection-induced tissue damage and vascular leakage are responsible for IgM spillage into alveolar spaces.
It is increasingly recognized that IgM plays a protective role during infections, yet the mechanisms of protection have not been consistently defined. IgM efficiently fixes complement and promotes bacteria agglutination and phagocytosis. Although, Ft is resistant to complement-mediated lysis [39] [51], it has been demonstrated that IgM-mediated C3 deposition enhances Ft LVS phagocytosis by neutrophils [40]. Our results support the conclusion that the protection conferred by passive transfer of anti-Ft IgM is mediated by enhanced agglutination/opsonization.
Although it is widely accepted that IgM represents an important first line of defense against infection [36], the pathways leading to production of these antibodies during infection and many aspects of their biology remain unclear. Different B cell subsets, including marginal zone (MZ) B cells and B1 B cells, have been shown to be the main source of the IgM rapidly generated against T-independent antigens during viral or bacterial infections [52]. Studies have indicated that among B1 B cells, the B1a subset is primarily responsible for production of natural IgM while B1b B cells produce pathogen-specific immune IgM [53,54]. However, recent studies have challenged this simplistic classification by showing pathogen-specific antibody production by B1a B cells [43].
Here we show that the reduced serum titer of Ft LPS-specific IgM in IL-1-deficient mice correlated with a significant reduction in the percentage of B1a B cells in the spleen and peritoneal cavity in these mice. Purification of B1a B cells from infected mice confirmed that these cells are responsible for production of the protective anti-Ft LPS IgM. This conclusion is in agreement with the work of Cole et al. [43] that elegantly showed that vaccination of mice with purified Ft LPS rapidly elicits expansion of a rare population of B1a B cells as well as production of anti-Ft LPS protective antibodies. In a follow-up study [55], the same authors showed that the protection conferred by Ft LPS immunization required antibody production and macrophages activation. It should be noted that neither study showed whether the protective anti-Ft LPS antibodies were IgM and whether transfer of sera containing them was sufficient for protection, as demonstrated in our experiments. Our study complements that of Cole et al. [43] by showing that expansion of B1a B cells and the production of anti-Ft LPS antibodies occurs during a natural infection with Ft LVS, as opposed to the artificial setting of vaccination. In their study [43], Cole et al. concluded that generation of the anti-Ft LPS antibodies was independent of innate immunity because this response was observed in TLR4 - or TLR2-deficient mice. Interestingly, despite similar titers of anti-Ft LPS antibodies in C57BL/6J and Tlr2-/- mice, immunization with Ft LPS was not protective in Tlr2-/- mice. Of note, Ft LPS does not activate TLR4 or TLR2 [5] [6] [7]. On the other hand, Ft LVS has been shown to activate TLR2 [10] [12] and, consistent with this, our results show that production of anti-Ft LPS IgM, as well as IL-1β, depends on TLR2 and the inflammasome adaptor ASC. Thus, it appears that generation of anti-Ft LPS antibodies can proceed in absence of TLR stimulation in the Ft LPS vaccination setting. However, during infection with Ft LVS, activation of TLR2 and the inflammasome significantly boosts this response in an IL-1β-dependent fashion. The disparities between vaccination and natural infection are likely due to the fact that vaccination with purified LPS delivers a signal sufficiently strong to activate B1a B cells even in absence of TLR engagement and cytokine production. In contrast, in the natural infection setting “free LPS” may be present in much lower amount and, under these circumstances, the adjuvant effect of IL-1β becomes essential for efficient expansion of B1a B cells. Interestingly, it has been reported that mice deficient in Bruton’s tyrosine kinase, which lack B1a B cells, were more susceptible to lung infection with the Ft virulent strain SchuS4 due to an increase in macrophages and NK/NKT cells [56].
