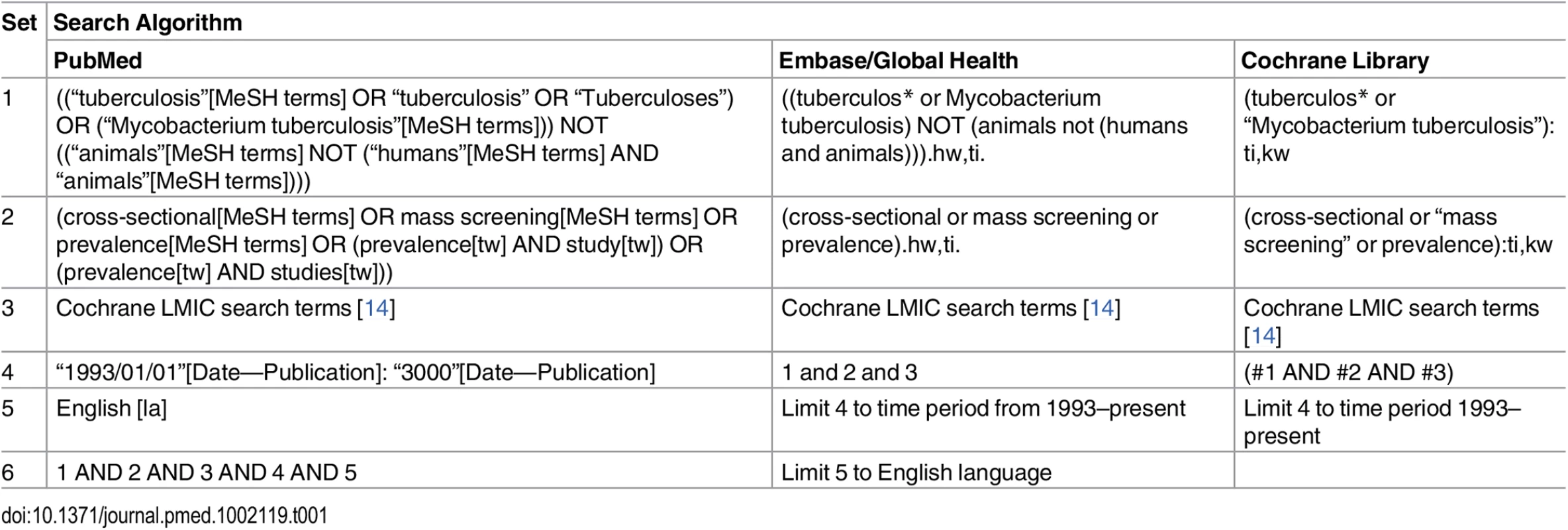
-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSex Differences in Tuberculosis Burden and Notifications in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis
In this systematic review and meta-analysis, Katherine Horton and colleagues examine the differences in tuberculosis burden and notifications between men and women living in low - and middle-income countries.
Published in the journal: . PLoS Med 13(9): e32767. doi:10.1371/journal.pmed.1002119
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002119Summary
In this systematic review and meta-analysis, Katherine Horton and colleagues examine the differences in tuberculosis burden and notifications between men and women living in low - and middle-income countries.
Introduction
Over the past twenty years, tuberculosis (TB) case notifications among men have exceeded those among women in most settings [1]. In 2014, the male-to-female (M:F) ratio in smear-positive pulmonary TB case notification was 1.7 globally and ranged from 1.0 in the Eastern Mediterranean Region to 2.1 in the Western Pacific Region [2]. The excess of notified cases among men has often been explained as a result of barriers faced by women in seeking care for and being diagnosed with TB [3,4]. However, notification data alone are insufficient to determine whether this is true, or whether sex differences in case notifications reflect an excess in the burden of disease among men and even a disadvantage among men in seeking and accessing TB care.
Prevalence surveys offer a robust measure of disease burden in the community, reducing or eliminating the care-seeking biases that affect case notifications: a higher proportion of men in case notifications could reflect either higher incidence of TB disease or more complete registration for treatment by men. Prevalence surveys predominantly identify infectious TB patients with previously undiagnosed TB disease who have, therefore, not contributed to routine notification data before participation in the survey. As such, comparison of the characteristics of diagnosed TB patients (notification data) with those of undiagnosed TB patients (prevalence survey data) provides a unique insight into diagnosis and treatment access barriers. For example, finding a similar male predominance in undiagnosed TB (prevalence surveys) patients as in notified TB cases would support the hypothesis that men genuinely have a higher burden of TB disease, while finding a greater male predominance in undiagnosed TB patients than in notified TB cases would suggest male-specific access barriers or male sex being a risk factor for TB disease.
A previous analysis in 2000 found that male TB prevalence exceeded female TB prevalence in 27 (93%) of 29 prevalence surveys conducted in 14 countries between 1953 and 1997 [5]. The same analysis calculated the patient diagnostic rate (the inverse of the prevalence-to-notification ratio) and found that female cases were more likely to be notified than male cases in 21 (72%) surveys.
Despite these findings, men are often overlooked in discussions of gender and TB. While global TB reports and meetings on gender acknowledge the fact that the majority of TB cases and TB-associated deaths occur among men, greater focus is usually placed on women. More broadly in global health discussions, there is a tendency to use the word “gender” when really “women” is meant, as exemplified by the Millennium Development Goals [6] and Sustainable Development Goals [7]. Subsequently, an emphasis on men runs contrary to global norms [8], and strategies to assess and address men’s barriers to TB care are notably absent from the global research agenda.
The World Health Organization’s End TB Strategy emphasises the importance of equity in access to diagnosis and treatment [9]; men should not be excluded from this target. The End TB Strategy has also prioritised systematic screening of high-risk groups to ensure early diagnosis of individuals with TB [10]. If TB prevalence remains higher among men than women, as in previous analysis [5], men should be considered a high-risk group for TB [11], and national TB programmes should more actively target men with routine diagnostic and/or screening services. This action is necessary to reduce the burden of TB in the whole population more effectively [12] and to ensure that principles of gender equity are upheld.
This review set out to systematically investigate sex differences in the prevalence of bacteriologically positive TB and smear-positive TB in adult participants in cross-sectional surveys conducted in low - and middle-income countries to determine whether sex ratios in adult case notifications reflect population sex differences in disease and to compare prevalence-to-notification (P:N) ratios for men and women. The current study adds to previous analysis [5] by including surveys conducted since the widespread availability of anti-TB chemotherapy in low-resource settings and the implementation of the directly observed treatment short course (DOTS) strategy, as well as the rise of the HIV/AIDS pandemic and the implementation of interventions against it—all factors that may have different effects on TB in men and women. The current study also provides more detailed meta-analyses of sex differences in TB prevalence and P:N ratios.
Methods
Search Strategy
In accordance with the published protocol [13], studies describing national and sub-national TB prevalence surveys in adult populations (age ≥ 15 y) in low - and middle-income countries published between 1 January 1993 and 15 March 2016 were identified through searches of PubMed, Embase, Global Health, and the Cochrane Database of Systematic Reviews (Table 1). The WHO Global Tuberculosis Report 2015 [2] and abstract books from the Union World Conference on Lung Health (2012–2015) were also searched by hand, as were the reference lists of included studies. Researchers in the field and at WHO were contacted to assist with identification of relevant studies.
Two authors (K. C. H. and P. M.) independently reviewed titles and abstracts in parallel to identify relevant studies for full-text review. A third author (E. L. C.) resolved any discrepancies. The same authors reviewed full texts to determine whether studies met inclusion criteria and then extracted data on study methodology and TB prevalence in parallel using piloted electronic forms.
Study authors were contacted for additional information if studies did not report the number of participants and the number of bacteriologically positive and/or smear-positive TB cases by sex for adult participants. Authors were also contacted if sex-specific prevalence data were not available by age group.
Inclusion and Exclusion Criteria
The review included cross-sectional prevalence surveys conducted in low - and middle-income countries [15]. Studies conducted among symptomatic or care-seeking individuals, children, individuals of a single sex, occupational settings, or other sub-populations (e.g., only HIV-positive individuals) were excluded. Studies reporting prevalence of Mycobacterium tuberculosis infection but not TB disease were excluded. Individuals under 15 y of age were excluded since diagnosis of childhood TB is more complicated than diagnosis of adult disease, especially within the context of community-based surveys [16]. Studies including both adults and children were included in the qualitative review but were excluded from quantitative analyses unless the study reported participation and prevalence for adults. Studies published in languages other than English were excluded due to limited resources for translation. Where more than one report was identified for a single survey, the most complete source was included and the others were excluded.
Study Quality
The risk of bias in included studies was assessed in parallel by K. C. H. and P. M. Each study was ranked on eight criteria from a tool developed to assess the risk of bias in prevalence surveys [17]. These criteria assessed factors related to the selection of the study population, the risk of nonresponse bias, data collection methods, and case definitions. The eight criteria were summarised to give an assessment of the overall risk of bias.
Definitions
Study participants were defined as individuals who were interviewed and/or underwent initial screening procedures, according to study-specific procedures. Participation was defined as the number of participants divided by the number of individuals who were eligible or invited to participate. High relative male participation was defined as a M:F ratio in participation ≥ 0.90.
Case definitions for TB were based on internationally recognised terminology, where available, and study-specific definitions otherwise. Bacteriologically positive TB was defined as positive smear microscopy, culture, or WHO-approved rapid diagnostic results (such as from Xpert MTB/RIF) [18].
Sex-specific prevalence of bacteriologically and smear-positive TB was defined as the number of individuals with bacteriologically or smear-positive TB divided by the number of study participants, by sex. Reported prevalence was used to estimate the number of cases or the number of participants where one of these values was missing. No adjustments were made for nonparticipation or nonsampling.
Sex-specific P:N ratios were calculated as the ratio of smear-positive TB prevalence per 100,000 individuals to smear-positive TB case notifications per 100,000 individuals among adults [5,19]. WHO case notification data [20] and United Nations population estimates [21] were matched to each prevalence survey by country and year. For surveys that took place over more than one calendar year, the annual case notification rate was averaged over all survey years (excluding years with no reported data). No adjustments were made for sub-national surveys.
