-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMaternal age and severe maternal morbidity: A population-based retrospective cohort study
Using population-based data including all singleton births to women residing in Washington State 2003 to 2013, Sarka Lisonkova and colleagues calculated age-specific rates of adverse maternal and neonate outcomes.
Published in the journal: . PLoS Med 14(5): e32767. doi:10.1371/journal.pmed.1002307
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002307Summary
Using population-based data including all singleton births to women residing in Washington State 2003 to 2013, Sarka Lisonkova and colleagues calculated age-specific rates of adverse maternal and neonate outcomes.
Introduction
One of the United Nations’ Millennium Development Goals of 2000 was to reduce maternal mortality by 75% in 15 y [1], a challenge that spurred an interest in maternal mortality and morbidity [2,3]. This challenge was not met by many industrialized countries [4–6]. Moreover, recent reports suggest an increase in maternal deaths in the United States, with the maternal mortality ratio rising from 12 per 100,000 live births in 1990 to 14 per 100,000 live births in 2015 [6–9]. Several explanations have been offered for the increase, including improved ascertainment of maternal deaths, especially those resulting from indirect obstetric causes and late deaths occurring after 42 d postpartum [10], and a temporal increase in chronic health conditions in the child-bearing population. Past decades have seen a rise in the number of parturient women with hypertension, diabetes, chronic heart disease, obesity [11–15], and advanced maternal age [16–21], reflecting an increased complexity of obstetric care, requiring careful prenatal monitoring and timely obstetric interventions.
One of the most remarkable recent demographic changes in industrialized countries has been an upward shift in maternal age [16–21]. In the US, for example, the proportion of births to teenage mothers declined from 12.8% in 1990 to 7.0% in 2013, while the proportion of births to women over 40 y increased from 1.2% to 2.8% [16,17]. The age-specific fertility rate declined from 59.9 births per 1,000 women in 1990 to 24.2 in 2014 for women aged 15–19 y, while the rate doubled among women aged 40–44 y (from 5.5 to 10.6 per 1,000 women) and quadrupled among women aged ≥45 y (from 0.2 to 0.8 births per 1,000 women) [17]. In the United Kingdom, the fertility rate declined from 33.3 per 1,000 women in 1990 to 14.5 in 2015 for women under 20 y but increased from 5.3 to 15.2 per 1,000 women for women ≥40 y over the same period [21]. In 2015, for the first time since 1947, the fertility rate among women aged 40 y or more rose above the rate in women under 20 y in the UK [21].
The effect of older maternal age on elevated rates of cesarean delivery, fetal death, and neonatal mortality and morbidity is well recognized [19,22–28]. While these infant outcomes have been extensively studied using broadly accessible information from birth certificates (e.g., stillbirth and linked live-birth infant death files from the US National Center for Health Statistics) and birth registries, little information is available on the consequences for the health of the mother. This gap leads to clinical uncertainty in risk identification. Our objective was to examine the association between maternal age and severe maternal morbidity using detailed information from a unique link of two population databases—live birth/fetal death certificates and maternal hospitalization files—in Washington State, US. We hypothesized that, besides the well-recognized elevated rates of fetal and neonatal mortality and morbidity at the extremes of maternal age, the risks of severe maternal morbidity would also be significantly increased.
Methods
All analyses were performed on publicly accessible de-identified data. An exemption from ethics approval was granted by the Department of Social and Health Services of Washington State. This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (S1 Checklist).
We studied all singleton live births and stillbirths to mothers aged 15–60 y in Washington State from 1 January 2003 to 31 December 2013. We used information from two linked population databases: (a) live birth/fetal death certificates with information on demographics and pregnancy and birth characteristics and (b) hospitalization files including information on specific maternal and infant morbidity. The Birth Events Record Database (BERD) (the database has since been renamed: Linked Birth—CHARS, 1987–2013; http://www.doh.wa.gov/DataandStatisticalReports/VitalStatisticsData/OrderDataFiles#Linked) includes information on maternal characteristics (maternal age, race, education, marital status, body mass index [BMI], chronic hypertension, diabetes mellitus, obstetric history, etc.); pregnancy, labor, and delivery characteristics (gestational diabetes, hypertension in pregnancy, gestational age at delivery, mode of delivery, prolonged labor, etc.); and birth outcomes (fetal and neonatal death, Apgar score at 5 min, birth weight, occurrence of neonatal seizures, neonatal intensive care unit [NICU] admission, etc.). The BERD data were linked with the Comprehensive Hospital Abstract Reporting System (CHARS) database, which includes all hospitalizations in Washington State and up to nine diagnostic and six procedure codes related to each hospitalization episode. We used maternal delivery hospitalization records for information on maternal death and maternal morbidity during delivery and the type of health insurance coverage, and infant birth hospitalization records for detailed information on severe neonatal morbidity and congenital anomalies diagnosed at birth. We did not include multiple pregnancies because the BERD dataset did not provide linkage between births from the same pregnancy (even though they were flagged as twins, triplets, etc.), and their inclusion in our study would therefore compromise the analysis (i.e., mothers of twins would be included twice in the dataset). In addition, multiple pregnancies are clinically distinct from singleton pregnancies (e.g., they have lower average gestational age at delivery, and some complications occur only among multiples, including twin-to-twin transfusion syndrome). Mothers aged <15 y were not included in the study, as they have distinct problems with respect to child abuse, low socioeconomic status, etc.