The role of IL-1 for antibody production has been extensively analyzed in conventional B cells, and seems to vary according to the experimental setting [57]. In contrast, whether IL-1 regulates the development of B1 B cells and production of IgM by this cell type has not been investigated. Decreased IgM production in Il-1r1-/- mice has been reported, although the involvement of B1 B cells subsets was not examined in that study [58]. Our study reveals that rapid production of anti-Ft LPS IgM by B1a B cells depends on IL-1β and suggests, for the first time, a role for IL-1β in the development of B1a B cells during a bacterial infection. Several questions are prompted by our study: Is IL-1β action on B1a B cells direct or mediated by other factors/cells that may regulate migration and activation of these cells? Is the requirement for IL-1β absolute or limited to an enhancing role for a timely production of IgM by B1a B cells? Although the B1a cell population was the only one quantitatively altered in absence of IL-1β, is production of IgM by B1b or MZ B cells regulated by IL-1β in other settings? The IL-1 family member IL-33 has been shown to be required for B1 B cells development [59]. Interestingly, IL-1RAcP, the signaling subunit shared by IL-1 receptor and ST2, the IL-33 receptor, was differentially expressed by B1a and B1b subsets. Whether IL-1RI is differentially expressed in B cell subsets remains to be determined. Future studies should examine the role of IL-1β in B1 B cells activation and IgM production during different bacterial and viral infections
Materials and Methods
Ethics statement
All the animal experiments described in the present study were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal studies were conducted under protocols approved by the Rosalind Franklin University of Medicine and Science Institutional Animal Care and Use Committee (IACUC) (protocol # B12-07). All efforts were made to minimize suffering and ensure the highest ethical and humane standards.
Mice
C57BL/6, Tlr2-/-, Il-1r1-/-, Il-18-/- were purchased from Jackson lab and bred in our facility. Il-18-/--Il-1r1-/- double deficient mice (DKO) were obtained by crossing the parental single knockout mice. Asc-/- mice were obtained from V. Dixit (Genentech). Il-1a-/- and Il-1a-/-/Il-1b-/- were from I. Iwakura, Il-1b-/- from D. Chaplin. All mouse strains were on C57BL/6 genetic background and were bred under specific pathogen-free conditions in our facility. Age-(8–12 weeks old) and sex-matched animals were used in all experiments. Generally, experimental groups were composed of at least 5 mice. All the animal experiments described in the present study were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal studies were conducted under protocols approved by Institutional Animal Care and Use Committees of the Rosalind Franklin University of Medicine and Science.
Bacteria, mice infection and treatments
For all experiments the Francisella tularensis LVS was used. GFP-expressing Ft LVS was provided by Mark Miller (UTHSC). Bacteria were grown in MH broth (Muller Hinton supplemented with 0.1% glucose, 0.1% cysteine, 0.25% ferric pyrophosphate, and 2.5% calf serum) to mid-logarithmic phase, their titer was determined by plating serial dilutions on complete MH agar, and stocks were maintained frozen at −80°C. No loss in viability was observed over prolonged storage. For infections, frozen stocks were diluted in sterile PBS to the desired titer. Aliquots were plated on complete MH agar to confirm actual cfu. Mice were anesthetized with isoflurane using a Surgivet apparatus and 50 ml of bacteria suspension were applied to the nare. In some experiments, mice were injected i.p. daily with recombinant mouse IL-1β (1 μg) or IFNγ (1 μg). IL-1ra (Biovitrum) was administered by alternating s.c. and i.p. injections every 12 hours (60 mg/kg body weight).
Determination of bacteria growth in tissue culture and organs
Organs aseptically collected were weighted and homogenized in 1 ml PBS containing 0.5% saponin and 3% BSA. Serial dilutions were plated on complete MH agar plates.
BALF collection and cytokine measurements
BALF were collected from euthanized mice by intratracheal injection and aspiration of 1 ml PBS. Cytokine levels in BALF or serum were measured by ELISA using the following paired antibodies kits: mIL-1α, mIL-1β, mIFNγ (eBioscience), mIL-18 (MBL Nagoya, Japan).