National estimates of TB and HIV burden were matched to each prevalence survey by country and year. For surveys that took place over more than one calendar year, estimates were averaged over all survey years (excluding years with no reported data). High TB prevalence was defined using the median value for included studies, which was an estimated national TB prevalence ≥ 300 per 100,000 individuals [22]. High HIV prevalence was defined as estimated national HIV prevalence ≥ 1% in the general population [23,24], and high HIV prevalence in incident TB was defined as estimated HIV prevalence ≥ 20% in new and relapse TB cases [22,25].
Data Analysis
Prevalence of bacteriologically positive TB and smear-positive TB was calculated for included studies by sex. Prevalence of bacteriologically positive TB by sex and age was also calculated, where possible. Sub-group prevalence was estimated for sub-groups based on survey characteristics including WHO geographical region, survey setting (national versus sub-national), national estimates of TB and HIV burden (both in the general population; the latter also in incident TB), study quality, initial screening procedures, case definitions, and relative male participation. Clopper-Pearson confidence intervals [26] and M:F ratios were calculated for all prevalence estimates. P:N ratios for smear-positive TB were estimated with confidence intervals based on the estimated variance using a continuity correction of 0.5 in the corresponding prevalence estimates.
Heterogeneity was assessed using the I2 statistic [27]. Due to substantial heterogeneity between studies, random-effects models were used for meta-analyses, weighting for the inverse of the variance. Random-effects weighted summary M:F ratios were calculated for participation, prevalence of bacteriologically positive TB and smear-positive TB, age-specific prevalence of bacteriologically positive TB, and P:N ratios.
Meta-regression was performed for M:F ratios in prevalence and M:F ratios in P:N ratios to examine associations with the survey characteristics mentioned above, plus the starting year of each survey. Univariate meta-regression of M:F ratios in prevalence was conducted separately for bacteriologically positive TB and smear-positive TB. If either univariate meta-regression suggested evidence of an association with a particular characteristic, that characteristic was included as a variable in the multivariate meta-regression models for both bacteriologically positive and smear-positive TB. Similarly, multivariate meta-regression of M:F ratios in P:N ratios was based on evidence of associations in univariate analysis.
All analyses were performed using R version 3.2.2 [28] (S1 Data; S1 Analysis).
Results
Study Characteristics
Of 7,502 potentially relevant English-language studies screened by title and abstract, 148 were reviewed in full; of these, 65 were excluded after full-text review (S1 Table) and 83 were eligible for inclusion (Fig 1; S2 Table) [29–111]. Included studies describe 88 surveys in 28 countries: 36 surveys in 13 countries in the African Region, three surveys in two countries in the Region of the Americas, four surveys in two countries in the Eastern Mediterranean Region, 28 surveys in five countries in the South-East Asia Region, and 17 surveys in six countries in the Western Pacific Region (Fig 2). There were 22 nationally representative surveys and 66 sub-national surveys, with at least 20 of the latter conducted in urban settings and eight among tribal populations. Over 3.1 million adult participants were included; 16 surveys did not report the number of adult participants.
Fig. 1. PRISMA flow diagram. 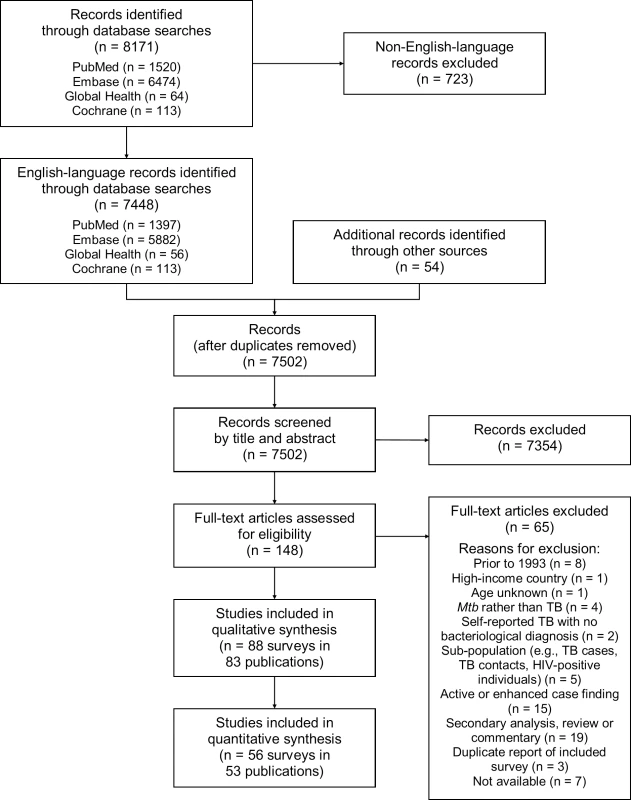
Mtb, Mycobacterium tuberculosis. Fig. 2. Global map showing countries in which prevalence surveys have been conducted. 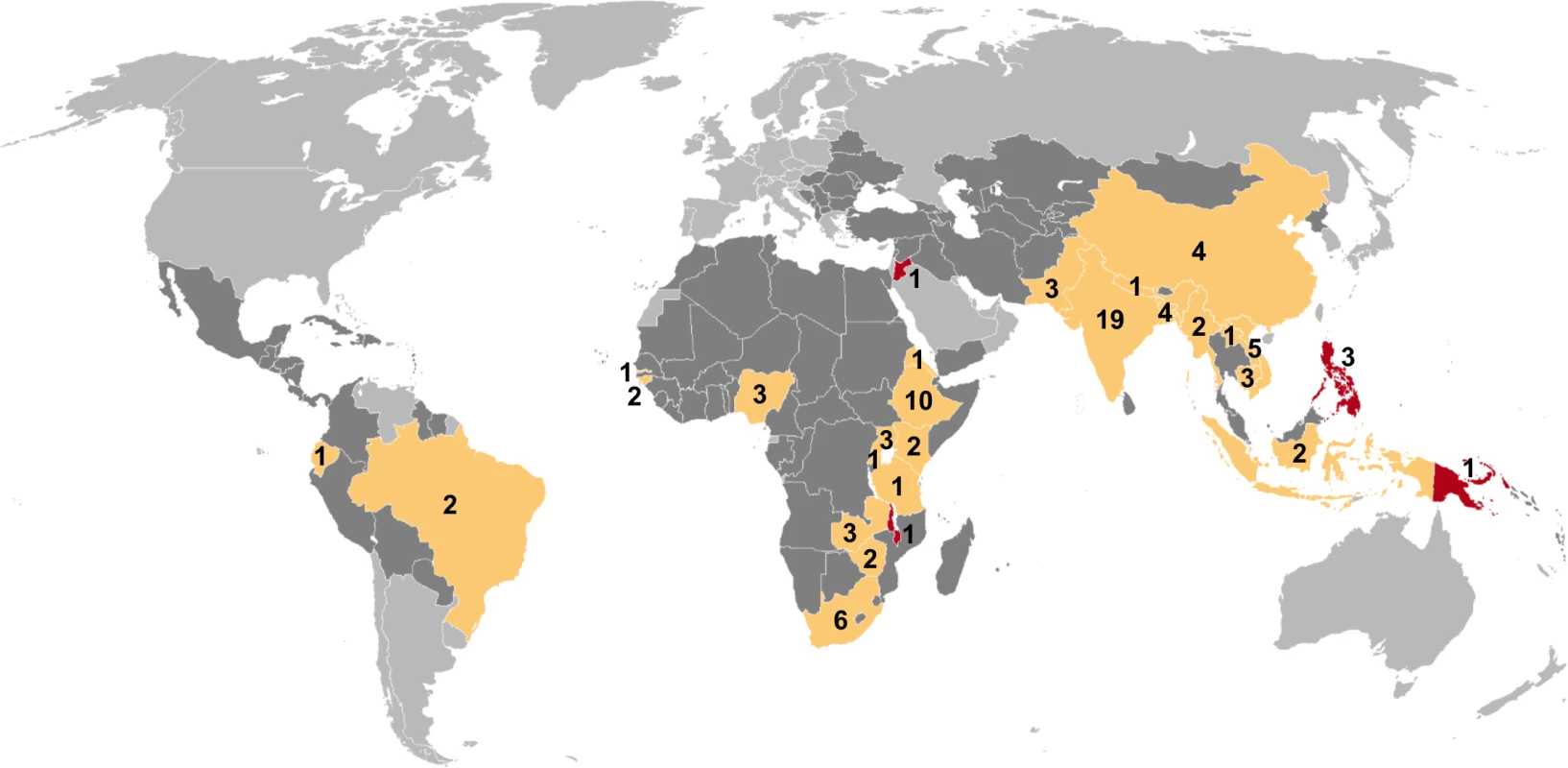
Yellow indicates low- and middle-income countries for which sex-disaggregated data are available from at least one prevalence survey (n = 24). Red indicates low- and middle-income countries in which at least one prevalence survey has been conducted but sex-disaggregated data are not available (n = 4). Dark gray indicates low- and middle-income countries where no prevalence survey has been identified (n = 107). Labels show the total number of surveys identified within each country for which at least one prevalence survey was identified (n = 88). Study Quality
The risk of bias assessment identified 33 (43%) surveys with low risk of bias, 32 (42%) with moderate risk of bias, and 12 (16%) with high risk of bias (S1 Fig). Eleven surveys for which only an abstract was available were characterised as unknown risk of bias due to limited information on study methodology [34,54,57,62,63,75,76,79,80,95,104]. The quantitative analyses included a slightly higher proportion of surveys with low risk of bias than the qualitative summary. In all, 84% to 94% of the surveys in the quantitative analyses had low to moderate risk of bias (S2 Fig).
Participation by Sex
Female participation equalled or exceeded male participation in all of the 28 surveys for which participation was reported by sex (Fig 3). Of 687,926 men eligible or invited to participate, 521,934 (75.9%) participated, while 611,901 (82.5%) of 741,705 eligible or invited women participated. The overall random-effects weighted M:F ratio in participation was 0.90 (95% CI 0.86–0.93; range 0.50 to 1.00).
