-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDevelopment of a treatment of pregnant women suffering from epilepsy in the region of Ostrava between the years 1991 and 2006
Vývoj terapie těhotných žen s epilepsií v ostravském regionu v letech 1991 až 2006
Práce monitoruje dlouhodobý trend v používání antiepileptik během těhotenství a sleduje jejich koncentrace při porodu. Jako zdroj informací byly použity údaje ze žádanek a koncentrace antiepileptik stanovené v rámci rutinního terapeutického monitorování při porodu v letech 1991–2006. Ve skupině 235 těhotenství jsme během tří pětiletých period sledovali použití mono - versus polyterapie antiepileptiky, četnost výskytu jednotlivých antiepileptik i jejich kombinací a naměřené koncentrace. Monoterapie antiepileptiky byla předepisována v letech 1991–1995 v 61 %, v letech 1996–2000 v 68 % a v letech 2001–2006 u 76 % těhotných. Nejvíce užívanými byly během prvního období fenytoin, karbamazepin a primidon, během druhého období karbamazepin, fenytoin a kyselina valproová a ve třetím období karbamazepin, lamotrigin a kyselina valproová. Většina naměřených koncentrací byla pod terapeutickým rozmezím s výjimkou karbamazepinu ve všech periodách a primidonu v druhém a třetím období. Během 15letého sledování jsme prokázali signifikantní posun v léčbě epilepsie těhotných z poly - na monoterapii a z prvo - a druho-generačních antiepileptik na látky druhé a třetí generace. Ve srovnání s daty švédských autorů došlo u nás k posunu o 10 let později a podíl monoterapie byl stále mírně nižší, avšak naše údaje byly srovnatelné s údaji EURAPu. Výsledky ukazují správný vývoj terapie těhotných epileptiček v ostravském regionu České republiky.
Klíčová slova:
těhotenství – epilepsie – antiepileptika – terapeutické monitorování
Autoři: I. Kacířová; M. Grundmann; B. Kořístková; H. Brozmanová
Působiště autorů: Department of Clinical Pharmacology, Medical Faculty, University Ostrava and University Hospital, Ostrava
Vyšlo v časopise: Čes. slov. Farm., 2010; 59, 172-178
Kategorie: Původní práce
Souhrn
The paper discusses long-term trends in the utilization of antiepileptic drugs during pregnancy and reports an analysis of their concentrations at delivery. The request forms for routine therapeutic drug monitoring and concentrations of antiepileptic drugs at delivery collected between the years 1991–2006 were used as the data source. Monotherapy versus polytherapy, the utilization of individual antiepileptic drugs and combinations, and concentrations were monitored in a group of 235 pregnancies within three 5-year periods. Monotherapy was used in 61% in the years 1991–1995, in 68% in the years 1996-2000, and in 76% in the years 2001–2006. During the1st period, the most frequently prescribed agents were phenytoin, carbamazepine and primidone, during the 2nd period, carbamazepine, phenytoin, and valproic acid, and during the 3rd period, carbamazepine, lamotrigine, and valproic acid. Concentrations were mostly under the therapeutics range with an exception of carbamazepine and primidone. Our data demonstrate a significant shift from poly - to monotherapy and from the first - and second-generation to the second - and third-generation of antiepileptic drugs in pregnant women suffering from epilepsy. The shift from poly - to monotherapy has taken place 10 years later and, moreover, the rate of monotherapy was lower than in Sweden but the result was in a good agreement with the EURAP report. Our results evidence a good progress in the treatment of pregnant women suffering from epilepsy in the region of Ostrava.
Key words:
pregnancy – epilepsy – antiepileptic drugs – therapeutic drug monitoringIntroduction
Epilepsy is a neurological disorder with a prevalence of approximately 0.5–1.5% in general population 1) and women suffering from epilepsy have been estimated to account for 0.3–0.4% of all pregnancies 2). Until 1993, six so-called old-generation (or first - and second-generation) antiepileptic drugs (AEDs) (phenytoin, phenobarbital, primidone, ethosuximide, carbamazepine and valproate) accounted for the overwhelming majority of prescriptions written for the treatment of epilepsy. Since 1993, felbamate, gabapentin, lamotrigine, topiramate, tiagabine, oxcarbazepine, levetiracetam, zonisamide and pregabalin (so-called new-generation or third-generation antiepileptic drugs) have been approved for the treatment of epilepsy 3). In the Czech Republic, carbamazepine for focal (partial) or secondarily generalised tonic-clonic seizures, valproate and lamotrigine for all type of seizures when the epileptic syndrome is not precisely identified, but the type of seizures is known, are recommended as the first choice of AEDs 4).
Treatment decisions for pregnant women suffering from epilepsy are complicated by the lack of evidence-based data on the comparative teratogenic potential of different AEDs. However, the most recent consensus guidelines issued by the American Academy of Neurology, the American College of Obstetricians and Gynecologists, and the International League against Epilepsy recommend:
- a) optimization treatment prior to conception,
- b) to use monotherapy if possible,
- c) to choose the most effective AED for the seizure type and syndrome,
- d) to use the lowest effective dose,
- e) to supplement treatment with folate,
- f) to treat the child with vitamin K at birth and possibly the mother late in pregnancy for AEDs that interfere with vitamin K.