Determination of antibody titers and competitive ELISA
Blood was collected aseptically from the submandibular vein. Ft LPS-specific immunoglobulin levels in sera or BALF were measured by ELISA. Serial dilutions of sera were plated in 96 wells plates coated with Ft LVS lysate (10 μg/ml). HRP-conjugated rat anti-mouse IgM or IgG (Southern Biotech Associates, Birmingham, AL) was added followed by TMB substrate and measurement of absorbance at 450 nm. For competitive ELISA (Fig. 6B) plates were coated with Ft LVS lysate (10 μg/ml), purified Ft LVS LPS (10 μg/ml), or E. coli LPS (0111:B4, 10 μg/ml). Immune serum was preincubated with PBS, E. coli LPS or Ft LVS LPS (1 μg) for 30 minutes before dilution and addition to coated plates.
Western blot
Ft LVS lysate (50 μg) was separated by 12% PAGE electrophoresis, transferred to PVDF membranes, and probed with pre-immune or immune serum (1 : 300 dilution) followed by anti-mouse IgM-HRP (Upstate Biotechnologies) and developed by ECL Femto (Pierce).
Passive immunization
Preimmune serum or sera from different mouse strains infected for 7 days were dialyzed against PBS (100 kDa mw cut-off) and 300 μl were intraperitoneally administered to mice 18 hours before infection. To deplete IgM, serum was incubated 1 hours at 4°C with anti-mouse IgM agarose (Sigma).
Bacteria agglutination, infectivity, and phagocytosis
Ft LVS was grown O/N in complete MH broth. 10 μl of bacterial culture were incubated with 10 μl of serum for 10 minutes at RT. Agglutination was confirmed by microscopy and flow cytometry. To measure infectivity of agglutinated Ft LVS in vitro, BMM were infected with bacteria preincubated with different sera at a MOI 500 and infection allowed to proceed for 2 hours. Cells were then washed 3 times with PBS and cultured for additional 18 hours in DMEM-10% FCS containing gentamicin and kanamycin (400 μg/ml). Media were aspirated and cells lysed in PBS containing 0.5% saponin and 3% BSA. Serial dilutions were plated on complete MH agar. To measure phagocytosis, BMDC were incubated for 4 hours with GFP-expressing Ft LVS agglutinated with different sera. Some sera were heat inactivated at 56 C for 30 minutes. Gentamicin and kanamycin (400 μg/ml) were then added to the medium for 4 more hours to kill extracellular bacteria and cells were analyzed by flow cytometry. The percentage of GFP positive cells (those that phagocytosed Ft LVS) was calculated by Overton subtraction.
Flow cytometry
Cells obtained from BALF, peritoneal lavage, spleen, or mediastinal lymph nodes were resuspended in FACS buffer (1% BSA, 0.05% NaN3 in PBS) containing anti-CD16/CD32 (clone 2.4G2) and counted. For analysis cells were incubated for 30’ on ice with the following antibody cocktails: for myeloid cells, CD11b-PECy7, Ly6G-PE, F4/80-APC, CD11c-FITC and NKp46-BV421; for GC B cells, B220-PercpCy5.5, CD4-APC, FAS-PE and GL-7-FITC; for marginal zone (MZ) and Follicular B cells, CD19-BV421, CD23-PECy7 and CD21-APC; for B1 cells, CD19-BV421, IgM-PECy7, IgD-APC, CD11b-AF700 and CD5-PE; for TFH cells, CXCR5-biotin followed by streptavidin-APC, CD4-PercpCy5.5, BTLA-PE and PD1-FITC.
GC B cells are defined as CD4- B220+ cells expressing both FAS and GL-7; TFH cells are CD4+ CXCR5+ BTLA+ [60]; Fol B cells are defined as CD19+ CD23+ CD21-/intermediate; MZ B cells are the CD19+ CD21+ CD23-/low cells [61]. B cells (CD19+) were gated on lymphocyte gate followed by singlet gate (FSC-A vs FSC/H) and identified as B2 or B1 cells according to the surface expression of IgD or IgM, respectively. B1 B cells subsets are defined as described in [54] [61] as CD19+ IgMhigh IgD-/low. B1 B cells were selected and gated for the expression of CD11b and CD5. B1a cells are defined as CD5+, B1b cells as CD5-. FMO or isotype controls were used to set the gate. Data were acquired with a BD LSR II flow-cytometer (BD biosciences) and analyzed with FlowJo 7.6.5 software (Treestar Inc).