Fig. 3. Male-to-female ratios of participation among eligible or invited individuals (n = 29). 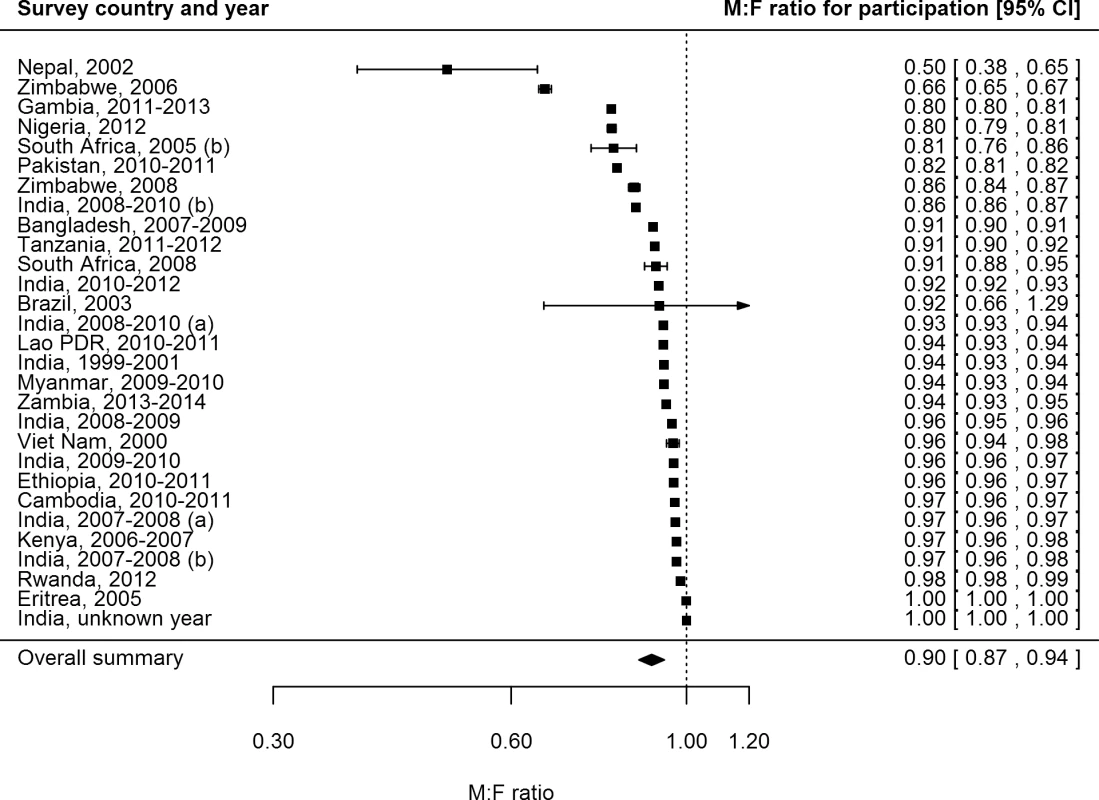
Analysis includes surveys that report the number of individuals who were eligible for screening and the number of individuals screened by sex. See S2 Table for survey details and references. Lao PDR, Lao People’s Democratic Republic. TB Prevalence by Sex
The prevalence of bacteriologically positive TB was reported by sex in 56 surveys with 2.2 million participants in 24 countries [29,30,32,33,35,36,38–44,47–51,53,55,56,58–60,65–67,69–74,82,84,85,87,89–94,97,101,102,104,105,107,110–112]. Forty surveys with 1.7 million participants in 22 countries reported the prevalence of smear-positive TB by sex [35,40,43,44,48–51,53,55,56,58–60,65–67,69–71,73,74,85,87,89,90,92,94,97,101,102,105,107,110,111]. The overall random-effects weighted prevalence per 100,000 individuals was 488 (95% CI 382–623) among men and 231 (95% CI 166–321) among women for bacteriologically positive TB and 314 (95% CI 245–403) among men and 129 (95% CI 89–189) among women for smear-positive TB (S3 Table).
Excluding the Region of the Americas—because it had only two small sub-national surveys included in the quantitative analysis—the prevalence of bacteriologically positive TB and smear-positive TB was highest in the African Region. There was strong evidence that male and female prevalence of bacteriologically positive TB per 100,000 individuals was higher in settings with high HIV prevalence in the general population (high versus low HIV prevalence settings: for men, 1,162, 95% CI 735–1,834, versus 360, 95% CI 275–471, p < 0.001; for women, 735, 95% CI 448–1202, versus 157, 95% CI 110–223, p < 0.001). This same relationship (higher prevalence of undiagnosed TB in settings with high HIV prevalence) was also apparent when HIV data from diagnosed TB patients, rather than the general population, were used (for men: 907, 95% CI 582–1,413, versus 359, 95% CI 270–477, p = 0.001; for women: 553, 95% CI 341–896, versus 153, 95% CI 105–224, p < 0.001) (S4 Table). Prevalence of smear-positive TB per 100,000 individuals was also higher in settings with high HIV prevalence in the general population (high versus low HIV prevalence settings: for men, 548, 95% CI 303–990, versus 275, 95% CI 208–364, p = 0.039; for women, 273, 95% CI 131–568, versus 110, 95% CI 71–169, p = 0.036) and in settings with high HIV prevalence in diagnosed TB patients for women (229, 95% CI 126–416, versus 103, 95% CI 64–165, p = 0.040) but not for men (459, 95% CI 289–727, versus 270, 95% CI 200–366, p = 0.060) (S4 Table).
Male-to Female Ratios in TB Prevalence
The overall random-effects weighted M:F prevalence ratio was 2.21 for bacteriologically positive TB (95% CI 1.92–2.54; range 0.62 to 6.18; 56 surveys in 24 countries) and 2.51 for smear-positive TB (95% CI 2.07–3.04; range 0.25 to 5.91; 40 surveys in 22 countries). Random-effects weighted M:F prevalence ratios for bacteriologically positive TB and smear-positive TB were significantly greater than one in all regions except the Region of the Americas, where analyses included only two small sub-national surveys (Fig 4).
Fig. 4. Male-to-female ratios in bacteriologically positive (n = 56) and smear-positive (n = 40) TB prevalence by WHO region. 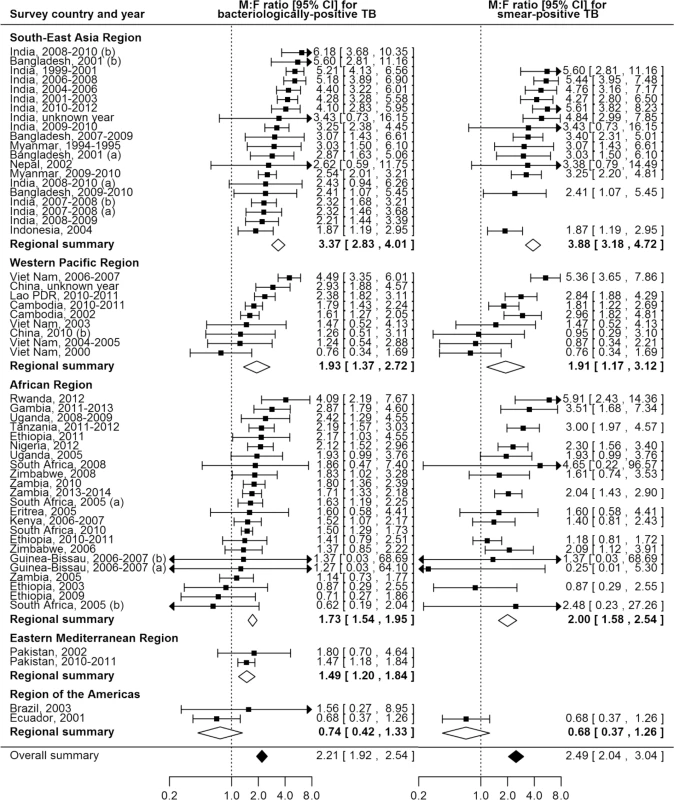
Regional and overall summaries from random-effects models for bacteriologically positive and smear-positive TB. See S2 Table for survey details and references. Lao PDR, Lao People’s Democratic Republic. Among countries with multiple surveys, an excess of male TB cases was observed in all studies in eight (73%) of 11 countries. Exceptions with inconsistent results were Ethiopia, South Africa, and Viet Nam, although overall random-effects weighted M:F prevalence ratios exceeded one for each of these countries.
Univariate Meta-regression of Male-to-Female Ratios in Prevalence
In univariate meta-regression of M:F ratios in bacteriologically positive TB (Table 2), there was strong evidence that M:F prevalence ratios were 1.95 times higher in the South-East Asia Region than in the African Region (95% CI 1.54–2.48; 56 surveys). M:F prevalence ratios were lower in settings with high HIV prevalence in the general population (0.67, 95% CI 0.49–0.90; 54 surveys) or in incident TB (0.69, 95% CI 0.53–0.93; 54 surveys).