Nevertheless, no recommendations related to different AEDs teratogenetic risk are offered 5). Pregnancy can affect the pharmacokinetics of AEDs at all levels, absorption, distribution, metabolism and elimination, resulting in declining plasma concentrations of AEDs as the pregnancy progresses. The alterations can be expected but their magnitude is difficult to predict. Therefore regular therapeutic drug monitoring (TDM) of AEDs during pregnancy and postpartum is recommend 6). There are only a few papers following-up the development of AEDs therapy during pregnancy. Wide et al. 7) analyzed AEDs therapy in a Swedish population over a period of 25 years. From 1999 some collected data of 42 countries are available in International Antiepileptic Drugs and Pregnancy Registry (EURAP) 8).
Long-term trends in the utilization of antiepileptic drugs during pregnancy in the region of Ostrava in the Czech Republic was the primary aim of our study, an analysis of maternal concentrations of AEDs at delivery being the secondary aim.
EXPERIMENTAL PART
Methods
The request forms for routine therapeutic drug monitoring and maternal AEDs plasma levels measured at delivery were used as the data source. The study involved all samples from pregnant women treated with AEDs and collected in our department between the years 1991–2006. During three study periods (1991–1995, 1996–2000, 2001–2006), AEDs monotherapy versus polytherapy, the utilization of individual AEDs and the utilization of combinations of AEDs, and plasma levels were analyzed. Plasma concentrations of AEDs were measured by gas chromatography and liquid chromatography 9–12). Collected data were statistically analyzed by χ2 test.
