-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvaluation of alternative calculation methods for determining LDL cholesterol
Zhodnotenie alternatívnych metód pre výpočet LDL cholesterolu
Úvod:
Vzhľadom na známe limity Friedewaldovej formuly boli navrhnuté aj alternatívne metódy výpočtu LDL cholesterolu (LDL‑C). V tejto práci sme posudzovali užitočnosť týchto metód.Metódy:
Do súboru sme zaradili deväťdesiat tri osôb bez ischemickej choroby srdca. LDL-C sme priamo stanovili homogénnou metódou, vypočítali Friedewaldovou formulou LDL-C = TC – HDL – (TG/2.2) (LDL1) a alternatívne ako LDL-C = 0,41 TC – 0,32 TG + 1,70 apoB – 0,27 (LDL2) a LDL-C = 0,94 TC – 0,94 HDL – 0,435 TG (LDL3). Výsledky: Všetky tri výpočty podhodnotili priamo stanovené hladiny LDL-C, v celom súbore a aj v skupinách podľa hladín TG (TG < 1,7 a v rozsahu 1,7–4,5 mmol/l, p < 0,001 pre všetky porovnania). Zaznamenali sme však významne vyššiu odchýlku vo všetkých troch výpočtoch u osôb s hladinou 1,7 ≤ TG < 4,5 mmol/l. Najnižšiu odchýlku v oboch sledovaných skupinách sme zaznamenali pre Friedewaldovu formulu. Absolútna odchýlka bola 7,6 % pre LDL1, 18,3 % pre LDL2 a 13,6 % pre LDL3. Výpočtami stanovené hladiny LDL-C korelovali s metódou priameho stanovenia v rozmedzí r = 0,82 – 0,90 (p < 0,0001 pre všetky s výnimkou LDL2 pre skupinu 1,7 ≤ TG < 4,5 mmol/l, kde p = 0,0011).Záver:
Friedewaldova formula sa javí v našej štúdii optimálnejšou metódou výpočtu LDL-C ako ďalšie dve alternatívne metódy, avšak podhodnocuje priamo stanovené LDL-C.Kľúčové slová:
LDL-C – alternatívne metódy výpočtu – Friedewaldova formula
Authors: B. Vohnout 1,2; A. Vachulová 1; P. Blažíček 3; A. Dukát 1; G. Fodor 4; J. Lietava 1
Authors place of work: nd Dept. of Internal Medicine, School of Medicine, Comenius University, Bratislava, Slovakia, head prof. Andrej Dukát, M. D., Ph. D. 1; Research Laboratories, “John Paul II” Center for High Technology Research and Education in Biomedical Sciences, Catholic University of the Sacred Heart, Campobasso, Italy, head prof. Giovanni de Gaetano, M. D., Ph. D. 2; Dept. of Clinical Biochemistry, Military Hospital, Bratislava, Slovakia, head Pavol Blažíček, M. Sc. 3; Prevention and Rehabilitation Centre, University of Ottawa Heart Institute, Ottawa, Canada, head prof. George Fodor, M. D., Ph. D. 4
Published in the journal: Vnitř Lék 2008; 54(10): 961-964
Category: Původní práce
Summary
Backgro und:
Due to limitati ons of the Fri edewald formula, alternative methods for calculating low - density lipoprotein cholesterol (LDL‑C) were suggested. We evalu ated utility of these methods. Methods: Ninety three subjects free of coronary he art dise ase were considered. LDL‑C was me asured by the homogeneo us method, and calculated by the Fri edewald formula LDL‑C = TC – HDL – (TG/ 2.2) (LDL1) and alternative formulas LDL‑C = 0.41 TC – 0.32 TG + 1.70 apoB – 0.27 (LDL2) and LDL‑C = 0.94 TC – 0.94 HDL – 0.435 TG (LDL3).Results:
All three formulas underestimated the me asured LDL‑C, both in the whole gro up and in subgro ups according to TG levels (TG < 1.7 and in a range of 1.7–4.5 mmol/ l, p < 0.001 for all). We fo und significantly higher bi as for all three formulas in subjects with 1.7 ≤ TG < 4.5 mmol/ l levels. The Fri edewald formula showed the lowest assay bi as in all the gro ups investigated. The me an absolute bi as for LDL1 was 7.6 %, 18.3 % for LDL2 and 13.6 % for LDL3, respectively. Line ar regressi on analysis showed correlati on of calculated LDL‑C values with the direct method in the range of r = 0.82 – 0.90 (p < 0.0001 for all, except of LDL2 in 1.7 ≤ TG < 4.5 mmol/ l gro up where p = 0.0011).Conclusi ons:
The Fri edewald formula seems to be a better estimator of LDL‑C in o ur study than the other two alternative formulas; however, it underestimated the LDL‑C levels.Key words:
LDL‑C – alternative calculati on formula – Fri edewald formulaIntroducti on
Low - density lipoprotein cholesterol (LDL‑C) is a well established risk factor of atherosclerosis and cardi ovascular dise ase (CVD), and CVD risk calculati on and tre atment go als both in primary and secondary preventi ons are based on LDL‑C levels. The refe-rence method for LDL‑C me asurement is direct me asurement by the β - qu antificati on method [1]. Beca use the direct method is time - consuming and involves ultracentrifugati on and a che-mical precipitati on step that is not available in ro utine laboratori es, the Fri edewald formula for LDL‑C calculati on is used as a standard method in clinical practice [2]. There are, however, several limitati ons of the formula. It multipli es the errors derived from total cholesterol (TC), triglyceride (TG) and HDL‑C me asurements, is based on an assumpti on of fixed mass rati o of plasma TG to very - low density lipoprotein cholesterol (VLDL‑C), is not valid if TG > 4.5 mmol/ l and in pati ents with type III hyperlipoproteinemi a, and requires fasting specimens. Moreover, the reli ability of the Fri edewald calculati on decre ases considerably with incre asing TG concentrati ons, even in specimens with TG concentrati on of 2.26 – 4.52 mmol/ l [3]. In the last decades, there have been several attempts to suggest alternative ways for LDL‑C calculati on [4,5]. Recently, a new generati on of homogeneo us methods for LDL detecti on, have also been introduced. Homogeneo us assays seem to be able to meet current Nati onal Cholesterol Educati on Program (NCEP) requirements for LDL‑C testing for precisi on (CV < 4%) and accuracy (bi as < 4%) [3]. The aim of this study was to evalu ate the alternative calculati on methods for determining LDL cholesterol and its’ utility regarding TG levels.
Methods
Ninety three subjects older than 18 ye ars, with TG levels less than 4.5 mmol/ l, and free of coronary he art dise ase (CHD) participating in a cross - secti onal screening for cardi ovascular risk factors in Slovaki a were involved in the analysis. We report results for the whole gro up and in subgro ups with normotriglyceridemi a and TG levels in range of 1.7 – 4.5 mmol/ l. The main characteristics of the gro up are in the table 1. Veno us blo od samples were collected after overnight fasting witho ut cubital compressi on. Serum levels of total cholesterol (TC) and triglycerides (TG) were me asured enzymatically, apoB levels were me asured by an immunoturbidimetric method, HDL cholesterol (HDL‑C) was determined directly by commerci al kit (Genzyme) on an a uto analyser (Hitachi 911). LDL‑C was me asured by homogeneo us method (LDL), based on the eliminati on principle (Direct LDL‑Cholesterol Randox, Randox Laboratori es Ltd, Crumlin, UK). The assay was performed according to the manufacturer’s recommendati on. Using control sera level I, II and III, the within‑run imprecisi on was 0.69, 0.57, and 0.50 ( %) and between - run imprecisi on was 1.67, 1.21 and 1.69 ( %), respectively. LDL‑C was calculated by the Fri edewald formula LDL‑C = TC – HDL – (TG/ 2.2) (LDL1) [2] and alternative formulas LDL‑C = 0.41 TC – 0.32 TG + 1.70 apoB – 0.27 (LDL2) [4] and LDL‑C = 0.94 TC – 0.94 HDL – 0.435 TG (LDL3) [5], expressed in mmol/ l.
Tab. 1. Main characteristics of the study population. 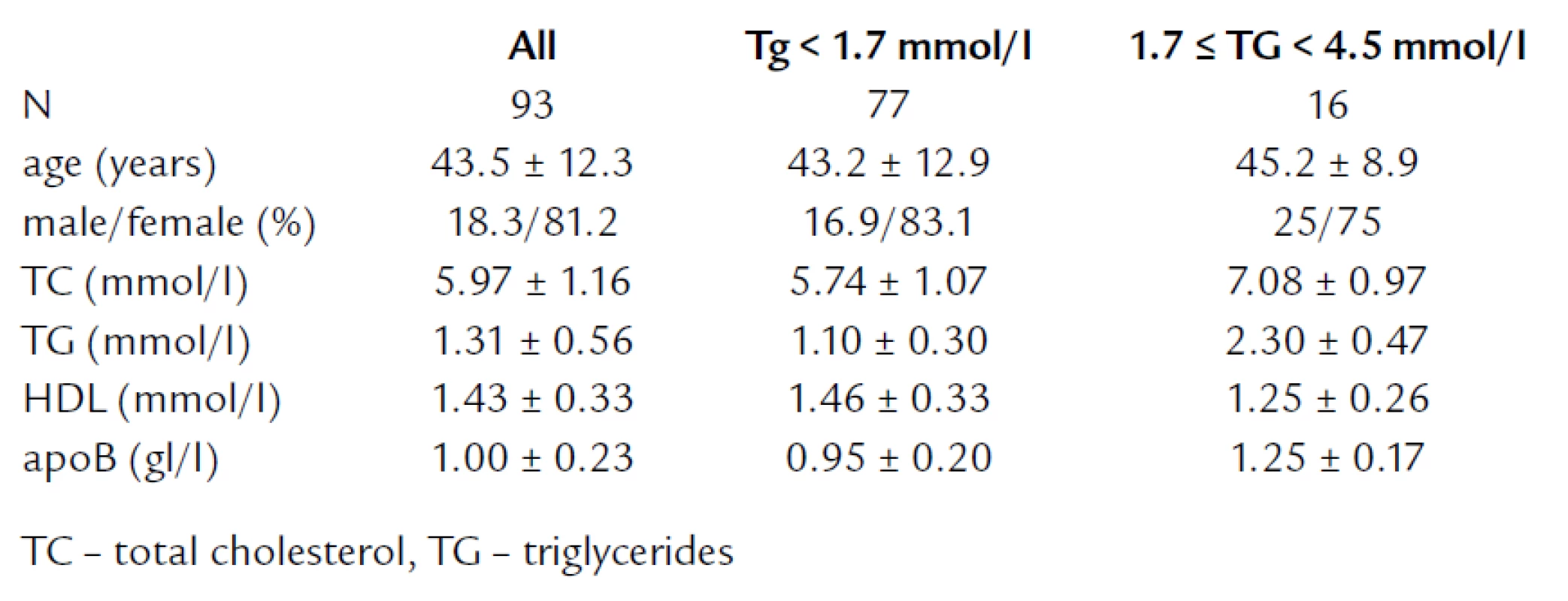
Values are expressed as me an ± SD. Line ar regressi on analysis was used for the determinati on of correlati on between parameters. The H0 hypothesis was that slope = 1 and intercept = 0, and was tested in the regressi on analy-sis. Assay bi as expressed as delta (Δ) was calculated as the direct me asurement of LDL result minus the formulas’ calculati on result. The differences bet-ween the direct me asurement and calculated values referred as a delta values were evalu ated using a signed rank test. Intergro up differences in assay bi as were compared by using a Mann‑Whitney two - sample ranksum test. A two - tailed P value of less than 0.05 was considered to indicate statistical significance. All computati ons were carri ed o ut with the SAS statistical package (SAS System for Windows V8) and with STATA (STATA/ SE 9.0 for Windows). The study was approved by the local Ethics Committee of the Medical Scho ol of Comeni us University in Bratislava and all subjects signed informed consent.