Tab. 2. Univariate and multivariate random-effects meta-regression results for male-to-female ratios in bacteriologically positive TB and smear-positive TB. 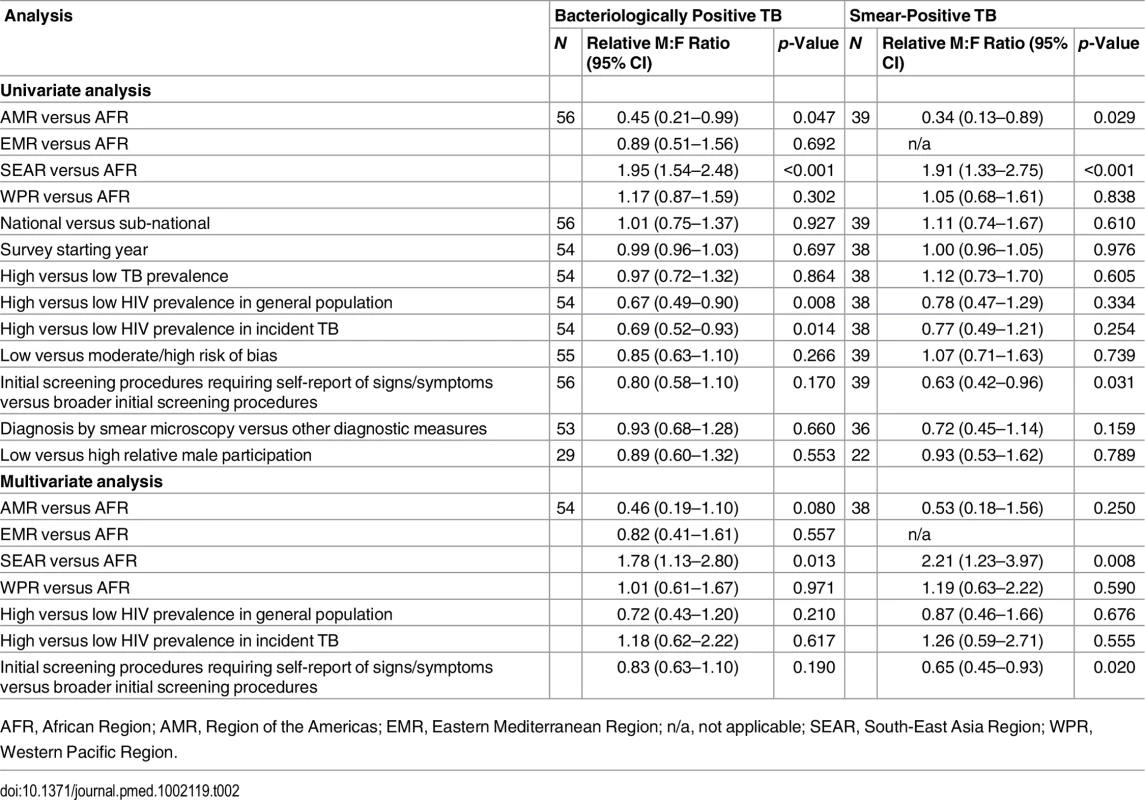
AFR, African Region; AMR, Region of the Americas; EMR, Eastern Mediterranean Region; n/a, not applicable; SEAR, South-East Asia Region; WPR, Western Pacific Region. M:F prevalence ratios were also higher in the South-East Asia Region than in the African Region in univariate meta-regression of smear-positive TB (1.91, 95% CI 1.33–2.75; 39 surveys). In this analysis there was also evidence that M:F prevalence ratios were lower in surveys that required individuals to report signs or symptoms of TB during initial screening procedures (0.63, 95% CI 0.42–0.96; 39 surveys) compared to surveys within which initial screening procedures included criteria such as chest X-ray, self-reported history of TB, or self-reported contact with a TB case, instead of or in addition to self-reported signs or symptoms.
In univariate meta-regression models for M:F ratios in bacteriologically positive TB and M:F ratios in smear-positive TB, none of the following survey characteristics were associated with differences in M:F ratios in TB prevalence: survey setting (national versus sub-national), survey starting year, TB prevalence, risk of bias, case definitions, or relative sex ratios in participation.
Multivariate Meta-regression of Male-to-Female Ratios in Prevalence
In multivariate meta-regression of M:F ratios in bacteriologically positive TB, there was evidence that M:F ratios remained higher in the South-East Asia Region than in the African Region after adjusting for HIV prevalence and initial screening procedures, although the relative M:F ratio between these two regions was slightly lower than in univariate analysis (1.78, 95% CI 1.13–2.80; 54 surveys).
There was evidence in the multivariate meta-regression of M:F ratios in smear-positive TB that M:F ratios were 2.21 times higher in the South-East Asia Region than in the African region (95% CI 1.23–4.04; 38 surveys). There was also evidence in the multivariate meta-regression that M:F ratios in surveys that required individuals to self-report signs or symptoms of TB in initial screening procedures were lower than those in surveys with broader initial screening procedures (0.65, 95% CI 0.45–0.93; 38 surveys).
TB Prevalence by Sex and Age
Data on the prevalence of bacteriologically positive TB by sex and age were available for 19 surveys in 13 countries [32,33,35,36,43,44,50,51,53,58,60,65–67,70,71,97,101,107]. Random-effects weighted M:F ratios in prevalence appear to increase with age from 1.28 (95% CI 0.85–1.92; range 0.29 to 5.06) among individuals aged 15–24 y to 3.18 (95% CI 2.24–4.53; range 0.57 to 11.34) among individuals aged 45–54 y (Fig 5).
Fig. 5. Random-effects weighted male-to-female prevalence ratios for bacteriologically positive TB by age group (n = 19). 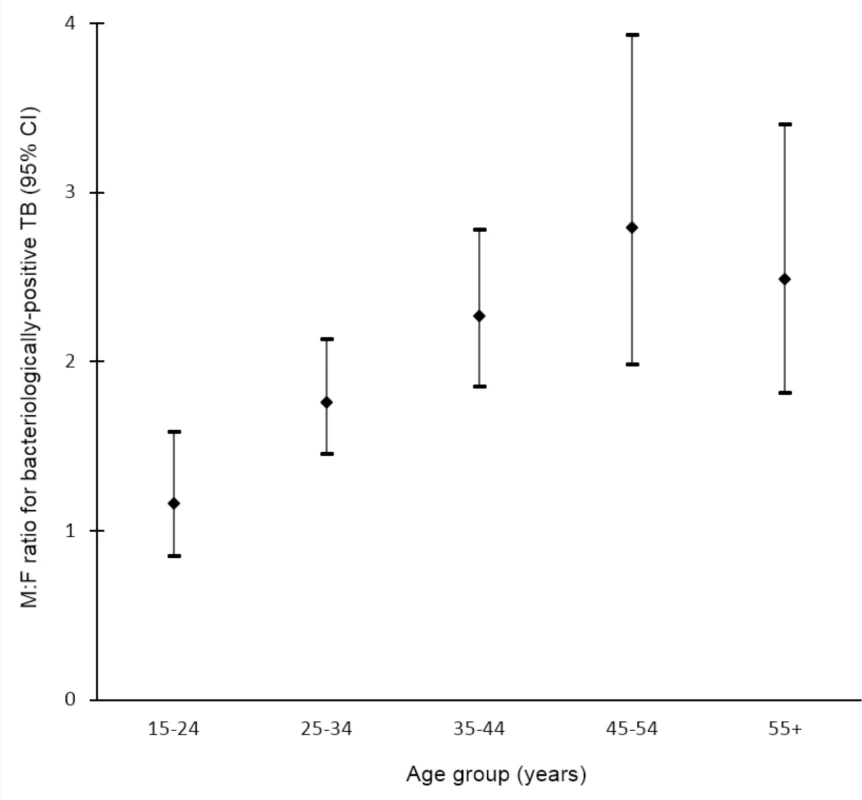
Analysis includes surveys that report the number of individuals screened and the number of bacteriologically positive TB cases by sex and age. Horizontal axis shows age groups in years. Vertical axis shows random-effects weighted M:F ratios in prevalence of bacteriologically positive TB per 100,000 individuals with 95% confidence intervals. Prevalence-to-Notification Ratios by Sex
P:N ratios for smear-positive TB exceeded one for both men and women in 25 (74%) of 34 surveys in 20 countries with available data (Fig 6). The median number of prevalent cases per notified case was 2.6 (interquartile range 1.3–3.4) for men and 1.6 (interquartile range 1.2–2.7) for women, and the overall random-effects weighted M:F ratio for P:N ratios was 1.55 (95% CI 1.25–1.91).
Fig. 6. Male-to-female ratios in prevalence-to-notification ratios (n = 34). 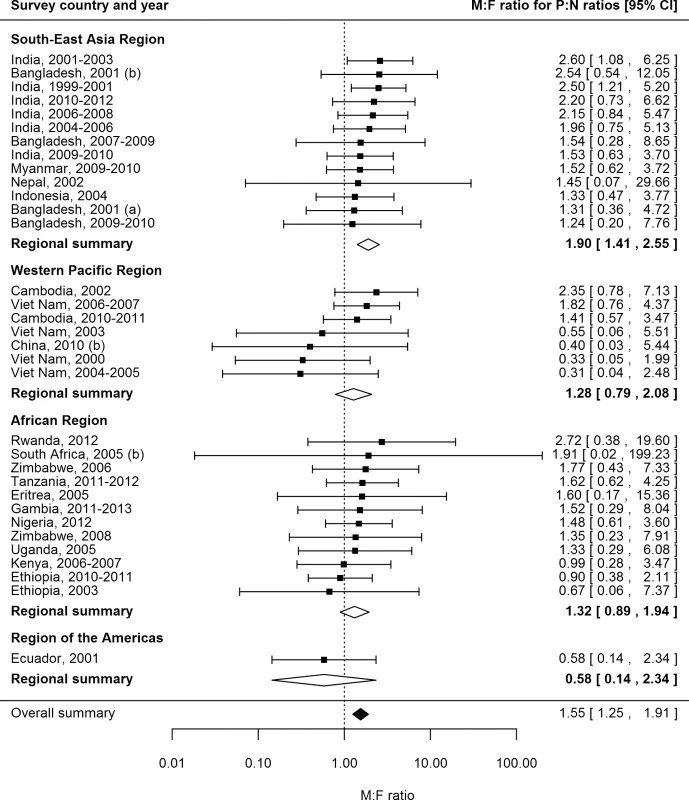
Analysis includes surveys that report the prevalence of smear-positive TB by sex and for which corresponding national notification and population data are available. See S2 Table for survey details and references. Univariate Meta-regression for Male-to-Female Ratios in Prevalence-to-Notification Ratios
There was no evidence in univariate meta-regression that any of the study or setting characteristics examined were associated with M:F ratios in P:N ratios (S5 Table). Due to the lack of evidence of associations in univariate analyses, multivariate meta-regression was not performed for M:F ratios in P:N ratios.
Discussion
Meta-analysis of 56 TB prevalence surveys including 2.2 million participants in 28 countries provides strong evidence that TB prevalence is higher among men than women, with a higher M:F ratio than that reported for case notification data. The number of prevalent cases per notified case of smear-positive TB was also higher among men than women, adding evidence that men may be less likely than women to seek or access care in many settings. Further evidence of men’s barriers to seeking or accessing care is provided by results showing that men were less likely than women to participate in prevalence surveys and that relatively fewer prevalent cases were found among men in surveys that required participants to self-report signs or symptoms in initial screening procedures.