Cohort
The study group consisted of 235 women. Maternal age and weight are presented in Table 1. A total daily dose of AEDs and a daily dose related to the body weight are stated in Table 2 and Table 3.
Tab. 1. Characteristics of the cohort: maternal age (years) and weight (kg); weight has not been recorded in all cases 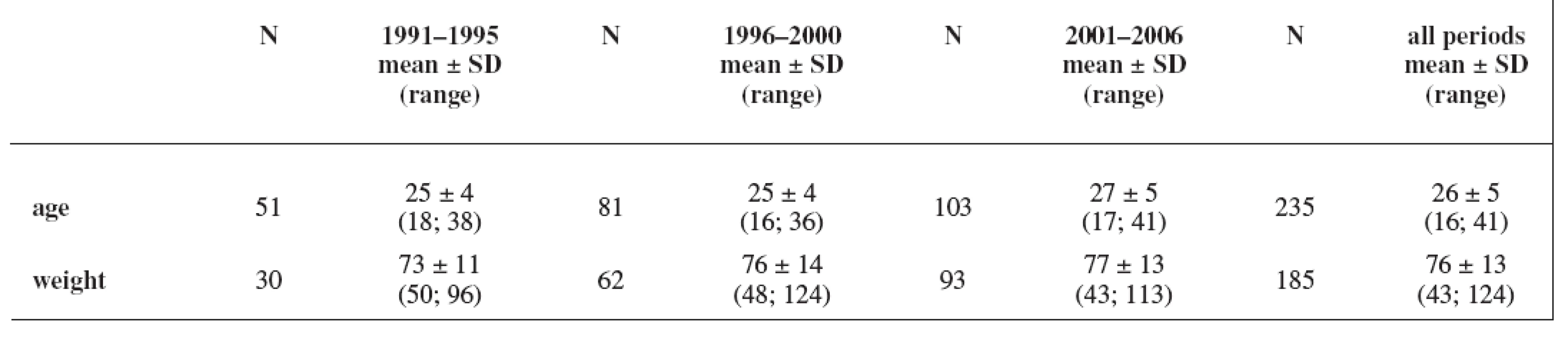
Tab. 2. Total daily dose (mg); values have not been recorded in all cases 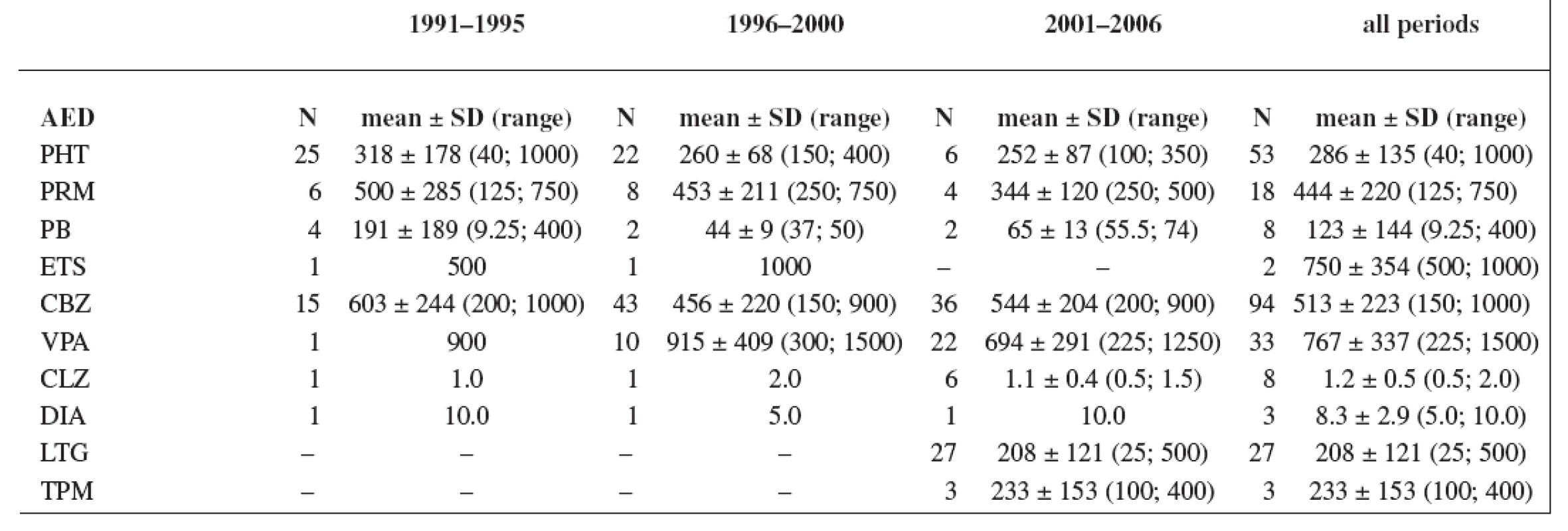
Tab. 3. Daily dose related to the body weight (mg/kg); values have not been recorded in all cases 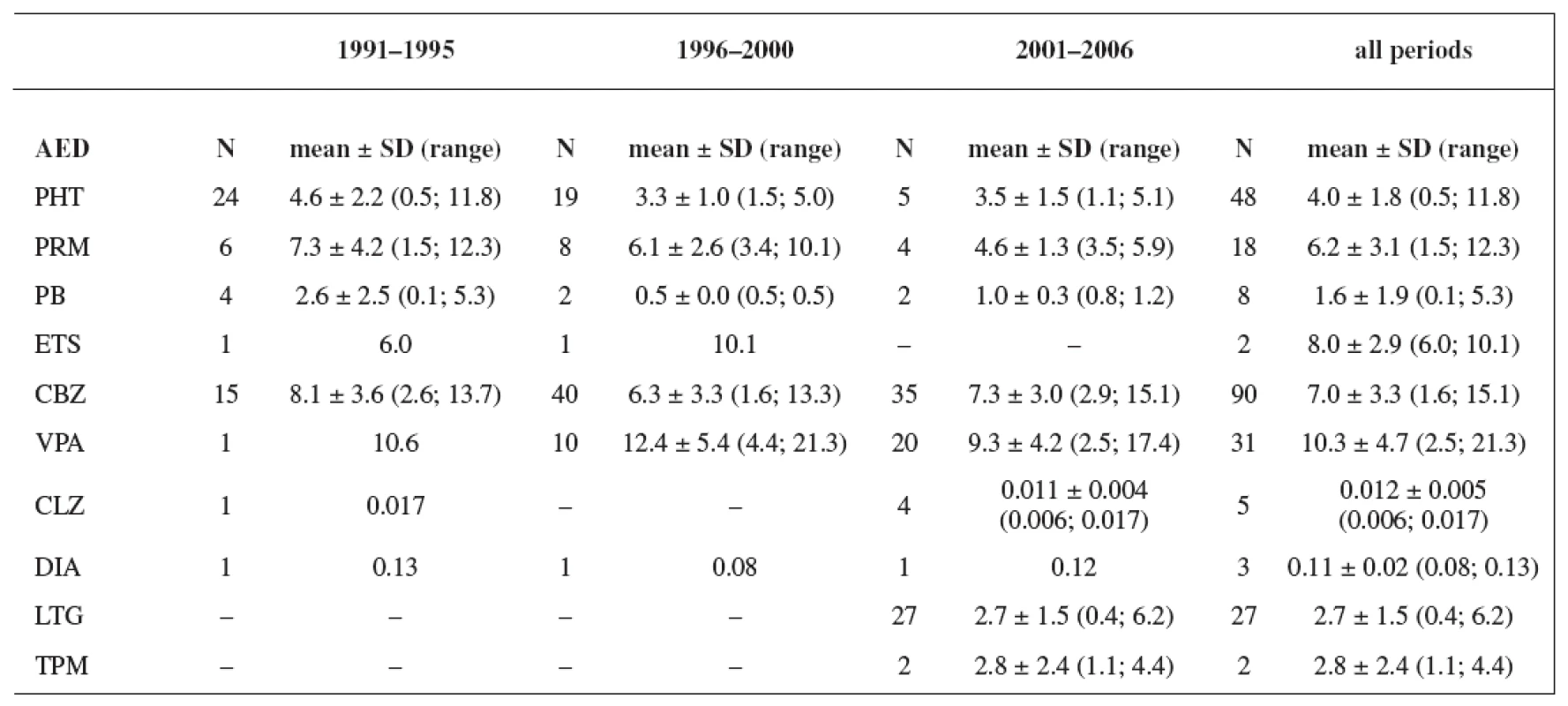
Results
Monotherapy was used during the first period in 61% of women, during the second period in 68% of women, and in the third period in 76% of women. Utilization of monotherapy in the third period was significantly higher than in the first period (p < 0.05) (Fig. 1). The most frequently prescribed AEDs independently of mono - and polytherapy during the first study period were phenytoin (PHT) (45.3%), carbamazepine (CBZ) (24.0%) and primidone (PRM) (9.3%), during the second period CBZ (50.5%), PHT (21.0%) and valproic acid (VPA) (11.4%), and during the third period CBZ (34.6%), lamotrigine (LTG) (24.4%) and VPA (21.3%), respectively. The most widely administered AEDs and combinations in relation to mono - and polytherapy were found during the firststudy period PHT (33.3%), CBZ (13.7%) and CBZ+PHT (13.7%), during the second period CBZ (44.4%), PHT (13.6%) and CBZ+VPA (8.6%), and during the third period CBZ (31.0%), LTG (22.3%) and VPA (12.6%), respectively (Table 4).