B1a B cell sorting and purification
B1a B cells were purified from the spleen of mice infected for 7 days with Ft LVS according to [56]. Single cell suspensions of splenic cells were enriched by negative selection for B cells using the B cell isolation kit (StemCell). The CD19+/CD5+ B1 a B cells were sorted with a BD FACSAria II u flow cytometer. B1a B cells were resuspended in DMEM-10% FCS at a density of 1.5 x106 and cultured in 96 well plate for 24 hours.
Statistical analysis
All data were expressed as mean + S.D. Survival curves were compared using the log rank Kaplan-Meier test. Mann-Whitney U test or unpaired t-test were used for analysis of the rest of data as specified in figure legends. Significance was set at p<0.05. Statistical analyses were performed using the GraphPad Prism 5.0.
Supporting Information
Zdroje
1. McLendon MK, Apicella MA, Allen LA (2006) Francisella tularensis: taxonomy, genetics, and Immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol 60 : 167–185. 16704343
2. Steiner DJ, Furuya Y, Metzger DW (2014) Host-pathogen interactions and immune evasion strategies in Francisella tularensis pathogenicity. Infect Drug Resist 7 : 239–251. doi: 10.2147/IDR.S53700 25258544
3. Elkins KL, Cowley SC, Bosio CM (2007) Innate and adaptive immunity to Francisella. Ann N Y Acad Sci 1105 : 284–324. 17468235
4. Jones CL, Napier BA, Sampson TR, Llewellyn AC, Schroeder MR, et al. (2012) Subversion of host recognition and defense systems by Francisella spp. Microbiol Mol Biol Rev 76 : 383–404. doi: 10.1128/MMBR.05027-11 22688817
5. Cole LE, Elkins KL, Michalek SM, Qureshi N, Eaton LJ, et al. (2006) Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J Immunol 176 : 6888–6899. 16709849
6. Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, et al. (2006) Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun 74 : 6730–6738. 16982824
7. Barker JH, Weiss J, Apicella MA, Nauseef WM (2006) Basis for the failure of Francisella tularensis lipopolysaccharide to prime human polymorphonuclear leukocytes. Infect Immun 74 : 3277–3284. 16714555
8. Ancuta P, Pedron T, Girard R, Sandstrom G, Chaby R (1996) Inability of the Francisella tularensis lipopolysaccharide to mimic or to antagonize the induction of cell activation by endotoxins. Infect Immun 64 : 2041–2046. 8675305
9. Dreisbach VC, Cowley S, Elkins KL (2000) Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun 68 : 1988–1996. 10722593
10. Katz J, Zhang P, Martin M, Vogel SN, Michalek SM (2006) Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun 74 : 2809–2816. 16622218
11. Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, et al. (2006) Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun 74 : 3657–3662. 16714598
12. Li H, Nookala S, Bina XR, Bina JE, Re F (2006) Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J Leukoc Biol 80 : 766–773. 16895974
13. Thakran S, Li H, Lavine CL, Miller MA, Bina JE, et al. (2008) Identification of Francisella tularensis lipoproteins that stimulate the toll-like receptor (TLR) 2/TLR1 heterodimer. J Biol Chem 283 : 3751–3760. 18079113
14. Henry T, Monack DM (2007) Activation of the inflammasome upon Francisella tularensis infection: interplay of innate immune pathways and virulence factors. Cell Microbiol 9 : 2543–2551. 17662071
15. Cunha LD, Zamboni DS (2013) Subversion of inflammasome activation and pyroptosis by pathogenic bacteria. Front Cell Infect Microbiol 3 : 76. doi: 10.3389/fcimb.2013.00076 24324933
16. Atianand MK, Duffy EB, Shah A, Kar S, Malik M, et al. (2011) Francisella tularensis reveals a disparity between human and mouse NLRP3 inflammasome activation. J Biol Chem 286 : 39033–39042. doi: 10.1074/jbc.M111.244079 21930705
17. Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27 : 519–550. doi: 10.1146/annurev.immunol.021908.132612 19302047
18. Kingry LC, Petersen JM (2014) Comparative review of Francisella tularensis and Francisella novicida. Front Cell Infect Microbiol 4 : 35. doi: 10.3389/fcimb.2014.00035 24660164
19. Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA (1991) Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun 59 : 2922–2928. 1879918
20. Elkins KL, Winegar RK, Nacy CA, Fortier AH (1992) Introduction of Francisella tularensis at skin sites induces resistance to infection and generation of protective immunity. Microb Pathog 13 : 417–421. 1297917
21. Mariathasan S, Weiss DS, Dixit VM, Monack DM (2005) Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med 202 : 1043–1049. 16230474
22. Collazo CM, Sher A, Meierovics AI, Elkins KL (2006) Myeloid differentiation factor-88 (MyD88) is essential for control of primary in vivo Francisella tularensis LVS infection, but not for control of intra-macrophage bacterial replication. Microbes Infect 8 : 779–790. 16513388
23. Huang MT, Mortensen BL, Taxman DJ, Craven RR, Taft-Benz S, et al. (2010) Deletion of ripA alleviates suppression of the inflammasome and MAPK by Francisella tularensis. J Immunol 185 : 5476–5485. doi: 10.4049/jimmunol.1002154 20921527
24. Ulland TK, Buchan BW, Ketterer MR, Fernandes-Alnemri T, Meyerholz DK, et al. (2010) Cutting edge: mutation of Francisella tularensis mviN leads to increased macrophage absent in melanoma 2 inflammasome activation and a loss of virulence. J Immunol 185 : 2670–2674. doi: 10.4049/jimmunol.1001610 20679532
25. Jayakar HR, Parvathareddy J, Fitzpatrick EA, Bina XR, Bina JE, et al. (2011) A galU mutant of Francisella tularensis is attenuated for virulence in a murine pulmonary model of tularemia. BMC Microbiol 11 : 179. doi: 10.1186/1471-2180-11-179 21819572
26. Peng K, Broz P, Jones J, Joubert LM, Monack D (2011) Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell Microbiol 13 : 1586–1600. doi: 10.1111/j.1462-5822.2011.01643.x 21883803
27. Ulland TK, Janowski AM, Buchan BW, Faron M, Cassel SL, et al. (2013) Francisella tularensis live vaccine strain folate metabolism and pseudouridine synthase gene mutants modulate macrophage caspase-1 activation. Infect Immun 81 : 201–208. doi: 10.1128/IAI.00991-12 23115038
28. Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA (1998) Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest 101 : 711–721. 9449707
29. Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, et al. (1995) Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 378 : 88–91. 7477296
30. Anthony LS, Ghadirian E, Nestel FP, Kongshavn PA (1989) The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog 7 : 421–428. 2516219
31. Anthony LS, Morrissey PJ, Nano FE (1992) Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol 148 : 1829–1834. 1541823
32. Chen W, KuoLee R, Shen H, Conlan JW (2004) Susceptibility of immunodeficient mice to aerosol and systemic infection with virulent strains of Francisella tularensis. Microb Pathog 36 : 311–318. 15120157
33. Duckett NS, Olmos S, Durrant DM, Metzger DW (2005) Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun 73 : 2306–2311. 15784575
34. Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, et al. (1998) Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med 187 : 1463–1475. 9565638
35. Oguri S, Motegi K, Iwakura Y, Endo Y (2002) Primary role of interleukin-1 alpha and interleukin-1 beta in lipopolysaccharide-induced hypoglycemia in mice. Clin Diagn Lab Immunol 9 : 1307–1312. 12414765
36. Racine R, Winslow GM (2009) IgM in microbial infections: taken for granted? Immunol Lett 125 : 79–85. doi: 10.1016/j.imlet.2009.06.003 19539648
37. Ehrenstein MR, Notley CA (2010) The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 10 : 778–786. doi: 10.1038/nri2849 20948548
38. Sorokin VM, Pavlovich NV, Prozorova LA (1996) Francisella tularensis resistance to bactericidal action of normal human serum. FEMS Immunol Med Microbiol 13 : 249–252. 8861038
39. Ben Nasr A, Klimpel GR (2008) Subversion of complement activation at the bacterial surface promotes serum resistance and opsonophagocytosis of Francisella tularensis. J Leukoc Biol 84 : 77–85. doi: 10.1189/jlb.0807526 18430786
40. Schwartz JT, Barker JH, Long ME, Kaufman J, McCracken J, et al. (2012) Natural IgM mediates complement-dependent uptake of Francisella tularensis by human neutrophils via complement receptors 1 and 3 in nonimmune serum. J Immunol 189 : 3064–3077. doi: 10.4049/jimmunol.1200816 22888138
41. Baumgarth N (2011) The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 11 : 34–46. doi: 10.1038/nri2901 21151033
42. Cerutti A, Cols M, Puga I (2013) Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 13 : 118–132. doi: 10.1038/nri3383 23348416
43. Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, et al. (2009) Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci U S A 106 : 4343–4348. doi: 10.1073/pnas.0813411106 19251656
44. Broz P, Monack DM (2011) Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev 243 : 174–190. doi: 10.1111/j.1600-065X.2011.01041.x 21884176
45. Cowley SC, Elkins KL (2011) Immunity to Francisella. Front Microbiol 2 : 26. doi: 10.3389/fmicb.2011.00026 21687418
46. Rhinehart-Jones TR, Fortier AH, Elkins KL (1994) Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun 62 : 3129–3137. 8039881
47. Fulop M, Mastroeni P, Green M, Titball RW (2001) Role of antibody to lipopolysaccharide in protection against low - and high-virulence strains of Francisella tularensis. Vaccine 19 : 4465–4472. 11483272
48. Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW (2007) Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol 179 : 532–539. 17579074
49. Lavine CL, Clinton SR, Angelova-Fischer I, Marion TN, Bina XR, et al. (2007) Immunization with heat-killed Francisella tularensis LVS elicits protective antibody-mediated immunity. Eur J Immunol 37 : 3007–3020. 17960662
50. Forestal CA, Malik M, Catlett SV, Savitt AG, Benach JL, et al. (2007) Francisella tularensis has a significant extracellular phase in infected mice. J Infect Dis 196 : 134–137. 17538893
51. Clay CD, Soni S, Gunn JS, Schlesinger LS (2008) Evasion of complement-mediated lysis and complement C3 deposition are regulated by Francisella tularensis lipopolysaccharide O antigen. J Immunol 181 : 5568–5578. 18832715
52. Martin F, Oliver AM, Kearney JF (2001) Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14 : 617–629. 11371363
53. Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, et al. (2003) The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol 170 : 3819–3827. 12646649
54. Haas KM, Poe JC, Steeber DA, Tedder TF (2005) B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23 : 7–18. 16039575
55. Cole LE, Mann BJ, Shirey KA, Richard K, Yang Y, et al. (2011) Role of TLR signaling in Francisella tularensis-LPS-induced, antibody-mediated protection against Francisella tularensis challenge. J Leukoc Biol 90 : 787–797. doi: 10.1189/jlb.0111014 21750122
56. Crane DD, Griffin AJ, Wehrly TD, Bosio CM (2013) B1a cells enhance susceptibility to infection with virulent Francisella tularensis via modulation of NK/NKT cell responses. J Immunol 190 : 2756–2766. doi: 10.4049/jimmunol.1202697 23378429
57. Nakae S, Asano M, Horai R, Iwakura Y (2001) Interleukin-1 beta, but not interleukin-1 alpha, is required for T-cell-dependent antibody production. Immunology 104 : 402–409. 11899425
58. Schmitz N, Kurrer M, Bachmann MF, Kopf M (2005) Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol 79 : 6441–6448. 15858027
59. Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, et al. (2011) IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol 186 : 2584–2591. doi: 10.4049/jimmunol.1002103 21239718
60. Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, et al. (2009) Bcl6 mediates the development of T follicular helper cells. Science 325 : 1001–1005. doi: 10.1126/science.1176676 19628815
61. Malkiel S, Kuhlow CJ, Mena P, Benach JL (2009) The loss and gain of marginal zone and peritoneal B cells is different in response to relapsing fever and Lyme disease Borrelia. J Immunol 182 : 498–506. 19109181
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