The excess male prevalence observed in surveys conducted between 1953 and 1997 [5] persists in more recent surveys, despite widespread implementation of the DOTS strategy and interventions against the HIV pandemic that have decreased overall TB prevalence. Regional summary M:F ratios in the current study were similar to those previously reported for South-East Asia (3.8 versus 3.2), where sex differences were greatest, and the Western Pacific (1.9 versus 2.0). However, in the current study, the summary M:F ratio for the African Region was twice that previously reported (2.0 versus 1.0), suggesting that sex disparities in TB prevalence in this region have increased over the past fifty years. The emergence of HIV during this time has had a substantial impact on TB epidemiology, especially in the African Region. However, while the prevalence of HIV is slightly higher among women than men [113], this study shows that the prevalence of TB is higher among men, even in countries with generalised HIV epidemics. Men also face a relative disadvantage in accessing and remaining in HIV care [114–117], and so men’s risk of TB is likely to be further increased as a result of undiagnosed and untreated HIV co-infection and missed opportunities for TB screening within HIV care.
Comparisons of sex ratios in TB prevalence and notification highlight sex differences in time to diagnosis and imply that in many settings women are more likely than men to have a timely TB diagnosis. While these results could be attributed to men seeking care in private facilities and therefore being less likely to be included in case notification numbers, this explanation would require that the proportion of men who seek care in the public sector be only two-thirds the proportion of women who seek care in that sector. Instead, there is wider evidence that men are less well-served than women by health services [118,119]. Within the context of HIV, which has a similarly lengthy pathway to diagnosis, there is also substantial evidence that men experience greater attrition and worse outcomes [114–117]. Men are less likely than women to access antiretroviral therapy, and in many countries this disparity has increased over time [114]. Similar evidence showing men’s disadvantage in the TB care pathway is building [120–122]. Focusing specifically on access to diagnosis, male TB patients often delay care-seeking longer than female TB patients [123], and this review adds support that timely entry into the TB care pathway may be more difficult for men than women in many settings.
Lower prevalence survey participation among men and evidence of lower M:F prevalence ratios in studies that require individuals to self-report signs or symptoms of TB in initial screening procedures imply that symptom screening in community-based active case finding may be a less effective tool for identifying TB disease in men than women. It is not known whether this is due to men refusing to report symptoms or whether the sub-clinical phase of disease may be longer for men [124]. Further investigation is needed to examine men’s acceptance of screening and reporting of symptoms, even when barriers related to visiting a healthcare facility are removed.
Findings from this review suggest that case detection efforts, whilst not ignoring women, should be greatly strengthened for men. This will require a detailed understanding of the barriers that men face in accessing care. Previous studies have highlighted factors such as loss of income and financial barriers, as well as stigma, that affect men’s healthcare decisions [125,126]. Care-seeking decisions are further influenced by perceptions of masculinity that discourage admission of illness, and systems of care that take away men’s sense of control and leave feelings of inadequacy [127,128]. Interventions to improve case detection among men must recognise and address these barriers. Healthcare providers should be sensitive to men’s needs and consider offering dedicated clinic times and outreach services for men. TB diagnostic services that incorporate men’s peer networks or workplaces to promote wellness and reduce stigma may also be effective. In South Africa, a men-only after-hours clinic situated close to a transport hub has been effective in improving men’s uptake of HIV testing and adherence to antiretroviral therapy [129]. Comparable opportunities for TB strategies that offer convenient access to care while maintaining men’s sense of control should be explored.
This review summarises evidence on sex ratios in TB prevalence from a large number of prevalence surveys across geographic regions, an approach which introduces a number of potential sources of bias. Surveys varied greatly in their methodology, particularly in screening criteria and case definitions, and levels of participation varied within and between studies. However, over 84% of the surveys in the analyses had low to moderate risk of bias.
Prevalence as a measure of disease burden has limitations as it provides an estimate at a single point in time and cannot distinguish between disease as a result of recent infection and disease from reactivation, limiting understanding of current transmission. Comparing the rate of prevalent cases to notified cases is a crude measurement, especially comparing all surveys to national case notification rates, regardless of study setting. Stratifying by age and rural or urban setting would improve P:N ratios; however, data on these characteristics were not available at the time of analysis. Prevalence data by sex and HIV status were too infrequently available to be reported here. To our knowledge, no surveys that conducted drug susceptibility testing reported the results of those analyses by sex, so it is not possible to comment on whether the sex differences reported here are also relevant to drug-resistant TB. Given the significant sex differences reported in prevalence, future surveys should analyse and report all results by sex to facilitate greater understanding of the relationship between gender and TB.
Men have a higher prevalence of TB and, in many settings, remain infectious in the community for a longer period of time than women. Men are therefore likely to generate a greater number of secondary infections than women, and social mixing patterns have suggested that, as a result, men are responsible for the majority of infections in men, women, and children [12]. Addressing men’s burden of disease and disadvantage in TB care is therefore an issue not only for men’s health but for broader TB prevention and care. Given the compelling evidence presented here, global discourse and policy on key underserved populations need to include a focus on men. Recommendations to address issues of gender and TB cannot continue to insist on addressing the needs of women and girls [130] while ignoring the inequity faced by men and boys, who carry the higher burden of disease, often with less access to timely diagnosis and treatment. With a clear need and high burden, improving diagnosis and treatment among men is essential to achieving the ambitious targets of the End TB Strategy.
Supporting Information
Zdroje
1. World Health Organization. Global tuberculosis control: WHO report 2011. WHO/HTM/TB/2011.16. 2011 [cited 2 Aug 2016]. Available: http://apps.who.int/iris/bitstream/10665/44728/1/9789241564380_eng.pdf.
2. World Health Organization. Global tuberculosis report 2015. WHO/HTM/TB/2015.22. 2015 [cited 2 Aug 2016]. Available: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf.
3. Long NH, Johansson E, Lonnroth K, Eriksson B, Winkvist A, Diwan V. Longer delays in tuberculosis diagnosis among women in Vietnam. Int J Tuberc Lung Dis. 1999;3 : 388–393. 10331727
4. Weiss MG, Auer C, Somma D, Abouihia A, Jawahar M, Karim F, et al. Gender and tuberculosis: cross-site analysis and implications of a multi-country study in Bandladesh, India, Malawi, and Colombia. 2006 [cited 2 Aug 2016]. Available: http://www.who.int/entity/tdr/publications/documents/sebrep3.pdf?ua=1.
5. Borgdorff M, Nagelkerke N, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis. 2000;4 : 123–132. 10694090
6. United Nations General Assembly. United Nations millennium declaration. A/RES/55/2. 2000 Sep 8 [cited 2 Aug 2016]. Available: http://www.un.org/millennium/declaration/ares552e.htm.
7. United Nations General Assembly. Transforming our world: the 2030 agenda for sustainable development. A/RES/70/1. 2015 Sep 25 [cited 2 Aug 2016]. Available: http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E.
8. Hawkes S, Buse K. Gender and global health: evidence, policy, and inconvenient truths. Lancet. 2013;381 : 1783–1787. doi: 10.1016/S0140-6736(13)60253-6 23683645
9. World Health Organization. Global strategy and targets for tuberculosis prevention, care and control after 2015. EB134/12. 2013 Nov 29 [cited 2 Aug 2016]. Available: http://apps.who.int/gb/ebwha/pdf_files/EB134/B134_12-en.pdf.
10. World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. 2013 [cited 2 Aug 2016]. Available: http://www.who.int/tb/publications/Final_TB_Screening_guidelines.pdf.
11. Lönnroth K, Corbett E, Golub J, Godfrey-Faussett P, Uplekar M, Weil D, et al. Systematic screening for active tuberculosis: rationale, definitions and key considerations. Int J Tuberc Lung Dis. 2013;17 : 289–298. doi: 10.5588/ijtld.12.0797 23407219
12. Dodd PJ, Looker C, Plumb I, Bond V, Schaap A, Shanaube K, et al. Age - and sex-specific social contact patterns and incidence of Mycobacterium tuberculosis infection. Am J Epidemiol. 2016;183 : 156–166. doi: 10.1093/aje/kwv160 26646292
13. Horton K, MacPherson P, White R, Houben R, Corbett L. Gender differences in tuberculosis prevalence in low - and middle-income countries. CRD42015022163. PROSPERO International Prospective Register of Systematic Reviews. 2015 [cited 2 Aug 2016]. Available: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015022163.
14. Cochrane. LMIC filters. 2012 [cited 2 Aug 2016]. Available: http://epoc.cochrane.org/lmic-filters.
15. The World Bank. World Bank country and lending groups. 2015 [cited 26 May 2015]. Available: http://data.worldbank.org/about/country-and-lending-groups.
16. World Health Organization. Tuberculosis prevalence surveys: a handbook. WHO/HTM/TB/2010.17. 2011 [cited 2 Aug 2016]. Available: http://apps.who.int/iris/bitstream/10665/44481/1/9789241548168_eng.pdf?ua=1&ua=1.
17. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65 : 934–939. doi: 10.1016/j.jclinepi.2011.11.014 22742910
18. World Health Organization. Definitions and reporting framework for tuberculosis—2013 revision (updated December 2014). 2013 [cited 2 Aug 2016]. Available: http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf.
19. Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. National TB prevalence surveys in Asia, 1990–2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20 : 1128–1145. doi: 10.1111/tmi.12534 25943163
20. World Health Organization. Tuberculosis (TB): case notifications. 2015 [cited 26 May 2015]. Available: http://www.who.int/tb/country/data/download/en/.
21. United Nations Department of Economic and Social Affairs. World population prospects: the 2012 revision. New York: United Nations Department of Economic and Social Affairs; 2013.
22. World Health Organization. Tuberculosis (TB): WHO TB burden estimates. 2015 [cited 26 May 2015]. Available: http://www.who.int/tb/country/data/download/en/.