Fig. 1. Mono- versus polytherapy of AEDs 
Tab. 4. A: Utilization of individual AEDs during study periods; B: Utilization of monotherapy and combinations of AEDs during study periods 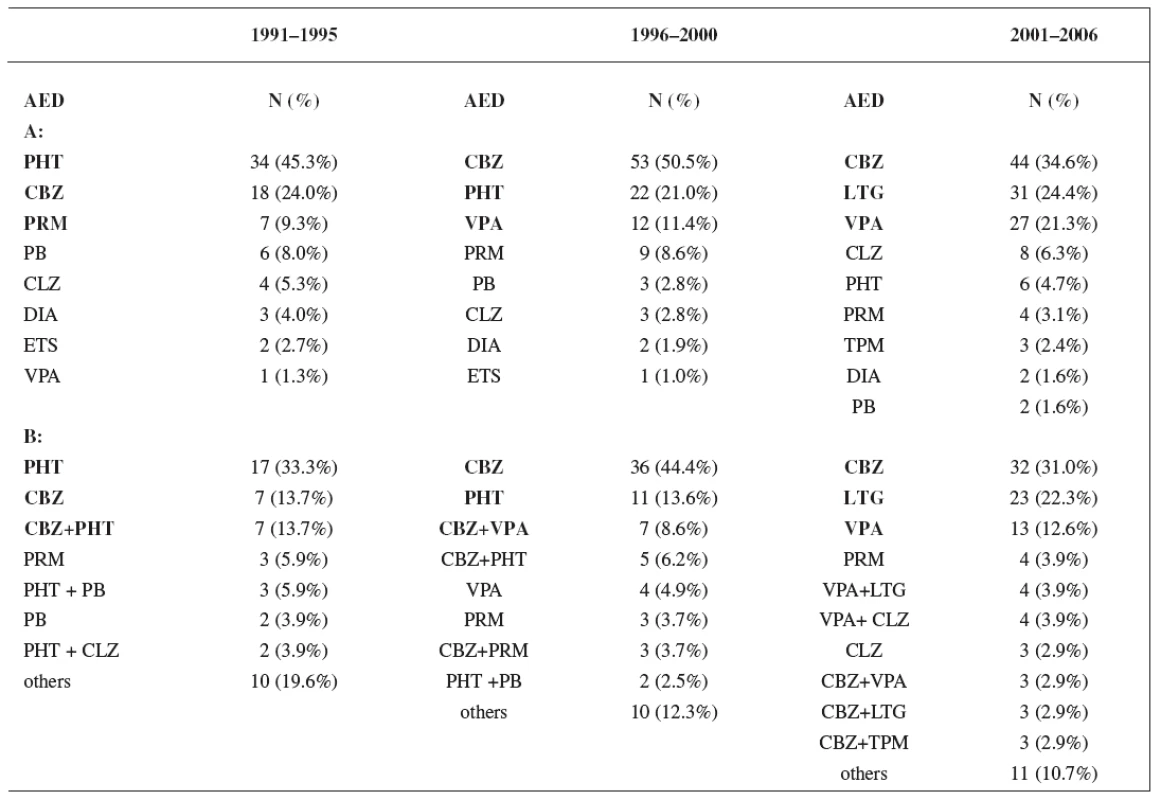
In all three study periods plasma levels of individual AEDs were mostly under the therapeutic range (estimated for non-pregnant women) with an exception of CBZ (during the first period 50% of women were in the therapeutics range, during the second period 40% of women were in the therapeutics range, and during the third period 45% of women were in the therapeutics range) and PRM (during the second period 44% of women were in the therapeutics range and 11% of women were above the therapeutic range, and during the third period 50% of women were in the therapeutics range) (Tables 5 and 6). Non-detectable concentrations have been found in 6.0% of patients, most frequently for lamotrigine followed by phenytoin, carbamazepine and primidone, indicating possible non-compliance.