The originally proposed analysis was to examine maternal age in the following categories: 15–19, 20–24, 25–29 (reference category), 30–34, 35–39, 40–44, and ≥45 y, with additional analyses of the outcomes with higher incidence among women ≥50 y. This categorization is commonly used in the literature, and the age categories span equal numbers of years; maternal age ≥35 y is generally considered advanced maternal age, while maternal age ≥40 y is considered “very advanced” maternal age. In the revised analysis, we included the maternal age category 45–49 y to examine severe morbidities with higher incidence rates, and a category ≥50 y for a composite maternal death/severe morbidity outcome (see below). Maternal death was identified from the CHARS database using the hospital discharge code for death. ICD-9-CM diagnostic and procedure codes were used to identify severe maternal morbidity. Severe maternal morbidity was identified using criteria developed by the Canadian Perinatal Surveillance System [29] based on high case fatality (e.g., sepsis), vital organ function damage (e.g., acute renal failure), high resource utilization (e.g., major surgical procedures), and important adverse sequelae (e.g., peripartum hysterectomy) (see S1 Table). We included additional conditions recognized as severe maternal morbidity by the US Centers for Disease Control and Prevention [30,31]: life-saving procedures such as mechanical ventilation, conversion of cardiac rhythm, etc. Severe maternal morbidity was divided into the following categories: (1) antepartum hemorrhage that required transfusion, e.g., placenta previa, placental abruption; (2) respiratory morbidity, e.g., obstetric pulmonary embolism, respiratory arrest; (3) thromboembolism, e.g., deep venous thrombosis; (4) cerebrovascular morbidity, e.g., cerebral venous thromboembolism, intracranial hemorrhage; (5) all acute cardiac morbidity, e.g., acute myocardial infarction, cardiac arrest, heart failure, peripartum cardiomyopathy; (6) severe postpartum hemorrhage (PPH) requiring transfusion; (7) maternal sepsis, e.g., major puerperal infection, septicemia during labor; (8) renal failure; (9) obstetric shock; (10) complications of anesthesia and obstetric interventions, including shock due to anesthesia, cardiac and pulmonary complications; and (11) severe morbidity denoted by the need for potentially life-saving procedures, e.g., transfusion, hysterectomy, mechanical ventilation. These categories aimed to describe specific groups of severe morbid conditions and were not mutually exclusive. A composite outcome of maternal death/severe morbidity included any of these conditions or procedures; other potentially life-threatening conditions, such as disseminated intravascular coagulation (DIC), eclampsia, uterine rupture, or acute liver failure (see S1 Table); admission to an intensive care unit (ICU); and maternal mortality. In addition, we aimed to describe the maternal age-specific incidence of specific morbidities, such as amniotic fluid embolism (AFE) and peripartum cardiomyopathy, and the rate of admission to an ICU.
Neonatal death was defined as infant death within 28 d after birth. Perinatal mortality was defined as stillbirth (intrapartum or antepartum fetal death after 20 wk of gestation) or neonatal death. Severe neonatal morbidity was identified from the CHARS database using the ICD-9-CM diagnostic codes and included conditions such as bronchopulmonary dysplasia, respiratory distress syndrome, retinopathy of prematurity, intraventricular hemorrhage grade 3 or more, intracranial hemorrhage, neonatal sepsis, necrotizing enterocolitis, and severe birth trauma (S1 Table). The occurrence of any of these conditions, perinatal mortality, or neonatal seizures composed a composite outcome of perinatal death/severe neonatal morbidity. In addition, we compared maternal age-specific incidence of other adverse birth outcomes, including preterm birth (<37 wk) and very preterm birth (<34 wk), small for gestational age (<10th percentile), large for gestational age (>90th percentile), Apgar score < 3 at 5 min, and NICU admission. SGA and LGA percentiles were derived from the US population [32].
Crude odds ratios (ORs) and 95% confidence intervals (CIs) for each maternal age category (relative to 25–29 y) were used to describe the association between maternal age and severe morbidities; we did not perform overall statistical significance tests yielding p-values. Logistic regression was used to adjust for demographic and pre-pregnancy characteristics, including race (non-Hispanic white, African-American, Hispanic, Native American, and other—including Asian), marital status (single/widowed/separated versus married/common law), pre-pregnancy BMI, smoking during pregnancy (no versus yes), parity, maternal education (high school graduate or more education versus less than high school graduation), type of health insurance (Medicaid, private, self-pay, other), fetal sex (female versus male), and year of childbirth. Parity and BMI were modelled using splines with a maximum of three knots. Sensitivity analyses were performed to adjust for underlying medical conditions (chronic hypertension, diabetes mellitus) and assisted conception, to examine their impact on the results. In addition, other pregnancy and labor characteristics (e.g., mode of delivery, labor induction) were added as covariates. These factors can be considered as mediators on the causal pathway between maternal age and maternal severe morbidity and adverse perinatal outcomes, and require strong assumptions about the directionality of associations, and were therefore not adjusted for in the main analyses. However, adjusting for these factors adds additional insight into the risks of severe maternal morbidity among older women who do not have any apparent chronic condition or indication for cesarean delivery.
Besides ORs and adjusted ORs (AORs) and their 95% CIs, we also present crude and adjusted risk differences (ARDs) with their 95% CIs to convey the magnitude of absolute risk increases. The adjusted age-specific risk difference indicates the additional number of mothers with adverse outcome above the baseline rate that would be expected if the same demographic and pre-pregnancy characteristics as the reference group (25–29 y) were present.
Analyses were carried out using SAS 9.4. Missing values for BMI (approximately 10%) were imputed using the multiple imputation procedure (PROC MI) in SAS. Other missing values that constituted <3% of the total for a factor are not included in the tables, but are presented in S2 Table. Except for BMI missing values (which were imputed), complete-case multivariable analyses were performed.
Results
Overall, 952,212 mothers gave birth (live or stillbirth) in Washington State between 1 January 2003 and 31 December 2013. We excluded births that occurred out of state, multiple births, births before 20 wk gestation, births to females aged <15 or >60 y (35,598 mothers, 3.7%), births that occurred out of hospital (24,716 mothers, 2.6%), and births that could not be matched with hospital records (64,609 mothers, 6.8%). The remaining 828,269 births were included in the study. Women who delivered out of hospital or whose birth could not be matched with hospital records were not substantially different from those included in the study, except for type of health insurance (they were more likely to have “other” insurance—other government insurance, student insurance, Indian Health Care, other health insurance programs, or no insurance; S2 Table).
The largest proportion of births was to mothers 25–29 y of age (28.9%), 25.3% of births were to mothers aged 30–35 y, and 22.5% of births were to mothers aged 20–24 y. Births to teenage mothers comprised 7.6%, while births to older mothers aged 35–39, 40–44, and ≥45 y comprised 12.7%, 2.8%, and 0.2% of all singleton births, respectively. Teenage mothers were more likely to be Hispanic or African-American, while older mothers (35–39, 40–44, and ≥45 y) were more likely to be non-Hispanic white or “other” than were younger mothers (Table 1).
Tab. 1. Maternal age-specific demographic and pregnancy characteristics, singleton births, Washington State, US, 2003–2013. 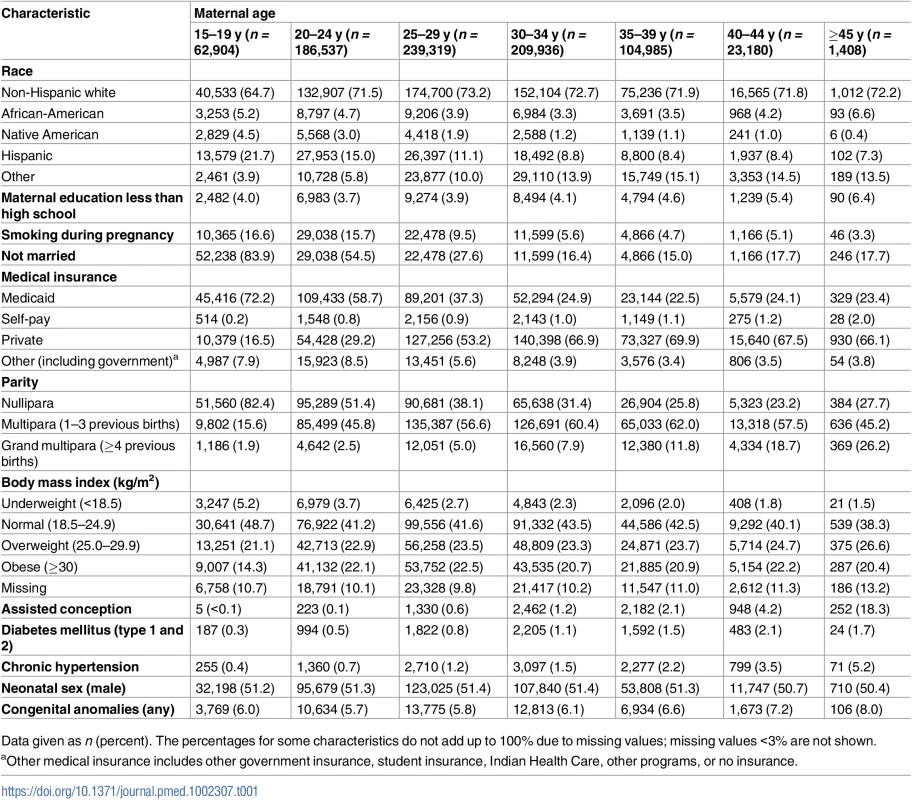
Data given as n (percent). The percentages for some characteristics do not add up to 100% due to missing values; missing values <3% are not shown. The proportions of non-married, nulliparous, and underweight women declined with maternal age, while the proportions of women with assisted conception, obesity, diabetes mellitus, and chronic hypertension increased with age (Table 1). Cesarean delivery was more common among older women—especially primary and repeat cesarean delivery without labor (Table 2).
Tab. 2. Maternal age-specific labor and delivery characteristics, singleton births, Washington State, US, 2003–2013. 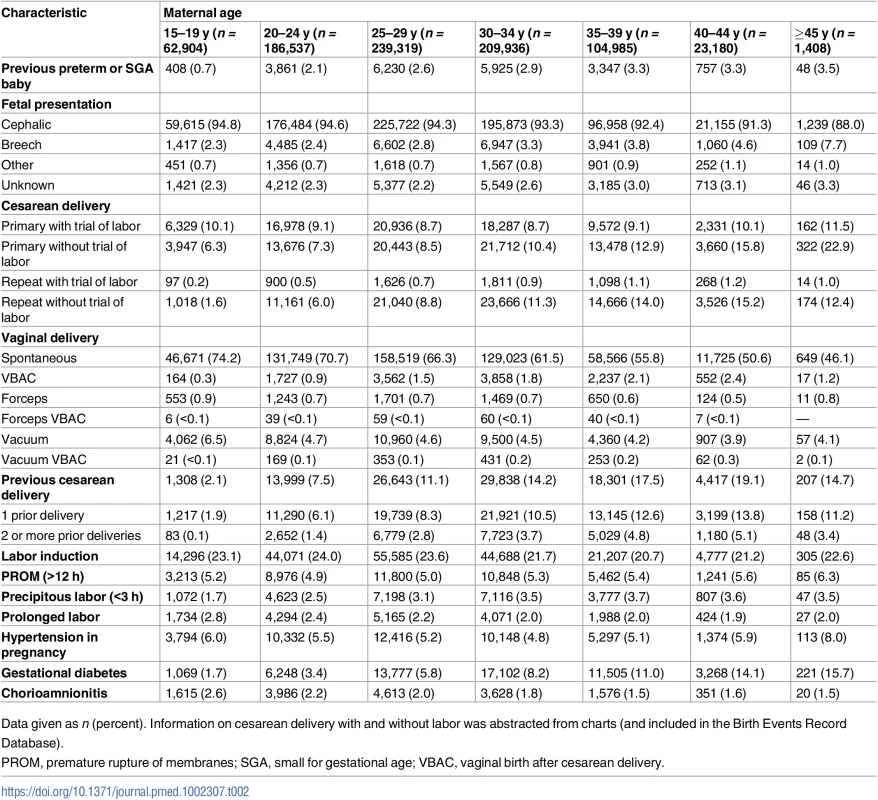
Data given as n (percent). Information on cesarean delivery with and without labor was abstracted from charts (and included in the Birth Events Record Database). The incidence of gestational diabetes increased markedly with maternal age, from 1.7% among teen mothers to 15.7% among the oldest mothers (45–60 y). Nearly all women had more than two prenatal care visits, with little variation between teenage mothers (97.7%) and the oldest mothers (98.4%).
The overall rate of severe maternal morbidity/mortality was 1.6 per 100 deliveries. Most severe maternal morbidity showed a J-shaped association with maternal age (Fig 1A; Table 3), with elevated rates among teenage mothers, low rates among mothers 20–34 y of age, and a sharp increase among mothers over 39 y of age.
Fig. 1. Age-specific rates of severe maternal morbidity and perinatal mortality/severe neonatal morbidity. 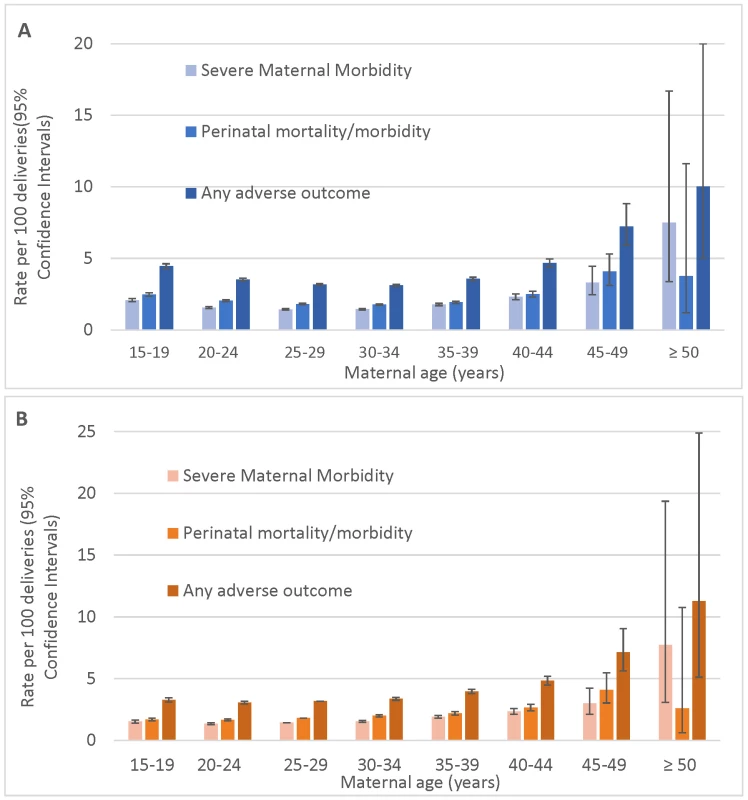
(A) Unadjusted rates and (B) rates adjusted for demographic and pre-pregnancy factors; singleton births, Washington State, US, 2003–2013. Bars show 95% CIs. Tab. 3. Maternal mortality and severe maternal morbidity and rate per 10,000 singleton births, Washington State, US, 2003–2013. 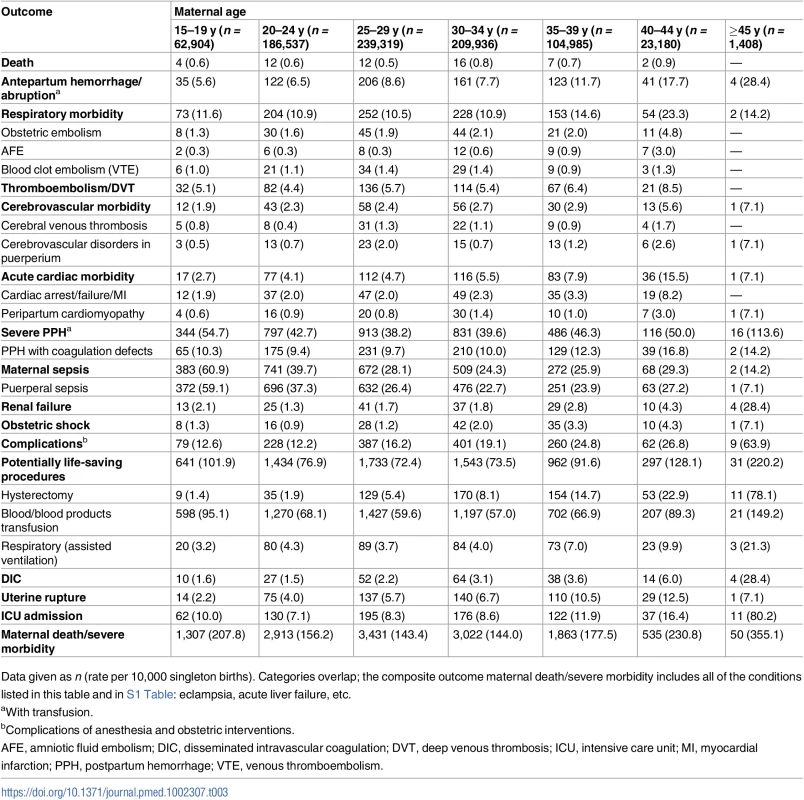
Data given as n (rate per 10,000 singleton births). Categories overlap; the composite outcome maternal death/severe morbidity includes all of the conditions listed in this table and in S1 Table: eclampsia, acute liver failure, etc. An exception was the rate of maternal sepsis, which was highest among teen mothers (65.8 per 10,000 deliveries) and declined with maternal age (to 14–35 per 10,000 deliveries among mothers over 34 y of age). Some severe morbid conditions had the lowest rate among teenage mothers, with the rate increasing with maternal age, e.g., obstetric embolism, AFE, acute cardiac morbidity, uterine rupture, and hysterectomy. The rates of severe PPH, renal failure, DIC, complications of obstetric interventions, and potentially life-saving procedures increased rapidly in women above 39 y. Similarly, the rate of ICU admission increased sharply among mothers over 44 y of age (80.2 per 10,000 deliveries, compared to 7.1 per 10,000 deliveries among mothers 20–24 y). The rate of the composite outcome of maternal death or severe morbidity was 2.1% among teen mothers, 1.5% among mothers aged 25–29 y, and 3.6% among mothers over 44 y of age. The J-shaped association was also evident between maternal age and fetal/infant outcomes (S3 Table), except for the rates of large for gestational age and macrosomia, which were lowest among teen mothers.
Teen mothers were not at significantly elevated risk of severe maternal morbidity compared to mothers aged 25–29 y after adjustment for maternal demographic and pre-pregnancy factors (Table 4; S1 Fig). They were significantly less likely to experience cerebrovascular morbidity, acute cardiac morbidity, and complications of obstetric interventions. However, teen mothers were at elevated risk of sepsis (AOR = 1.2, 95% CI 1.1–1.4). After adjustment, the ORs for most types of severe maternal morbidity remained significantly increased among women over 39 y of age. In particular, mothers 40–44 y had a 3-fold higher risk of shock (AOR = 2.9, 95% CI 1.3–6.6), an 8-fold increased risk of AFE (AOR = 8.0, 95% CI 2.7–23.7), and considerably elevated acute cardiac morbidity (AOR = 3.8, 95% CI 2.5–5.7), including cardiomyopathy (AOR = 4.7, 95% CI 1.9–11.4; Table 4). Mothers 45–49 y had higher risk of renal failure (AOR = 15.9, 95% CI 4.8–52), complications of obstetric interventions (AOR = 4.7, 95% CI 2.3–9.5), and ICU admission (AOR = 4.8, 95% CI 2.0–11.9).
Tab. 4. The association between maternal age and severe maternal morbidity and adverse birth outcomes among singleton births, Washington State, US, 2003–2013. 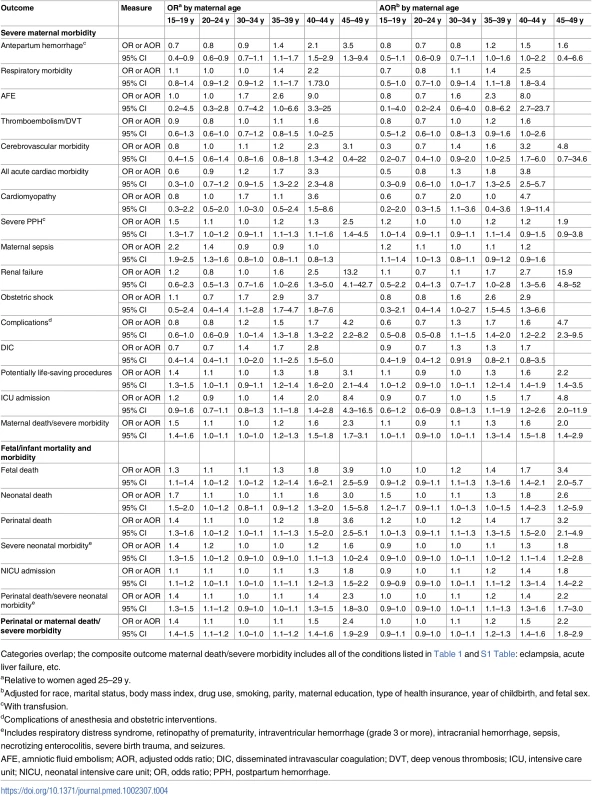
Categories overlap; the composite outcome maternal death/severe morbidity includes all of the conditions listed in Table 1 and S1 Table: eclampsia, acute liver failure, etc. While the unadjusted absolute rates of adverse maternal outcomes continued to increase with age among women over 39 y of age, the rates of adverse perinatal outcomes plateaued at maternal age ≥45 y (Fig 1A). The adjusted rates of mortality/severe morbidity were substantially lower (Fig 1B). The absolute rates of fetal and neonatal death and morbidity were elevated among teen mothers, and the neonatal death rate remained higher after adjustment for demographic and pre-pregnancy factors, while the rate of NICU admission was significantly lower among teen mothers after adjustment.