Results
Me ans of directly me asured and calculated LDL‑C, together with regressi on models and correlati on coefici ents are in the table 2. All three formulas significantly underestimated the LDL‑C levels compared to directly me asured LDL both in the whole gro up and in two subgro ups divided according to their TG levels (p < 0.001 for all Δ values). In fact, only 15.1% of values calculated by the Fri edewald formula and 2.2% by LDL3 formula were equ al or higher than values me asured by the direct method. LDL2 formula underestimated the LDL in all the cases. When we compared assay bi as according to the TG levels, we fo und significantly higher bi as for all three formulas in subjects with 1.7 ≤ TG < 4.5 mmol/ l levels. The Fri edewald formula showed the lowest assay bi as in all the gro ups investigated. The me an absolute bi as for LDL1 was 7.6%, 18.3% for LDL2 and 13.6% for LDL3, respectively. Line ar regressi on analysis showed significant correlati on of calculated LDL‑C values with the direct method with a range of r = 0.82 – 0.90 (p < 0.0001 for all, except of LDL2 in 1.7 ≤ TG < 4.5 mmol/ l gro up where p = 0.0011).
Tab. 2. Description of LDL-C data by different methods of estimation. 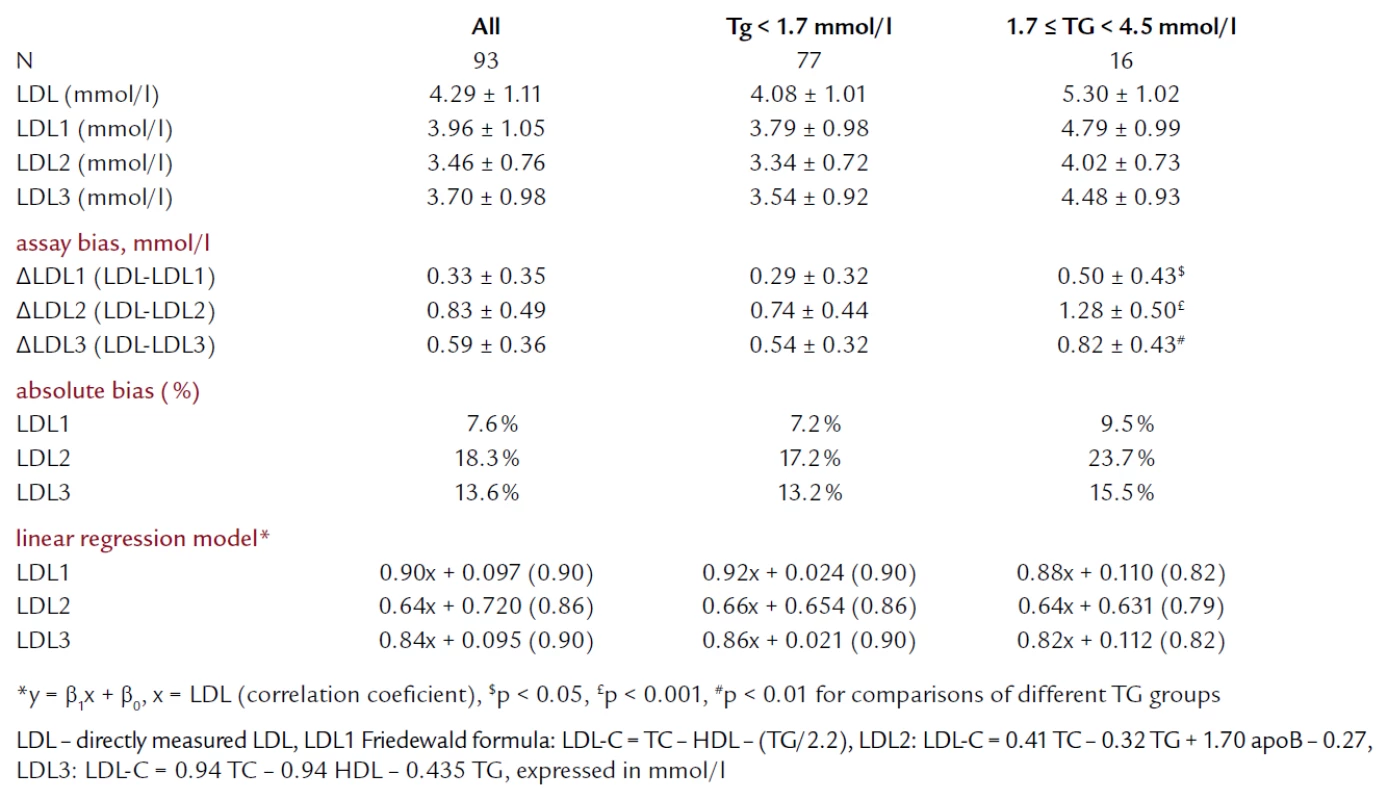
Discussi on
A ca usative relati on of LDL‑C to CHD has been well established in epidemi ological studi es and clinical tri als. The accurate determinati on of LDL‑C is thus an important component of the assessment of the CHD risk. In o ur study we evalu ated alternative formulas for the LDL‑C calculati on in comparison with a homogeneo us LDL‑C assay. LDL2 formula was based on a calculati on using TC, TG and apoB levels. ApoB is beli eved to be a better predictor of CHD risk than LDL‑C [6] and several advantages of LDL‑C calculati on by using the apoB based formula have been suggested. The formula can be used in hypertriglyceridemic pati ents and HDL‑C me asurement by precipitati on can be avo ided. Moreover, the formula has been suggested to be superi or to the Fri edewald calculati on in estimating LDL‑C levels [7 – 9].
In o ur study, all of the formulas significantly underestimated the LDL‑C levels me asured by the homogeneo us LDL‑C assay. Our finding of underestimati on of LDL‑C by the Fri edewald formula is in agreement with a large Canadi an study reve aling a tendency of the Fri edewald formula to underestimate LDL‑C in comparison to β - qu antificati on with a me an percent error of calculated LDL‑C less than 5% in subjects with TG < 4.5 mmmol/ l [10]. The apoB based LDL2 formula showed the biggest assay bi as and worst correlati on with the homogeneo us LDL‑C assay. This is in contrast with results published by Bairaktari et al [7 – 9], where the apoB based formula was superi or to Fri edewald when compared with β - qu antificati on method. This can be at le ast parti ally explained by different populati ons included in the studi es (hemodi alysis pati ents) and by different reference method of LDL‑C me asurement used. However, the homogeneo us LDL‑C assay by Roche has been shown to have a tendency to underestimated LDL‑C levels compared to β-qu antificati on method [3], suggesting that the potenti al imprecisi on of the homogeneo us LDL‑C assay used in o ur study can shift o ur result towards underestimati on of true LDL‑C values by the calculati on formulas. Altho ugh the apoB based LDL2 formula in o ur study was inferi or to other two calculati on formulas, apoB itself is a close reflecti on of the number of LDL particles, can be me asured with a high precisi on, and it has been suggested being a better index of cardi ovascular risk than LDL cholesterol [6]. ApoB me asurement can be especi ally useful in pati ents with hypertriglyceridemi a and in disorders associ ated with hypertriglyceridemi a, such as di abetes mellitus or metabolic syndrome. In such disorders me asurement of LDL cholesterol may not be an accurate reflecti on of LDL particles, due to an incre ased number of small dense LDL particles. We have recently shown incre ased apoB levels despite of comparable LDL cholesterol levels in subjects with TG in a range of 2.0 – 4.5 mmol/ l compared to normotriglyceridemic subjects [11].
When we compared o ur data accor-ding to TG status, we have fo und hig-her assay bi as and worse correlati onsbetween the calculated LDL‑C and me asured ones in subjects with 1.7 ≤ TG < 4.5 mmol/ l. These findings are in accordance to previ o usly published data for the Fri edewald and LDL3 formulas [3,8], however, it has been suggested that apoB based LDL2 formula is less affected by incre ased TG levels [8,9].
Our study has several limitati ons. First of all, we used a homogeneo us LDL‑C assay and not the reference β - qu antificati on method to me asure LDL‑C levels. However, homogeneo us assays seem to be able to meet current NCEP requirements for LDL‑C testing for both precisi on and accuracy. Se-cond, the results of the hypertriglyceridemic gro up is based on 16 observati ons only, therefore we can not exclude spuri o us findings due to small number of observati ons. Moreover, we did not se arch for presence of type III hyperli-pidemi a for which the Fri edewald formula is not valid. Even that type III hyperlipidemi a is an uncommon genetic disorder of lipoprotein metabolism we thus can not exclude a presence of type III pati ent(s) in o ur set of subjects.
In conclusi on, the Fri edewald formula seems to be a better estimator of LDL‑C in o ur study than the other two alternative formulas, both in normo and hypertriglyceridemic subjects (with TG < 4.5 mmol/ l), but it underestimated the LDL‑C levels me asured by direct method for LDL‑C me asurement (Randox, UK). We sho uld however consider, that all the current clinical guidelines and recommendati ons for lipid monitoring, tre atment and cardi ovascular dise ase preventi on are based on data from large epidemi ological studi es and clinical tri als, that have estimated LDL cholesterol by the Fri edewald formula. Therefore in a ro utine clinical practice underestimati on of LDL cholesterol levels by the Fri edewald formula compared to the direct LDL me asurement in fact does not cre ate a significant problem.
Acknowledgements
The study was supported by the VEGA grant of Slovak Ministry of Educati on (AFAV 1/ 7236/ 20) and the Grant Agency of Scho ol of Medicine, Comeni us University, Bratislava, Slovaki a
Branislav Vohno ut, M.D., Ph.D.
www.rm.unicatt.it
e‑mail: branislav.vohno ut@rm.unicatt.it
Doručeno do redakce: 15. 5. 2008
Přijato po recenzi: 19. 6. 2008
Zdroje
1. Na uck M, Rifai N. Analytical performance and clinical efficacy of three ro utine procedures for LDL cholesterol me asurement compared with the ultracentrifugati on - dextran sulfate-Mg2+ method. Clin Chim Acta 2000; 294 : 77 – 92.
2. Fri edewald WT, Levy RI, Fredrickson DS. Estimati on of the concentrati on of the low - density lipoprotein cholesterol in plasma witho ut the use of preparative ultra centrifugati on. Clin Chem 1972; 18 : 499 – 509.
3. Na uck M, Warnick GR, Rifai N. Methods for me asurement of LDL‑cholesterol: a critical assessment of direct me asurement by homogeneo us assays versus calculati on. Clin Chem 2002; 48 : 236 – 254.
4. Planella T, Cortes M, Martinez - Bru C et al. Calculati on of LDL‑cholesterol by using apolipoprotein B for classificati on of nonchylomicronemic dyslipemi a. Clin Chem 1997; 43 : 808 – 815.
5. Hattori Y, Suzuki M, Tsushima M et al. Development of approximate formula for LDL‑chol, LDL‑apo B and LDL‑chol/ LDL‑apo B as indices of hyperapobetalipoproteinemi a and small dense LDL. Atherosclerosis 1998; 138 : 289 – 299.
6. Sniderman AD, Furberg CD, Keech A et al. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin tre atment. Lancet 2003; 361 : 777 – 780.
7. Bairaktari E, Elisaf M, Tzallas Ch et al. Evalu ati on of five methods for determining low - density lipoprotein cholesterol (LDL‑C) in hemodi alysis pati ents. Clin Bi ochem 2001; 34 : 593 – 602.
8. Bairaktari ET, Tzallas C, Kali entzido u M et al. Evalu ati on of alternative calculati on methods for determining low - density lipoprotein cholesterol in hemodi alysis pati ents. Clin Bi ochem 2004; 37 : 937 – 940.
9. Bairaktari E, Hatzidimo u K, Tzallas C et al. Estimati on of LDL cholesterol based on the Fri edewald formula and on apo B levels. Clin Bi ochem 2000; 33 : 549 – 555.
10. Tremblay AJ, Morrissette H, Gagne JM et al. Validati on of the Fri edewald formula for the determinati on of low - density lipoprotein cholesterol compared with beta‑qu antificati on in a large populati on. Clin Bi ochem 2004; 7 : 785 – 790.
11. Vachulová A, Vohno ut B, Blažíček P et al. Vzťah kalkulovaného LDL cholesterolu k hladinám apolipoproteínu B u subjektov s normálnymi a zvýšenými hladinami tri acylglycerolov. Cardi ol 2005; 14 : 88 – 91.
Štítky
Diabetologie Endokrinologie Interní lékařství
Článek vyšel v časopiseVnitřní lékařství
Nejčtenější tento týden
2008 Číslo 10- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Pregabalin je účinné léčivo s příznivým bezpečnostním profilem pro pacienty s neuropatickou bolestí
-
Všechny články tohoto čísla
- Hemopurification in sepsis: current view
- Cardiac Arrhythmias in Obstructive Sleep Apnea
- The isolated form of cardiac amyloidosis in the form of beginning infiltrative cardiomyopathy without restrictive physiology
- Myopathy and mixed hyperlipoproteinemia as the first symptom of systemic AL-amyloidosis
- XIV. medzinárodný kongres pre výživu a metabolizmus pri chorobách obličiek, 11.–15. júna 2008, Marseille (Francúzsko)
- Z odborné literatury
- Zahajovací zdravice XV. kongresu České internistické společnosti České lékařské společnosti J. E. Purkyně, Brno, 24. 9. 2008
- Internistická moudrost – matka celé klinické medicíny. Metamorfózy internistova myšlení a praktického konání
- Komentář k novým Doporučením o diagnostice a léčbě plicní embolie Evropské kardiologické společnosti (EKS)(Torbicki et al 2008) ve světle Doporučení diagnostiky,léčby a prevence plicní embolie České kardiologické společnosti – verze 2007
- Stanovení LDL‑cholesterolu – stále nevyřešený problém: vypočíst, nebo změřit? – editorial
- Nukleární kardiologie: postradatelná, či nepostradatelná? – editorial
- Srdeční amyloidóza – editorial
- Srdeční amyloidóza – podceňovaná hrozba? – editorial
- Immediate and long‑term results of conventionally performed radiofrequency catheter ablations of paroxysmal atrial fibrillation
- Evaluation of alternative calculation methods for determining LDL cholesterol
- Biomarkers of myocardial ischemia and necrosis in 2008
-
Zobrazovací metody v diagnostice viability myokardu.
Část 1. Interpretace nálezů při zobrazování viability myokardu pomocí SPECT a PET - Colorectal carcinoma and diabetes mellitus
- Dual antiplatelet treatment
- Hemophilia
- Vnitřní lékařství
- Archiv čísel
- Aktuální číslo
- Pouze online
- Informace o časopisu
Nejčtenější v tomto čísle- Dual antiplatelet treatment
- Cardiac Arrhythmias in Obstructive Sleep Apnea
- Stanovení LDL‑cholesterolu – stále nevyřešený problém: vypočíst, nebo změřit? – editorial
- Hemopurification in sepsis: current view
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