Tab. 5. AEDs concentrations (mg/L; CLZ-μg/L) 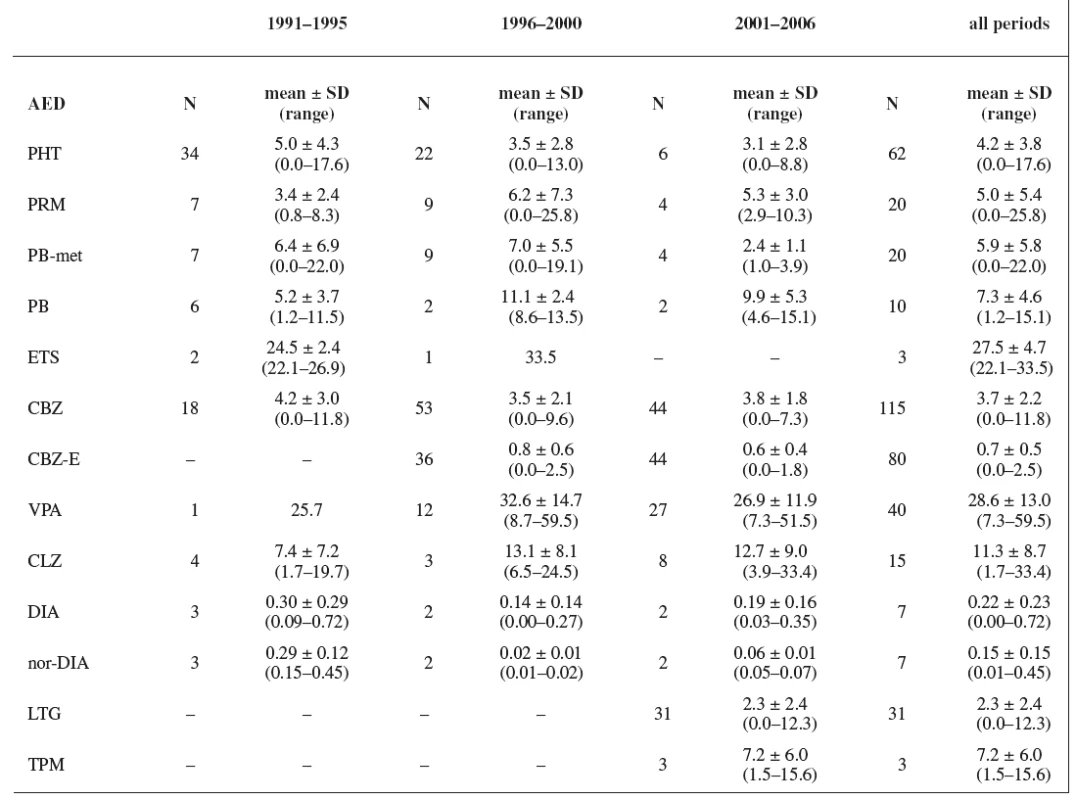
Tab. 6. Rate of AEDs concentrations in the therapeutic range 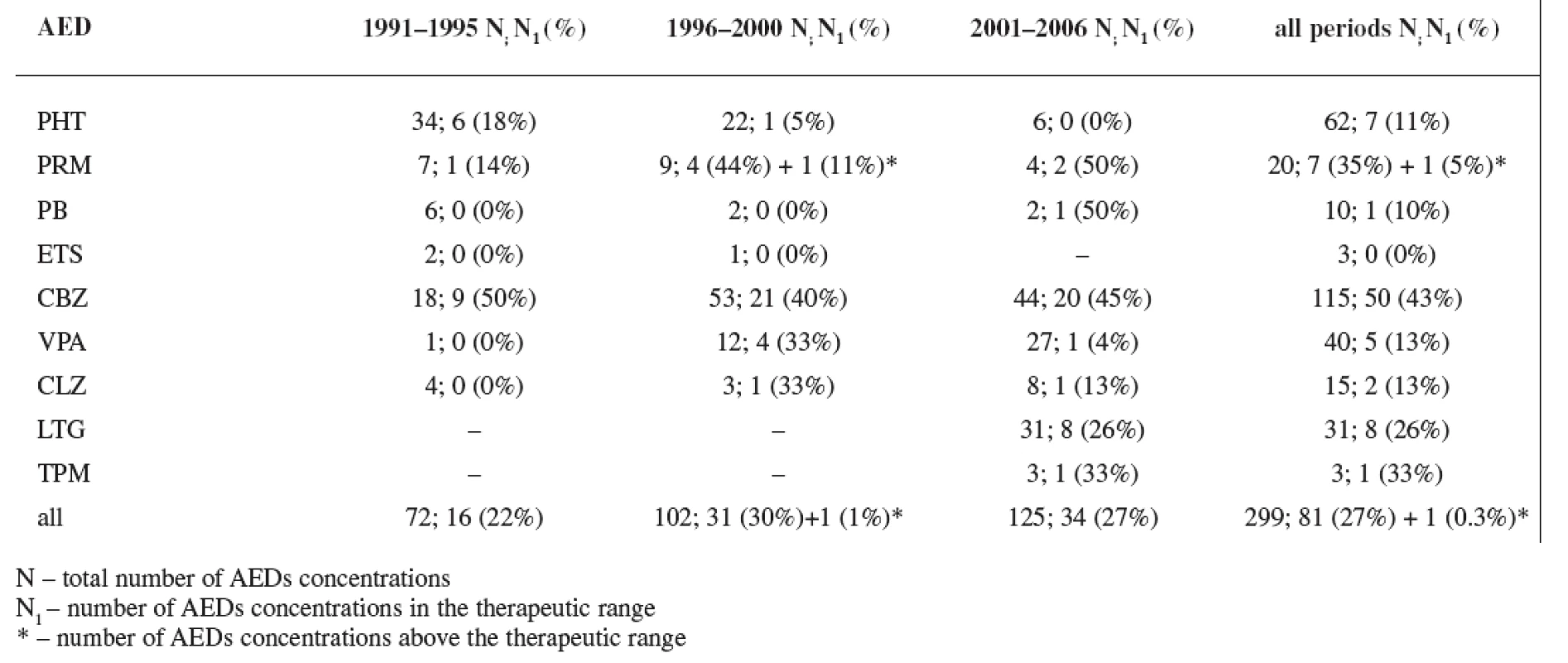
Discussion
Our data showed a significant shift over the years from AEDs polytherapy to monotherapy, which is in good agreement with the recommendations of the American Academy of Neurology, the American College of Obstetricians and Gynecologists, and the International League against Epilepsy 5) for the treatment of pregnant women suffering from epilepsy as well as for the treatment of epilepsy in general population 14). The result of our third study period was comparable with the EURAP group 8) but the rate of monotherapy we found lower