Additional analyses showed that severe maternal morbidity and/or mortality increased dramatically among the oldest mothers (≥50 y, n = 80). The AOR compared to mothers 25–29 y increased from 1.6 at 40–44 y (95% CI 1.5–1.8) to 2.0 at 45–49 y (95% CI 1.4–2.9), and to 5.2 at 50 y or more (95% CI 2.1–13.1). Such a trend among mothers ≥50 y was also observed for a composite outcome of any perinatal or maternal death/severe morbidity (AOR = 3.6, 95% CI 1.6–7.8).
Sensitivity analyses revealed that the association between maternal age 45–49 y and some severe maternal morbidities attenuated slightly after adjustment for chronic conditions (diabetes mellitus and chronic hypertension) and assisted conception; the AOR for renal failure was 11.4 (95% CI 3.3–39.3). Additional adjustment for labor characteristics and mode of delivery (S4 Table) further attenuated the association of age with some morbidities; however, a significant increase in many severe morbidities remained among women aged 40 y or more. The risk of renal failure remained significantly elevated among those ≥45 y even after additional adjustment for pre-pregnancy and pregnancy hypertension and for preeclampsia (including eclampsia and superimposed preeclampsia and eclampsia).
The adjusted risk difference (ARD) in overall severe maternal morbidity compared to mothers 25–29 y and those 45–49 y was 1.6% (95% CI 0.7%–2.8%) and increased to 6.4% for those ≥50 y (95% CI 1.7%–18.2%; S5 Table). The ARD for the composite outcome of any perinatal or maternal death/severe morbidity was 4.0% for women 45–49 y (95% CI 2.4%–5.9%) and 8.1% for women ≥50 y (95% CI 1.9%–21.6%).
Discussion
Our results show elevated rates of severe maternal morbidity at the extremes of maternal age. While the elevated risk among teenage mothers was mostly due to an increased rate of sepsis, rates of all other causes of severe maternal morbidity were elevated among older mothers (≥40 y). The absolute rates and ORs were lower after adjustment for demographic and pre-pregnancy factors but remained elevated for sepsis among teenage mothers and for all other morbidities among older women. Our results confirm that perinatal mortality and neonatal morbidity are elevated among teenage mothers and older mothers, as compared with mothers aged 25–29 y [22–25]. The association between teenage motherhood and most adverse perinatal outcomes disappeared after adjustment for maternal demographic factors, with the exception of the neonatal death rate, which remained 50% higher.
Incidence rates of maternal morbidity in industrialized countries vary greatly depending on the definition of “morbidity,” ranging from 0.4% to 31.1% [29–31,33–40], with the highest rates including any morbidity outside a normal delivery [40]. In the US, the population-based incidence of severe maternal morbidity increased from 0.73% of hospital deliveries in 1998/1999 to 1.63% in 2010/2011 [30,34], while hospital-based incidence rates ranged from 0.51% to 2.45% in 2012/2013 [31]. The incidence rate of severe maternal morbidity has been reported as 1.4% in both Canada (in 2007) [29] and Australia (in 2004) [35], 0.7% in the Netherlands (in 2004–2006) [36], 0.5% in England (in 2012/2013) [37], and 0.4% in Scotland (in 2001/2002) [38]. These differences in severe morbidity rates are mainly due to varying definitions of severe morbidity [29–31,33,36–40]. However, discrepancies may also arise due to differences in the study populations, which can be defined geographically (including all births in the country/region) [30,34–37] or as hospital-based or insurance-provider-based obstetric populations [31,38]. The number of maternal deaths in our study is relatively low in comparison with the US maternal mortality ratio reported by WHO [5,6]. However, the WHO reports include all maternal deaths during pregnancy and postpartum, while our study captured only deaths occurring during delivery hospitalization.
Aging leads to non-specific deterioration of most physiological functions [41], with chronological age being a marker for (but not equivalent to) biological or reproductive age. Elevated cardiac, cerebrovascular, and respiratory morbidity in older mothers can be partly attributed to physiological changes associated with aging, including reduced cardiac reserve, muscle atrophy, atherosclerosis and other changes in the vasculature, and reduced lung function. Such changes may not be clinically apparent in the absence of pregnancy, but the added physiological burden of pregnancy can reveal a decline in organ function. This conclusion is supported by our findings that adjustment for chronic hypertension and diabetes mellitus decreased the risk of renal failure and acute cardiac morbidity among women 35 y old or older. Pre-pregnancy risk factors, including higher BMI and assisted conception, may also be partly responsible for elevated severe morbidity among older mothers; elevated risks adjusted for pre-pregnancy factors were substantially attenuated among older women. Previous obstetric history (e.g., prior cesarean delivery) and aging of reproductive organs may lead to dysfunctional labor and higher rates of obstetric interventions (e.g., cesarean delivery), which increases the risk of complications and therefore severe morbidity.
Higher rates of renal failure among older mothers are notable. Studies from North America reported a temporal increase in the rate of renal failure from 2.3 per 10,000 delivery hospitalizations in 1998–1999 to 4.5 in 2008–2009 in the US [34], and from 1.6 per 10,000 deliveries in 2003 to 2.7 in 2010 in Canada [42]. The latter study also showed that the observed increase was predominantly among women with hypertensive disorders in pregnancy, e.g., preeclampsia, while the rate of acute renal failure was 2.8 times higher among mothers ≥40 y compared with those 20–24 y. Our results indicate an even larger (16-fold) increased risk among mothers 45 y or older. However, even after additional adjustment for pre-pregnancy and pregnancy hypertension, preeclampsia, and cesarean delivery, the risk of renal failure remained significantly elevated among women ≥45 y.
Our finding of an elevated risk of sepsis (including septicemia during labor, major puerperal infection, systemic inflammatory response, and septic shock) among teen mothers is also notable. Sepsis is recognized as one of the leading causes of maternal mortality [43]. Prior studies of the association between young maternal age and sepsis are sparse. A case—control study from Scotland reported a 5-fold increase in the adjusted odds of sepsis among mothers <25 y old compared with women older than 34 y [44], suggesting an even higher risk than we observed. Untreated sepsis may lead to a cascade of sequential organ failure. It is possible that the signs of generalized infection (hypotension, tachycardia, fever, oliguria, and hypoxemia) are masked among young mothers by other compensatory mechanisms, leading to a delay in timely recognition and antibiotic treatment. Young healthy women can compensate and mask symptoms such as decreased level of consciousness and pathologic hypotension. These findings warrant further research into specific risk factors, etiopathogeneses, and compensatory mechanisms among young mothers. They also warrant increased clinical vigilance and a reduced threshold for considering early antibiotics in young mothers presenting with possible signs of infection.
An increased risk of adverse perinatal outcomes with advancing maternal age is well documented in many studies [45–55]; some have also reported elevated risks of antepartum hemorrhage, PPH, preeclampsia, and ICU admission [45,53]. However, we are aware of no previous comprehensive assessment of associations between maternal age and potentially life-threatening maternal morbidity.
Strengths of our study include a large population database collected consistently over a prolonged study period and a unique breadth of relevant information from two linked databases. These strengths enabled us to examine associations between maternal age and severe maternal morbidity due to a wide variety of specific clinical conditions. Moreover, they provided better control for confounding by maternal demographic and clinical variables than previous studies when studying associations with adverse fetal/infant outcomes. For example, we were able to adjust for maternal BMI (even though we had to impute approximately 10% of values that were missing) and assisted conception. Such adjustment is not possible in most population-based studies, which are therefore prone to residual confounding by these variables. BMI was derived from the mother’s reported height and pre-pregnancy weight; this information has been included in the latest revision of the national standard birth certificate in the US (2003) [55]. BMI information is not validated using maternal pre-pregnancy measurement of height and weight, but patterns of obesity among non-Hispanic white, non-Hispanic black, non-Hispanic Asian, and Hispanic women from the National Health and Nutrition Examination Survey are comparable to those in the birth certificate data [56]. In addition, overall patterns of pre-pregnancy obesity by state from the birth certificates generally correspond with those of all adult women from the Behavioral Risk Factor Surveillance System [55,57]. Education was self-reported information abstracted from the medical charts. We used the indicator variable “less than high school graduation” as a proxy for low socioeconomic status. Teenage mothers were naturally less likely to have graduated from high school (due to their young age), which indicates that these women were more likely to be disadvantaged with respect to their socioeconomic status.
To maintain the consistency of our data sources, we limited the study to a time period when the latest version of the US birth certificate (introduced in 2003 in Washington State) was used to collect information on pregnancy, labor, delivery, and postpartum events, while hospitals continued to use ICD-9-CM codes. Using both validated data sources increases the detection rate of maternal morbid conditions [58,59]. Moreover, any potential underreporting would bias our results towards the null, as differential coding of major life-threatening medical events based on maternal age is unlikely.
Our study also has several limitations. First, despite our large sample size, we did not have sufficient statistical power to examine the association between maternal age and maternal death or very rare severe morbidities; we therefore created larger categories of morbid conditions and a composite outcome. Second, the degree of severity of some maternal morbidity was not possible to ascertain from available ICD-9-CM codes. For example, the code for eclampsia (ICD-9-CM code 642.6) did not include superimposed eclampsia (eclampsia after chronic hypertension). A specific code for superimposed eclampsia was not available in ICD-9-CM—only a code identifying mild/severe superimposed preeclampsia/eclampsia (ICD-9-CM code 642.7). This latter condition was not included in our composite outcome of severe maternal morbidity, as it would artificially inflate rates among older women, who are more likely to have chronic hypertension (and hence superimposed preeclampsia/eclampsia). The exclusion of this condition likely led to underestimated rates and ORs of severe maternal morbidity among older women. Third, errors and omissions in diagnostic coding are inevitable in all large administrative databases. In our study, however, these should have resulted in non-differential misclassification, as coding practices are unlikely to differ by maternal age. The observed rates of severe morbidity may therefore be underestimates, and the ORs biased towards the null. Finally, we were unable to adjust for income, which tends to be higher among women who delay childbearing (because some women delay childbearing in order to advance their career and achieve financial security) [60,61]. However, we did adjust for marital status, education, race, and the type of medical insurance—all of which are highly associated with income. We assessed a relatively large number of associations, and some may be statistically significant owing to chance. We excluded multiple births as they have specific pregnancy complications (with higher rates of preterm birth and maternal/perinatal morbidity), which were beyond the scope of this paper. This may affect the generalizability of our results to multifetal pregnancies. We were unable to account for potential correlations among outcomes among women who delivered more than once during the study period, because consecutive pregnancies are not linked in the BERD dataset. This could affect the risk estimates, especially for recurrent maternal morbidities such as severe PPH.
Conclusion
Although severe maternal morbidity is uncommon, older women experience increased risks of severe morbidity during pregnancy. Counselling of older mothers should include information on both fetal/neonatal risks and maternal risks. Health care providers should be aware of the severe morbidities that can arise among older mothers and that can impact obstetrical care delivery and resource utilization during labor, delivery, and postpartum.
Supporting Information
Zdroje
1. Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet. 2016;387(10017):462–74. doi: 10.1016/S0140-6736(15)00838-7 26584737
2. Chou D, Daelmans B, Jolivet RR, Kinney M, Say L, Every Newborn Action Plan (ENAP) and Ending Preventable Maternal Mortality (EPMM) working groups. Ending preventable maternal and newborn mortality and stillbirths. Priority interventions for maternal and newborn care. BMJ. 2015;351:h4255. doi: 10.1136/bmj.h4255 26371222
3. D’Alton M. Where is the “M” in maternal-fetal medicine. Obstet Gynecol. 2010;116 : 1401–4. doi: 10.1097/AOG.0b013e3181fd2556 21099610
4. Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384 : 980–1004. doi: 10.1016/S0140-6736(14)60696-6 24797575
5. World Health Organization, United Nations Children’s Fund, United Nations Population Fund, World Bank, United Nations Population Division. Trends in maternal mortality: 1990 to 2013. Estimates by WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. Geneva: World Health Organization; 2014 [cited 2017 Apr 21]. http://www.who.int/reproductivehealth/publications/monitoring/maternal-mortality-2013/en/.
6. World Health Organization, United Nations Children’s Fund, United Nations Population Fund, World Bank, United Nations Population Division. Trends in maternal mortality: 1990 to 2015. Estimates by WHO, UNICEF, UNFPA, The World Bank and the United Nations Population Division. Geneva: World Health Organization; 2015 [cited 2017 Apr 21]. http://www.who.int/reproductivehealth/publications/monitoring/maternal-mortality-2015/en/.
7. Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125 : 5–12. doi: 10.1097/AOG.0000000000000564 25560097
8. American Congress of Obstetricians and Gynecologists. ACOG statement on maternal mortality. Washington (District of Columbia): American Congress of Obstetricians and Gynecologists; 2015 May 4 [cited 2017 Apr 21]. http://www.acog.org/About-ACOG/News-Room/Statements/2015/ACOG-Statement-on-Maternal-Mortality.
9. Chescheir N. Enough already. Obstet Gynecol. 2015;125 : 1–4.
10. Joseph KS, Lisonkova S, Muraca GM, Razaz N, Sabr Y, Mehrabadi A, et al. Contribution of improved surveillance to temporal increases in maternal mortality in the United States. Obstet Gynecol. 2017;129(1):91–100.
11. Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States: 1998–2006. Obstet Gynecol. 2009;113(6):1299–306. doi: 10.1097/AOG.0b013e3181a45b25 19461426
12. Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, et al. Diabetes trends among delivery hospitalizations in the United States, 1994–2004. Diabetes Care. 2010;33(4):768–73. doi: 10.2337/dc09-1801 20067968
13. Kuklina EV, Callaghan WM. Chronic heart disease and severe obstetric morbidity among hospitalizations for pregnancy in the USA: 1995–2006. BJOG. 2011;118(3):345–52. doi: 10.1111/j.1471-0528.2010.02743.x 21091604
14. Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005–2008). J Rural Health. 2012;28 : 392–7. doi: 10.1111/j.1748-0361.2012.00411.x 23083085
15. Heslehurst N, Ells LJ, Simpson H, Batterham A, Wilkinson J, Summerbell CD. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG. 2007;114(2):187–94. 17305899
16. Osterman MJK, Kochanek KD, MacDorman MF, Strobina DM, Guyer BG. Annual summary of vital statistics: 2012–2013. Paediatrics. 2015;135(6):1115–25.
17. Mathews TJ, Hamilton BE. First births to older women continue to rise. NCHS Data Brief No. 152. Hyattsville (Maryland): National Center for Health Statistics; 2014.
18. Zeitlin J, Mohangoo A, Delnord M. European perinatal health report: health and care of pregnant women and babies in Europe in 2010. Paris: Euro-Peristat; 2010 [cited 2017 Apr 21]. http://www.europeristat.com/reports/european-perinatal-health-report-2010.html.
19. Johnson JA, Tough S, Society of Obstetricians and Gynaecologists of Canada. Delayed child-bearing. J Obstet Gynaecol Can. 2012;34(1):80–93. 22260768
20. Public Health Agency of Canada. Perinatal health indicators for Canada 2013: a report of the Canadian Perinatal Surveillance System. Ottawa: Public Health Agency of Canada; 2013 [cited 2017 Apr 21]. http://publications.gc.ca/collections/collection_2014/aspc-phac/HP7-1-2013-eng.pdf.
21. Office for National Statistics. Births in England and Wales: 2015. Live births, stillbirths, and the intensity of childbearing measured by the total fertility rate. London: Office for National Statistics; 2016 [cited 2017 Apr 21]. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthsummarytablesenglandandwales/2015.
22. Timofeev J, Reddy UM, Huang CC, Driggers RW, Landy HJ, Laughon SK. Obstetric complications, neonatal morbidity, and indications for caesarean delivery by maternal age. Obstet Gynecol. 2013;122(6):1184–95. doi: 10.1097/AOG.0000000000000017 24201681
23. Lisonkova S, Janssen PA, Sheps SB, Lee SK, Dahlgren LS. The effect of maternal age on adverse birth outcomes: does parity matter? J Obstet Gynaecol Can. 2010;32(6):541–8. 20569534
24. Reddy UM, Ko CW, Willinger M. Maternal age and the risk of stillbirth throughout pregnancy in the United States. Am J Obstet Gynecol. 2006;195(3):764–70. doi: 10.1016/j.ajog.2006.06.019 16949411
25. Salmeen K, Zlatnik M. The oldest gravidas: a review of pregnancy risks in women over 45. Obst Gynecol Surv. 2011;66(9):580–90.
26. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105 : 1410–8. doi: 10.1097/01.AOG.0000163256.83313.36 15932837
27. Callaway LK, Lust K, McIntyre HD. Pregnancy outcomes in women of very advanced maternal age. Aust N Z J Obstet Gynaecol. 2005;45(1):12–6. doi: 10.1111/j.1479-828X.2005.00333.x 15730358
28. Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. 2005;105 : 983–90. doi: 10.1097/01.AOG.0000158118.75532.51 15863534
29. Joseph KS, Liu S, Rouleau J, Kirby RS, Kramer MS, Sauve R, et al. Severe maternal morbidity in Canada, 2003 to 2007: surveillance using routine hospitalization data and ICD-10CA codes. J Obstet Gynaecol Can. 2010;32 : 837–46. 21050516
30. Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. Atlanta: Centers for Disease Control and Prevention; 2016 [cited 2017 Apr 21]. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html.
31. Main EK, Abreo A, McNulty J, Gilbert W, McNally C, Poeltler D, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol. 2016;214 : 643.e1–10.
32. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan MD. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8.
33. Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC, et al. Maternal mortality and mortality in the United States: where are we now? J Womens Health. 2014;23(1):3–9.
34. Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120 : 1029–36. 23090519
35. Roberts CL, Ford JB, Algert CS, Bell JC, Simpson JM, Morris JM. Trends in adverse maternal outcomes during childbirth: a population-based study of severe maternal morbidity. BMC Pregnancy Childbirth. 2009;9 : 7. doi: 10.1186/1471-2393-9-7 19243578
36. Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population-based study of 371,000 pregnancies. BJOG. 2008;115(7):842–50. doi: 10.1111/j.1471-0528.2008.01713.x 18485162
37. Nair M, Kurinczuk JJ, Knight M. Establishing a national maternal morbidity outcome indicator in England: a population-based study using routine hospital data. PLoS ONE. 2016; 11(4):e0153370. doi: 10.1371/journal.pone.0153370 27054761
38. Brace V, Penney G, Hall M. Quantifying severe maternal morbidity: a Scottish population study. BJOG. 2004;111(5):481–4. doi: 10.1111/j.1471-0528.2004.00101.x 15104614
39. Say L, Pattinson RC, Gulmezoglu AM. WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss). Reprod Health. 2004;1(1):3. doi: 10.1186/1742-4755-1-3 15357863
40. Danel I, Berg C, Johnson CH, Atrash H. Magnitude of maternal morbidity during labor and delivery: United States, 1993–1997. Am J Public Health. 2003;93(4):631–4. 12660209
41. Cohen WR. Does maternal age affect pregnancy outcome? BJOG. 2014;121 : 252–4. doi: 10.1111/1471-0528.12563 24428449
42. Mehrabadi A, Liu S, Batholomew S, Hutcheon JA, Magee LA, Kramer MS, et al. Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: population based retrospective cohort study. BMJ. 2014;349:g4731. doi: 10.1136/bmj.g4731 25077825
43. Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk JJ, editors. Saving lives, improving mothers’ care: lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012. Oxford: National Perinatal Epidemiology Unit; 2014.
44. Acosta C, Bhattacharya S, Tuffnell D, Kurinczuk J, Knight M. Maternal sepsis: a Scottish population-based case—control study. BJOG. 2012;119 : 474–83. 22251396
45. Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, Knight M. Pregnancy at very advanced maternal age: a UK population-based cohort study. BJOG. 2016 Sep 1.
46. McCall SJ, Nair M, Knight M. Factors associated with maternal mortality at advanced maternal age: a population-based case-control study. BJOG. 2016 Jul 13.
47. Schimmel MS, Bromiker R, Hammerman C, Chertman L, Ioscovich A, Granovsky-Grisaru S, et al. The effects of maternal age and parity on maternal and neonatal outcome. Arch Gynecol Obstet. 2015;291(4):793–8. doi: 10.1007/s00404-014-3469-0 25227657
48. Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, et al. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG. 2014;121(Suppl 1):49–56.
49. Mutz-Dehbalaie I, Scheier M, Jerabek-Klestil S, Brantner C, Windbichler GH, Leitner H, et al. Perinatal mortality and advanced maternal age. Gynecol Obstet Invest. 2014;77(1):50–7. doi: 10.1159/000357168 24356234
50. Barton JR, Sibai AJ, Istwan NB, Rhea DJ, Desch CN, Sibai BM. Spontaneously conceived pregnancy after 40: influence of age and obesity on outcome. Am J Perinatol. 2014;31(9):795–8. doi: 10.1055/s-0033-1359716 24338114
51. Klemetti R, Gissler M, Sainio S, Hemminki E. Associations of maternal age with maternity care use and birth outcomes in primiparous women: a comparison of results in 1991 and 2008 in Finland. BJOG. 2014;121(3):356–62. doi: 10.1111/1471-0528.12415 23944685
52. Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013;42(6):634–43. 23630102
53. Carolan MC, Davey MA, Biro M, Kealy M. Very advanced maternal age and morbidity in Victoria, Australia: a population based study. BMC Pregnancy Childbirth. 2013;13 : 80. doi: 10.1186/1471-2393-13-80 23537152
54. Kenny LC, Lavender T, McNamee R, O’Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS ONE. 2013;8(2):e56583. doi: 10.1371/journal.pone.0056583 23437176
55. Branum A, Kirmeyer SE, Gregory ECW. Pre-pregnancy body mass index by maternal characteristics and state: data from the birth certificate, 2014. Natl Vital Stat Rep. 2016; 65(6):1–11. 27508894
56. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief No. 219. Hyattsville (Maryland): National Center for Health Statistics; 2015 [cited 2017 Apr 21]. http://www.cdc.gov/nchs/data/databriefs/db219.pdf.
57. National Center for Chronic Disease Prevention and Health Promotion. BRFSS prevalence & trends data. Atlanta: Centers for Disease Control and Prevention; 2017 [cited 2017 Apr 21]. http://www.cdc.gov/brfss/brfssprevalence/.
58. Lydon-Rochelle MT, Holt VL, Cardenas V, Nelson JC, Easterling TR, Gardella C, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193(1):125–34. doi: 10.1016/j.ajog.2005.02.096 16021070
59. Lydon-Rochelle MT, Holt VL, Nelson JC, Cardenas V, Gardella C, Easterling TR, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol. 2005;19 : 460–71. doi: 10.1111/j.1365-3016.2005.00682.x 16269074
60. Tough S, Tofflemire K, Benzies K, Fraser-Lee N, Newburn-Cook C. Factors influencing childbearing decisions and knowledge of perinatal risks among Canadian men and women. Matern Child Health J. 2007;11 : 189–98. doi: 10.1007/s10995-006-0156-1 17237994
61. Bayrampour H, Heaman M. Comparison of demographic and obstetric characteristics of Canadian primiparous women of advanced maternal age and younger age. J Obstet Gynaecol Can. 2011;33(8):820–9. 21846437
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 5- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Rotavirus vaccine will have an impact in Asia
- Towards control of the global HIV epidemic: Addressing the middle-90 challenge in the UNAIDS 90–90–90 target
- Ebola exposure, illness experience, and Ebola antibody prevalence in international responders to the West African Ebola epidemic 2014–2016: A cross-sectional study
- Long-term inpatient disease burden in the Adult Life after Childhood Cancer in Scandinavia (ALiCCS) study: A cohort study of 21,297 childhood cancer survivors
- Contribution of systemic and somatic factors to clinical response and resistance to PD-L1 blockade in urothelial cancer: An exploratory multi-omic analysis
- Association between expansion of primary healthcare and racial inequalities in mortality amenable to primary care in Brazil: A national longitudinal analysis
- Vitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: A Mendelian randomization study
- Maternal age and severe maternal morbidity: A population-based retrospective cohort study
- Measuring personal beliefs and perceived norms about intimate partner violence: Population-based survey experiment in rural Uganda
- A universal testing and treatment intervention to improve HIV control: One-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial
- Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: A model-based analysis
- First-trimester artemisinin derivatives and quinine treatments and the risk of adverse pregnancy outcomes in Africa and Asia: A meta-analysis of observational studies
- Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of infection in Ethiopia: A randomized controlled trial
- Impact evaluation of different cash-based intervention modalities on child and maternal nutritional status in Sindh Province, Pakistan, at 6 mo and at 1 y: A cluster randomised controlled trial
- Tobacco control: Developing an innovative and effective global strategy
- Data sharing in clinical trials: An experience with two large cancer screening trials
- Mortality and kidnapping estimates for the Yazidi population in the area of Mount Sinjar, Iraq, in August 2014: A retrospective household survey
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mortality and kidnapping estimates for the Yazidi population in the area of Mount Sinjar, Iraq, in August 2014: A retrospective household survey
- Vitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: A Mendelian randomization study
- Maternal age and severe maternal morbidity: A population-based retrospective cohort study
- Estimation of the cost-effectiveness of HIV prevention portfolios for people who inject drugs in the United States: A model-based analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