23. World Health Organization. Global Health Observatory data repository: prevalence of HIV among adults aged 15 to 49—estimates by WHO region. 2015 [cited 26 May 2015]. Available: http://apps.who.int/gho/data/node.main.622?lang=en.
24. Joint United Nations Programme on HIV/AIDS. UNAIDS terminology guidelines. 2011 Oct [cited 2 Aug 2016]. Available: http://files.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2118_terminology-guidelines_en.pdf.
25. World Health Organization. Global tuberculosis report 2014. WHO/HTM/TB/2014.08. 2014 [cited 2 Aug 2016]. Available: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf.
26. Clopper C, Pearson E. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934 : 404–413. doi: 10.1093/biomet/26.4.404
27. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327 : 557. doi: 10.1136/bmj.327.7414.557 12958120
28. R Core Team. R: a language and environment for statistical computing. Version 3.2.2. Vienna: R Foundation for Statistical Computing; 2015.
29. Aggarwal AN, Gupta D, Agarwal R, Sethi S, Thakur JS, Anjinappa SM, et al. Prevalence of pulmonary tuberculosis among adults in a north Indian district. PLoS ONE. 2015;10:e0117363. doi: 10.1371/journal.pone.0117363 25695761
30. Akhtar S, White F, Hasan R, Rozi S, Younus M, Ahmed F, et al. Hyperendemic pulmonary tuberculosis in peri-urban areas of Karachi, Pakistan. BMC Public Health. 2007;7 : 70. doi: 10.1186/1471-2458-7-70 17477870
31. Alvi AR, Hussain SF, Shah MA, Khalida M, Shamsudin M. Prevalence of pulmonary tuberculosis on the roof of the world. Int J Tuberc Lung Dis. 1998;2 : 909–913. 9848612
32. Ayles H, Schaap A, Nota A, Sismanidis C, Tembwe R, De Haas P, et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS ONE. 2009;4:e5602. doi: 10.1371/journal.pone.0005602 19440346
33. Ayles H, Muyoyeta M, Du Toit E, Schaap A, Floyd S, Simwinga M, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382 : 1183–1194. doi: 10.1016/s0140-6736(13)61131-9 23915882
34. Banda R, Munthali A, Mpunga J. Findings from the first Malawi TB prevalence survey. 46th Union World Conference on Lung Health; 2015 Dec 2–6; Cape Town, South Africa.
35. Banu S, Rahman MT, Uddin MK, Khatun R, Ahmed T, Rahman MM, et al. Epidemiology of tuberculosis in an urban slum of Dhaka City, Bangladesh. PLoS ONE. 2013;8:e77721. doi: 10.1371/journal.pone.0077721 24204933
36. Basta PC, Coimbra CE Jr, Escobar AL, Santos RV, Alves LC, Fonseca L de S. Survey for tuberculosis in an indigenous population of Amazonia: the Surui of Rondonia, Brazil. Trans R Soc Trop Med Hyg. 2006;100 : 579–585. doi: 10.1016/j.trstmh.2005.07.014 16274716
37. Basta PC, Coimbra CE Jr, Welch JR, Correa Alves LC, Santos RV, Bastos Camacho LA. Tuberculosis among the Xavante Indians of the Brazilian Amazon: an epidemiological and ethnographic assessment. Ann Hum Biol. 2010;37 : 643–657. doi: 10.3109/03014460903524451 20113213
38. Berhe G, Enqueselassie F, Hailu E, Mekonnen W, Teklu T, Gebretsadik A, et al. Population-based prevalence survey of tuberculosis in the Tigray region of Ethiopia. BMC Infect Dis. 2013;13 : 448. doi: 10.1186/1471-2334-13-448 24073793
39. Bhat J, Rao VG, Gopi PG, Yadav R, Selvakumar N, Tiwari B, et al. Prevalence of pulmonary tuberculosis amongst the tribal population of Madhya Pradesh, Central India. Int J Tuberc Lung Dis. 2009;38 : 1026–1032. doi: 10.1093/ije/dyp222
40. Bjerregaard-Andersen M, da Silva ZJ, Ravn P, Ruhwald M, Andersen PL, Sodemann M, et al. Tuberculosis burden in an urban population: a cross sectional tuberculosis survey from Guinea Bissau. BMC Infect Dis. 2009;10 : 96. doi: 10.1186/1471-2334-10-96
41. Chadha VK, Kumar P, Anjinappa SM, Singh S, Narasimhaiah S, Joshi MV, et al. Prevalence of pulmonary tuberculosis among adults in a rural sub-district of South India. PLoS ONE. 2012;7:e42625. doi: 10.1371/journal.pone.0042625 22956993
42. Claassens M, van Schalkwyk C, den Haan L, Floyd S, Dunbar R, van Helden P, et al. High prevalence of tuberculosis and insufficient case detection in two communities in the Western Cape, South Africa. PLoS ONE. 2013;8:e58689. doi: 10.1371/journal.pone.0058689 23560039
43. Corbett EL, Bandason T, Cheung YB, Makamure B, Dauya E, Munyati SS, et al. Prevalent infectious tuberculosis in Harare, Zimbabwe: burden, risk factors and implications for control. Int J Tuberc Lung Dis. 2009;13 : 1231–1237. 19793427
44. Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, Churchyard GJ, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376 : 1244–1253. doi: 10.1016/s0140-6736(10)61425-0 20923715
45. Demissie M, Zenebere B, Berhane Y, Lindtjorn B. A rapid survey to determine the prevalence of smear-positive tuberculosis in Addis Ababa. Int J Tuberc Lung Dis. 2002;6 : 580–584. 12102296
46. den Boon S, White NW, van Lill SW, Borgdorff MW, Verver S, Lombard CJ, et al. An evaluation of symptom and chest radiographic screening in tuberculosis prevalence surveys. Int J Tuberc Lung Dis. 2006;10 : 876–882. 16898372
47. Deribew A, Abebe G, Apers L, Abdissa A, Deribe F, Woldemichael K, et al. Prevalence of pulmonary TB and spoligotype pattern of Mycobacterium tuberculosis among TB suspects in a rural community in Southwest Ethiopia. BMC Infect Dis. 2012;12 : 54. doi: 10.1186/1471-2334-12-54 22414165
48. Dhanaraj B, Papanna MK, Adinarayanan S, Vedachalam C, Sundaram V, Shanmugam S, et al. Prevalence and risk factors for adult pulmonary tuberculosis in a metropolitan city of south India. PLoS ONE. 2015;10:e0124260. doi: 10.1371/journal.pone.0124260 25905900
49. Gasana M, Uwizeye C, Migambi P, Klinkenberg E, Ndahindwa V. Report of the first national pulmonary tuberculosis prevalence survey in Rwanda. Kigali (Rwanda): Ministry of Health; 2014.
50. Gopi PG, Subramani R, Radhakrishna S, Kolappan C, Sadacharam K, Devi TS, et al. A baseline survey of the prevalence of tuberculosis in a community in south India at the commencement of a DOTS programme. Int J Tuberc Lung Dis. 2003;7 : 1154–1162. 14677890
51. Gupta RK, Suri SP, Jamwal DS, Verma AK. Prevalence of tuberculosis in a rural population aged 15 years and above in R.S. Pura block of district Jammu. JK Pract. 2013;18 : 41–44.
52. Guwatudde D, Zalwango S, Kamya MR, Debanne SM, Diaz MI, Okwera A, et al. Burden of tuberculosis in Kampala, Uganda. Bull World Health Organ. 2003;81 : 799–805. 14758406
53. Hamid Salim MA, Declercq E, Van Deun A, Saki KA. Gender differences in tuberculosis: a prevalence survey done in Bangladesh. Int J Tuberc Lung Dis. 2004;8 : 952–957. 15305476
54. Hamusse S, Demissie M, Lindtjorn B. Prevalence and incidence of smear positive pulmonary tuberculosis in the Hetosa District of Arsi Zone, Oromia Regional State, Central Ethiopia. 46th Union World Conference on Lung Health; 2015 Dec 2–6; Cape Town, South Africa.
55. Hoa NB, Sy DN, Nhung NV, Tiemersma EW, Borgdorff MW, Cobelens FG. National survey of tuberculosis prevalence in Viet Nam. Bull World Health Organ. 2010;88 : 273–280. doi: 10.2471/blt.09.067801 20431791
56. Horie T, Lien LT, Tuan LA, Tuan PL, Sakurada S, Yanai H, et al. A survey of tuberculosis prevalence in Hanoi, Vietnam. Int J Tuberc Lung Dis. 2007;11 : 562–566. 17439682
57. John S, Gidado M, Tahir D, Nyako N, Ray T. Active tuberculosis case finding among nomadic pastoralists of northern Nigeria. Int J Tuberc Lung Dis. 2013;17(12 Suppl 2):S446–S447.
58. Joshi YP, Mishra PN, Joshi DD. Prevalence of pulmonary tuberculosis in far Western Nepal. JNMA J Nepal Med Assoc. 2005;44 : 47–50. 16554871
59. Kolappan C, Subramani R, Radhakrishna S, Santha T, Wares F, Baskaran D, et al. Trends in the prevalence of pulmonary tuberculosis over a period of seven and half years in a rural community in south India with DOTS. Indian J Tuberc. 2013;60 : 168–176. 24000495
60. Law I, Sylavanh P, Bounmala S, Nzabintwali F, Paboriboune P, Iem V, et al. The first national TB prevalence survey of Lao PDR (2010–2011). Trop Med Int Health. 2015;20 : 1146–1154. doi: 10.1111/tmi.12536 25939366
61. Legesse M, Mamo G, Ameni G, Medhin G, Bjune G, Abebe F. Community-based prevalence of undiagnosed mycobacterial diseases in the Afar Region, north-east Ethiopia. Int J Mycobacteriol. 2013;2 : 94–102. doi: 10.1016/j.ijmyco.2013.04.001 26785896
62. Ley SD. Tuberculosis active case detection in sentinel sites across Papua New Guinea. Am J Trop Med Hyg. 2011;1 : 74.