than in the latest data set of Wide et al. 7) in the years 1995–1997 (Table 7). Earlier results of Wide et al. 7) showed monotherapy of AEDs in the first data set (1973–1981) only in 47% of the mothers (polytherapy in 53%) and in the second data set (1984–1994) monotherapy was used in 82% of the mothers (polytherapy in 18%).
Tab. 7. Comparison of monotherapy rate in three studies 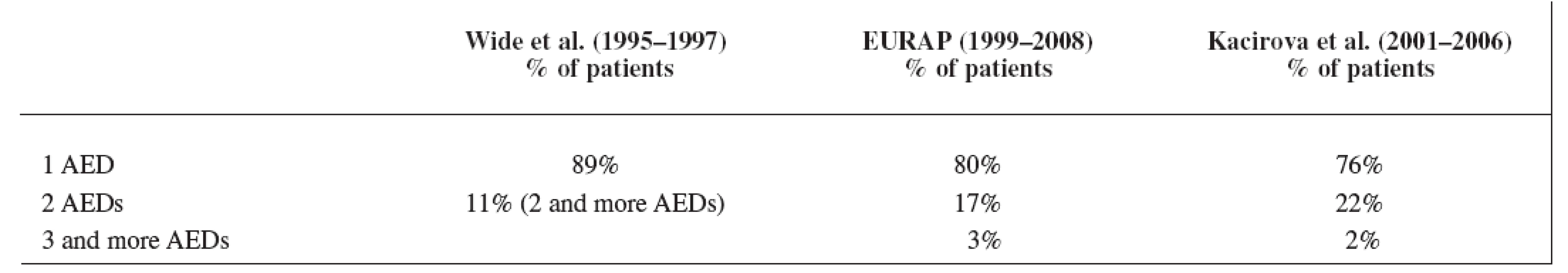
Carbamazepine was the most often used antiepileptic drug in all three compared studies, followed by lamotrigine and valproic acid in the EURAP 8) and in our study group (Table 8). In the study of Wide et al. 7), carbamazepine was followed by valproic acid and phenytoin due to earlier time of analysis.
Tab. 8. Comparison of the most often used AEDs in three studies 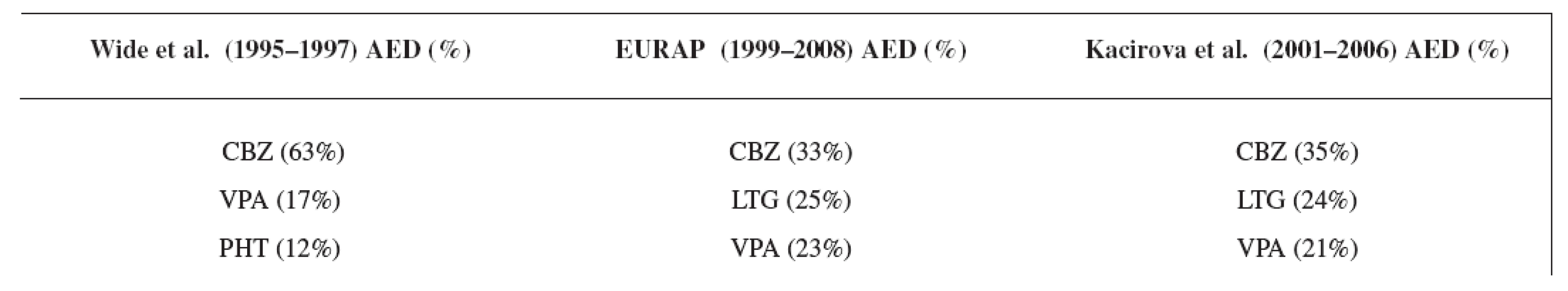
Plasma levels of AEDs during pregnancy were recorded only by Wide et al. 13) between the years 1985 and 1995. During the third trimester, plasma concentrations of CBZ (N = 39) ranged from 11 to 32 μmol/L (2.6–7.6 mg/L) with a mean of 20 μmol/L (4.7 mg/L) and plasma concentrations of PHT (N = 22) ranging from 5 to 78 μmol/L (1.3–19.7 mg/L) with a mean 20 μmol/L (5.1 mg/L). The mean of CBZ and PHT plasma levels measured during our first study period was similar as that recorded by Wide et al. 13). The results of our study concerning to the plasma levels are more complex and include TDM of primidone, phenobarbital, phenobarbital as a metabolite of primidone, ethosuximide, phenytoin, valproic acid, carbamazepine, epoxy-metabolite of carbamazepine, clonazepam, diazepam and its metabolite nordiazepam, lamotrigine and topiramate. The most of the measured values were under the usual therapeutic range for non-pregnant patients, which was caused either by gestational alterations in the pharmacokinetics of AEDs at all levels (absorption, distribution, metabolism and elimination) 6) or by non-compliance of pregnant women. These changes could be a reason for the failure to attain the expected effect of AEDs treatment 14).
In conclusion, our data demonstrate a significant shift from AEDs poly - to monotherapy and from the first - and second - generation AEDs (PHT, CBZ and PRM) to the second - and third-generation AEDs (CBZ, LTG and VPA) in the treatment of pregnanty women suffering from epilepsy between the years 1991 and 2006 in our study group. The result is in good agreement with the EURAP report 8). The shift from AEDs poly - to monotherapy has taken place ten years later than in the period described by Wide et al. 7) and, moreover, the rate of monotherapy in the third period of our study was lower than in the latest data set of Wide et al. 7) of Sweden who had excellent access to prenatal care 15). One of the reasons of discrepancy between the Swedish and our results could be gestational alterations in the pharmacokinetics of AEDs resulting in declining plasma concentrations of AEDs as pregnancy progresses 6), which correlate with the plasma levels under the usual therapeutic range for the most of AEDs in our study group. This changes may result in a failure of AED monotherapy 14) followed by an addition of another antiepileptic drug (polytherapy) instead of dose adjustment. For example, in the case of lamotrigine, an increased seizure frequency in the second trimester was associated with a lower ratio of current LTG concentration to the baseline target concentration, and a ratio < 0.65 was a significant predictor of seizure worsening 16). Nevertheless, our results in the third study period evidence a good progress in the treatment of pregnant women suffering from epilepsy in the region of Ostrava in the Czech Republic. Therapeutic drug monitoring of AEDs during pregnancy and after delivery can be helpful to optimize the treatment in women suffering from epilepsy in this period of unstable kinetics.
Therapeutic range
phenytoin: 10–20 mg/L
primidone: 5–15 mg/L
phenobarbital: 15–35 mg/L
ethosuximide: 40–100 mg/L
carbamazepine: 4–12 mg/L (in years 1991–2001)
4–9 mg/L (in years 2002–2006)
valproic acid: 40–100 mg/L (in years 1991–2000)
50–100 mg/L (in years 2001–2006)
clonazepam: 20–80 μg/L
lamotrigine: 3–14 mg/L
topiramate: 5–20 mg/LAbreviations
- AEDs – antiepileptic drugs
- CBZ – carbamazepine
- CBZ-E – carbamazepine - 10,11-epoxide
- CLZ – clonazepam
- DIA – diazepam
- ETS – ethosuximide
- EURAP – International Antiepileptic Drugs and Pregnancy Registry
- LTG – lamotrigine
- N – number
- nor-DIA – nordiazepam
- PB – phenobarbital
- PB-met – phenobarbital as a metabolite of primidone
- PHT – phenytoin
- PRM – primidone
- TDM – therapeutic drug monitoring
- TPM – topiramate
- VPA – valproic acid
Address for correspondence:
MUDr. Ivana Kacířová
Ústav klinické farmakologie FN
17. listopadu 1790, 708 52 Ostrava
e-mail: ivana.kacirova@fnspo.cz
Zdroje
1. Genton, P., Semah, F., Trinka, E.: Valproic Acid in Epilepsy. Pregnancy-Related Issues. Drug. Saf., 2006; 29, 1–21.
2. Tomson, T., Battino, D.: Pharmacokinetics and Therapeutic Drug Monitoring of Newer Antiepileptic Drugs During Pregnancy and the Puerperium. Clin. Pharmacokinet., 2007; 46, 209–219.
3. Bourgeois, B. F. D.: Broader is Better. The Ranks of Broad-Spectrum Antiepileptic Drugs are Growing. Neurology, 2007; 69, 1734–1736.
4. EpiStop: Minimální diagnostické a terapeutické standardy. Terapie epilepsie podle typu záchvatů. Praha: Maxdorf 2007; 26 (www.epistop.cz).
5. Meador, K. J., Baker, G. A., Finnell, R. H., Kalayjian, L. A., Liporace, J. D., Loring, D. W. et al. for the NEAD Study Group: In utero antiepileptic drug exposure: Fetal death and malformations. Neurology, 2006; 67, 407–412.
6. Sabers, A., Tomson, T.: Managing antiepileptic drugs during pregnancy and lactation. Curr. Opin. Neurol., 2009; 22, 157–161.
7. Wide, K., Winbladh, B., Tomson, T., Källén, B.: Body dimensions of infants exposed to antiepileptic drugs in utero: observations spanning 25 years study. Epilepsia, 2000; 41, 854–861.
8. EURAP. An International Antiepileptic Drugs and Pregnancy Registry. Interim Report May 2008. www. eurapinternational.org.
9. Brozmanova, H., Grundmann, M.: First experiences with therapeutic monitoring of antiepileptics. Cesk. Fysiol., 1985; 34, 416.
10. Grundmann, M., Brozmanova, H., Bobcikova, P.: The changes of metabolism of primidone during comedication with phenytoin and carbamazepine. Ther. Drug. Monit., 1999; 21, 451.
11. Malakova, J., Brozmanova, H., Vorisek, V., Prochazkova, V., Palicka, V.: A capillary GC method using nitrogen phosphorus detection for determination of topiramate in patients with epilepsy. Chromatographia, 2007; 66, 363–367.
12. Budakova, L., Brozmanova, H., Grundmann, M., Fischer, J.: Simultaneous determination of antiepileptic drugs and two active metabolites by HPLC. J. Separ. Sci., 2008; 31, 1–8.
13. Wide, K., Winbladh, B., Tomson, T., Sars-Zimmer, K., Berggren, E.: Psychomotor development and minor anomalies in children exposed to antiepileptic drugs in utero. A prospective population based study. Dev. Med. Child. Neurol., 2000; 42, 87–92.
14. Donath, V.: Zlyhanie farmakologickej antiepileptickej liečby. Cesk. Slov. Neurol. N., 2008; 71/104, 134–138.
15. Finnell, R.H., Burn, J.: Effect of anti-epileptic drugs on intrauterine growth. Lancet, 2000; 356, 1537–1538.
16. Pennell, P. B., Peng, L., Newport, D. J., Ritchie, J. C., Koganti, A., Holley, D. K., Newman, M., Stowe, Z. N.: Lamotrigine in pregnancy: Clearance, therapeutic drug monitoring, and seizure frequency. Neurology, 2008; 70 (22, part 2 of 2), 2130–2136.
Štítky
Farmacie Farmakologie
Článek NOVÉ KNIHY
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2010 Číslo 4- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Antimikróbne pôsobiace látky produkované baktériami mliečneho kvasenia
-
Standardní receptura pro přípravu léčivých přípravků v lékárnách IV
Čím může být užitečná sbírka Formularium hospitale
- Řízené uvolňování léčiv z lékových forem obalených vodnými celulosovými a akrylátovými polymerními disperzemi
- Development of a treatment of pregnant women suffering from epilepsy in the region of Ostrava between the years 1991 and 2006
- Technológia tabliet s riadeným uvoľňovaním s obsahom veľmi dobre rozpustných liečiv
-
Pracovní den Sekce přírodních léčiv České farmaceutické společnosti ČLS JEP
Hradecký den léčivých rostlin
Hradec Králové, 11. červen 2010
- Farmaceuti rokovali v Ružomberku
- NOVÉ KNIHY
- Doc. RNDr. Marián Bukovský, PhD. jubiluje
- Jaroslav Květina – osmdesátiletý
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle-
Standardní receptura pro přípravu léčivých přípravků v lékárnách IV
Čím může být užitečná sbírka Formularium hospitale
- Technológia tabliet s riadeným uvoľňovaním s obsahom veľmi dobre rozpustných liečiv
- Řízené uvolňování léčiv z lékových forem obalených vodnými celulosovými a akrylátovými polymerními disperzemi
- Antimikróbne pôsobiace látky produkované baktériami mliečneho kvasenia
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



