-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
The zebrafish adult pigment pattern has emerged as a useful model for understanding the development and evolution of adult form as well as pattern-forming mechanisms more generally. In this species, a series of horizontal melanophore stripes arises during the larval-to-adult transformation, but the genetic and cellular bases for stripe formation remain largely unknown. Here, we show that the seurat mutant phenotype, consisting of an irregular spotted pattern, arises from lesions in the gene encoding Immunoglobulin superfamily member 11 (Igsf11). We find that Igsf11 is expressed by melanophores and their precursors, and we demonstrate by cell transplantation and genetic rescue that igsf11 functions autonomously to this lineage in promoting adult stripe development. Further analyses of cell behaviors in vitro, in vivo, and in explant cultures ex vivo demonstrate that Igsf11 mediates adhesive interactions and that mutants for igsf11 exhibit defects in both the migration and survival of melanophores and their precursors. These findings identify the first in vivo requirements for igsf11 as well as the first instance of an immunoglobulin superfamily member functioning in pigment cell development and patterning. Our results provide new insights into adult pigment pattern morphogenesis and how cellular interactions mediate pattern formation.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002899
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002899Summary
The zebrafish adult pigment pattern has emerged as a useful model for understanding the development and evolution of adult form as well as pattern-forming mechanisms more generally. In this species, a series of horizontal melanophore stripes arises during the larval-to-adult transformation, but the genetic and cellular bases for stripe formation remain largely unknown. Here, we show that the seurat mutant phenotype, consisting of an irregular spotted pattern, arises from lesions in the gene encoding Immunoglobulin superfamily member 11 (Igsf11). We find that Igsf11 is expressed by melanophores and their precursors, and we demonstrate by cell transplantation and genetic rescue that igsf11 functions autonomously to this lineage in promoting adult stripe development. Further analyses of cell behaviors in vitro, in vivo, and in explant cultures ex vivo demonstrate that Igsf11 mediates adhesive interactions and that mutants for igsf11 exhibit defects in both the migration and survival of melanophores and their precursors. These findings identify the first in vivo requirements for igsf11 as well as the first instance of an immunoglobulin superfamily member functioning in pigment cell development and patterning. Our results provide new insights into adult pigment pattern morphogenesis and how cellular interactions mediate pattern formation.
Introduction
Pigment patterns are among the most striking of vertebrate traits and nowhere are these patterns more diverse than in teleost fishes [1]–[4]. In this group, a stunning array of pigment patterns function in predation avoidance, shoaling, and mate choice and are thought to have played important roles in speciation [5]–[8]. Among teleosts, the zebrafish Danio rerio has emerged as a useful model organism for uncovering the genetic and cellular bases of pigment pattern development.
The zebrafish adult pigment pattern comprises a series of dark horizontal stripes that include black melanophores, alternating with lighter “interstripes” that include yellow–orange xanthophores; a third class of pigment cells, the iridescent iridophore occurs in both stripes and interstripes. Development of this pattern occurs during the larval-to-adult transformation between ∼2–4 weeks post-fertilization [9]–[12]. At this time, latent precursor cells of presumptive neural crest origin migrate from peripheral nerves and possibly other locations to the hypodermis, between the epidermis and the myotome, where differentiation occurs and the initially intermingled cells organize into stripes [10], [13].
Mutational analyses have identified several loci that are required for the development of adult melanophores [9], [14]–[17], xanthophores [18], and iridophores [19], [20], and these and other approaches have revealed important roles for cellular interactions, particularly between melanophores and xanthophores, in organizing the adult stripe pattern [21]–[24]. Remarkably, these interactions meet the predictions of Turing models of pattern formation that rely on dynamics driven by processes of reaction diffusion with lateral inhibition [25], [26]. Nevertheless, the molecular mechanisms that drive cellular behaviors during stripe formation have remained obscure.
Of particular interest for understanding the genetic mechanisms and cellular behaviors underlying stripe formation are mutants that retain all three classes of pigment cells while nevertheless developing abnormal adult pigment patterns. To date, two such mutants have been analyzed most extensively. The jaguar mutant exhibits fewer stripes than wild-type fish and, within these stripes, melanophores and xanthophores are intermingled [27]–[29]. The jaguar phenotype arises from mutations in kir7.1, encoding an inwardly rectifying potassium channel, expressed and required by cells of the melanophore lineage [28], [30]. By contrast, the leopard mutant [9], [12], [22], [29] exhibits spots rather than stripes of melanophores, a defect arising from mutations in the gap junction gene, connexin41.8, which is expressed by melanophores and xanthophores [31]. The presumed functions of both gene products raised the possibility that physiological ion fluxes contribute to pattern formation; indeed wild-type, but not jaguar (kir7.1) mutant melanophores depolarize as a result of contacts with xanthophores in vitro [28]. Nevertheless, it has remained unclear to what extent genes classically known to regulate other morphogenetic processes are required specifically during pigment stripe formation.
In this study, we analyze the seurat mutant phenotype, consisting of an irregular spotting pattern similar to that of the leopard mutant. We chose the seurat mutant because, unlike some adult pigment mutants [19], [32], [33], defects are found in both body and fin pigment patterns, suggesting the affected locus may function normally in a core aspect of pattern formation. We show that seurat corresponds to immunoglobulin superfamily member 11 (igsf11), encoding a cell surface receptor containing two immunoglobulin-like domains. We find that igsf11 is expressed by the melanophore lineage, promotes the migration and survival of these cells during adult stripe development, and mediates adhesive interactions in vitro. Our results are the first demonstration of igsf11 functions in vivo, and, more generally, are the first to implicate a major family of “classical” cell adhesion molecule in adult pigment stripe formation. In turn, these findings set the stage for future investigations into how physiological and morphogenetic mechanisms affecting cell migration and survival interact to generate the adult pigment phenotype of zebrafish and other teleosts.
Results
seurat requirement for patterning adult melanophores
We isolated the recessive, homozygous viable allele seuratutr15e1 from the inbred ABwp genetic background during a forward genetic screen for ENU-induced mutations affecting adult pigment pattern development. In comparison to the wild-type, seurat homozygotes develop fewer adult melanophores, which form irregular spots rather than stripes (Figure 1A, 1B); embryonic and early larval pigment patterns are indistinguishable between wild-type and seurat mutants (not shown). We isolated two additional ENU-induced alleles, seuratwp15e2 and seuratwp15e3, in the wik genetic background by non-complementation screening against seuratutr15e1. These additional alleles were phenotypically indistinguishable from one another and exhibited less severe phenotypes than seuratutr15e1 (Figure 1C; Figure S1). Gross deficiencies in xanthophore or iridophore numbers were not apparent. For all phenotypic analyses below, we used the stronger allele, seuratutr15e1 (hereafter seurat).