63. Lolong D, Pangaribuan I, Musadad A, Dwihardiani M, Mustikawati D. Results from the national TB prevalence survey of Indonesia. Int J Tuberc Lung Dis. 2014;18(11 Suppl 1):S43.
64. Lorent N, Choun K, Thai S, Kim T, Huy S, Pe R, et al. Community-based active tuberculosis case finding in poor urban settlements of Phnom Penh, Cambodia: a feasible and effective strategy. PLoS ONE. 2014;9:e92754. doi: 10.1371/journal.pone.0092754 24675985
65. Mao TE, Okada K, Yamada N, Peou S, Ota M, Saint S, et al. Cross-sectional studies of tuberculosis prevalence in Cambodia between 2002 and 2011. Bull World Health Organ. 2014;92 : 573–581. doi: 10.2471/BLT.13.131581 25177072
66. Middelkoop K, Bekker LG, Myer L, Whitelaw A, Grant A, Kaplan G, et al. Antiretroviral program associated with reduction in untreated prevalent tuberculosis in a South African township. Am J Respir Crit Care Med. 2010;182 : 1080–1085. doi: 10.1164/rccm.201004-0598OC 20558626
67. Cambodia Ministry of Health. Report of the national TB prevalence survey, 2002. Phnom Penh: Cambodia Ministry of Health; 2005.
68. Ethiopia Ministry of Health. First Ethiopian national population based tuberculosis prevalence survey. Addis Ababa: Ethiopia Ministry of Health; 2011.
69. Myanmar Ministry of Health. Sputum positive point prevalence survey (1994). Yangon: Myanmar Ministry of Health; 1994.
70. Myanmar Ministry of Health. Report on national TB prevalence survey 2009–2010, Myanmar. Yangon: Myanmar Ministry of Health; 2011.
71. Nigeria Ministry of Health. First national TB prevalence survey 2012, Nigeria. Abuja: Nigeria Ministry of Health; 2012.
72. Zambia Ministry of Health. National tuberculosis prevalence survey 2013–2014 technical report. Lusaka: Zambia Ministry of Health; 2015.
73. Tanzania Ministry of Health and Social Welfare. The first national tuberculosis prevalence survey. Primary analysis. Final report. Dar es Salaam: Tanzania Ministry of Health and Social Welfare; 2013.
74. Gambia Ministry of Health and Social Welfare. The Gambian survey of tuberculosis prevalence (GAMSTEP). Banjul: Gambia Ministry of Health and Social Welfare; 2014.
75. Muhammed S, Joltin C, Banuru Muralidhara P, Nair A. Active case finding in urban slums: experience from a pilot under Axshya project in India. Int J Tuberc Lung Dis. 2014;18(11 Suppl 1):S334.
76. Mukhopadhyay S, Cornelius S, Biswal S, Edward V, Jose M, Shukla V, et al. Improving tuberculosis case detection in difficult-to-reach villages of Chhattisgarh and Madhya Pradesh, India, through a door-to-door tuberculosis campaign. Int J Tuberc Lung Dis. 2014;17(12 Suppl 2):S344.
77. Murhekar MV, Kolappan C, Gopi PG, Chakraborty AK, Sehgal SC. Tuberculosis situation among tribal population of Car Nicobar, India, 15 years after intensive tuberculosis control project and implementation of a national tuberculosis programme. Bull World Health Organ. 2004;82 : 836–843. 15640919
78. Nduba V, Van’t Hoog AH, Mitchell E, Onyango P, Laserson K, Borgdorff M. Prevalence of tuberculosis in adolescents, western Kenya: implications for control programs. Int J Infect Dis. 2015;35 : 11–17. doi: 10.1016/j.ijid.2015.03.008 25770911
79. Nguyen T, Nguyen P, Nhung N, Nguyen B, Tran K, Ho J, et al. Prevalent tuberculosis detected by active case finding among adults in the community in Ca Mau, Viet Nam. 46th Union World Conference on Lung Health; 2015 Dec 2–6; Cape Town, South Africa.
80. Onazi O, Gidado M, Onoh M, Yisa J, Obasanya J, Eneogu R, et al. Innovative approaches for increased case finding: the role of house-to-house in TB case finding. Int J Tuberc Lung Dis. 2014;18(11 Suppl 1):S461.
81. Pronyk PM, Joshi B, Hargreaves JR, Madonsela T, Collinson MA, Mokoena O, et al. Active case finding: understanding the burden of tuberculosis in rural South Africa. Int J Tuberc Lung Dis. 2001;5 : 611–618. 11467367
82. Qadeer E, Fatima R, Tahseen S, Samad Z, Kalisvaart N, Tiemersma E, et al. Prevalence of pulmonary tuberculosis among the adult population in Pakistan 2010–2011. Islamabad (Pakistan): National TB Control Program; 2013.
83. Rao VG, Bhat J, Yadav R, Gopi PG, Selvakumar N, Wares DF. Prevalence of pulmonary tuberculosis among the Bharia, a primitive tribe of Madhya Pradesh, central India. Int J Tuberc Lung Dis. 2010;14 : 368–370. 20132630
84. Rao VG, Gopi PG, Bhat J, Selvakumar N, Yadav R, Tiwari B, et al. Pulmonary tuberculosis: a public health problem amongst the Saharia, a primitive tribe of Madhya Pradesh, Central India. Int J Infect Dis. 2010;14:e713–6. doi: 10.1016/j.ijid.2010.02.2243 20605504
85. Rao VG, Bhat J, Yadav R, Gopalan GP, Nagamiah S, Bhondeley MK, et al. Prevalence of pulmonary tuberculosis—a baseline survey in central India. PLoS ONE. 2012;7:e43225. doi: 10.1371/journal.pone.0043225 22952651
86. Rekha Devi K, Narain K, Mahanta J, Deori R, Lego K, Goswami D, et al. Active detection of tuberculosis and paragonimiasis in the remote areas in North-Eastern India using cough as a simple indicator. Pathog Glob Health. 2013;107 : 153–156. doi: 10.1179/2047773213y.0000000086 23683370
87. Romero-Sandoval NC, Flores-Carrera OF, Sanchez-Perez HJ, Sanchez-Perez I, Mateo MM. Pulmonary tuberculosis in an indigenous community in the mountains of Ecuador. Int J Tuberc Lung Dis. 2007;11 : 550–555. 17439680
88. Rumman KA, Sabra NA, Bakri F, Seita A, Bassili A. Prevalence of tuberculosis suspects and their healthcare-seeking behavior in urban and rural Jordan. Am J Trop Med Hyg. 2008;79 : 545–551. 18840742
89. Sebhatu M, Kiflom B, Seyoum M, Kassim N, Negash T, Tesfazion A, et al. Determining the burden of tuberculosis in Eritrea: a new approach. Bull World Health Organ. 2007;85 : 593–599. 17768517
90. Sekandi JN, Neuhauser D, Smyth K, Whalen CC. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int J Tuberc Lung Dis. 2009;13 : 508–513. 19335958
91. Sekandi JN, List J, Luzze H, Yin XP, Dobbin K, Corso PS, et al. Yield of undetected tuberculosis and human immunodeficiency virus coinfection from active case finding in urban Uganda. Int J Tuberc Lung Dis. 2014;18 : 13–19. doi: 10.5588/ijtld.13.0129 24365547
92. Shargie EB, Yassin MA, Lindtjorn B. Prevalence of smear-positive pulmonary tuberculosis in a rural district of Ethiopia. Int J Tuberc Lung Dis. 2006;10 : 87–92. 16466043
93. Sharma SK, Goel A, Gupta SK, Mohan K, Sreenivas V, Rai SK, et al. Prevalence of tuberculosis in Faridabad district, Haryana State, India. Indian J Med Res. 2015;141 : 228–235. 25900959
94. Soemantri S, Senewe FP, Tjandrarini DH, Day R, Basri C, Manissero D, et al. Three-fold reduction in the prevalence of tuberculosis over 25 years in Indonesia. Int J Tuberc Lung Dis. 2007;11 : 398–404. 17394685
95. Soni T, Sagili K, Thapa B, Chadha S, Wilson N. Active case finding of tuberculosis among marginalised and vulnerable populations from two districts in India: a retrospective cohort study. Int J Tuberc Lung Dis. 2014;18(11 Suppl 1):S330.
96. Tadesse T, Demissie M, Berhane Y, Kebede Y, Abebe M. Two-thirds of smear-positive tuberculosis cases in the community were undiagnosed in Northwest Ethiopia: population based cross-sectional study. PLoS ONE. 2011;6:e28258. doi: 10.1371/journal.pone.0028258 22164256
97. Thorson A, Hoa NP, Long NH, Allebeck P, Diwan VK. Do women with tuberculosis have a lower likelihood of getting diagnosed? Prevalence and case detection of sputum smear positive pulmonary TB, a population-based study from Vietnam. J Clin Epidemiol. 2004;57 : 398–402. doi: 10.1016/j.jclinepi.2002.11.001 15135842
98. Tupasi TE, Radhakrishna S, Rivera AB, Pascual ML, Quelapio MI, Co VM, et al. The 1997 nationwide tuberculosis prevalence survey in the Philippines. Int J Tuberc Lung Dis. 1999;3 : 471–477. 10383058
99. Tupasi TE, Radhakrishna S, Quelapio MI, Villa ML, Pascual ML, Rivera AB, et al. Tuberculosis in the urban poor settlements in the Philippines. Int J Tuberc Lung Dis. 2000;4 : 4–11. 10654637
100. Tupasi T, Radhakrishna S. Significant decline in the tuberculosis burden in the Philippines ten years after initiating DOTS. Int J Tuberc Lung Dis. 2009;13 : 1224–1230. 19793426
101. van’t Hoog AH, Laserson KF, Githui WA, Meme HK, Agaya JA, Odeny LO, et al. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med. 2011;183 : 1245–1253. doi: 10.1164/rccm.201008-1269OC 21239690
102. Vree M, Hoa NB, Sy DN, Co NV, Cobelens FG, Borgdorff MW. Low tuberculosis notification in mountainous Vietnam is not due to low case detection: a cross-sectional survey. BMC Infect Dis. 2007;7 : 109. doi: 10.1186/1471-2334-7-109 17880701
103. Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet. 2014;383 : 2057–2064. doi: 10.1016/s0140-6736(13)62639-2 24650955
104. Wang Y. Analysis of tuberculosis screening results in six remove villages in Yunnan Province. Int J Tuberc Lung Dis. 2013;17(12 Suppl 2):S464–S465.
