-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCommunity-based strategies to strengthen men’s engagement in the HIV care cascade in sub-Saharan Africa
Monica Sharma and colleagues discuss evidence-based approaches to improving HIV services for men in sub-Saharan Africa.
Published in the journal: . PLoS Med 14(4): e32767. doi:10.1371/journal.pmed.1002262
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1002262Summary
Monica Sharma and colleagues discuss evidence-based approaches to improving HIV services for men in sub-Saharan Africa.
Summary points
Men in sub-Saharan Africa are less likely than women to engage in HIV services across the care cascade, resulting in poorer clinical outcomes.
Health care facilities have achieved limited HIV testing and treatment coverage in men, with barriers including confidentiality concerns, distance to the facility, inconvenient hours, and perceptions that facilities provide women-centered services. Other barriers to male engagement include stigma, poverty, and feelings of compromised masculinity associated with seeking health care.
Community-based HIV interventions can overcome barriers associated with facilities and increase men’s engagement in care. Social and livelihood interventions can reduce stigma and poverty.
Community-based testing interventions (particularly home and mobile) have high acceptability and reach more men than health care facility-based approaches. For men testing HIV positive, providing immediate antiretroviral therapy (ART) is associated with high retention and viral suppression. This strategy of “collapsing the cascade” provides streamlined services and reduces loss to follow-up.
Community-based interventions should be tailored to the needs of men to maximize uptake, including flexible hours, multiple follow-up visits, and convenient and private access to care. Integrating HIV testing into screening for chronic disease can reduce stigma and increase program efficiency. More research is needed on male-centered approaches to increase men’s engagement in HIV services, particularly later in the cascade. Interventions targeted to men who have sex with men are urgently needed.
The current state of evidence strongly suggests that community-based test-and-treat strategies can reduce the gender disparity in HIV testing and treatment by achieving higher levels of ART coverage and viral suppression in HIV-positive men.
Introduction
The successful scale-up of antiretroviral therapy (ART) in sub-Saharan Africa (SSA) will require policy makers to address the gender gap in HIV testing and treatment access. Men in SSA are less likely than women to undergo HIV testing and more likely to start ART at advanced disease stages and interrupt or drop out of ART [1]. These disparities have resulted in a life expectancy gap of up to 10 years between HIV-positive men and women [2–5]. Low male testing and treatment rates also increase HIV transmission to female partners. For example, pregnant women in SSA have high HIV testing coverage through antenatal care (ANC) yet have twice the HIV incidence of nonpregnant women [6]. This can be partially attributable to low testing rates in their male partners. In Kenya during 2013, 88% of pregnant women were tested for HIV compared to 4.5% of their male partners [7,8].
Men who have sex with men (MSM) are an underserved group who should not be overlooked. Despite having an HIV prevalence up to eight times higher than the general population, HIV interventions for MSM in SSA are scarce [9,10]. Numerous policy barriers exist that restrict availability and access to HIV-related services for MSM, including police harassment and criminal laws [11].
Stigma is one potential explanation for low male engagement in the HIV care cascade. HIV-positive persons are often perceived by their community as disabled and incapable of contributing economically to society [12]. Men may be more affected by this stigma as they are traditionally the family “breadwinners.” Poverty and food insecurity, common in SSA, can exacerbate HIV-related stigma as engagement in the labor force is crucial for community survival [12]. Poverty can also directly affect engagement in care as individuals lack resources required to attend facilities. Further barriers to visiting clinics include confidentiality concerns, costs (transport, wait time, and lost wages), inconvenient hours, and the perception that clinics are places for women [13].
Lack of high-quality data on male HIV prevalence and health-seeking behavior can hinder prevention strategies
Accurate HIV prevalence estimates are crucial for designing and evaluating prevention programs. However, HIV surveillance programs such as Demographic and Health Surveys (DHS) are expensive to implement. Instead, much of SSA relies on prevalence estimates extrapolated from ANC data, which exclude men altogether [14]. Countries with DHS tend to have more accurate HIV prevalence estimates. However, measuring male HIV prevalence can be difficult as disproportionately more men decline DHS participation (although nonparticipation is an issue for both sexes). Studies show that DHS often underestimate HIV prevalence, indicating nonparticipation is related to knowledge of HIV status [15–17]. An analysis of 12 DHS found that 10 of the 12 surveys underestimated male HIV prevalence, with bias-corrected estimates up to 9% higher than original DHS prevalence estimates [15]. Another study evaluating DHS in Zambia found that the sex gap in HIV prevalence disappeared after correcting for nonparticipation, with prevalence in men increasing from 12% to 21% [16]. Underestimating HIV prevalence can lead to prevention policies that do not adequately address the male HIV burden. Advanced methods to adjust HIV prevalence for nonparticipation including instrumental variables and Heckman-type selection models can generate more accurate estimates [15,17,18]. These methods will become increasingly important as knowledge of HIV status increases in SSA, which can increase the bias in DHS estimates [14]. Additionally, bias correction is one of the few viable options for obtaining accurate HIV prevalence estimates in settings such as the Swaziland HIV Incidence Measurement Survey (SHIMS), which had lower response rates for men (65%) compared to women (81%) despite up to nine repeated home visits to reduce nonparticipation in the survey [19].
Data on HIV prevalence and health-seeking behaviors (voluntary medical male circumcision [VMMC] and ART uptake and adherence) also inform mathematical models that evaluate HIV interventions. A validation study assessing the ability of 10 mathematical models to predict HIV burden in South Africa found that 8 out of 10 models projected declining male HIV prevalence, whereas empirical data showed increasing prevalence; this may be partly because the models assumed equal ART uptake among men and women [20]. Future models should account for sex differences in HIV testing, ART initiation, and retention to more accurately estimate the impact of interventions.
Ascertaining where losses to follow-up occur across the HIV care cascade is also crucial to targeting interventions. However, it is challenging to generate accurate HIV-related mortality estimates since many HIV-positive men never access care. Additionally, clinics often do not track outcomes for persons who have dropped out of care, making it difficult to determine if men have transferred to another facility or stopped treatment [21]. A study conducted in South Africa was able to assess mortality throughout the HIV continuum using a population-based cohort and determining cause of death in all persons, including those not engaged in HIV care [22]. The authors found that the gap between female and male life expectancy doubled from 2003 to 2011 and twice as many women initiated ART than men. Over half (57%) of male HIV mortality in 2011 occurred among individuals who never sought care, highlighting the importance of HIV testing and linkage strategies. However, substantial proportions of men were lost throughout the cascade, including after ART initiation (33%), so interventions to maintain retention are vital; otherwise, increasing HIV testing and linkage could shift the distribution of deaths to later in the cascade instead of preventing them entirely. Improving ART adherence and retention can also reduce the risk of developing and transmitting drug-resistant virus.
Community-based HIV testing and counseling (HTC) strategies are successful at reaching men
Facility-based HTC has achieved limited coverage in men. Studies highlight men’s desire to test outside clinics [23], with some men preferring to test at home [24], while others have concerns about the confidentiality of home testing [25] and consider mobile HTC to be more private [26]. As there is no one-size-fits-all approach, a variety of community-based HTC strategies will likely be needed to achieve high testing coverage (Table 1). Meta-analyses find that community HTC achieves higher population coverage than facility testing [27], with home and mobile HTC increasing coverage in men (mobile having a larger effect than home HTC) [26]. Community HTC reaches more first-time testers, suggesting expanded coverage to persons who may not otherwise undergo facility testing [28]. Additionally, community HTC identifies asymptomatic HIV-positive men at higher cluster of differentiation 4 (CD4) cell counts [28]. This can facilitate earlier linkage to ART, preventing morbidity, mortality, and transmission.
Tab. 1. Community-based HIV HTC interventions to increase male testing and engagement in care. 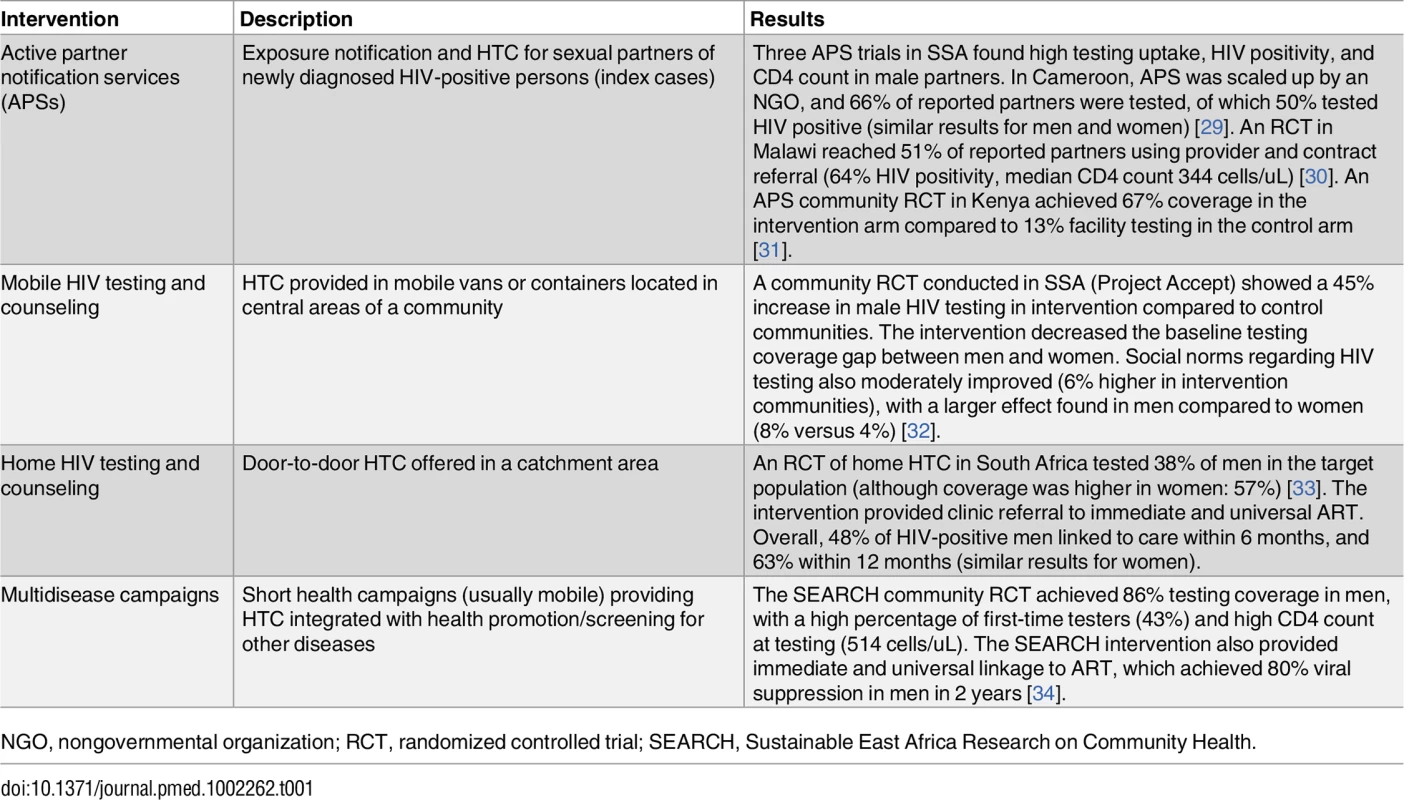
NGO, nongovernmental organization; RCT, randomized controlled trial; SEARCH, Sustainable East Africa Research on Community Health. However, community HTC should be tailored to the needs of men to optimize coverage and effectiveness. For example, a home HTC intervention in Botswana reached 85% of women in the target population compared with 50% of men, likely because the intervention was conducted during the workday, so employed men were missed [35]. Similarly, a home HTC trial (largely conducted during weekdays) successfully contacted more women than men in the target population to offer HTC (74% versus 50%). However, HTC acceptance rates among men contacted were similar to the women who were contacted (77%) [33]. Home HTC with flexible hours and multiple follow-up visits has the potential to test more men. Similarly, a randomized controlled trial (RCT) of workplace HTC for men had 53% uptake when testing was offered on-site compared to 19% for off-site testing, underscoring the importance of convenient testing access [36]. Finally, partner notification (notifying sexual partners of newly diagnosed HIV-positive individuals of their potential exposure and offering HTC) reaches more men when using active (provider tracing or contract referral) compared to passive notification (index cases are asked to notify sexual partners and refer them to the clinic). In a partner notification study in Malawi, men represented only 15% of those presenting to the clinic for testing through passive referral compared to 50% of those tested through active tracing, with no increase in adverse events (e.g., intimate partner violence) [30].
Mobile HTC is a promising strategy for reaching men. A large community RCT in Tanzania and Zimbabwe (Project Accept) reported male testing coverage of 44%–53% in sites randomized to mobile testing compared to 4%–9% facility testing coverage in control sites, decreasing the sex gap in testing coverage found at baseline [37]. Integrating HTC into mobile multidisease campaigns has also had success in reaching men [38]. Community health campaigns can achieve rapid HTC coverage in short time frames (e.g., 2 weeks) [39,40]. The Sustainable East Africa Research on Community Health (SEARCH) campaign conducted in Uganda and Kenya provided HTC; men’s health stations (including prostate cancer screening and VMMC information); screening and treatment for diabetes, hypertension, and malaria; and home HTC for persons who did not participate in the campaign (ClinicalTrials.gov: NCT01864603). This hybrid approach tested an impressive 86% of male community residents, with the vast majority testing through the campaigns (80%) [38]. Combining HTC with other health interventions can reduce HIV-related stigma while improving program efficiency. Studies find that men prefer HIV services that are not separate from other health care services [41].
HTC for male partners of pregnant woman is another strategy to expand male testing. Messaging that testing can protect one’s unborn child can motivate men to undergo HTC [42]. Additionally, couples HTC and status disclosure can increase women’s adherence to ART and regimens for prevention of mother-to-child transmission [43]. Interventions to encourage male partners to come to the ANC for couples HTC have had varied success [44]. Home HTC for male partners of pregnant women can overcome barriers of male ANC attendance and achieve high testing coverage. The Home-Based Partner Education and Testing (HOPE) intervention in Kenya found that 87% of male partners had been tested 6 weeks postpartum in the home-testing arm compared to 39% in the ANC arm [45]. HIV self-testing kits are also potentially effective in reaching men. An RCT in Kenya found that distributing HIV self-tests to pregnant and postpartum women achieved 91% testing coverage in male partners within 3 months compared to 51% in the control arm (male invitation for clinic testing). Self-reported couples testing and disclosure was higher in the self-testing (75%) arm compared to the control arm (35%) [46]. Couples-based approaches may increase male testing regardless of female pregnancy status. For example, a six-session couples behavioral intervention in South Africa found higher couples testing in the intervention compared to the control arm (45% versus 12%) [47].
Community-based educational initiatives to reduce stigma, decrease high-risk sexual behavior, increase HIV testing, and challenge gender norms have had some success (Table 2). The SASA! trial was particularly effective: it was associated with an increase in male HIV testing, condom use, and male appreciation for women’s work [48]. Studies show that social networks are important influences on male HIV testing uptake. Men who believe that at least one close friend has tested for HIV have twice the odds of having undergone HIV testing [49]. More research is needed on social interventions to increase testing coverage in men in SSA. Particularly, training peer leaders to encourage HIV testing should be explored in future studies. Additionally, support from men’s primary female partners has been shown to help overcome barriers to ART initiation and promote adherence [50]. Future interventions should evaluate leveraging social support from primary partners to encourage male engagement in care.
Tab. 2. Community-based educational initiatives for HIV prevention. 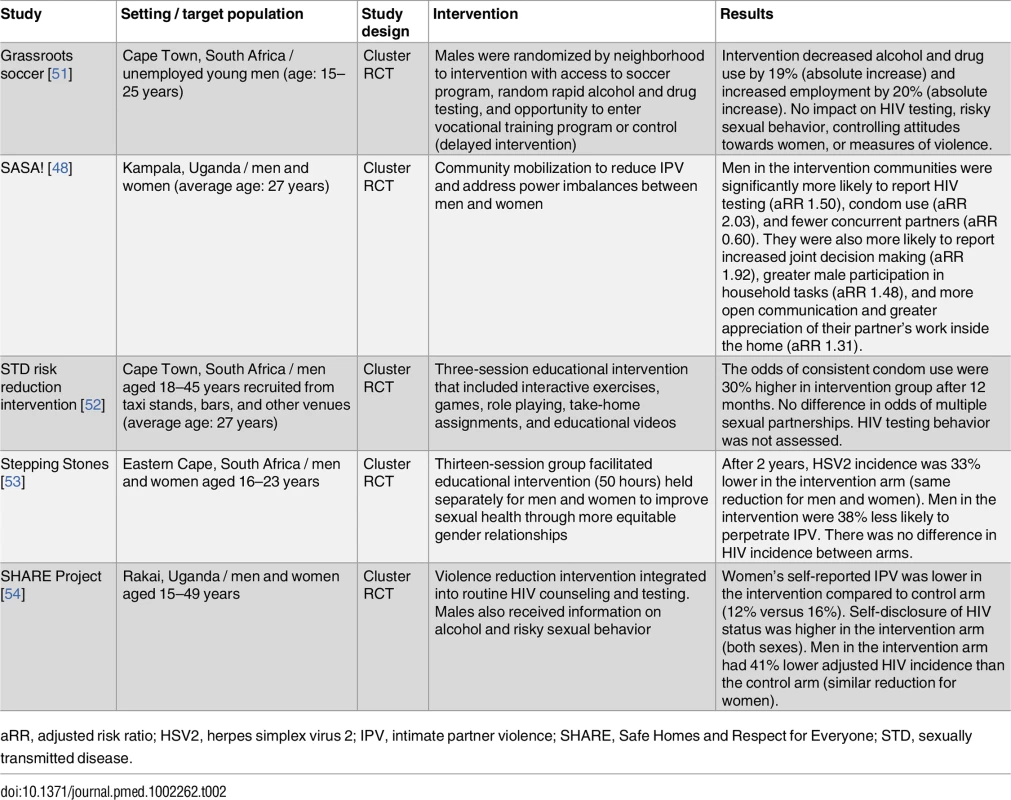
aRR, adjusted risk ratio; HSV2, herpes simplex virus 2; IPV, intimate partner violence; SHARE, Safe Homes and Respect for Everyone; STD, sexually transmitted disease. HIV self-testing is a relatively low-cost strategy that achieves high uptake in men, particularly young men [27,55–57]. Self-testing was the preferred option for future HIV testing among men surveyed in Malawi [55]. HIV self-testing can overcome concerns regarding confidentiality of provider-administered HTC and stigma associated with facility testing [55]. However, HIV self-testing is a screening tool and HIV positive results require confirmatory testing. Further research is needed on quality, accuracy, distribution channels, and linkage to care after HIV self-testing.
The literature on HIV testing strategies for MSM in SSA is very limited [10,58,59]. Studies show high HIV positivity combined with a high proportion of first-time testers, highlighting the need for service expansion. Successful testing strategies for MSM are community based (particularly mobile), as many MSM are marginalized without adequate access to conventional health systems [59]. Community HTC for MSM has higher acceptability and greater HIV yield than clinic referral [10]. In addition, HIV self-testing is a potential strategy to reach MSM; it is considered convenient and private [60]. Further, self-testing is useful for groups who benefit from frequent retesting [55].
Community-based facilitated linkage and retention strategies can increase male engagement in HIV care
The success of HTC strategies depends on their ability to link and engage HIV-positive individuals in care. Extra support may be needed to ensure linkage in community-based modalities, as they are conducted outside of health care systems [27]. Community HTC with facilitated linkage (e.g., counsellor follow-up) has achieved higher linkage than HTC without such support [27]. However, facility linkage rates after community HTC remain lower than what is needed for epidemic control [33,61]. Although community HTC reduces barriers to receiving an HIV test, individuals still must travel and wait at a clinic to obtain treatment. Community-based ART initiation is a convenient alternative to facility linkage. An RCT in Malawi found higher ART uptake with optional home ART initiation compared to facility initiation, with no difference in retention at 6 months [57]. Notably, a recent systematic review found no current or planned trials evaluating community-based ART initiation in men in SSA; likewise, there is a lack of interventions for MSM [62].
Similar to ART initiation, retention is improved with streamlined services. Interviews with persons who have dropped out of care find that lack of transportation is the most common reason for dropout, followed by lack of money and work commitments [21]. Distance from clinic has been negatively associated with ART retention [63]. Food insecurity and long wait times are also reported as barriers to ART pickup [50]. Community-based ART delivery (dispensing ART outside health care systems) may overcome these barriers and increase retention. Home, workplace, or mobile ART pickup sites can be particularly convenient for men. An ongoing RCT, Delivery Optimization for Antiretroviral Therapy (DO ART; ClinicalTrials.gov: NCT02929992), is investigating the effectiveness of community ART initiation and delivery on ART uptake, time to initiation, and viral suppression in South Africa and Uganda.
Similarly, the SEARCH hybrid mobile and home HTC campaign achieved high ART linkage in rural East Africa by “collapsing the cascade” or providing immediate universal treatment for all HIV-positive persons. The campaign offered participants ART visits every 3 months with flexible scheduling, viral load counseling, and treatment for other diseases using a stigma-reducing chronic care model. At baseline, fewer men than women were virally suppressed (44% versus 50%, respectively), but this gap decreased after 2 years of follow-up, with little difference in viral suppression by gender (80% of men and 83% of women) [34].
Since men are more likely than women to be lost to follow-up after testing HIV positive, peer support groups can encourage engagement in care. Studies show men can experience more stigma than women after an HIV diagnosis; they can feel that their masculinity is compromised by admitting they are sick and asking for help [64,65]. Fear of losing respect or being perceived as a failure for acquiring HIV is also a barrier [66]. Support groups and peer counselling can be difficult for men as they may be viewed as activities for women. Peer support groups for men should emphasize responsible fatherhood and skills training. Messaging that ART can allow men to regain their health, restore masculinity compromised by HIV, and provide for their families (particularly children) can be powerful motivators for engagement in care [64,65]. Receiving livelihood support (e.g., farm work, nutritional support, and school fee grants) from providers or support groups can motivate men to initiate and adhere to treatment and also restore masculinity by allowing them to provide for their family [65,66]. Livelihood interventions can also reduce the social stigma caused by an HIV diagnosis [67,68]. Additionally, support groups that challenge gender norms and stigma can change attitudes about masculinity and HIV and increase engagement in care [69]. Effective messages will likely vary by men’s age or the intervention setting. Although ART peer support groups have been successful in motivating engagement and retention, many groups are majority (65%–71%) female [70–72]. Qualitative research on male engagement and retention on ART is sparse.
Financial incentives can increase HIV testing and ART uptake by (1) providing a near-immediate reward for a health behavior and (2) offsetting costs associated with clinic attendance (travel and missed work) [73]. They can also reduce structural HIV risk factors by alleviating poverty and increasing access to education. A study in South Africa investigating incentivized compared to nonincentivized mobile testing for reaching men found higher HIV prevalence (16% versus 5.5%), more first-time testers (60% versus 42%), and more advanced HIV disease (15% versus 8%) in men accessing incentivized compared to nonincentivized services [74]. Fewer studies have examined the effects of cash transfers later in the HIV care cascade, although two trials are ongoing: Link4Health—a combination approach of accelerated ART initiation, counselling, and financial incentives for linkage and retention in Swaziland (ClinicalTrials.gov: NCT01904994)—and ENGAGE4HEALTH—evaluating accelerated ART initiation, short message service (SMS) reminders, and noncash financial incentives for retention and linkage for both sexes in Mozambique (ClinicalTrials.gov: NCT01930084) [73]. More research is needed on conditional and unconditional cash transfers and lottery systems for increasing male engagement in the care cascade. Additionally, some beneficial health behaviors are known to diminish after the cessation of cash transfers, so studies are needed to evaluate the long-term effects of financial incentives on ART adherence [73].
Linkage to care is challenging for MSM since health care workers in SSA typically have little or no training in addressing their health care needs [75]. Further, MSM report discrimination, harassment, and denial of services [76]. A study from Kenya found that providing health care workers with a web-based 2-day MSM sensitivity training decreased homophobic attitudes and increased knowledge of MSM-related health issues (maintained 3 months postintervention). Encouragingly, the strongest impact was seen in those with the most negative attitudes towards MSM [77]. However, such training is rare, and the health care workers who attended the training reported experiencing secondary stigma from other health care workers who were not trained [75]. Therefore, widespread structural interventions are needed to make a substantial impact on health care workers’ attitudes towards MSM. Further, little is known about ART adherence and clinical outcomes in HIV-positive MSM [75]. One of the few studies evaluating this topic found that ART adherence in Kenya was substantially lower in MSM compared to heterosexual men [78]. However, only one intervention to promote engagement in care and adherence to ART in HIV-positive MSM is currently being conducted in SSA [79]. Successful strategies will require multiple approaches including pre-exposure prophylaxis (PrEP), treatment as prevention, and behavioral risk reduction [80].
Conclusions
More work remains to be done to reduce the gender disparity in HIV testing, linkage, and retention in care in SSA. Multicomponent interventions are needed to reduce stigma and address issues of masculinity and health-seeking behavior. Additionally, leveraging social support and providing poverty alleviation can change norms around testing and treatment while addressing other factors contributing to low engagement in care (e.g., food insecurity and poverty). Community-based testing, and potentially community-based ART initiation and medication resupply, can overcome barriers associated with clinics and strengthen male engagement across the care cascade. A variety of community HTC modalities can be implemented simultaneously to achieve maximum coverage. Targeted messaging to motivate men (e.g., protection of one’s sexual partner and future children, and restoration of health through ART) can increase testing uptake and linkage [81]. Community-based “test and treat” strategies may reduce loss to follow-up associated with clinic-based ART initiation. Further, an integrated approach combining testing and treatment with other HIV interventions (VMMC and PrEP) and chronic disease screening can increase intervention program efficiency while reducing stigma. Further research is needed on community-based interventions that motivate male engagement in care, particularly later in the cascade (i.e., ART initiation and retention).
Zdroje
1. Mills EJ, Beyrer C, Birungi J, Dybul MR. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med. 2012;9(2):e1001167. doi: 10.1371/journal.pmed.1001167 22346735
2. Nsanzimana S, Remera E, Kanters S, Chan K, Forrest JI, Ford N, et al. Life expectancy among HIV-positive patients in Rwanda: a retrospective observational cohort study. Lancet Glob Health. 2015;3(3):e169–77. doi: 10.1016/S2214-109X(14)70364-X 25701995
3. Geng EH, Bwana MB, Muyindike W, Glidden DV, Bangsberg DR, Neilands TB, et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr. 2013;63(2):e64–71. doi: 10.1097/QAI.0b013e31828af5a6 23429504
4. Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10(4):e1001418. doi: 10.1371/journal.pmed.1001418 23585736
5. Tsai AC, Siedner MJ. The Missing Men: HIV Treatment Scale-Up and Life Expectancy in Sub-Saharan Africa. PLoS Med. 2015;12(11):e1001906. doi: 10.1371/journal.pmed.1001906 26599825
6. Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–8. doi: 10.1016/S0140-6736(05)67481-8 16198767
7. WHO. Global Update on the health sector response to HIV, 2014. http://apps.who.int/iris/bitstream/10665/128494/1/9789241507585_eng.pdf?ua=1. Accessed: 10/16/2016.
8. UNAIDs. Kenya AIDS Response Progress Report, 2014. http://www.unaids.org/sites/default/files/country/documents/KEN_narrative_report_2014.pdf Accessed: 1/20/2017.
9. Wheeler T, Wolf RC, Kapesa L, Cheng Surdo A, Dallabetta G. Scaling-up HIV responses with key populations in West Africa. J Acquir Immune Defic Syndr. 2015;68 Suppl 2:S69–73.
10. Adebajo S, Eluwa G, Njab J, Oginni A, Ukwuije F, Ahonsi B, et al. Evaluating the effect of HIV prevention strategies on uptake of HIV counselling and testing among male most-at-risk-populations in Nigeria; a cross-sectional analysis. Sex Transm Infect. 2015 Dec. 91(8):555–60. doi: 10.1136/sextrans-2014-051659 25921019
11. Duvall S, Irani L, Compaore C, Sanon P, Bassonon D, Anato S, et al. Assessment of policy and access to HIV prevention, care, and treatment services for men who have sex with men and for sex workers in Burkina Faso and Togo. J Acquir Immune Defic Syndr. 2015;68 Suppl 2:S189–97.
12. Tsai AC, Bangsberg DR, Weiser SD. Harnessing poverty alleviation to reduce the stigma of HIV in Sub-Saharan Africa. PLoS Med. 2013;10(11):e1001557. doi: 10.1371/journal.pmed.1001557 24319400
13. Leichliter JS, Paz-Bailey G, Friedman AL, Habel MA, Vezi A, Sello M, et al. 'Clinics aren't meant for men': sexual health care access and seeking behaviours among men in Gauteng province, South Africa. SAHARA J. 2011;8(2):82–8. doi: 10.1080/17290376.2011.9724989 23237685
14. Manda S, Masenyetse L, Cai B, Meyer R. Mapping HIV prevalence using population and antenatal sentinel-based HIV surveys: a multi-stage approach. Popul Health Metr. 2015;13 : 22. doi: 10.1186/s12963-015-0055-z 26336361
15. Hogan DR, Salomon JA, Canning D, Hammitt JK, Zaslavsky AM, Barnighausen T. National HIV prevalence estimates for sub-Saharan Africa: controlling selection bias with Heckman-type selection models. Sex Transm Infect. 2012;88 Suppl 2:i17–23.
16. Barnighausen T, Bor J, Wandira-Kazibwe S, Canning D. Correcting HIV prevalence estimates for survey nonparticipation using Heckman-type selection models. Epidemiology. 2011;22(1):27–35. doi: 10.1097/EDE.0b013e3181ffa201 21150352
17. McGovern ME, Marra G, Radice R, Canning D, Newell ML, Barnighausen T. Adjusting HIV prevalence estimates for non-participation: an application to demographic surveillance. J Int AIDS Soc. 2015;18(1):19954.
18. Clark SJ, Houle B. Validation, replication, and sensitivity testing of Heckman-type selection models to adjust estimates of HIV prevalence. PLoS ONE. 2014;9(11):e112563. doi: 10.1371/journal.pone.0112563 25402333
19. Bicego GT, Nkambule R, Peterson I, Reed J, Donnell D, Ginindza H, et al. Recent patterns in population-based HIV prevalence in Swaziland. PLoS ONE. 2013;8(10):e77101. doi: 10.1371/journal.pone.0077101 24143205
20. Eaton JW, Bacaer N, Bershteyn A, Cambiano V, Cori A, Dorrington RE, et al. Assessment of epidemic projections using recent HIV survey data in South Africa: a validation analysis of ten mathematical models of HIV epidemiology in the antiretroviral therapy era. Lancet Glob Health. 2015;3(10):e598–608. doi: 10.1016/S2214-109X(15)00080-7 26385301
21. Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53(3):405–11. doi: 10.1097/QAI.0b013e3181b843f0 19745753
22. Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, et al. Mass HIV Treatment and Sex Disparities in Life Expectancy: Demographic Surveillance in Rural South Africa. PLoS Med. 2015;12(11):e1001905. doi: 10.1371/journal.pmed.1001905 26599699
23. Leblanc NM, Andes KL. An exploration of men's knowledge, attitudes, and perceptions of HIV, HIV risk, and willingness to test for HIV in Yendi District, Northern Ghana. J Assoc Nurses AIDS Care. 2015;26(3):281–95. doi: 10.1016/j.jana.2014.09.006 25456835
24. Osoti AO, John-Stewart G, Kiarie JN, Barbra R, Kinuthia J, Krakowiak D, et al. Home-based HIV testing for men preferred over clinic-based testing by pregnant women and their male partners, a nested cross-sectional study. BMC Infect Dis. 2015;15 : 298. doi: 10.1186/s12879-015-1053-2 26223540
25. Ostermann J, Njau B, Brown DS, Muhlbacher A, Thielman N. Heterogeneous HIV testing preferences in an urban setting in Tanzania: results from a discrete choice experiment. PLoS ONE. 2014;9(3):e92100. doi: 10.1371/journal.pone.0092100 24643047
26. Hensen B, Taoka S, Lewis JJ, Weiss HA, Hargreaves J. Systematic review of strategies to increase men's HIV-testing in sub-Saharan Africa. AIDS. 2014;28(14):2133–45. doi: 10.1097/QAD.0000000000000395 25062091
27. Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77–85. doi: 10.1038/nature16044 26633769
28. Suthar AB, Ford N, Bachanas PJ, Wong VJ, Rajan JS, Saltzman AK, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10(8):e1001496. doi: 10.1371/journal.pmed.1001496 23966838
29. Henley C, Forgwei G, Welty T, Golden M, Adimora A, Shields R, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis. 2013;40(12):909–14. doi: 10.1097/OLQ.0000000000000032 24220349
30. Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56(5):437–42. 22046601
31. Cherutich P, Golden MR, Wamuti B, Richardson BA, Asbjornsdottir KH, Otieno FA, et al. Assisted partner services for HIV in Kenya: a cluster randomised controlled trial. Lancet HIV. 2017 Feb;4(2):e74–e82. doi: 10.1016/S2352-3018(16)30214-4 27913227
32. Coates TJ, Kulich M, Celentano DD, Zelaya CE, Chariyalertsak S, Chingono A, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014;2(5):e267–77. doi: 10.1016/S2214-109X(14)70032-4 25103167
33. Iwuji CC, Orne-Gliemann J, Larmarange J, Okesola N, Tanser F, Thiebaut R, et al. Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial. PLoS Med. 2016;13(8):e1002107. doi: 10.1371/journal.pmed.1002107 27504637
34. Petersen M. SEARCH test and treat study in Uganda and Kenya exceeds the UNAIDS 90-90-90 cascade target by achieving 81% population-level viral suppression after 2 years. [Oral] AIDS 2016 conference. Durban, South Africa. 7/20/2016.
35. Novitsky V, Bussmann H, Okui L, Logan A, Moyo S, van Widenfelt E, et al. Estimated age and gender profile of individuals missed by a home-based HIV testing and counselling campaign in a Botswana community. J Int AIDS Soc. 2015;18 : 19918. doi: 10.7448/IAS.18.1.19918 26028155
36. Corbett EL, Dauya E, Matambo R, Cheung YB, Makamure B, Bassett MT, et al. Uptake of workplace HIV counselling and testing: a cluster-randomised trial in Zimbabwe. PLoS Med. 2006;3(7):e238. doi: 10.1371/journal.pmed.0030238 16796402
37. Sweat M, Morin S, Celentano D, Mulawa M, Singh B, Mbwambo J, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis. 2011;11(7):525–32. doi: 10.1016/S1473-3099(11)70060-3 21546309
38. Chamie G, Clark TD, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, et al. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV. 2016;3(3):e111–9. doi: 10.1016/S2352-3018(15)00251-9 26939734
39. Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Uptake of community-based HIV testing during a multi-disease health campaign in rural Uganda. PLoS ONE. 2014;9(1):e84317. doi: 10.1371/journal.pone.0084317 24392124
40. Lugada E, Millar D, Haskew J, Grabowsky M, Garg N, Vestergaard M, et al. Rapid implementation of an integrated large-scale HIV counseling and testing, malaria, and diarrhea prevention campaign in rural Kenya. PLoS ONE. 2010;5(8):e12435. doi: 10.1371/journal.pone.0012435 20865049
41. Mak J, Mayhew SH, von Maercker A, Colombini M. Men. Sex Health. 2016 Jun;13(3):265–74. doi: 10.1071/SH15244 27028455
42. Matthews LT, Crankshaw T, Giddy J, Kaida A, Smit JA, Ware NC, et al. Reproductive decision-making and periconception practices among HIV-positive men and women attending HIV services in Durban, South Africa. AIDS Behav. 2013;17(2):461–70. doi: 10.1007/s10461-011-0068-y 22038045
43. Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011;56(1):76–82. doi: 10.1097/QAI.0b013e3181fdb4c4 21084999
44. van den Berg W, Brittain K, Mercer G, Peacock D, Stinson K, Janson H, et al. Improving men's participation in preventing mother-to-child transmission of HIV as a maternal, neonatal, and child health priority in South Africa. PLoS Med. 2015;12(4):e1001811. doi: 10.1371/journal.pmed.1001811 25849433
45. Krakowiak D, Kinuthia J, Osoti AO, Asila V, Gone MA, Mark J, et al. Home-Based HIV Testing Among Pregnant Couples Increases Partner Testing and Identification of Serodiscordant Partnerships. J Acquir Immune Defic Syndr. 2016;72 Suppl 2:S167–73.
46. Masters SH, Agot K, Obonyo B, Napierala Mavedzenge S, Maman S, Thirumurthy H. Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial. PLoS Med. 2016;13(11):e1002166. doi: 10.1371/journal.pmed.1002166 27824882
47. Darbes L, McGrath NM, Johnson MO, Hosegood V, Fritz K, Ngubane T, et al. Positive impact of a randomized controlled trial of the Uthando Lwethu ("Our Love") intervention on rates of couples HIV testing in rural South Africa. [Oral]. AIDS 2016. Durban, South Africa.
48. Kyegombe N, Abramsky T, Devries KM, Starmann E, Michau L, Nakuti J, et al. The impact of SASA!, a community mobilization intervention, on reported HIV-related risk behaviours and relationship dynamics in Kampala, Uganda. J Int AIDS Soc. 2014;17 : 19232. doi: 10.7448/IAS.17.1.19232 25377588
49. Yamanis TJ, Dervisevic E, Mulawa M, Conserve DF, Barrington C, Kajula LJ, et al. Social Network Influence on HIV Testing Among Urban Men in Tanzania. AIDS Behav. 2016 Aug 9.
50. Conroy A, Leddy A, Johnson M, Ngubane T, van Rooyen H, Darbes L. “I told her this is your life”: how primary partners help overcome barriers related to ART adherence in South Africa. [Oral]. AIDS 2016, Durban, South Africa.
51. Rotheram-Borus MJ, Tomlinson M, Durkin A, Baird K, DeCelles J, Swendeman D. Feasibility of Using Soccer and Job Training to Prevent Drug Abuse and HIV. AIDS Behav. 2016 Sep;20(9):1841–50. doi: 10.1007/s10461-015-1262-0 26837624
52. Jemmott JB 3rd, Jemmott LS, O'Leary A, Ngwane Z, Icard LD, Heeren GA, et al. Cluster-randomized controlled trial of an HIV/sexually transmitted infection risk-reduction intervention for South African men. Am J Public Health. 2014;104(3):467–73. doi: 10.2105/AJPH.2013.301578 24432923
53. Jewkes R, Nduna M, Levin J, Jama N, Dunkle K, Puren A, et al. Impact of stepping stones on incidence of HIV and HSV-2 and sexual behaviour in rural South Africa: cluster randomised controlled trial. BMJ. 2008;337:a506. doi: 10.1136/bmj.a506 18687720
54. Wagman JA, Gray RH, Campbell JC, Thoma M, Ndyanabo A, Ssekasanvu J, et al. Effectiveness of an integrated intimate partner violence and HIV prevention intervention in Rakai, Uganda: analysis of an intervention in an existing cluster randomised cohort. Lancet Glob Health. 2015;3(1):e23–33. doi: 10.1016/S2214-109X(14)70344-4 25539966
55. Choko AT, Desmond N, Webb EL, Chavula K, Napierala-Mavedzenge S, Gaydos CA, et al. The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. PLoS Med. 2011;8(10):e1001102. doi: 10.1371/journal.pmed.1001102 21990966
56. Kurth AE, Lally MA, Choko AT, Inwani IW, Fortenberry JD. HIV testing and linkage to services for youth. J Int AIDS Soc. 2015;18(2 Suppl 1):19433.
57. MacPherson P, Lalloo DG, Webb EL, Maheswaran H, Choko AT, Makombe SD, et al. Effect of optional home initiation of HIV care following HIV self-testing on antiretroviral therapy initiation among adults in Malawi: A randomized clinical trial. JAMA. 2014;312(4):372–9. doi: 10.1001/jama.2014.6493 25038356
58. Mine M, Chishala S, Makhaola K, Tafuma TA, Bolebantswe J, Merrigan MB. Performance of rapid HIV testing by lay counselors in the field during the behavioral and biological surveillance survey among female sex workers and men who have sex with men in Botswana. J Acquir Immune Defic Syndr. 2015;68(3):365–8. doi: 10.1097/QAI.0000000000000434 25394190
59. Mulongo S, Kapila G, Hatton T, Canagasabey D, Arney J, Kazadi T, et al. Applying innovative approaches for reaching men who have sex with men and female sex workers in the Democratic Republic of Congo. J Acquir Immune Defic Syndr. 2015;68 Suppl 2:S248–51.
60. Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and Acceptability on HIV Self-testing Among Key Populations: A Literature Review. AIDS Behav. 2015;19(11):1949–65. doi: 10.1007/s10461-015-1097-8 26054390
61. Genberg BL, Naanyu V, Wachira J, Hogan JW, Sang E, Nyambura M, et al. Linkage to and engagement in HIV care in western Kenya: an observational study using population-based estimates from home-based counselling and testing. Lancet HIV. 2015;2(1):e20–e26. doi: 10.1016/S2352-3018(14)00034-4 25621303
62. Govindasamy D, Meghij J, Kebede Negussi E, Clare Baggaley R, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low - and middle-income settings—a systematic review. J Int AIDS Soc. 2014;17 : 19032. doi: 10.7448/IAS.17.1.19032 25095831
63. Geng EH, Glidden DV, Bwana MB, Musinguzi N, Emenyonu N, Muyindike W, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS ONE. 2011;6(7):e21797. doi: 10.1371/journal.pone.0021797 21818265
64. Mburu G, Ram M, Siu G, Bitira D, Skovdal M, Holland P. Intersectionality of HIV stigma and masculinity in eastern Uganda: implications for involving men in HIV programmes. BMC Public Health. 2014 Oct 11;14 : 1061. doi: 10.1186/1471-2458-14-1061 25304035
65. Siu GE, Seeley J, Wight D. Dividuality, masculine respectability and reputation: how masculinity affects men's uptake of HIV treatment in rural eastern Uganda. Soc Sci Med. 2013;89 : 45–52. doi: 10.1016/j.socscimed.2013.04.025 23726215
66. Skovdal M, Campbell C, Madanhire C, Mupambireyi Z, Nyamukapa C, Gregson S. Masculinity as a barrier to men's use of HIV services in Zimbabwe. Global Health. 2011;7 : 13. doi: 10.1186/1744-8603-7-13 21575149
67. Tsai AC, Hatcher AM, Bukusi EA, Weke E, Lemus Hufstedler L, Dworkin SL, et al. A Livelihood Intervention to Reduce the Stigma of HIV in Rural Kenya: Longitudinal Qualitative Study. AIDS Behav. 2017 Jan;21(1):248–260. doi: 10.1007/s10461-015-1285-6 26767535
68. Kakuhikire B, Suquillo D, Atuhumuza E, Mushavi R, Perkins JM, Venkataramani AS, et al. A livelihood intervention to improve economic and psychosocial well-being in rural Uganda: Longitudinal pilot study. SAHARA J. 2016;13(1):162–9. doi: 10.1080/17290376.2016.1230072 27619011
69. Colvin C, Robins S. Positive Men in Hard, Neoliberal Times: Engendering Health Citizenship in South Africa. In Gender and HIV/AIDS: critical perspectives from the developing world. Edited by: Boesten J, Poku N. Farnham: Ashgate Publishing Limited; 2009 : 177–190.
70. Grimsrud A, Lesosky M, Kalombo C, Bekker LG, Myer L. Community-based Adherence Clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: a cohort study. J Acquir Immune Defic Syndr. 2016 Jan 1;71(1):e16–23. doi: 10.1097/QAI.0000000000000863 26473798
71. Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS ONE. 2010;5(6):e10923. doi: 10.1371/journal.pone.0010923 20532194
72. Chang LW, Nakigozi G, Billioux VG, Gray RH, Serwadda D, Quinn TC, et al. Effectiveness of peer support on care engagement and preventive care intervention utilization among pre-antiretroviral therapy, HIV-infected adults in Rakai, Uganda: a randomized trial. AIDS Behav. 2015;19(10):1742–51. doi: 10.1007/s10461-015-1159-y 26271815
73. Bassett IV, Wilson D, Taaffe J, Freedberg KA. Financial incentives to improve progression through the HIV treatment cascade. Curr Opin HIV AIDS. 2015;10(6):451–63. doi: 10.1097/COH.0000000000000196 26371461
74. Nglazi MD, van Schaik N, Kranzer K, Lawn SD, Wood R, Bekker LG. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2012;59(3):e28–34. doi: 10.1097/QAI.0b013e31824445f0 22173039
75. Dijkstra M, van der Elst EM, Micheni M, Gichuru E, Musyoki H, Duby Z, et al. Emerging themes for sensitivity training modules of African healthcare workers attending to men who have sex with men: a systematic review. Int Health. 2015;7(3):151–62. doi: 10.1093/inthealth/ihu101 25596188
76. van der Elst EM, Gichuru E, Omar A, Kanungi J, Duby Z, Midoun M, et al. Experiences of Kenyan healthcare workers providing services to men who have sex with men: qualitative findings from a sensitivity training programme. J Int AIDS Soc. 2013;16 Suppl 3 : 18741.
77. van der Elst EM, Smith AD, Gichuru E, Wahome E, Musyoki H, Muraguri N, et al. Men who have sex with men sensitivity training reduces homoprejudice and increases knowledge among Kenyan healthcare providers in coastal Kenya. J Int AIDS Soc 2013;16 Suppl 3 : 18748.
78. Charurat ME, Emmanuel B, Akolo C, Keshinro B, Nowak RG, Kennedy S, et al. Uptake of treatment as prevention for HIV and continuum of care among HIV-positive men who have sex with men in Nigeria. J Acquir Immune Defic Syndr. 2015;68 Suppl 2:S114–23.
79. Graham SM, Micheni M, Kombo B, Van Der Elst EM, Mugo PM, Kivaya E, et al. Development and pilot testing of an intervention to promote care engagement and adherence among HIV-positive Kenyan MSM. AIDS. 2015;29 Suppl 3:S241–9.
80. Sanders EJ, Jaffe H, Musyoki H, Muraguri N, Graham SM. Kenyan MSM: no longer a hidden population. AIDS. 2015;29 Suppl 3:S195–9.
81. Dageid W, Govender K, Gordon SF. Masculinity and HIV disclosure among heterosexual South African men: implications for HIV/AIDS intervention. Cult Health Sex. 2012;14(8):925–40. doi: 10.1080/13691058.2012.710337 22943462
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 4- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Implementation science: Relevance in the real world without sacrificing rigor
- Clinical decision tools are needed to identify HIV-positive patients at high risk for poor outcomes after initiation of antiretroviral therapy
- Silk garments plus standard care compared with standard care for treating eczema in children: A randomised, controlled, observer-blind, pragmatic trial (CLOTHES Trial)
- An open source pharma roadmap
- A new cascade of HIV care for the era of “treat all”
- Status and methodology of publicly available national HIV care continua and 90-90-90 targets: A systematic review
- Talking sensibly about depression
- Governing multisectoral action for health in low- and middle-income countries
- Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial
- Community-based strategies to strengthen men’s engagement in the HIV care cascade in sub-Saharan Africa
- Graft-derived cell-free DNA, a noninvasive early rejection and graft damage marker in liver transplantation: A prospective, observational, multicenter cohort study
- Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study
- Fresh fruit consumption in relation to incident diabetes and diabetic vascular complications: A 7-y prospective study of 0.5 million Chinese adults
- The effects of implementing a point-of-care electronic template to prompt routine anxiety and depression screening in patients consulting for osteoarthritis (the Primary Care Osteoarthritis Trial): A cluster randomised trial in primary care
- Identification of genes associated with dissociation of cognitive performance and neuropathological burden: Multistep analysis of genetic, epigenetic, and transcriptional data
- Demographic transition and the dynamics of measles in six provinces in China: A modeling study
- Changes in prices, sales, consumer spending, and beverage consumption one year after a tax on sugar-sweetened beverages in Berkeley, California, US: A before-and-after study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Silk garments plus standard care compared with standard care for treating eczema in children: A randomised, controlled, observer-blind, pragmatic trial (CLOTHES Trial)
- Talking sensibly about depression
- A new cascade of HIV care for the era of “treat all”
- Fresh fruit consumption in relation to incident diabetes and diabetic vascular complications: A 7-y prospective study of 0.5 million Chinese adults
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