Fig. 1. Defective adult pigment stripes in seurat mutants. 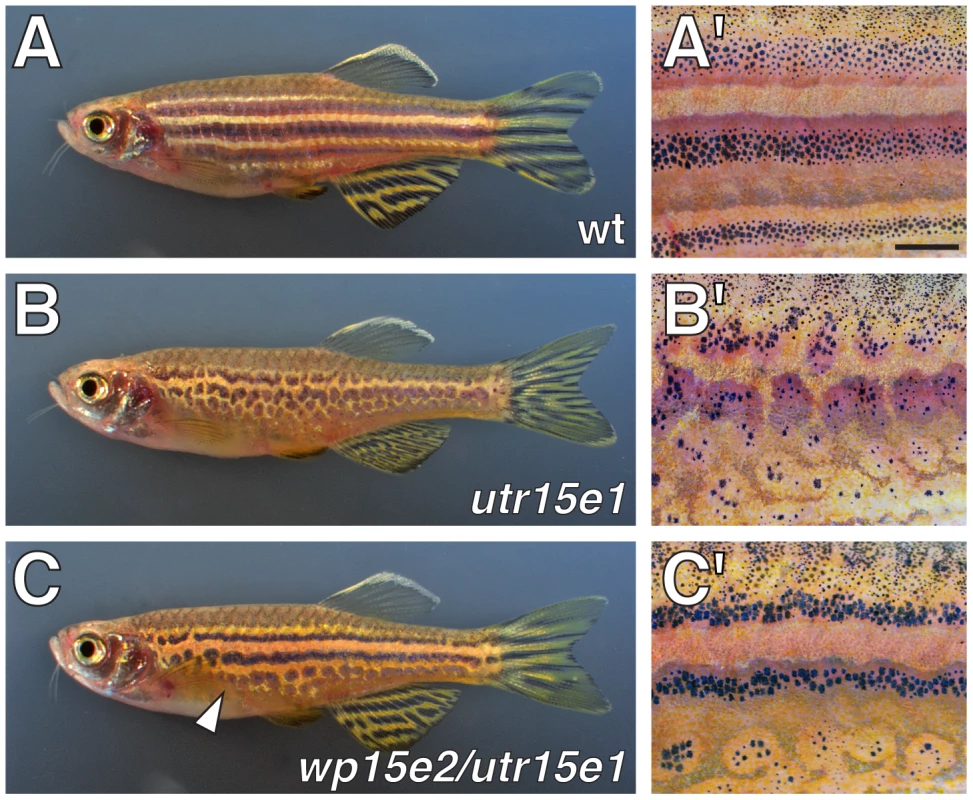
Shown are adult fish (A, B, C) and details of patterns (A′, B′, C′). (A, A′) Wild-type fish exhibit several dark stripes of melanophores and iridophores, as well as light “interstripes” of xanthophores and iridophores. (B, B′) Homozygous seuratutr15e1 mutants exhibit irregular spots of melanophores. (C, C′) seuratwp15e2/seuratutr15e1 fish exhibit a less severe stripe defect, most evident ventrally (arrowhead). seuratwp15e3/seuratutr15e1 exhibit a phenotype indistinguishable from seuratwp15e2/seuratutr15e1. At this stage, seuratwp15e2 and seuratwp15e3 are nearly indistinguishable from the wild-type when homozygous, though phenotypes are more apparent during the initial stages of stripe formation (not shown). Scale bar: in (A′), 1 mm for (A′–C′). Genetic mosaic analyses reveal a melanophore-autonomous role for seurat in stripe development
To test if seurat acts autonomously to the melanophore lineage in promoting adult pigment stripe formation, we transplanted cells at the blastula stage from phenotypically wild-type Tg(bactin:GFP) embryos to homozygous seurat mutant embryos and reared the resulting chimeras until adult pigment patterns had formed. If seurat acts within the melanophore lineage, we anticipated that wild-type (GFP+) melanophores would form patches more organized than the irregular spots formed by seurat mutant melanophores; regions of rescued pattern should include wild-type (GFP+) melanophores but also might include seurat mutant (GFP−) melanophores, some of which develop where stripes would normally form (Figure 1B and see below). Consistent with these predictions, we found that wild-type→seurat mutant chimeras in which wild-type melanophores developed exhibited large spots or rescued stripes, comprising both wild-type (GFP+) melanophores as well as some seurat mutant (GFP−) melanophores (Figure 2). We did not observe these organized patches of melanophores in chimeras that failed to develop wild-type melanophores despite the presence of wild-type epidermis, iridophores, or nerves; we did not observe chimeras that developed donor xanthophores.
Fig. 2. seurat is required autonomously to the melanophore lineage. 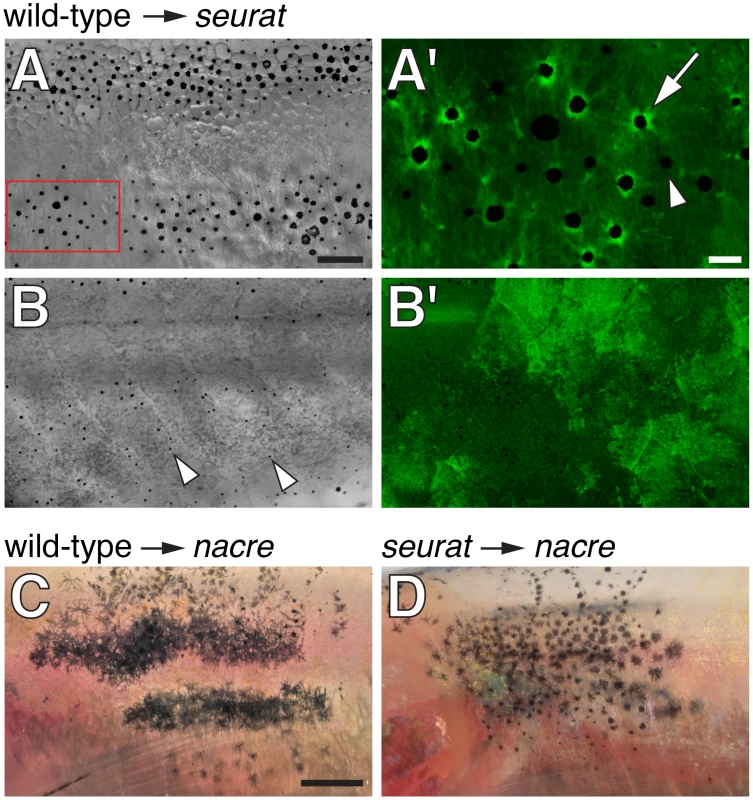
(A, B) Wild-type Tg(bactin:GFP) cells transplanted to seurat mutant hosts. Fish shown are juveniles (∼13 mm standardized standard length, SSL [11]) and were treated just prior to imaging with epinephrine, which contracts melanosomes towards cell bodies, thereby facilitating the detection of GFP fluorescence. (A) Chimeras that developed wild-type melanophores exhibited patches of restored stripes (n = 6). (A′) Detail of boxed region in A, showing GFP+ melanophores (e.g., arrow), as well as occasional GFP−, presumptive seurat mutant melanophores (e.g., arrowhead). (B) Chimeras in which wild-type melanophores failed to develop exhibited a seurat mutant pattern of dispersed melanophores (arrowheads; n>100). In the example shown here, wild-type GFP+ cells developed as epidermis (B′; shown at same magnification as B). (C) When wild-type melanophores differentiated in a nacre mutant background, patches of normal stripes developed (n = 3; [16]). (D) By contrast, when seurat mutant melanophores differentiated in nacre hosts, these cells retained a dispersed pattern, as in the seurat mutant (n = 8), indicating a failure of xanthophores, iridophores, or other cell types to rescue melanophore stripe organization. In additional experiments, in which nacre; Tg(bactin:GFP) cells were transplanted to seurat mutant hosts, the differentiation of nacre- GFP+ (seurat+) iridophores likewise failed to rescue melanophore stripes in the seurat mutant background (donor xanthophores did not develop in these chimeras; data not shown). Scale bars: in (A) 100 µm for (A,B,B′); in (A′) 20 µm for (A′); in (C) 500 µm for (C,D). To further assess the cell autonomy of seurat activities, we transplanted wild-type or seurat mutant cells to nacrew2 mutant embryos. nacre mutants fail to develop melanophores owing to a mutation in the mitfa transcription factor, which is required autonomously for specifying melanophore fate [15]. Any melanophores developing in these chimeras are thus donor-derived [16]. nacre mutants do, however, develop xanthophores and iridophores [27]. If seurat acts autonomously to the melanophore lineage, wild-type melanophores should form stripes in the nacre mutant background, whereas seurat mutant melanophores should fail to do so. Alternatively, if seurat effects on melanophore organization are non-autonomous, perhaps acting via xanthophores or another cell type, then both wild-type and seurat mutant melanophores should organize into stripes in the nacre mutant background [16], [21]. Phenotypes of seurat mutant→nacre mutant chimeras support an autonomous role for seurat within the melanophore lineage, as donor, seurat mutant melanophores failed to organize into stripes and instead developed in dispersed patterns, as in the seurat mutant (Figure 2C, 2D). Together these data support a model in which seurat acts within melanophores or their precursors to promote the organization of these cells into stripes.
seurat corresponds to immunoglobulin superfamily member 11
To identify the gene affected in seurat mutants, we mapped the mutant phenotype to a telomeric region of chromosome 15 between microsatellite markers Z10193 (45.6 Mb) and Z8551 (46.5 Mb) (Figure 3A). Fine-mapping using single nucleotide polymorphisms (S1, S2, S3, S4) within this region revealed a critical genetic interval containing six complete or partial open readings frames. By sequencing exons and cDNAs of each locus and comparing resulting sequences to pre-mutagenized ABwp and wik genetic backgrounds, as well as single nucleotide polymorphisms in the Ensembl database, we identified novel, ENU-induced lesions in immunoglobulin superfamily member 11 (igsf11; sc:d812) in each of the three seurat alleles (GenBank accession number JQ796184), and found no such lesions in the other candidate genes within this interval. Analyses of the inferred, 442 amino acid Igsf11 peptide sequence revealed a signal sequence, two immunoglobulin-like domains, a transmembrane domain, and a cytoplasmic domain (Figure 3B). The zebrafish peptide sequence exhibited 64% identity and 77% similarity to human IGSF11. In seuratutr15e1 a T→C transition leads to a substitution, S151P, located within the second immunoglobulin domain (Figure 3B; Figure S2). Mutations in the weaker alleles, seuratwp15e2 (T29P) and seuratwp15e3 (V28E) were found at the boundary between the predicted signal sequence and the beginning of the first immunoglobulin domain. These findings suggested that mutations in igsf11 cause the seurat mutant phenotypes.
Fig. 3. seurat mutants exhibit lesions in igsf11. 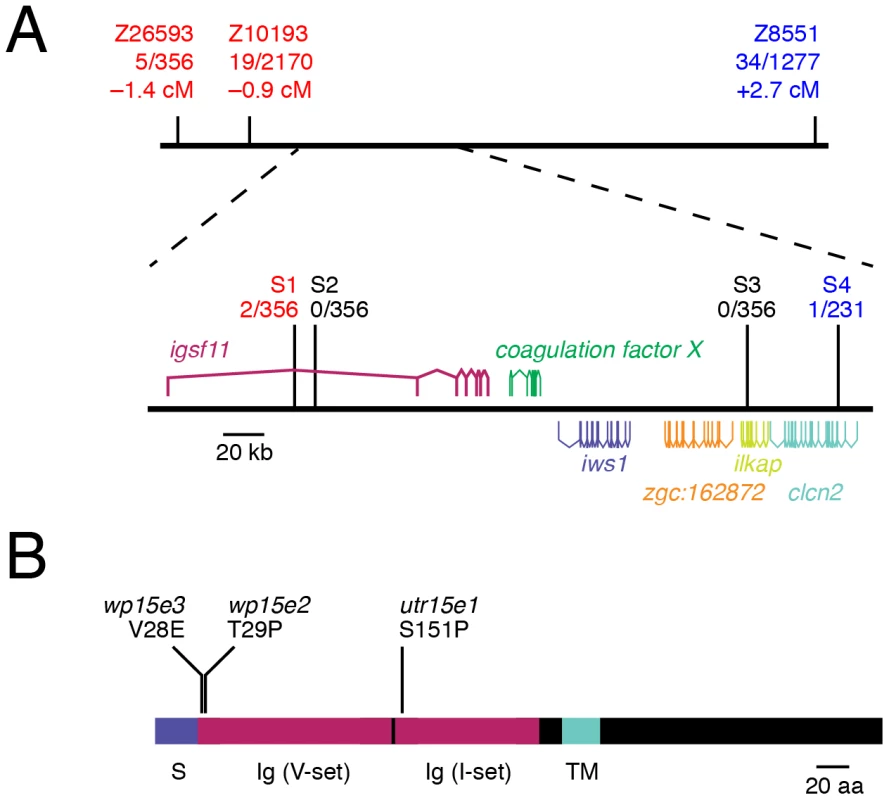
(A) Meiotic mapping of the seuratutr15e1 allele revealed a ∼210 kb critical genetic interval harboring several open reading frames of which only igsf11 exhibits ENU-induced lesions. Differences in numbers of individuals tested across markers reflect the absence of polymorphisms in some mapping families. (B) Schematic of inferred Igsf11 protein showing identified mutations and predicted domains. The lesion in the mapped allele, seuratutr15e1, occurred in exon 4, whereas lesions in seuratwp15e2 and seuratwp15e3 were found in exon 2. S, predicted signal sequence; TM, predicted transmembrane domain; Ig (V-set), immunoglobulin V-set domain; Ig (I-set), immunoglobulin I-set domain. seurat mutant melanophore patterning is rescued by wild-type igsf11 expressed in pigment cell lineages
To further test the allelism of igsf11 and seurat, we asked if the seurat mutant phenotype could be rescued by expressing wild-type igsf11 cDNA within pigment cells or their precursors. To this end, we constructed transgenes to drive igsf11 with the mitfa promoter [34], [35], which is expressed by precursors to adult melanophores (and possibly iridophores) and newly differentiated melanophores during the larval-to-adult transformation, as well as xanthophores and undifferentiated cells that may be precursors to multiple pigment cell classes in the late larva and adult (Figure S3). We then injected seuratutr15e1 embryos with mitfa:igsf11 or mitfa:nlsVenus-V2a-igsf11 transgenes and reared fish through completion of the adult pigment pattern. These genetically mosaic fish expressed nuclear-localizing Venus within the melanophore lineage and exhibited partially rescued stripes (Figure 4A–4C). After screening for germ line carriers, we additionally found that stable transgenic lines expressing mitfa:igsf11 exhibited stripes nearly indistinguishable from those of the wild-type (Figure 4D–4G). These results and those of positional cloning analyses confirm that seurat corresponds to igsf11. In conjunction with the results of cell transplantation analyses, these phenotypes also suggest that igsf11 promotes normal melanophore stripe development in part by acting through melanophores, or their undifferentiated and possibly multipotent precursors, though we do not exclude the possibility of contributory igsf11 functions within other lineages as well.
Fig. 4. seurat mutant phenotype rescued by wild-type igsf11 expressed in mitfa+ pigment cell precursors. 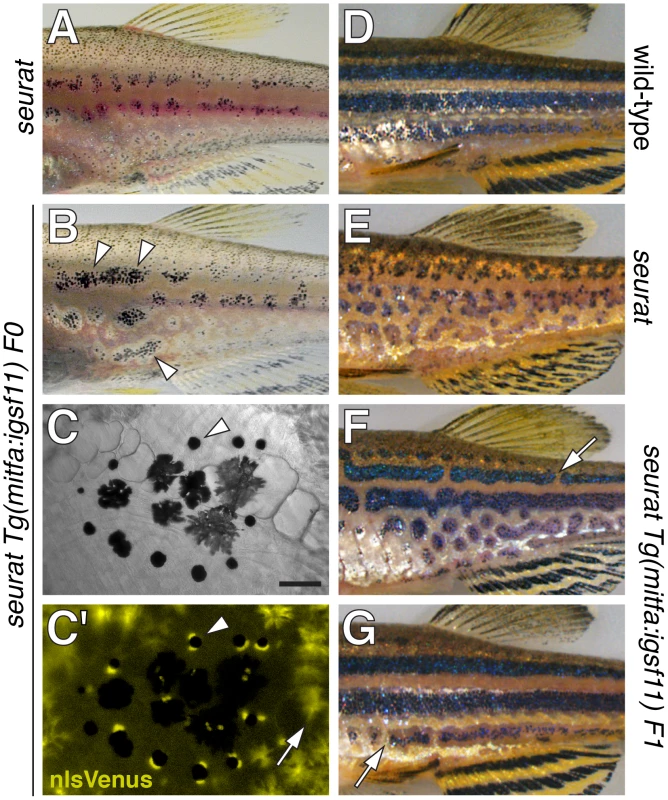
(A) Juvenile seurat mutant. (B) Sibling of fish in A injected with mitfa:nlsVenus-V2a-igsf11 transgene at the one cell stage exhibits patches of rescued melanophore stripes (arrowheads). (C,C′) Close-up showing individual melanophores (e.g., arrowhead) that express nlsVenus. This individual had been treated with epinephrine to contract melanin-containing organelles (melanosomes) towards the cell body, which facilitates fluorophore visualization; melanosomes of only some melanophores are in the fully contracted state. Arrow, xanthophores autofluorescence in the same channel as nlsVenus. (D) Adult wild-type. (E) Adult seurat mutant. (F, G) Two examples of seurat mutants transgenic for stably integrated mitfa:igsf11, which exhibit nearly wild-type stripes marked by occasional breaks (arrows). Individuals were genotyped by PCR to verify presence of the transgene. Scale bar: in (C) 60 µm for (C,C′). Expression of igsf11 by pigment cells, pigment cell precursors, epidermis, and other adult tissues
The above analyses suggest that igsf11 should be expressed by adult melanophores and perhaps their precursors, though widespread expression in the early embryo [36] suggests the potential for expression more broadly as well. During the larval-to-adult transformation, in situ hybridization revealed igsf11 transcripts in relatively rare, scattered cells in the hypodermis, where stripe formation takes places between the skin and muscle (Figure 5A, 5B), in extra-hypodermal locations where pigment cell precursors are found [34], and in cells within the spinal cord (Figure S4). A polyclonal antiserum raised against a zebrafish Igsf11 peptide (Figure S4) revealed an identical distribution of Igsf11-immunoreactive cells. To determine if scattered Igsf11+ cells might be pigment cell precursors, we examined Tg(mitfa:GFP)w47 fish [34], [35]. These analyses revealed that many mitfa:GFP+ cells coexpressed Igsf11 (Figure 5C, 5E), consistent with the autonomous activity of igsf11 within the pigment cell or melanophore lineages demonstrated by genetic mosaic analyses. Analyses at adult stages further revealed Igsf11 immunoreactivity of isolated melanophores (Figure 5D) and igsf11 transcripts expressed in isolated cells highly enriched for melanophores and xanthophores (Figure 5F). We did not detect gross differences in levels of Igsf11 immunoreactivity between wild-type and seurat mutants, either in sections of larvae or in isolated melanophores, consistent with similar translational efficiency of the wild-type protein and S151P mutant protein (data not shown). Finally, we also detected igsf11 transcript in several other adult tissues, including the eye, brain, heart, skin, fin, testis and ovary (Figure 5G), presumably reflecting expression by other cells types, or pigment cells or their precursors resident in some of these tissues. Together, RT-PCR, in situ hybridization, and immunohistochemistry support the conclusion that Igsf11 is expressed in adult pigment cells and their precursors in post-embryonic zebrafish.
Fig. 5. igsf11 is expressed by pigment cells and their precursors. 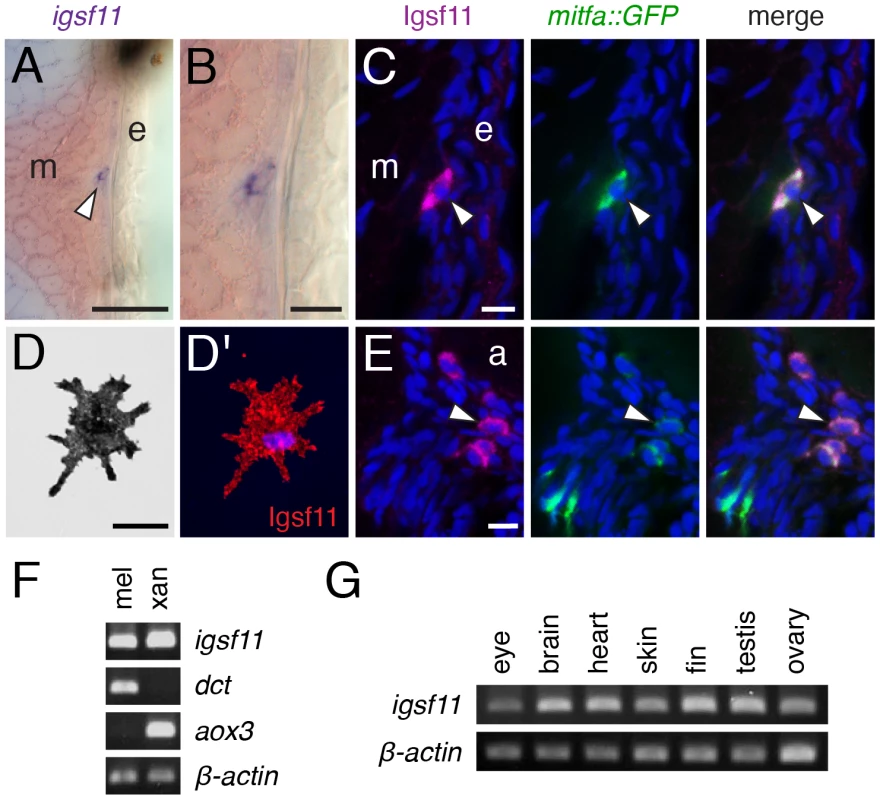
(A,B) In situ hybridization for igsf11 transcript during the larval-to-adult transformation, showing an igfs11-expressing cell near the hypodermis (A, and higher magnification in B). e, epidermis; m, myotome. (C) Similar location to that shown in (B), illustrating a cell within the hypodermis (arrowhead), coexpressing Igsf11 cell (magenta) and mitfa:GFP (green). Nuclei in all immunofluorescence images are counterstained with DAPI (blue). (D,D′) A melanophore isolated in vitro expresses Igsf11 (red). (E). Extra-hypodermal Igsf11+ cells (arrowhead) also coexpressed Igsf11 (magenta) and mitfa:GFP, though some mitfa:GFP+ cells were Igsf11− (lower left of panels). Shown here are cells just ventral to the aorta (a). (F) RT-PCR showed that cell populations isolated by differential centrifugation and highly enriched for melanophores (mel) and xanthophores (xan) express igsf11 transcript. dct, dopachrome tautomerase, expressed by melanophores; aox3, aldehyde oxidase 3, expressed by xanthophores. β-actin, loading control. (G) igsf11 expression was detected in several additional tissue types dissected from adult fish. Scale bars: in (A) 40 µm for (A); in (B) 10 µm for (B); in (C) 10 µm for (C,E); in (D) 20 µm for (D). Adhesive interactions mediated by Igsf11 in vitro
Immunoglobulin superfamily members mediate a wide range of adhesive interactions. To test if zebrafish Igsf11 also might contribute to adhesive interactions, and the potential of seurat mutations to disrupt such interactions, we transfected K562 human myeloid leukemia cells with wild-type or seurat mutant igsf11 cDNAs. In rotary cultures, cells expressing wild-type Igsf11 adhered to one another to form large aggregates within two hours (Figure 6; Figure S5). By contrast, mock transfected cells or cells transfected with S151P (seuratutr15e1) or T29P (seuratwp15e2) mutant igsf11 cDNAs failed to form large aggregates. These findings support a model in which Igsf11 can mediate adhesive interactions in vivo and further demonstrate that both mutant forms of Igsf11 are compromised for such activity.
Fig. 6. Igsf11 promotes aggregation of K562 myeloid leukemia cells in vitro. 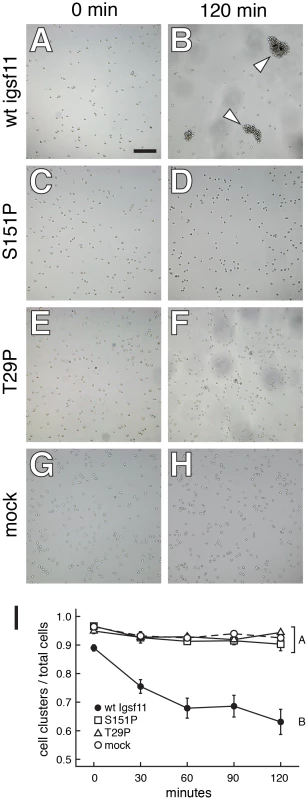
Cells were transfected with wild-type zebrafish igsf11 (A, B), S151P, seuratutr15e1 mutant igsf11 (C, D), T29P, seuratwp15e2 mutant igsf11 (E, F), or mock transfected (G, H). At the start of the experiment, the numbers of small cellular aggregates and total numbers of cells were similar (A, C, E, G). By 120 min, a relatively small number of aggregates containing numerous cells had formed in the cells transfected with wild-type igsf11 (arrowheads in B), though this was not the case in cells of other treatments (D, F, H). (I) Quantitation of the ratio of cellular aggregates to the total number of cells (mean±SE) confirmed that cells were increasingly found in fewer, larger aggregates when transfected with wild-type igsf11. At 120 min, degrees of aggregation differed significantly among treatments overall (F3,48 = 19.8, P<0.0001). Post hoc means comparisons indicated that aggregation behavior in cells transfected with wild-type igsf11 (B in the figure) differed significantly from that of cells transfected with mutant igsf11 or controls (A in the figure; Tukey Kramer post hoc comparisons, P<0.01); aggregation of cells transfected with mutant forms of igsf11 did not differ significantly from one another or from mock transfected cells. Values shown are least squares means adjusted to control for variation after controlling for minor but significant variation among replicates (P<0.01). igsf11 promotes the migration and survival of melanophores and their precursors
Our finding that Igsf11 can mediate adhesive interactions in vitro, and the well-known roles of adhesive interactions in promoting cell migration and survival, led us to ask if either of these morphogenetic behaviors were compromised in seurat mutants. We repeatedly imaged homozygous seurat mutants and heterozygous wild-type siblings through the larval-to-adult transformation. These image series indicated that melanophores in seurat mutants tend to be more punctate than in the wild-type and exhibit reduced rates of migration and an increased likelihood of death as compared to wild-type melanophores (Figure 7A; Videos S1, S2). seurat mutants also exhibited a progressively more severe deficiency in melanophore numbers as the larval–to–adult transformation progressed (Figure S6).
Fig. 7. igsf11-dependent migration and survival of melanophores. 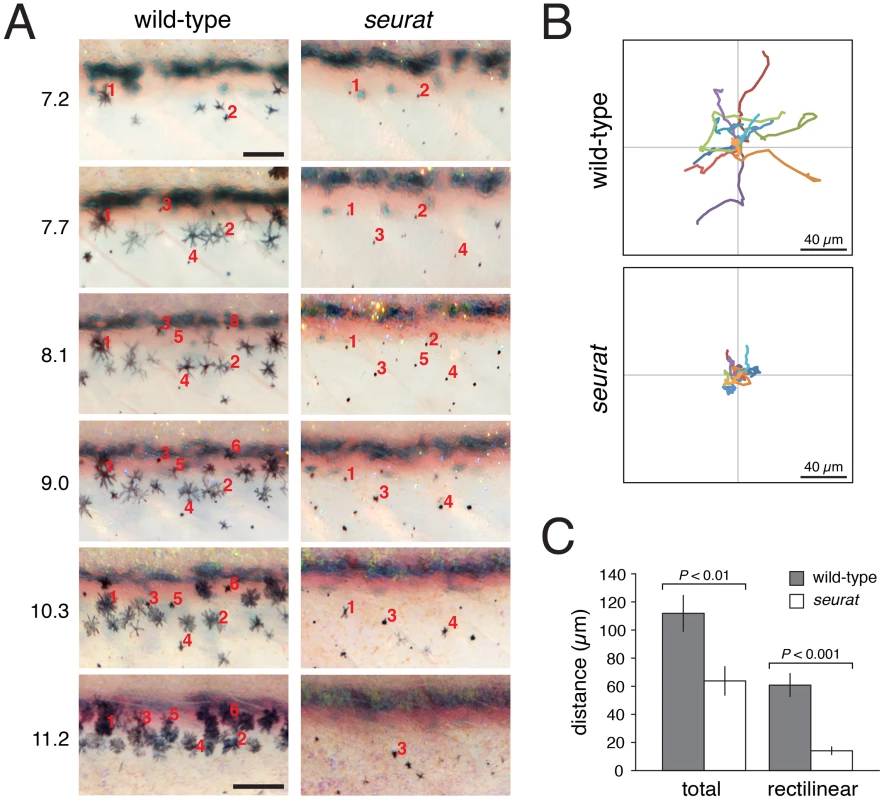
(A) Repeated images of developing wild-type and seurat mutant larvae between 14–28 days post-fertilization. Numbers to the left of images are SSL. In wild-type larvae, new adult melanophores differentiated already within stripes or translocated short distances as stripes formed (e.g., note changes in the relative positions of cells 2 vs. 4, and cell 3 vs. 1 and 5). In seurat mutants, however, little movement was observed and many melanophores died as evidenced by the presence of melanized cellular debris apparent at high magnification (not shown; [21], [32]. Images shown were rescaled to maintain the same field of view as the fish grew; scale bars at 7.2 SSL and 11.2 SSL represent 100 and 200 µm, respectively. (B) When cultured in vitro, wild-type melanophores migrated further than seurat mutant melanophores. Shown are tracks of 16 cells of each genotype. (C) Quantification of total and rectilinear distances moved by cells in vitro confirmed reduced motility of seurat mutant melanophores (t = 3.0, t = 5.4, respectively; d.f. = 26). Shown are means ± SE. To further assess a role for igsf11 in promoting melanophore migration we compared the motility of wild-type and igsf11 mutant melanophores in vitro. Similar to phenotypes in vivo, seurat mutant melanophores attached poorly to their substrate resulting in a more rounded appearance, and seurat mutant melanophores that did attach migrated significantly shorter distances than wild-type melanophores (Figure 7B, 7C; Videos S3, S4).
Finally, to determine if igsf11 is required for the migration and survival of melanophore precursors, in addition to differentiated melanophores, we crossed Tg(mitfa:GFP)w47 into the seurat mutant background and examined cell behaviors by ex vivo imaging [34]. As for differentiated melanophores, these analyses revealed significantly reduced migration and survival of mitfa:EGFP+ cells in seurat mutants as compared to the wild-type (Figure 8, Videos S5, S6, S7, S8).
Fig. 8. Melanophore precursors require igsf11 for their migration and survival. 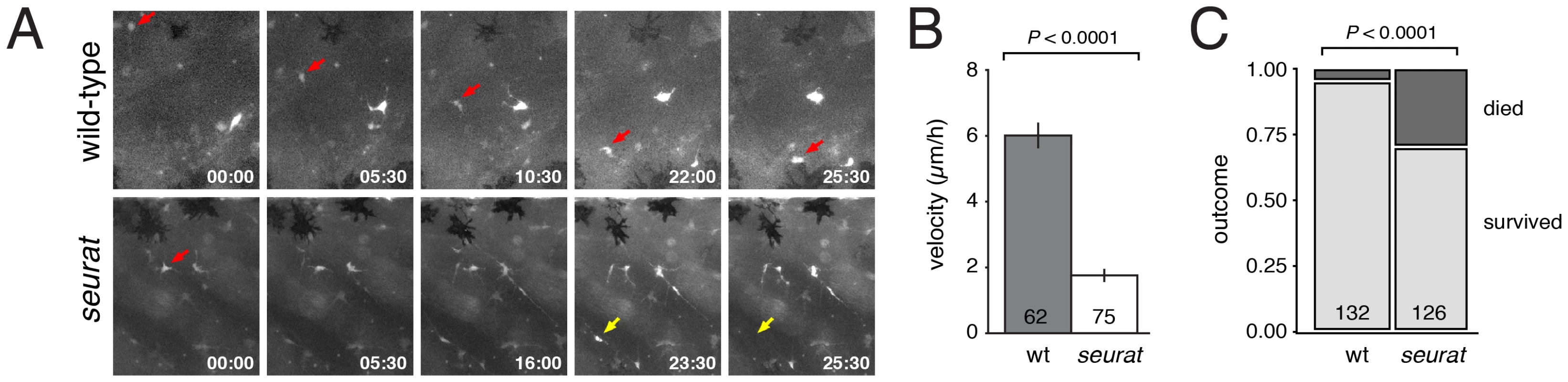
(A) Selected frames from time-lapse movies of mitfa:GFP+ cells in wild-type and seurat mutant explants. A single cell (red arrow) moved from dorsal to ventral over the duration of the movie. In a seurat mutant, many cells failed to migrate (e.g., red arrow) or died (yellow arrow) during the period of imaging. (B) Velocities (mean±SE) of mitfa:GFP+ cells were significantly reduced in seurat mutants compared to the wild-type (t = 11.2, d.f. = 135), as were total distances traveled (not shown). (C) seurat mutant melanophores were also significantly more likely to die than were wild-type melanophores (X2 = 29.8, d.f. = 1). Together, these analyses demonstrate a requirement for igsf11 in promoting the migration and survival of melanophores and their precursors, supporting a model in which melanophore organization into stripes is mediated in part through Igsf11-dependent adhesive interactions.
Discussion
The results of this study identify critical roles for the immunoglobulin superfamily member Igsf11 in the development of zebrafish adult pigment stripes. We found that lesions in igsf11 are responsible for the seurat mutant phenotype of irregular melanophore spots. We demonstrated that Igsf11 promotes adhesive interactions of heterologous cells in vitro, and that seurat mutant forms of Igsf11 harboring missense mutations are defective for this activity. By cell transplantation and cell-type specific rescue experiments, we additionally found that igsf11 acts autonomously to pigment cell lineages in promoting melanophore stripe formation. Finally, our analyses of cellular behaviors in vivo, in vitro, and in ex vivo explants indicate that igsf11 promotes both the migration and survival of melanophores and their precursors. Whereas roles for cell adhesion molecules in the development of specific pigment patterns have long been suspected [37]–[41], our study is the first to implicate a particular locus expressed by pigment cells in these processes.
Our study expands the known developmental roles of immunoglobulin superfamily (IgSF) proteins, which include such well-studied members as N-CAM, DSCAM and ICAM-1, and provides the first in vivo model system for dissecting the functions of Igsf11 specifically. The immunoglobulin superfamily is an especially diverse set of transmembrane proteins [42], [43], the functions of which have been analyzed most extensively in the nervous system, where they mediate axon guidance and fasciculation, target recognition, and dendrite patterning [44]–[47], as well as in the immune system, where they are required for mediating interactions between immune cells and their environments, and for mounting immune responses [48]–[50]. IgSF members also play important roles in regeneration [51] and in cancer, acting as tumor suppressors or enhancers of invasion [52], [53]. Although IgSF members are not known to be expressed abundantly by normal human melanocytes, several of these genes are dysregulated in melanoma and associated with melanoma progression and metastasis [54], [55] and immunoreactivity using an anti-N-CAM antibody has been detected in xanthophores of some species [56].
IGSF11 was first identified in mouse and human and shown to be expressed highly in brain and testes (for this reason being named originally Brain - and Testes-specific-IgSF, BT-IgSF) [57]. IGSF11 was also identified independently as a gene up-regulated frequently in intestinal-type gastric cancers [58]. Our finding that igsf11 promotes melanophore morphogenesis represents the first identified function for an igsf11 orthologue in vivo as well as the first evidence of an IgSF member contributing to normal pigment cell development and patterning.
Our finding that igsf11 is expressed and required by cells of the melanophore lineage and promotes adhesive interactions in vitro suggests two complementary models for the cellular bases of Igsf11-dependent interactions during adult pigment pattern formation. First, Igsf11 could mediate adhesive interactions specifically amongst differentiated melanophores as these cells organize into stripes. Second, Igsf11 could promote stripe development by mediating interactions between melanophores or their possibly multipotent precursors and their environments, either through homophilic or heterophilic adhesive interactions. Our analyses cannot yet speak to the first model, but results of the present study do support the second model of Igsf11-dependent interactions between melanophores and neighboring cell types. For example, we found that mitfa:GFP+ cells exhibited defects in migration and survival in seurat mutants, prior to melanization and stripe formation. Likewise, seurat mutant melanophores attached poorly to a collagen type IV substrate in serum-containing medium, and exhibited reduced motility independent of interactions with other melanophores.
The biochemical mechanisms for Igsf11-dependent interactions remain unknown. Mammalian IGSF11 mediates homophilic adhesive interactions in vitro [59] and such interactions could occur in vivo during adult pigment pattern formation. Yet, our findings that Igsf11 acts autonomously to melanophores or their precursors in cell transplantation and genetic rescue experiments, and that igsf11 transcripts and protein are not detected in the environment through which these cells migrate, suggest that Igsf11 interacts with one or more heterophilic binding partners to promote melanophore lineage morphogenesis. Indeed, the coxsackie and adenovirus receptor, encoded by CXADR, which is the closest homologue of IGSF11, mediates both homophilic and heterophilic adhesive interactions [60]. Consistent with the existence of additional Igsf11 ligands is our observation that different seurat mutant forms of Igsf11 equally abrogate cellular aggregation in vitro, despite having either severe or more mild pigment pattern defects in vivo; this outcome suggests that different mutant forms of Igsf11 may be differentially affected in their adhesive interactions with heterologous factors present in vivo but not in vitro. The existence of other Igsf11 interaction partners also seems likely from our demonstration that isolated wild-type and seurat mutant melanophores differed in their motility on Type IV collagen (though we cannot exclude the possibility that IgSF11 could have been present in serum). Future studies aim to elucidate the mechanisms responsible for Igsf11-dependent adhesion, including the identification of cis- and trans-interacting proteins.
In conclusion, our results identify a new gene required for adult pigment pattern formation and suggest an essential role for Igsf11-dependent adhesive interactions in promoting the morphogenesis of melanophores and their precursors during development of the adult form. It will be especially interesting to learn how pathways dependent on “classical” cell adhesion molecules of the sort identified here interact with physiological mechanisms mediated by kir7.1 and other factors [28] to orchestrate pigment pattern formation in zebrafish and other teleosts.
Materials and Methods
Ethics statement
All work in this study was conducted in accordance with guidelines and approved protocols for animal care and use at the University of Washington and Osaka University.
Isolation of seurat mutant alleles
seuratwp15e1 was isolated in a forward genetic, early pressure screen for N-ethyl-N-nitrosourea (ENU) induced mutations in the ABwp genetic background and was subsequently maintained in the same, unmutagenized background. Additional alleles, seuratwp15e2 and seuratwp15e2, were isolated as ENU-induced mutations in the wik genetic background by screening against seuratwp15e1, with subsequent backcrosses of non-complementing individuals to confirm allelism of new mutations.
Cell transplantation
Chimeric embryos were generated by transplanting cells at blastula stages (3.3–3.8 hours post-fertilization) and then rearing through late juvenile stages by which time an adult pattern has formed [11]. The Tg(bactin:GFP) transgenic line was provided by Ken Poss.
Positional cloning and sequence analyses
seurat was mapped to chromosome 15 by bulked segregant analyses of fish derived from mapping crosses constructed using seurat (ABwp genetic background) and wik, then subsequently mapped between microsatellite markers Z10193 and Z8551. Additional single nucleotide polymorphisms (S1, S2, S3, S4) were identified within this region of chromosome 15 (45.8∼46.1 Mb) and were used to narrow the critical genetic interval in additional mapping crosses generated in Tubingen and AB genetic backgrounds. Differences in total numbers of individuals tested reflect background-specific polymorphisms and numbers of informative individuals analyzed. Gene predictions were derived from Ensembl (Sanger Institute). cDNA sequences for all genes in the critical interval were compared to those of the un-mutagenized ABwp as well as other backgrounds. To test for lesions that might affect mRNA splicing, exons and flanking intronic sequences of these loci were examined from genomic DNA as well, though the presence of numerous repetitive elements in this telomeric region precluded complete sequencing of some splice junctions. Protein domains were predicted using Pfam, CLC Main Workbench 6.6.1 (CLC bio, Muehital Germany) and SignalP 4.0 [61] and by alignment and structural comparison with the closely related coxsackie and adenovirus receptor [60], [62]. Structures were illustrated using Cn3D (http://www.ncbi.nlm.nih.gov/Structure/CN3D/cn3d.shtml).
Rescue of seurat mutant phenotype
Two different plasmid DNAs were generated for rescue experiments. For one construct, mitfa:igsf11, the pT2AL200R150G vector [63] was modified by replacing the ef1a promoter with a 1.3 kb fragment of the mitfa promoter followed by the igsf11 coding sequence. The second construct, mitfa:nlsVenus-V2a-igsf11, was generated using the Gateway Tol2kit pDestTol2pA2 vector [64], and included a 2.2 kb fragment of the mitfa promoter followed by a composite open reading frame generated by overlap extension PCR [65] that consisted of a nuclear-localizing Venus fluorophore and the igsf11 coding sequence, linked by a V2a peptide breaking sequence to allow the production of separate peptide products [66]. Rescue constructs and Tol2 mRNA synthesized in vitro were injected into homozygous seuratutr15e1 embryos at the one-cell stage. Effects of transgenes were evaluated in these injected, mosaic fish, and in the non-mosaic F1 progeny of germ-line carriers for the mitfa:igsf11 transgene, in which genomic incorporation of the transgene was verified by PCR.
RT–PCR analysis of igsf11 expression
Zebrafish adult tissues were harvested following euthanasia by methyl methane sulfonate (MMS, Sigma) overdose. Total RNAs were obtained using the RNeasy Protect Mini Kit (Qiagen), and cDNA generated with SuperScript III CellsDirect cDNA Synthesis System (Invitrogen). 4.4 ng of the cDNAs (RNA equivalent) obtained from each organ were used in PCRs to detect expression of igsf11 expression or β-actin as a positive control. PCR amplifications were performed for 32 cycles for igsf11 and 27 cycles for β-actin at 95°C for 30 s, at 60°C for 30 s, and 72°C for 30 s.
To test for igsf11 expression in adult melanophores and xanthophores, fin pigment cells were isolated and cDNAs synthesized. Zebrafish were anesthetized with MMS and then fin regions containing melanophores and xanthophores, respectively, were dissected under a stereomicroscope. Fin clips were treated with solution containing 2.5 mg/ml trypsin liquid (Worthington), 1.2 mg/ml BSA (Sigma) and 1 mM EDTA (Wako) in PBS for 10 min at 28°C. Trypsin solution was then removed and the tissue rinsed several times with PBS, after which samples were incubated for 60 min at 28°C with solution containing 1 mg/ml collagenase I (Worthington), 0.1 mg/ml DNase I (Worthington), 0.1 mg/ml STI (Worthington), 1.2 mg/ml BSA, and 100 nM epinephrine (Sigma) in PBS. Suspension solutions were filtered with 25 µm mesh, followed by density-gradient centrifugation at 30× g for 15 min at room temperature in 50% Percoll (Sigma), precipitating separate populations of melanophores and xanthophores to ∼95% purity from other contaminating cell types such as epidermis. To evaluate cross-contamination of melanophores and xanthophores, we tested for expression of dct and aox3, which are specifically expressed by melanophores and xanthophores, respectively [18], [67]. PCR amplifications were performed for 40 cycles for igsf11, 34 cycles for dct and aox3, and 38 cycles for β-actin at 95°C for 30 s, at 60°C for 30 s, and 72°C for 30 s. Primer sets were designed to span introns. For igsf11: 5′-TCTGATCGCGGGCACCATCG-3′, 5′-TAGGTGTTGGTGGACGTCAGAGTG-3′; β-actin: 5′-CGGTTTTGCTGGAGATGATG-3′, 5′-CGTGCTCAATGGGGTATTTG-3′; dct: 5′-ATCAGCCCGCGTTCACGGTT-3′, 5′-ACACCGAGGTGTCCAGCTCTCC-3′; aox3: 5′-AGGGCATTGGAGAACCCCCAGT-3′ and 5′-ACACGTTGATGGCCCACGGT-3′.
Immunohistochemistry and in situ hybridization
Polyclonal antisera for zebrafish Igsf11 were generated in mouse. The peptide immunogen selected, PTYAWEKQESVPKLPHN, occurs within the second predicted immunoglobulin domain of Igsf11. This antiserum did not recognize a specific fragment of the predicted size in Western blots, therefore its specificity was assessed by injecting embryos at the one-cell stage with a morpholino oligonucleotide (Gene Tools, LLC) targeting the igsf11 translational start site (igsf11-MO: CATGTTTCCCAGCGAAAGTCGTCGT) to test for reduced immunoreactivity at 24 hours post-fertilization. Morpholino was injected at 2 ng or 4 ng per embryo, as determined by an absence of toxicity at these doses using a 5 base pair mismatch control morpholino (igsf11-MM: CATcTTTgCCAcCGAAAcTCcTCGT). Antiserum was used at 1∶500–1∶1000 following fixation in 4% paraformaldehyde and detected using goat anti-mouse Alexa 555 or Alexa 568 secondary antibodies. For immunohistochemistry of larvae, individuals of 7.0–9.0 standardized standard length (SSL; [11]) were fixed in 4% paraformaldehyde containing 1% DMSO in PBS, embedded in OCT, and sectioned by cryostat at 18–20 µm. In situ hybridization on vibratome sections followed [17] using a full length (1329 bp) igsf11 cDNA for synthesis of antisense and sense riboprobes.
Aggregation assay
The human myeloid leukemia cell line (K562) was maintained with RPMI-1640 medium (Sigma) containing 10% FBS (Invitrogen). pIRES2-igsf11 plasmid for nucleofection, a modified electroporation technique, was generated by cloning of full length Igsf11 fragment into pIRES2-AcGFP (Clontech). Cells transiently expressing Igsf11 proteins (wild-type or mutant) were obtained using a 4D-Nucleofector (Lonza) with pIRES2-igsf11 plasmid. Twenty four hours after the nucleofection, cultures were suspended into single-cells by repeated pipetting, centrifuged and then resuspended in HBSS (Gibco) containing 1 mM CaCl2 at a density of 6×104 cells/ml. 500 µl of cell suspensions were transferred into a 24-well culture plate. The plate was then rotated on a gyratory shaker (80 rpm) at 37°C for 2 h. Three independent experiments were performed and 5 random fields of view for cells in each treatment were imaged every 30 min. The degree of adhesion was evaluated as the ratio of the number of cell clusters over the total number of cells. Data were analyzed for effects of treatment and replicate by analyses of variance (ANOVA) in JMP 8.0.2 (SAS Institute, Cary NC) after arc sin transformation to control for heteroscedasticity of residuals that is common for ratio data [68]. Differences between specific treatments were assessed by Tukey Kramer post hoc comparisons. In additional experiments, transfected cells were split after centrifugation, with half of each sample used for aggregation assays and the other half used either for verifying the equivalence of transfection efficiencies of wild-type and mutant igsf11 constructs by fluorescence activated cell sorting (BD FACS Calibur, BD Biosciences) for GFP expression (5000 cells per sample). Similar levels of wild-type and mutant Igsf11 protein expression were also examined by immunocytochemistry.
Repeated imaging and melanophore counts of fish during larval-to-adult transformation
Larvae were viewed and imaged with an Olympus SZX-12 stereomicroscope and Axiocam HR camera. For time-course analyses, individual fish from a seurat/+ backcross were imaged daily and genotypes determined retrospectively. Fish were reared individually and imaged after brief anesthetization with MMS. Complete image series were obtained for 5 wild-type and 8 seurat mutant individuals. For determination of melanophore numbers, all melanophores were counted between the dorsal and ventral margins of the flank in a region bounded by the anterior margin of the dorsal fin and the posterior margin of the anal fin. Counts were obtained from individual larvae at selected standardized standard lengths during the larval-to-adult transformation with genotypes and binned sizes analyzed as fixed effects in analyses of variance. For depicting pattern development in animations shown in Videos S1 and S2, all images were aligned and rescaled to control for growth using Adobe Photoshop CS5.
Analyses of melanophore motility in vitro
Melanophores were isolated from adult fish as described above and then re-suspended in L15 (Sigma) without FBS. Cells were cultured in 96-well culture dishes that had been coated with type IV collagen (BD Biosciences). After one overnight incubation at 28°C, culture medium was changed with fresh L15 containing 5% FBS and the cells were imaged using an Olympus IX71 microscope equipped with an Olympus DP72 digital camera and Lumina Vision software (Mitani Corporation).
Melanophores that attached, survived at least 48 h, and did not interact with other melanophores were chosen for analysis. Melanophore centroid positions were obtained in ImageJ (http://rsb.info.nih.gov/ij/) and plotted every hour between 12–36 h after medium change. Rectilinear migration distance was defined as the length between the beginning and ending positions for each cell.
Analyses of motility and survival ex vivo
To image the morphogenetic behaviors of presumptive melanophore precursors ex vivo, 7.0 SSL larvae were rinsed with 10% Hanks medium and then anesthetized and then decapitated with a razor blade. Larval trunks were then placed on 0.4 µM transwell membranes (Milipore) in glass bottom dishes containing L15 medium, 3% fetal bovine serum, and penicillin/streptomycin. The trunks were equilibrated for 3 h at 28.5°C then imaged at 30 min intervals for 18–26 h on a Zeiss Observer inverted epifluorescence microscope with an Axiocam MRm camera. Z-stacks of 10–15 planes collected at 4 µm intervals were merged for final analyses.
Supporting Information
Zdroje
1. KelshRN (2004) Genetics and evolution of pigment patterns in fish. Pigment Cell Res 17 : 326–336.
2. StreelmanJT, PeichelCL, ParichyDM (2007) Developmental genetics of adaptation in fishes: The case of novelty. Annual Review of Ecology Evolution and Systematics 38 : 655–681.
3. ParichyDM (2006) Evolution of danio pigment pattern development. Heredity 97 : 200–210.
4. MillsMG, PattersonLB (2008) Not just black and white: Pigment pattern development and evolution in vertebrates. Semin Cell Dev Biol 20 : 72–81.
5. Houde AE (1997) Sex, Color, and Mate Choice in Guppies. Princeton, NJ: Princeton University Press.
6. EngeszerRE, WangG, RyanMJ, ParichyDM (2008) Sex-specific perceptual spaces for a vertebrate basal social aggregative behavior. Proc Natl Acad Sci U S A 105 : 929–933.
7. PriceAC, WeadickCJ, ShimJ, RoddFH (2008) Pigments, Patterns, and Fish Behavior. Zebrafish 5 : 297–307.
8. SeehausenO, SchluterD (2004) Male-male competition and nuptial-colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proceedings of the Royal Society of London Series B-Biological Sciences 271 : 1345–1353.
9. JohnsonSL, AfricaD, WalkerC, WestonJA (1995) Genetic control of adult pigment stripe development in zebrafish. Dev Biol 167 : 27–33.
10. ParichyDM, TurnerJM (2003) Zebrafish puma mutant decouples pigment pattern and somatic metamorphosis. Developmental Biology 256 : 242–257.
11. ParichyDM, ElizondoMR, MillsMG, GordonTN, EngeszerRE (2009) Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Developmental Dynamics 238 : 2975–3015.
12. KirschbaumF (1975) Untersuchungen über das Farbmuster der Zebrabarbe Brachydanio rerio (Cyprinidae, Teleostei). Wilhelm Roux's Arch 177 : 129–152.
13. HirataM, NakamuraK-i, KanemaruT, ShibataY, KondoS (2003) Pigment cell organization in the hypodermis of zebrafish. Developmental Dynamics 227 : 497–503.
14. ParichyDM, RawlsJF, PrattSJ, WhitfieldTT, JohnsonSL (1999) Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development 126 : 3425–3436.
15. ListerJA, RobertsonCP, LepageT, JohnsonSL, RaibleDW (1999) nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126 : 3757–3767.
16. BudiEH, PattersonLB, ParichyDM (2008) Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development 135 : 2603–2614.
17. LarsonTA, GordonTN, LauHE, ParichyDM (2010) Defective adult oligodendrocyte and Schwann cell development, pigment pattern, and craniofacial morphology in puma mutant zebrafish having an alpha tubulin mutation. Dev Biol 346 : 296–309.
18. ParichyDM, RansomDG, PawB, ZonLI, JohnsonSL (2000) An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development 127 : 3031–3044.
19. ParichyDM, MellgrenEM, RawlsJF, LopesSS, KelshRN, et al. (2000) Mutational analysis of endothelin receptor b1 (rose) during neural crest and pigment pattern development in the zebrafish Danio rerio. Dev Biol 227 : 294–306.
20. LopesSS, YangX, MullerJ, CarneyTJ, McAdowAR, et al. (2008) Leukocyte tyrosine kinase functions in pigment cell development. PLoS Genet 4: e1000026 doi:10.1371/journal.pgen.1000026.
21. ParichyDM, TurnerJM (2003) Temporal and cellular requirements for Fms signaling during zebrafish adult pigment pattern development. Development 130 : 817–833.
22. MaderspacherF (2003) Formation of the adult pigment pattern in zebrafish requires leopard and obelix dependent cell interactions. Development 130 : 3447–3457.
23. YamaguchiM, YoshimotoE, KondoS (2007) Pattern regulation in the stripe of zebrafish suggests an underlying dynamic and autonomous mechanism. Proc Natl Acad Sci U S A 104 : 4790–4793.
24. TakahashiG, KondoS (2008) Melanophores in the stripes of adult zebrafish do not have the nature to gather, but disperse when they have the space to move. Pigment Cell Melanoma Res 21 : 677–686.
25. NakamasuA, TakahashiG, KanbeA, KondoS (2009) Interactions between zebrafish pigment cells responsible for the generation of Turing patterns. Proc Natl Acad Sci U S A 106 : 8429–8434.
26. KondoS, MiuraT (2010) Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329 : 1616–1620.
27. MaderspacherF, Nusslein-VolhardC (2003) Formation of the adult pigment pattern in zebrafish requires leopard and obelix dependent cell interactions. Development 130 : 3447–3457.
28. InabaM, YamanakaH, KondoS (2012) Pigment pattern formation by contact-dependent depolarization. Science 335 : 677.
29. HaffterP, OdenthalJ, MullinsM, LinS, FarrellMJ, et al. (1996) Mutations affecting pigmentation and shape of the adult zebrafish. Dev Genes Evol 206 : 260–276.
30. IwashitaM, WatanabeM, IshiiM, ChenT, JohnsonSL, et al. (2006) Pigment pattern in jaguar/obelix zebrafish is caused by a Kir7.1 mutation: Implications for the regulation of melanosome movement. PLoS Genet 2: e197 doi:10.1371/journal.pgen.0020197.
31. WatanabeM, IwashitaM, IshiiM, KurachiY, KawakamiA, et al. (2006) Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep 7 : 893–897.
32. LangMR, PattersonLB, GordonTN, JohnsonSL, ParichyDM (2009) Basonuclin-2 requirements for zebrafish adult pigment pattern development and female fertility. PLoS Genet 5: e1000744 doi:10.1371/journal.pgen.1000744.
33. KawakamiK, AmsterdamA, ShimodaN, BeckerT, MuggJ, et al. (2000) Proviral insertions in the zebrafish hagoromo gene, encoding an F-box/WD40-repeat protein, cause stripe pattern anomalies. Curr Biol 10 : 463–466.
34. BudiEH, PattersonLB, ParichyDM (2011) Post-embryonic nerve-associated precursors to adult pigment cells: genetic requirements and dynamics of morphogenesis and differentiation. PLoS Genet 7: e1002044 doi:10.1371/journal.pgen.1002044.
35. CurranK, ListerJA, KunkelGR, PrendergastA, ParichyDM, et al. (2010) Interplay between Foxd3 and Mitf regulates cell fate plasticity in the zebrafish neural crest. Dev Biol 344 : 107–118.
36. ThisseB, ThisseC, WrightGJ (2008) Embryonic and Larval Expression Patterns from a Large Scale Screening for Novel Low Affinity Extracellular Protein Interactions. ZFIN Direct Data Submission (Available at: http://zfinorg, ZDB-PUB-080227-22, Accessed 2012 July 17).
37. MoreiraJ, DeutschA (2005) Pigment pattern formation in zebrafish during late larval stages: a model based on local interactions. Dev Dyn 232 : 33–42.
38. ParichyDM (1996) Salamander pigment patterns: how can they be used to study developmental mechanisms and their evolutionary transformation? Int J Dev Biol 40 : 871–884.
39. Parichy DM, Reedy MV, Erickson CA (2006) Chapter 5. Regulation of melanoblast migration and differentiation. In: Nordland JJ, Boissy RE, Hearing VJ, King RA, Oetting WS, et al.., editors. The Pigmentary System: Physiology and Pathophysiology 2nd Edition. New York, New York: Oxford University Press.
40. MacmillanGJ (1976) Melanoblast-tissue interactions and the development of pigment pattern in Xenopus larvae. J Embryol Exp Morphol 35 : 463–484.
41. EpperleinHH, LofbergJ (1990) The development of the larval pigment patterns in Triturus alpestris and Ambystoma mexicanum. Adv Anat Embryol Cell Biol 118 : 1–99.
42. CrossinKL, KrushelLA (2000) Cellular signaling by neural cell adhesion molecules of the immunoglobulin superfamily. Dev Dyn 218 : 260–279.
43. BarclayAN (2003) Membrane proteins with immunoglobulin-like domains–a master superfamily of interaction molecules. Semin Immunol 15 : 215–223.
44. RougonG, HobertO (2003) New insights into the diversity and function of neuronal immunoglobulin superfamily molecules. Annu Rev Neurosci 26 : 207–238.
45. KatidouM, VidakiM, StriginiM, KaragogeosD (2008) The immunoglobulin superfamily of neuronal cell adhesion molecules: lessons from animal models and correlation with human disease. Biotechnol J 3 : 1564–1580.
46. LongH, OuY, RaoY, van MeyelDJ (2009) Dendrite branching and self-avoidance are controlled by Turtle, a conserved IgSF protein in Drosophila. Development 136 : 3475–3484.
47. SiebertM, BanovicD, GoellnerB, AberleH (2009) Drosophila motor axons recognize and follow a Sidestep-labeled substrate pathway to reach their target fields. Genes Dev 23 : 1052–1062.
48. DermodyTS, KirchnerE, GuglielmiKM, StehleT (2009) Immunoglobulin superfamily virus receptors and the evolution of adaptive immunity. PLoS Pathog 5: e1000481 doi:10.1371/journal.ppat.1000481.
49. GoliasC, BatistatouA, BablekosG, CharalabopoulosA, PeschosD, et al. (2011) Physiology and pathophysiology of selectins, integrins, and IgSF cell adhesion molecules focusing on inflammation. A paradigm model on infectious endocarditis. Cell Commun Adhes 18 : 19–32.
50. MontgomeryBC, CortesHD, Mewes-AresJ, VerheijenK, StaffordJL (2011) Teleost IgSF immunoregulatory receptors. Dev Comp Immunol 35 : 1223–1237.
51. IrintchevA, SchachnerM (2011) The Injured and Regenerating Nervous System: Immunoglobulin Superfamily Members as Key Players. Neuroscientist
52. MohMC, ShenS (2009) The roles of cell adhesion molecules in tumor suppression and cell migration: a new paradox. Cell Adh Migr 3 : 334–336.
53. Wai WongC, DyeDE, CoombeDR (2012) The role of immunoglobulin superfamily cell adhesion molecules in cancer metastasis. Int J Cell Biol 2012 : 340296.
54. SatyamoorthyK, MuyrersJ, MeierF, PatelD, HerlynM (2001) Mel-CAM-specific genetic suppressor elements inhibit melanoma growth and invasion through loss of gap junctional communication. Oncogene 20 : 4676–4684.
55. HaassNK, SmalleyKS, LiL, HerlynM (2005) Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res 18 : 150–159.
56. FukuzawaT, ObikaM (1995) N-CAM and N-cadherin are specifically expressed in xanthophores, but not in the other types of pigment cells, melanophores, and iridiphores. Pigment Cell Res 8 : 1–9.
57. SuzuS, HayashiY, HarumiT, NomaguchiK, YamadaM, et al. (2002) Molecular cloning of a novel immunoglobulin superfamily gene preferentially expressed by brain and testis. Biochem Biophys Res Commun 296 : 1215–1221.
58. KatohM (2003) IGSF11 gene, frequently up-regulated in intestinal-type gastric cancer, encodes adhesion molecule homologous to CXADR, FLJ22415 and ESAM. Int J Oncol 23 : 525–531.
59. HaradaH, SuzuS, HayashiY, OkadaS (2005) BT-IgSF, a novel immunoglobulin superfamily protein, functions as a cell adhesion molecule. J Cell Physiol 204 : 919–926.
60. PatzkeC, MaxKE, BehlkeJ, SchreiberJ, SchmidtH, et al. (2010) The coxsackievirus-adenovirus receptor reveals complex homophilic and heterophilic interactions on neural cells. J Neurosci 30 : 2897–2910.
61. PetersenTN, BrunakS, von HeijneG, NielsenH (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8 : 785–786.
62. VerdinoP, WitherdenDA, HavranWL, WilsonIA (2010) The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science 329 : 1210–1214.
63. UrasakiA, MorvanG, KawakamiK (2006) Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174 : 639–649.
64. KwanKM, FujimotoE, GrabherC, MangumBD, HardyME, et al. (2007) The Tol2kit: A multisite gateway-based construction kit forTol2 transposon transgenesis constructs. Developmental Dynamics 236 : 3088–3099.
65. HoSN, HuntHD, HortonRM, PullenJK, PeaseLR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77 : 51–59.
66. ProvostE, RheeJ, LeachSD (2007) Viral 2A peptides allow expression of multiple proteins from a single ORF in transgenic zebrafish embryos. Genesis 45 : 625–629.
67. KelshR (2000) Genetic Analysis of Melanophore Development in Zebrafish Embryos. Developmental Biology 225 : 277–293.
68. Sokal RR, Rohlf FJ (1981) Biometry. New York, New York: W. H. Freeman and Company.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