105. Wei X, Zhang X, Yin J, Walley J, Beanland R, Zou G, et al. Changes in pulmonary tuberculosis prevalence: Evidence from the 2010 population survey in a populous province of China. BMC Infect Dis. 2014;14 : 21. doi: 10.1186/1471-2334-14-21 24410932
106. Woldesemayat EM, Datiko DG, Lindtjorn B. Follow-up of chronic coughers improves tuberculosis case finding: results from a community-based cohort study in Southern Ethiopia. PLoS ONE. 2015;10:e0116324. doi: 10.1371/journal.pone.0116324 25719541
107. Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175 : 87–93. doi: 10.1164/rccm.200606-759OC 16973982
108. Yadav R, Rao V, Bhat J, Gopi P, Selvakumar N, Wares D. Prevalence of pulmonary tuberculosis amongst the Baigas—a primitive tribe of Madhya Pradesh, Central India. Indian J Tuberc. 2010;57 : 114–116. 21114182
109. Yimer S, Holm-Hansen C, Yimaldu T, Bjune G. Evaluating an active case-finding strategy to identify smear-positive tuberculosis in rural Ethiopia. Int J Tuberc Lung Dis. 2009;13 : 1399–1404. 19861013
110. Zaman K, Yunus M, Arifeen SE, Baqui AH, Sack DA, Hossain S, et al. Prevalence of sputum smear-positive tuberculosis in a rural area in Bangladesh. Epidemiol Infect. 2006;134 : 1052–1059. doi: 10.1017/s0950268806006108 16569271
111. Zaman K, Hossain S, Banu S, Quaiyum MA, Barua PC, Salim MA, et al. Prevalence of smear-positive tuberculosis in persons aged ≥ 15 years in Bangladesh: results from a national survey, 2007–2009. Epidemiol Infect. 2012;140 : 1018–1027. doi: 10.1017/s0950268811001609 21880168
112. Kebede AH, Alebachew Wagaw Z, Tsegaye F, Lemma E, Abebe A, Agonafir M, et al. The first population-based national tuberculosis prevalence survey in Ethiopia, 2010–2011. Int J Tuberc Lung Dis. 2014;18 : 635–639. doi: 10.5588/ijtld.13.0417 24903931
113. Joint United Nations Programme on HIV/AIDS. The gap report. UNAIDS/JC2656. Geneva: Joint United Nations Programme on HIV/AIDS; 2014 [cited 2 Aug 2016]. Available: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf.
114. Auld A, Shiraishi R, Mbofana F, Couto A, Fetogang E, El-Halabi S, et al. Lower levels of antiretroviral therapy enrollment among men with HIV compared with women—12 countries, 2002–2013. MMWR Morb Mortal Wkly Rep. 2014;64 : 1281–1286. doi: 10.15585/mmwr.mm6446a2
115. Druyts E, Dybul M, Kanters S, Nachega J, Birungi J, Ford N, et al. Male sex and the risk of mortality among individuals enrolled in antiretroviral therapy programs in Africa: a systematic review and meta-analysis. AIDS. 2013;27 : 417–425. doi: 10.1097/QAD.0b013e328359b89b 22948271
116. Geng EH, Nash D, Kambugu A, Zhang Y, Braitstein P, Christopoulos KA, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7 : 234–244. doi: 10.1007/s11904-010-0061-5 20820972
117. Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, et al. Mass HIV treatment and sex disparities in life expectancy: demographic surveillance in rural South Africa. PLoS Med. 2015;12:e1001905. doi: 10.1371/journal.pmed.1001905 26599699
118. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380 : 2224–2260. doi: 10.1016/S0140-6736(12)61766-8
119. Wang H, Dwyer-Lindgren L, Lofgren KT, Rajaratnam JK, Marcus JR, Levin-Rector A, et al. Age-specific and sex-specific mortality in 187 countries, 1970–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380 : 2071–2094. doi: 10.1016/S0140-6736(12)61719-X
120. Uplekar M, Rangan S, Weiss M, Ogden J, Borgdorff M, Hudelson P. Attention to gender issues in tuberculosis control. Int J Tuberc Lung Dis. 2001;5 : 220–224. 11326820
121. MacPherson P, Houben R, Glynn JR, Corbett E, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low - and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ. 2014;92 : 77–152. doi: 10.2471/BLT.13.124800
122. Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15 : 871–885. doi: 10.5588/ijtld.10.0352 21496360
123. van den Hof S, Najlis CA, Bloss E, Straetemans M. A systematic review on the role of gender in tuberculosis control. KNCV Tuberculosis Foundation. 2010 [cited 2 Aug 2016]. Available: https://www.kncvtbc.org/uploaded/2015/09/Role_of_Gender_in_TB_Control.pdf.
124. Dowdy DW, Basu S, Andrews JR. Is passive diagnosis enough? The impact of subclinical disease on diagnostic strategies for tuberculosis. Am J Respir Crit Care Med. 2013;187 : 543–51. doi: 10.1164/rccm.201207-1217OC. 23262515
125. World Health Organization. Gender and tuberculosis Geneva, Switzerland: WHO, Department of Gender and Women’s Health; 2002 [cited 2 Aug 2016]. Available: http://apps.who.int/iris/bitstream/10665/68891/1/a85584.pdf.
126. Chikovore J, Hart G, Kumwenda M, Chipungu GA, Corbett L. ‘For a mere cough, men must just chew Conjex, gain strength, and continue working’: the provider construction and tuberculosis care-seeking implications in Blantyre, Malawi. Glob Health Action. 2015;8 : 26292. doi: 10.3402/gha.v8.26292 25833138
127. Chikovore J, Hart G, Kumwenda M, Chipungu GA, Desmond N, Corbett L. Control, struggle, and emergent masculinities: a qualitative study of men’s care-seeking determinants for chronic cough and tuberculosis symptoms in Blantyre, Malawi. BMC Public Health. 2014;14 : 1053. doi: 10.1186/1471-2458-14-1053 25301572
128. Mavhu W, Dauya E, Bandason T, Munyati S, Cowan F, Hart G, et al. Chronic cough and its association with TB–HIV co-infection: factors affecting help-seeking behaviour in Harare, Zimbabwe. Trop Med Int Health. 2010;15 : 574–579. doi: 10.1111/j.1365-3156.2010.02493.x 20214762
129. Cornell M, McIntyre J, Myer L. Men and antiretroviral therapy in Africa: our blind spot. Trop Med Int Health. 2011;16 : 828–829. doi: 10.1111/j.1365-3156.2011.02767.x 21418449
130. United Nations Development Programme. Checklist for integrating gender into the processes and mechanisms of the Global Fund to Fight AIDS, Tuberculosis and Malaria. 2015 [cited 2 Aug 2016]. Available: http://www.undp.org/content/dam/undp/library/HIV-AIDS/HIV%20MDGs%20and%20Development%20Planning/UNDP-Checklist%20for%20Integrating%20Gender-May21-2014.pdf.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ferinject: správně indikovat, správně podat, správně vykázat
- Optimální dávkování apixabanu v léčbě fibrilace síní
-
Všechny články tohoto čísla
- Reporting of Adverse Events in Published and Unpublished Studies of Health Care Interventions: A Systematic Review
- A Public Health Framework for Legalized Retail Marijuana Based on the US Experience: Avoiding a New Tobacco Industry
- Improving Research into Models of Maternity Care to Inform Decision Making
- Associations between Extending Access to Primary Care and Emergency Department Visits: A Difference-In-Differences Analysis
- Sex Differences in Tuberculosis Burden and Notifications in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis
- Pre-exposure Prophylaxis Use by Breastfeeding HIV-Uninfected Women: A Prospective Short-Term Study of Antiretroviral Excretion in Breast Milk and Infant Absorption
- A Comparison of Midwife-Led and Medical-Led Models of Care and Their Relationship to Adverse Fetal and Neonatal Outcomes: A Retrospective Cohort Study in New Zealand
- Scheduled Intermittent Screening with Rapid Diagnostic Tests and Treatment with Dihydroartemisinin-Piperaquine versus Intermittent Preventive Therapy with Sulfadoxine-Pyrimethamine for Malaria in Pregnancy in Malawi: An Open-Label Randomized Controlled Trial
- Tenofovir Pre-exposure Prophylaxis for Pregnant and Breastfeeding Women at Risk of HIV Infection: The Time is Now
- The Policy Dystopia Model: An Interpretive Analysis of Tobacco Industry Political Activity
- International Criteria for Acute Kidney Injury: Advantages and Remaining Challenges
- Chronic Kidney Disease in Primary Care: Outcomes after Five Years in a Prospective Cohort Study
- Potential for Controlling Cholera Using a Ring Vaccination Strategy: Re-analysis of Data from a Cluster-Randomized Clinical Trial
- Association between Adult Height and Risk of Colorectal, Lung, and Prostate Cancer: Results from Meta-analyses of Prospective Studies and Mendelian Randomization Analyses
- The Incidence Patterns Model to Estimate the Distribution of New HIV Infections in Sub-Saharan Africa: Development and Validation of a Mathematical Model
- Antimicrobial Resistance: Is the World UNprepared?
- A Médecins Sans Frontières Ethics Framework for Humanitarian Innovation
- Reduced Emergency Department Utilization after Increased Access to Primary Care
- "The Policy Dystopia Model": Implications for Health Advocates and Democratic Governance
- Interplay between Diagnostic Criteria and Prognostic Accuracy in Chronic Kidney Disease
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Sex Differences in Tuberculosis Burden and Notifications in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis
- International Criteria for Acute Kidney Injury: Advantages and Remaining Challenges
- Potential for Controlling Cholera Using a Ring Vaccination Strategy: Re-analysis of Data from a Cluster-Randomized Clinical Trial
- The Policy Dystopia Model: An Interpretive Analysis of Tobacco Industry Political Activity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání