-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
NEUROFIBROMATOSIS TYPE 1 AND OPTIC PATHWAY GLIOMA
Authors: A. Siwá 1; R. Autrata 1; K. Vejmělková 2; Z. Pavelka 2; K. Zitterbart 2
Authors place of work: Dětská oční klinika LF MU a FN Brno, přednosta prof. MUDr. Rudolf Autrata, CSc., MBA 1; Klinika dětské onkologie LF MU a FN Brno, přednosta prof. MUDr. Jaroslav Štěrba, Ph. D. 2
Published in the journal: Čes. a slov. Oftal., 75, 2019, No. 4, p. 200-208
Category: Původní práce
doi: https://doi.org/10.31348/2019/4/4Summary
Purpose: Evaluate the effectiveness of treatment of patients with optic pathway glioma.
Materials and Methods: Comparison of literature research on neurofibromatosis and optic pathway glioma with a cohort of pediatric patients treated at the Children‘s Ophthalmology Clinic of the University Hospital in Brno from January 2013 until June 2018.
Discussion: The main challenge of this and other retrospective studies is variable intervals between ophthalmologic examinations. In some pediatric patients it is also difficult to objectively assess visual functions. The main risk factors are age at the time of treatment and tumor localization. Tumor progression itself does not always correlate with worse visual acuity outcomes, and it remains to be evaluated whether some patients would be better off without treatment. As of now, there are no clinical biomarkers able to predict impending visual acuity loss.
Conclusion: The cohort outcome agrees with literature. Chemotherapy remains a treatment of choice and its most likely outcome is visual acuity stabilization. In order to properly evaluate the treatment’s effectiveness, better collaboration between medical specialists and regular standardized ophthalmology examinations are required.
Keywords:
neurofibromatosis – children – optic pathway glioma – Chemotherapy – treatment
NEUROFIBROMATOSIS TYPE 1
With an incidence of 1/3000 live born children, neurofibromatosis type 1 (NF1) is one of the most commonly occurring autosomal-dominant hereditary diseases in humans (30). Approximately one half of cases are hereditary, and the remaining 50% occur on the basis of new mutations (26). New mutations usually appear in paternal chromosomes. One of the explanations is that the higher age of the fathers at the time of conception leads to a long-term methylation of the DNA of some sperm cells, triggering point mutations which have the consequence of an increased incidence of sporadic NF1. “The cause of development of the pathology is mutation of a tumour-suppressor gene of NF1 (17q11.2) and subsequent malfunction of formation of the cytoplasmatic protein neurofibromin. In the case of NF1 this malfunction is linked with an incidence of multiple tumorous processes, which histologically correspond to hamartomas or benign tumours. Tumours of the central nervous system (CNS) are above all low grade gliomas (pilocytic astrocytoma, grade I), especially in the region of the visual pathway (33).”
CLINICAL PICTURE
Coffee stains (café-au-lait) – this skin symptom is almost always present. It is a multiple, light brown, smooth, sharply bordered pigmentation on the level of the skin. Stains are most often localised on the chest, but also on the limbs and face.
They originate through an accumulation of pigment melanoblasts on the basal layer of the epidermis, and a subsequent malfunction of the development of melanocytes (21). They occur in newborns, but their size and number may increase with age (19, 21, 30). For a definitive diagnosis of NF1 it is sufficient for there to be 6 stains with a diameter larger than 5 mm in children and 15 mm in adults (30, 40). No relationship exists between the number of stains and the severity of NF1 (27). Lisch nodules are a useful diagnostic criterion of NF1. These are multiple hamartomas on the iris and this usually concerns a bilateral finding.
They can be found in a small percentage of children, but they appear in more than 90% of individuals aged over 6 years (23, 27). Initially melanocytic hamartomas are light coloured, but darken with age (19, 21, 30). Freckling – varying number of small rounded macules with pronounced pigmentation. They are located in the underarm and groin, and appear between the 3rd and 5th year of life.
A neurofibroma may appear anywhere in the peripheral nerve. If more than 2 appear, they represent one of the diagnostic criteria of NF1. Neurofibromas manifest themselves before the onset of or during puberty, and may also be found in persons without a diagnosis of NF1. In the case of small lesions, skin neurofibromas can be removed with the aid of a laser, in the case of larger lesions priority is given to surgical removal, but here there is a risk of healing with a hypertrophic scar and recurrence of neurofibromas (33).
A plexiform neurofibroma (PN) may appear on all parts of the body where there are peripheral nerves. It is abundantly vascularised, not encapsulated and does not metastasise. It often manifests infiltrative behaviour (2). The transformation of a benign PN into a Malignant Peripheral Nerve Sheath Tumour (MPNST) represents an 8-13% lifelong risk (14). A symptom of malignant character of a PN may be pain, change of consistency and aggressive growth (2). Patients with a basic diagnosis of NF1 have a 5 year prognosis of 21% survival following the determination of a diagnosis of MPNST, without diagnosis of NF1 42%. MPNST is the most frequent cause of death in adults with a diagnosis of NF1 (14). PN occurs predominantly in orbito-palpebral form. The clinical symptoms may be expressed in newborns, but there is frequent manifestation in the first years of life and progression is generally evident during puberty. Affliction is always unilateral and occurs more frequently in boys. The region of predilection is the outer half of the upper eyelid and the ceiling of the eye socket. Typical manifestations are deformation and hypertrophy of the upper eyelid with palpable nodules, protrusion and dislocation of the eyeball in a downward direction, enlargement of the eye socket and bulging of the fossa temporalis due to long-term persistent expansion of the volume of soft tissues. Neurofibroma is the main cause of enlargement of the eye socket, the ceiling of which may manifest signs of severe skeletal dysplasia. In the case of a defect of the orbital ceiling, the meningoencephalocele bulges into the eye socket, transmitting brain pulsation to the contents of the orbit and giving rise to pulsating exophthalmos. The skin of the eyelid is pigmented, pliable (cutis lata), with angioectasias showing through. Affliction of the extraocular muscles and intraconal sensory and motor nerves may occur. On magnetic resonance imaging (MRI) T1 is hypointense and T2 hyperintense (2). In the case of neurofibromas, which are problematic in their localisation, are painful even at rest, grow rapidly and influence their surrounding area, a surgical procedure is most often applied. However, treatment of PN is problematic, and in children there is a recurrence rate of as high as 75% (32). Optic pathway glioma (OPG) from a histological perspective is a spongioblastoma, astrocytoma or oligodendrocytoma. The tumour originates through hyperplasia of the neuroglia in the trunk of the optics, penetrates through the pial sheath and spreads in the intervaginal spaces, where it causes massive proliferation of mesothelial cells. This represents a characteristic property of this tumour. OPG is manifested macroscopically as a spindle-shaped swelling of the optics in the eye socket or in the cranial cavity, or has a dumbbell-shaped appearance if present in both cavities. The initial symptom is malfunction of vision, which however is generally not recognised sufficiently in time. The first reason for examination in children is strabismus ex anopsia, more frequently convergent than divergent, and progressing axial protrusion of the eye without malfunction of motility, edema or reddening. Upon examination it is possible to observe a relative afferent pupillary defect (RAPD) on an eye with exophthalmos. On images obtained from computed tomography (CT) there is a typical spindle-shaped thickening of the nerve throughout the entire course of the orbit, smoothly and sharply contoured. On X-ray images it is expressed by a concentric extension of the canal beyond a diameter of 5.5 mm, without abrasion and destruction of the surrounding tissue. A value of 6 mm or more, as well as a lateral difference of 20%, thus greater than 1 mm, is considered a pathological value of extension. The diagnostic picture of chiasmal glioma is a perceptible pear-shaped base in the lateral projection, caused by an extension of the ventral part of the sella turcica as a consequence of pressure exerted by the glioma.
In the case of orbital gliomas, there may be a simple atrophy on the ocular fundus or an edema of the optic nerve disc. A finding of bilateral congested papilla or combination of congestion and atrophy points to an affliction of the hypothalamus and the floor of the 3rd brain ventricle, with blockade of cerebrospinal fluid circulation and internal hydrocephalus.
Chiasmal gliomas are divided into two clinical types. An anterior optic chiasmal tumour has the same clinical picture, diagnosis and prognosis as an optic glioma. A posterior hypothalamic chiasmal tumour represents a primary glioma of the hypothalamus, and therefore is of a different biological character. It concerns a very malignant tumour, infiltrating the chiasma secondarily, and its rapid and aggressive growth leads to a fatal outcome. Functional symptoms of chiasmal glioma include progressive malfunction of vision, blind spots in the visual field and disorder of colour perception. An image of bilateral congested papillas is expressed on the ocular fundus, progressing to atrophy of the papillas. There is often present internal hydrocephalus with paralysis of the abducens nerve, with diencephalic symptomatology and disorder of growth (2, 21, 29, 30, 31). The best method for estimating the extent of an OPG is MRI with gadolinium (38). Other diagnostic methods are CT, diffuse tensor imaging and diffuse tensor tractography. Upon MRI examination optic gliomas are T1 hypointense or T1 isointense and T2 hyperintense (35). At present glioma is most often treated by chemotherapy according to the SIOP protocol for low grade tumours, using carboplatin and vincristine. More on therapy and issues thereof in the retrospective study. Other clinical symptoms – up to 85% of children with NF1 have FASI (Foci of Abnormal Signal Intensity) or UBO (Unidentified Bright Object); hypersignal lesions in T2 balanced images on MRI of the brain and spinal cord (18, 32). This most probably concerns aberrant myelinisation with vacuolar change of myelin located in the region of the basal ganglions, globus pallidus, in the thalamus, cerebellum, in the brain stem and subcortical area, predominantly in the temporal region. They are not saturated following application of a contrast substance and do not exert pressure on the surrounding structures, by which they differ from low grade gliomas. FASI are asymptomatic from a lesion neurological perspective, but may be linked with cognitive malfunctions in NF1. In adulthood they have a tendency to reduce in size or disappear, and are rare in individuals over 30 years of age. A chance finding upon MRI is intraspinal tumours, which manifest themselves more slowly than intracranial tumours. Non-tumorous manifestations of NF1 include small growth with abnormal values of growth hormone, macrocephaly and skeletal anomalies upon a background of congenital dysplasia of longer bones, most frequently the tibia, manifested in newborn or breastfeeding age. Scoliosis also appears, which worsens with age. Systemic hypertension is most often renovascular, in which stenosis of the renal artery occurs on a background of fibromuscular dysplasia. These vascular changes may occur also elsewhere. A rare occurrence is the moya-moya syndrome, which is accompanied with the risk of ischemic stroke. It originates in connection with previous radiotherapy of tumours of the CNS (32). Mild mental retardation affects 29-35% of individuals with NF1 (13, 39). Specific developmental learning and behavioural disorders appear. Epilepsy occurs in up to 7% of children and is predominantly secondary upon expansive processes of the CNS (40, 18). An overview of the diagnostic criteria for NF1 is presented in table 1.
Fig. 1. Café-au-lait
Source: Paediatric Clinic, Faculty of Medicine, Masaryk University and University Hospital Brno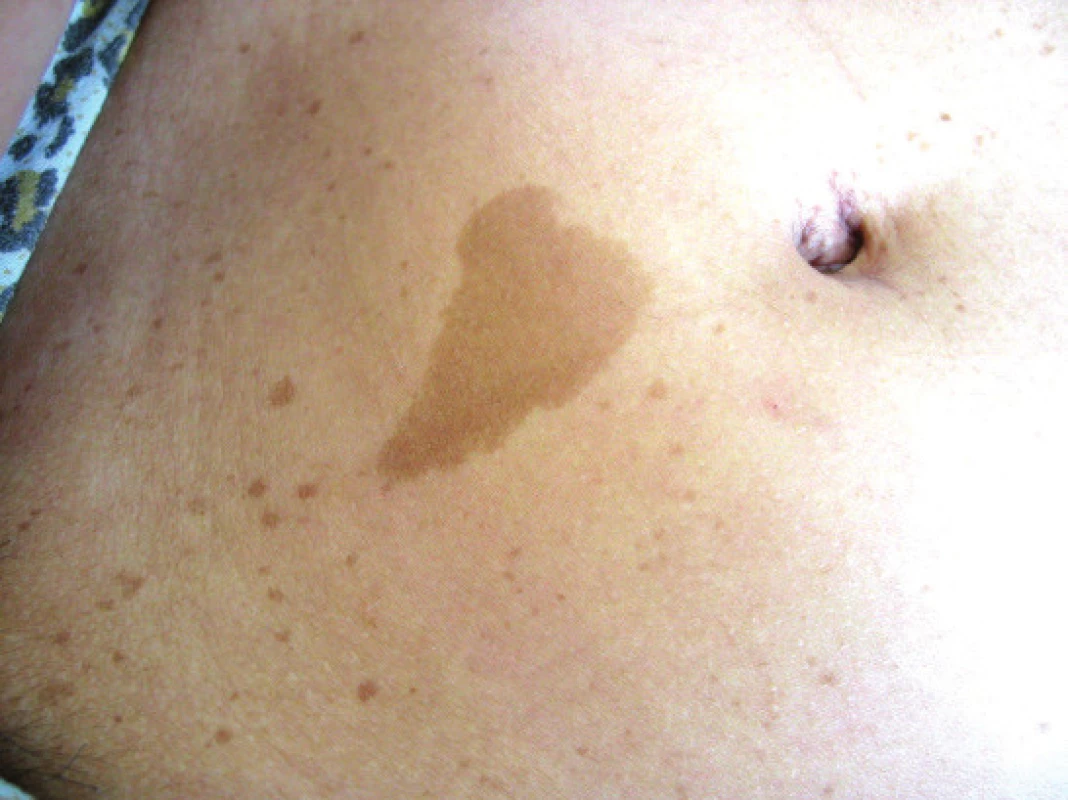
Fig. 2. Lisch nodules
Source: Children’s Ophthalmology Clinic, Faculty of Medicine, Masaryk University and University Hospital Brno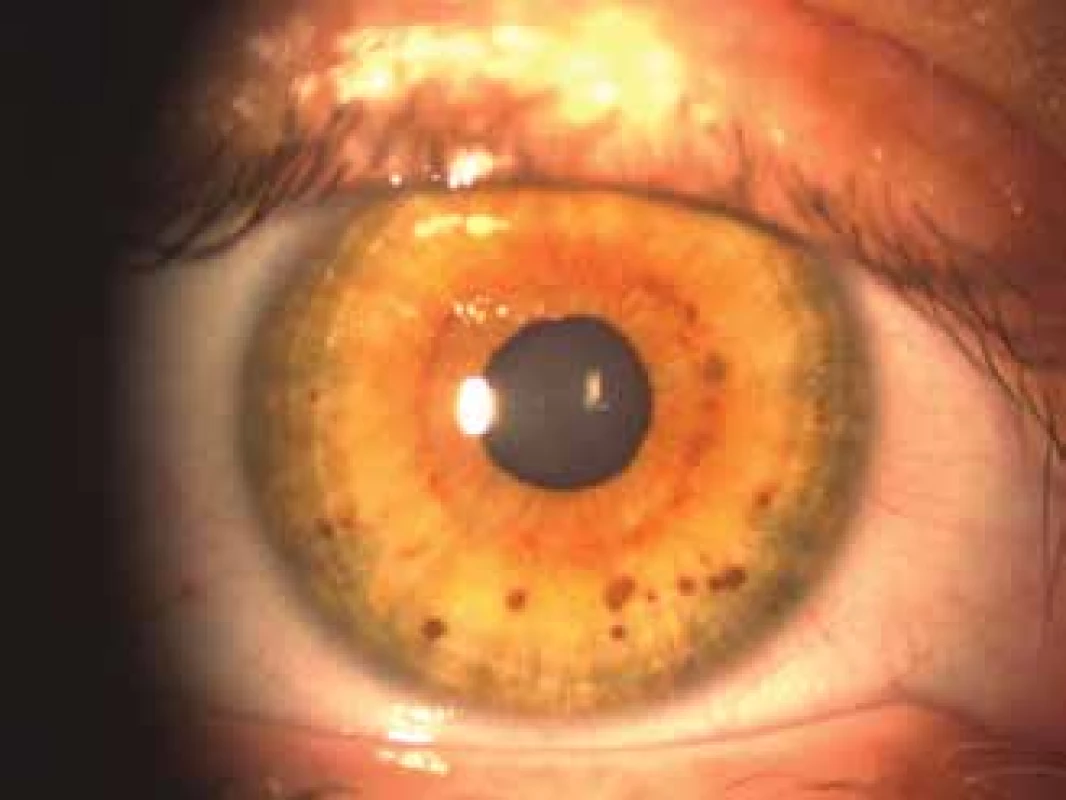
Fig. 3. Freckling in underarm
Source: Paediatric Clinic, Faculty of Medicine, Masaryk University and University Hospital Brno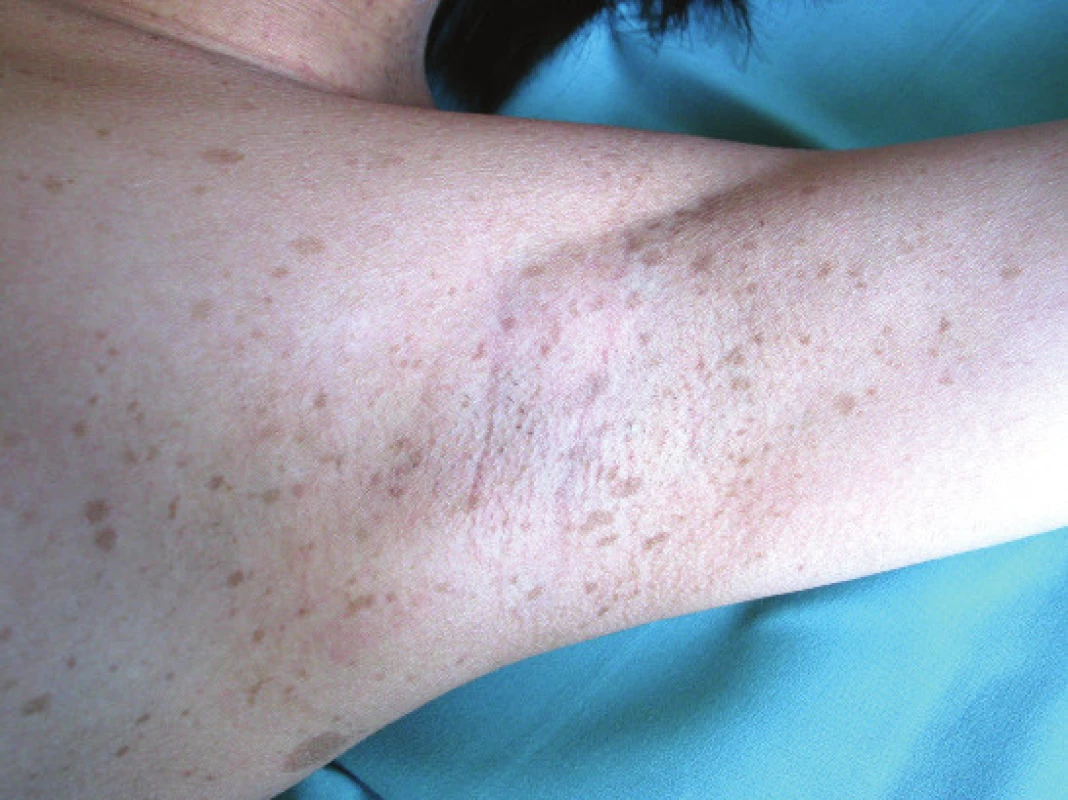
Fig. 4. Plexiform neurofibroma
Source: Paediatric Clinic, Faculty of Medicine, Masaryk University and University Hospital Brno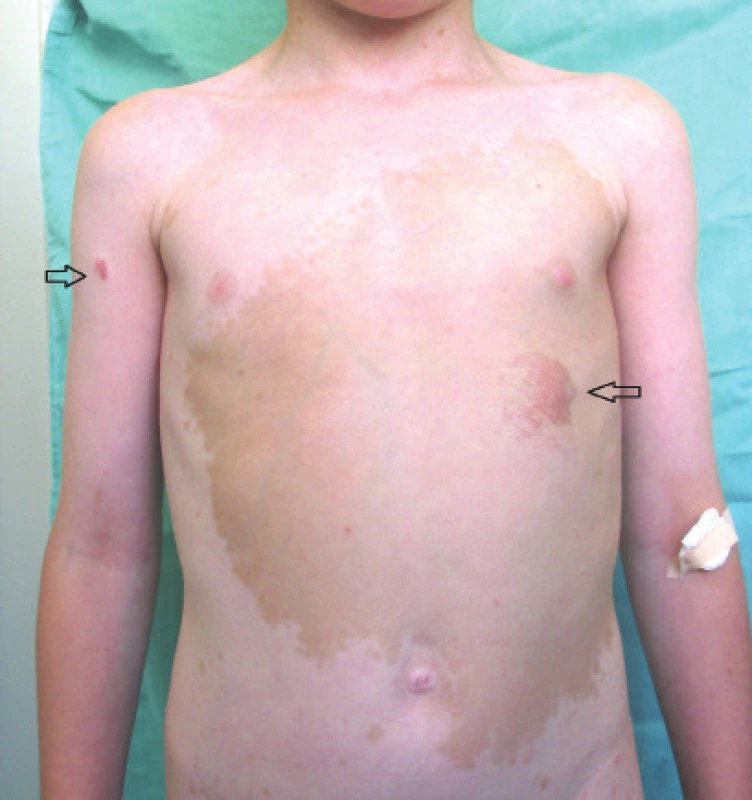
Tab. 1. Neurofibromatosis type 1 – diagnostic criteria
National Institute of Neurological Disorders and Stroke: Neurofibromatosis Fact Sheet (USA), 2011 (41)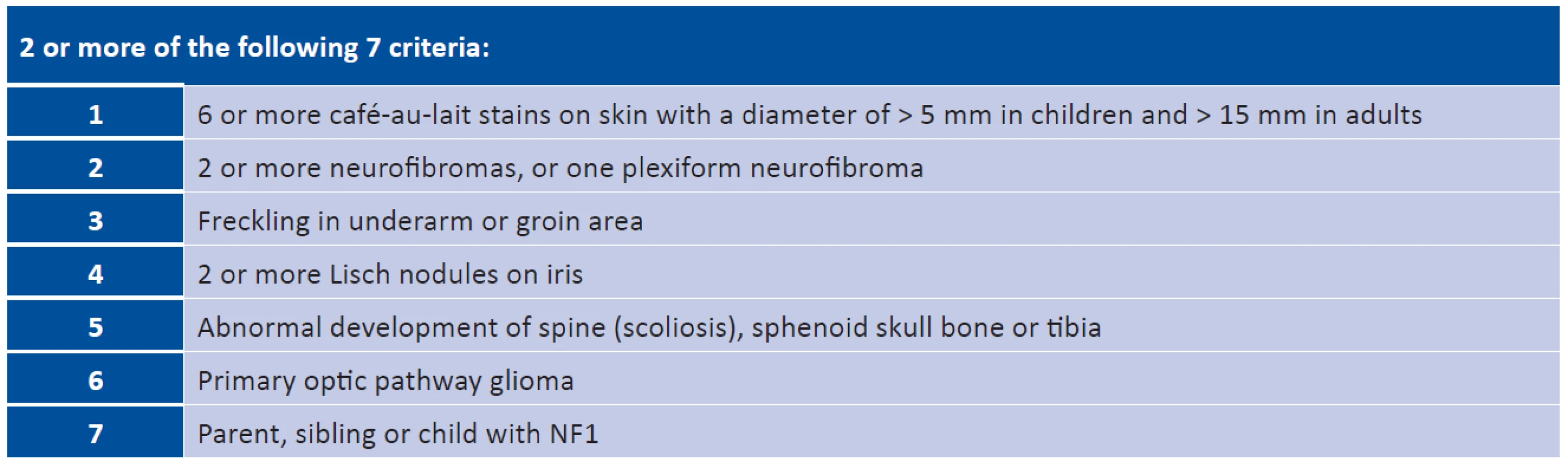
OPTIC PATHWAY GLIOMA
Optic pathway glioma (OPG) is the most common tumour of the optic pathway. It constitutes 3-5% of brain tumours in children (25, 35), 75% of optic tumours and 10% of tumours of the eye socket (2). It is ranked within the group of low grade gliomas (LGG), which are the most common brain tumours in children. The prognosis of survival for patients with LGG is 94% 10 years after diagnosis (20). Approximately one half of all LGGs are asymptomatic. The first choice of treatment is generally chemotherapy, after which the tumour is stabilised in 44% of cases, and radiotherapy, which brings about stabilisation in 62% of cases (20). However, these numbers do not relate to resulting visual acuity (VA) and quality of life. Girls are more susceptible to tumours than boys (12), and boys also manifest longer stability of the tumour following treatment by chemotherapy (20). However, the biological factors determining the progression of tumours remain to be clarified (4).
OPG may occur anywhere in the course of the visual pathway. In 1958 the Dodge classification was designed, which categorised tumours into three types according to localisation, and served to inform the choice of therapy and selection of patients for resection. The arrival of MRI enabled more precise localisation of OPG according to the modified Dodge classification, see fig. 7 (38). The most common functional symptoms of OPG are worsened VA and nystagmus (36). Disorders of the visual field, colour perception and strabismus may also occur. If the life of the patient is not threatened, treatment is indicated with the aim of halting the loss of VA and avoiding blindness, because these factors are linked with a marked deterioration of quality of life. The risk of loss of VA is increased if a tumour is bilateral, or if it appears in the chiasmal region (12, 18, 16, 35). In the case of a chiasmal tumour, propagation into the hypothalamus may furthermore occur, thereby afflicting the hypothalamic-pituitary-gonadal axis, causing premature puberty and diencephalic syndrome. OPG occurs in connection with NF1 (NF1+) or sporadically (NF1-). In the literature the proportion of sporadic OPG is within the range of 30-60% (11, 16, 17, 25, 29, 37). Symptoms appear in 91% of NF1+ patients, whereas in the sporadic type they appear in only 29% of cases (36). Their manifestations are diverse and require individual care.
Fig. 5. MR T2 TSE sectional trans. scan of optic glioma with slight protrusion of right eyeball. Source: Children’s Radiology Clinic, Faculty of Medicine, Masaryk University and University Hospital Brno 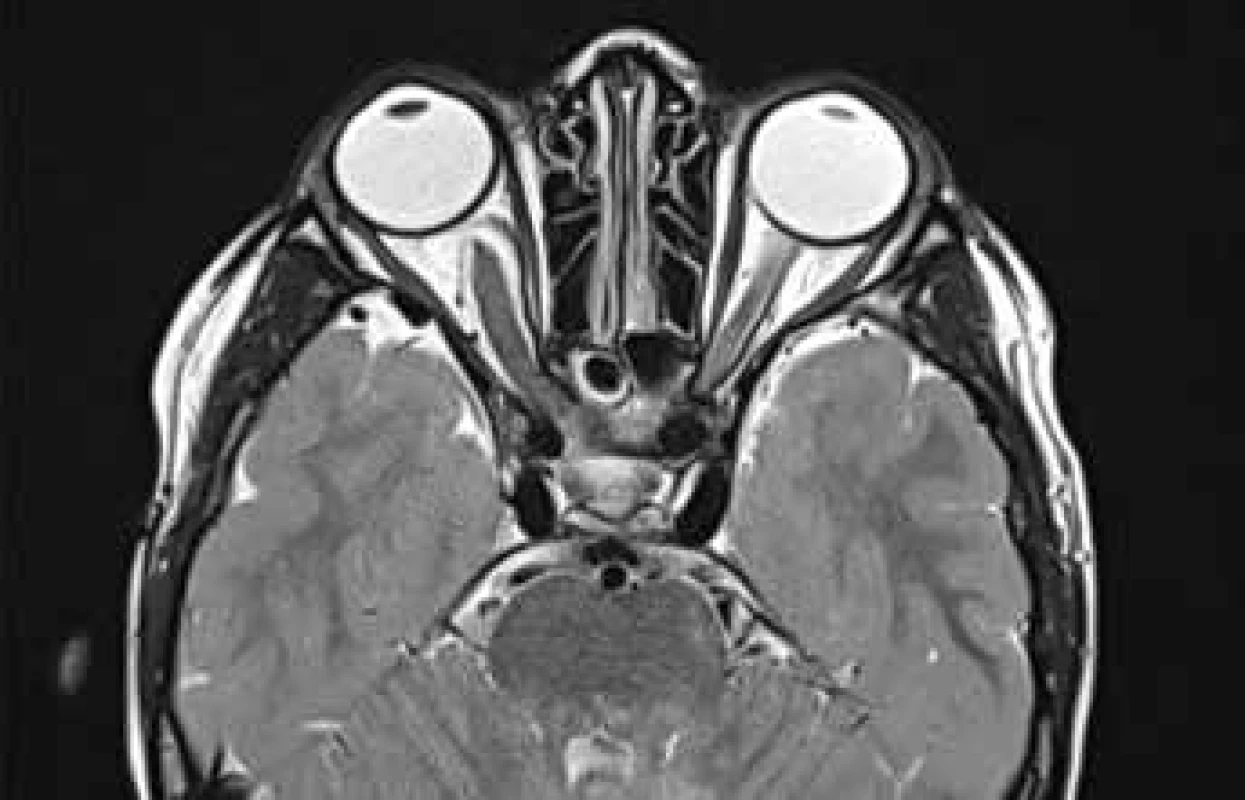
Fig. 6. Photograph of fundus of OD: edema of papilla and atrophy of papilla in patient with optic glioma Source: Children’s Ophthalmology Clinic, Faculty of Medicine, Masaryk University and University Hospital Brno 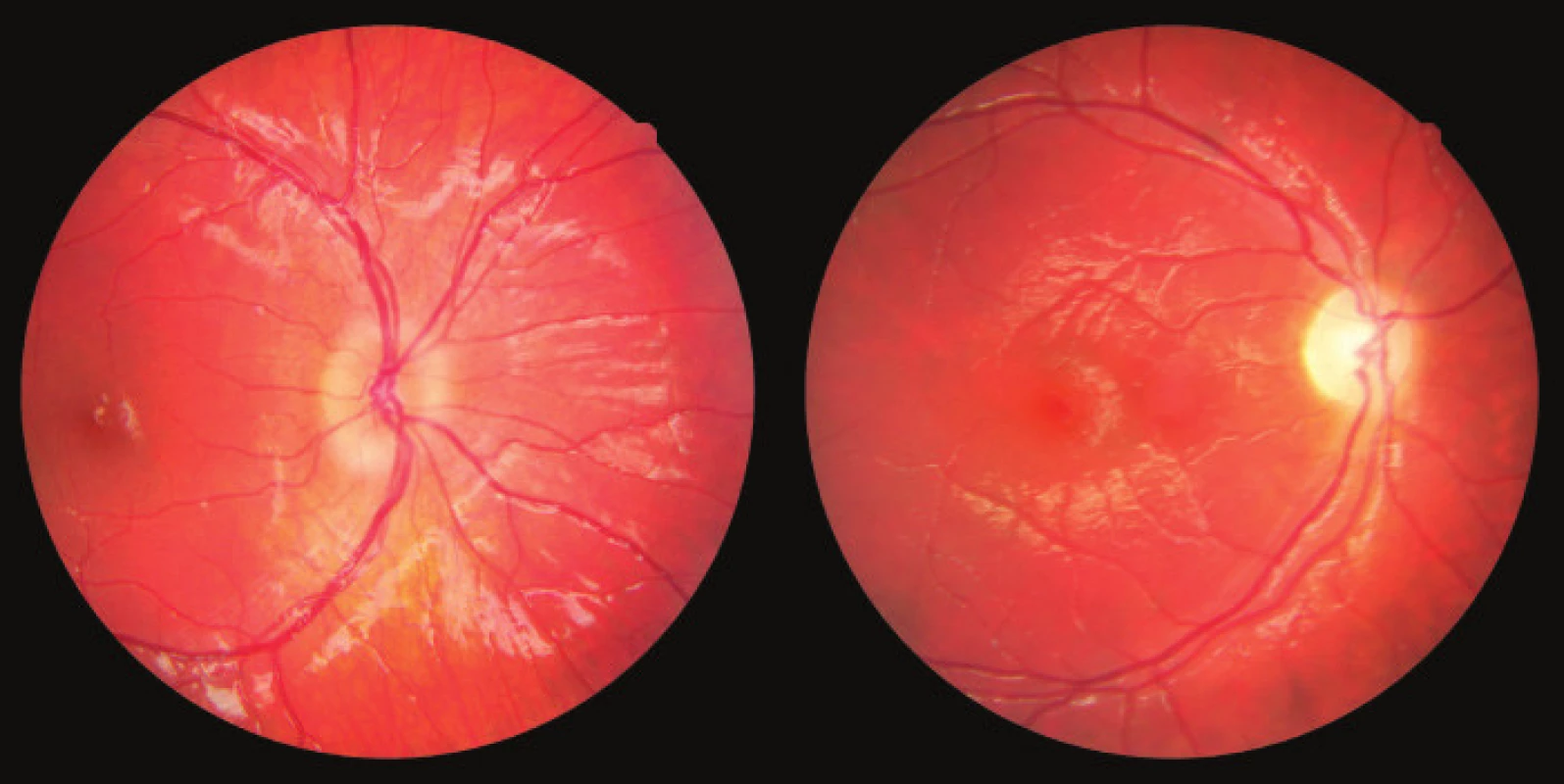
Fig. 7. Original and modified Dodge classification
Source: own illustration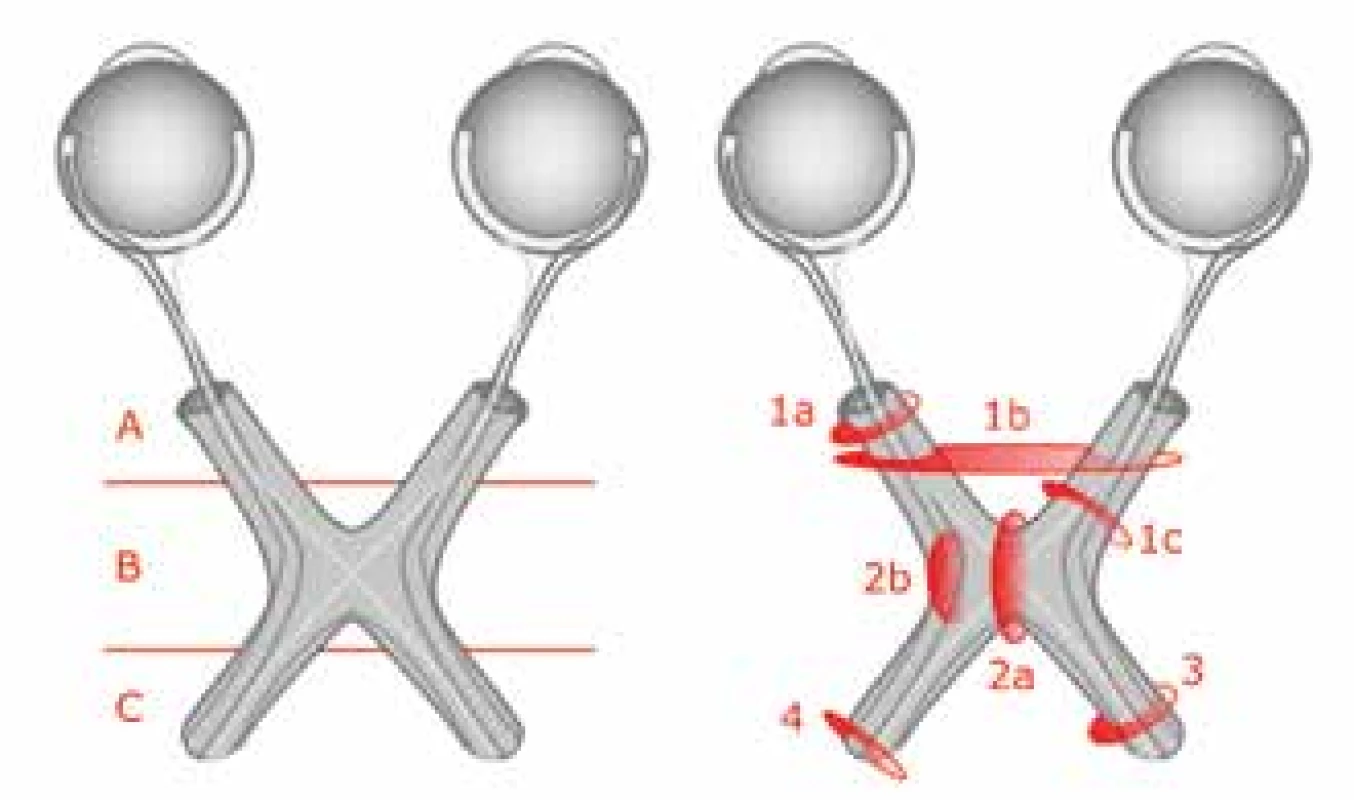
INCIDENCE
NF1 is the most important risk factor for OPG. In children with NF1, OPG usually occurs in 15-25% of cases (6, 17), but in one study this was as high as 76% (12). As a rule it appears in the first decade of life, but approximately 50% of patients are asymptomatic during this period (4, 18). NF1 mutation affects girls more often than boys (17, 36), and OPG occurs more frequently in girls upon diagnosis of NF1 (5). In girls the occurrence of a tumour is more probable in the post-chiasmal region, but girls show better results of treatment than boys (36). From the literature it ensues that deterioration of VA occurs in 20-70% of patients, despite therapy (5). A risk factor is age of under 2 years and over 5 years (17). In 46% of patients with NF1 both optic nerves are affected, whereas in the case of sporadic OPG this is 17%, and according to some studies sporadic OPG is located more frequently in the chiasma (36, 37, 38). If a tumour is present in the post-chiasmal region, the risk of loss of sight is 2 times higher (5). Moreover, in patients with NF1 and OPG there is a documented risk of secondary tumours following treatment by radiotherapy (37).
Sporadic OPG has a more aggressive character and worse prognosis for the development of VA. Even if at the beginning sporadic OPG has a worse prognosis for survival (37), the results of long-term observation indicate that after 15 years the difference between NF1+ and NF1 - patients is erased (34). Nystagmus is a characteristic symptom of sporadic form of OPG localised in the chiasmal region and in the hypothalamus (36, 38).
TREATMENT
Patients with OPG have a relatively good prognosis for survival, which nonetheless decreases in the course of time from 95% 5 years after diagnosis to 92% after 10 years, 81% after 15 and 76% after 18 years. The length of observation of patients in the literature ranges up to 10 years, and as a result it is possible that it does not fully capture the true nature of this pathology. In addition, the risk of death is 3 times higher in patients diagnosed before the first year of life and as much as 5 times higher upon the occurrence of intracranial hypertension, which offers itself as a prognostic factor of survival (34). Since OPGs in patients with NF1 are not of an aggressive character, the main aim of treatment is the preservation of VA (16). The choice of treatment is based on the age of the patient, localisation of tumours and expressed clinical symptoms. In certain cases resection is the chosen method, for the purpose of reducing the volume of the tumour. Resection is linked with a better prognosis for survival and stabilisation of the tumour, but may result in blindness, damage to the hypothalamus, vascular complications and deterioration of endocrine functions (1, 3). A study of 109 NF1+ patients with OPG points to localisation of the tumour and surgical procedure as risk factors for the progression of the tumour (12). In 2011 a resolution was issued at the international conference of paediatric neurosurgery in Paris, stating that a surgical procedure is not the standard primary therapy, even if resection is acceptable in certain cases with a lower risk (41). Biopsy is recommended, since it helps ensure certainty in diagnosis and is useful if an atypical finding appears on MRI (1). Chemotherapy is currently the standard procedure. In 60-80% of patients, OPG is diagnosed until up to 5 years of age, and chemotherapy is indicated if a deterioration of VA is recorded, or if there is unacceptable progression of the tumour (5, 9, 11, 25, 29, 34, 36, 37). Asymptomatic progression of the tumour alone is not sufficient for the commencement of treatment, since the OPG may be benign and the correlation between the progression of a tumour and VA is not reliable (5, 9, 11, 25, 28). Risk factors are low age and tumours in the chiasm or hypothalamus, in which a deterioration of VA has been recorded in 32% of cases (11). The best results of VA following chemotherapy are recorded in patients between the 2nd and 5th years of life (17). However, the effectiveness of chemotherapy on preserving sight has been disputed by numerous studies. The most frequent result of treatment, in approximately 50% of cases, is stabilisation of VA at its level before chemotherapy (9, 11, 28). In patients who commenced chemotherapy with baseline VA worse than 6/24, a further deterioration of VA was recorded (25). In one study it is even stated that none of the patients recorded an improvement of VA after chemotherapy (17). The most effective treatment for the preservation of VA appears to be radiotherapy (28). However, this treatment has a range of adverse effects, such as vascular and endocrine dysfunctions, and deterioration of cognitive functions in children younger than 5 years of age (3, 11). In patients younger than 5 years of age, chemotherapy is usually applied with the aim of deferring radiotherapy. However, NF1 syndrome causes learning and behavioural disorders, as well as mild mental retardation (13, 39). As a result, there is a question as to whether the risk of deterioration of cognitive functions in NF1 patients is a sufficient reason for deferring radiotherapy. Further clinical tests are required in order to confirm this hypothesis (3, 28).
RETROSPECTIVE STUDY
INTRODUCTION
The aim of this retrospective study is to analyse a cohort of patients with OPG and to compare the obtained data with international publications. In the healthcare documentation of the Children’s Ophthalmology Clinic and the Children’s Oncology Clinic of the Faculty of Medicine at the Masaryk University and University Hospital Brno, 47 children with OPG were identified. All the patients were observed at the Children’s Ophthalmology Clinic of the Faculty of Medicine at the Masaryk University and University Hospital Brno during the period from January 2013 to June 2018.
METHOD
The criteria for inclusion in the cohort covered: age at the time of diagnosis, NF1 status, sex, localisation of tumour according to MDC performed by radiologist and VA before and after treatment. In addition, the documentation had to include a description of the development of the radiological findings and clinical symptoms. VA was evaluated on a Snellen chart, in children of pre-school age pictorial optotypes or Pflüger optotypes were used. In children younger than 3 years of age, preferential vision was examined with the aid of a Cardiff and Single Book test. These criteria were met by 37 patients.
RESULTS
Of the 37 patients with OPG, 15 were girls and 22 boys. Sporadic OPG occurred in 27% of cases (n = 10), and was equally represented in both sexes. The majority of OPGs (73%) were linked with NF1, among the girls the incidence of NF1 was in 66% of cases (n = 10), in boys in 77% (n = 17). In the group of NF1+ patients the median age was 2 years and 9 months and in the NF1 - group the median was 4 years and 3 months. In both groups the optic nerve was most frequently affected. Sporadic OPG had a higher probability of manifestation in the optic nerve, chiasmal and post-chiasmal region. In both groups, the number of occurrences of the bilateral tumour was comparable, see graph 1 and table 2.
Graph 1. Distribution of tumours according to modified Dodge classification 
Tab. 2. Modified Dodge classification – explanation. Source: Taylor T. et al., 2008 (37) 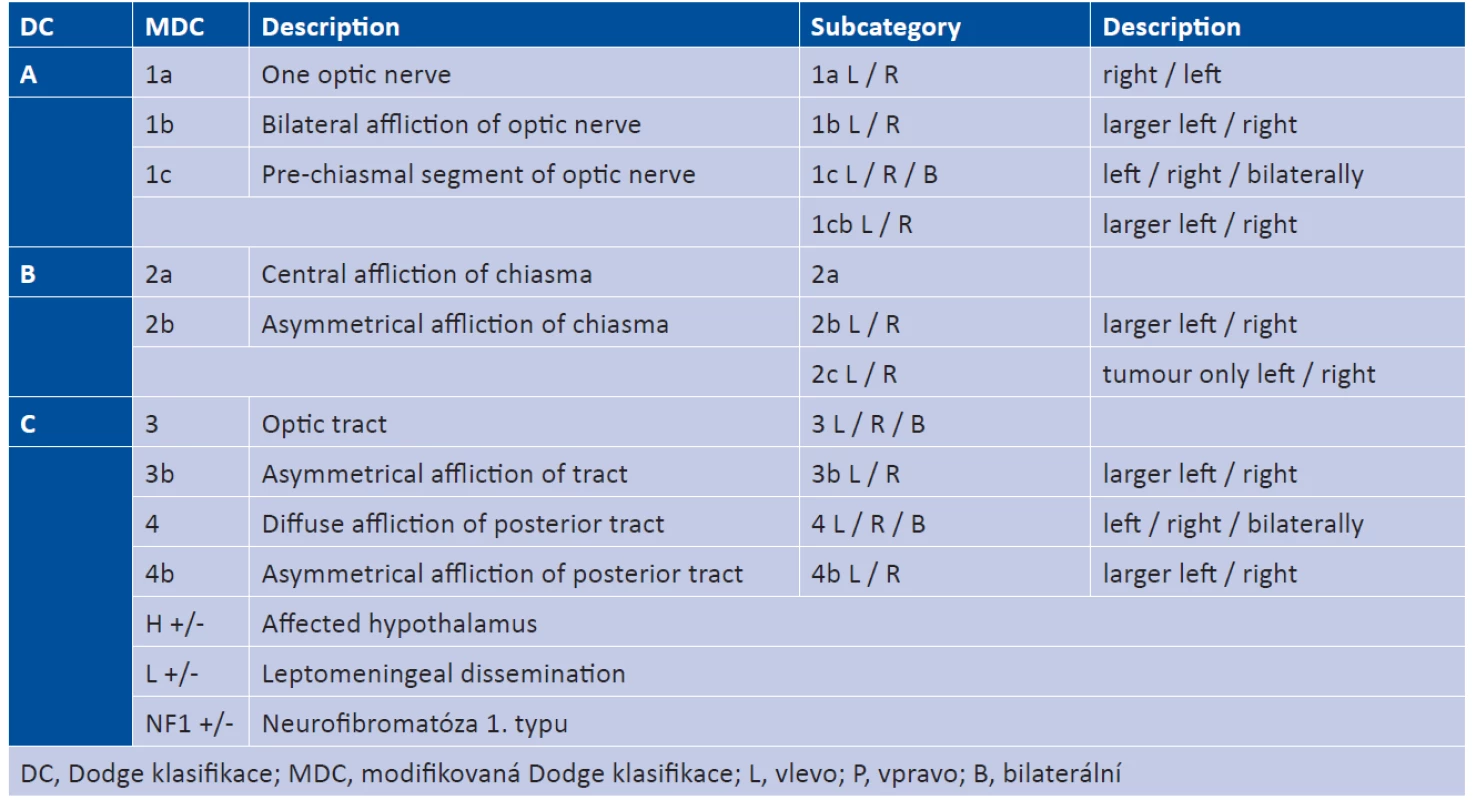
Nystagmus was a characteristic finding in the NF1 - group (n = 3 vs. n = 0 in NF1+). Hydrocephalus appeared in 3 patients, of whom one was from the NF1+ group.
The prognosis for VA in patients with a sporadic tumour is poor. At the last examination, out of 20 eyes (n = 10 patients) there were only 5 eyes with stable VA of 6/6 (25%). One eye recorded an improvement of VA from 6/24 to 6/6. 13 eyes (65%) were amaurotic, and according to the World Health Organization came within one of three categories of blindness. One eye had to be enucleated. The course of the pathology and the resulting VA were fundamentally better in the NF1+ patients. Out of 27 NF1+ patients, 59% (n = 17) were merely observed. Out of 34 eyes in this group, 20 eyes were with VA of 6/6 throughout the entire observation period and 7 eyes spontaneously improved by 1 to 2 rows. Stability of VA was recorded in 7 eyes. The remaining 10 patients (20 eyes) underwent treatment by chemotherapy. In 6 patients the SIOP protocol (carboplatin and vincristine) was selected, in 3 the ACNS protocol (carboplatin, vincristine, temolozomide), and one patient underwent monotherapy with carboplatin. The results of treatment in 3 eyes were a deterioration of VA and in one eye an improvement of VA by one row, 80% of eyes recorded stability. Eyes with baseline VA better than 6/24 recorded stabilisation after chemotherapy, while eyes with baseline VA of 6/24 or less recorded a deterioration of VA after treatment.
DISCUSSION
In the group of patients with NF1, in contrast with the literature OPG occurred more frequently in boys (63% vs. 37%). In this group neither increased incidence of OPG in the chiasm in comparison with other localisations, nor more frequent incidence of bilateral OPG in comparison with the NF1 - group were demonstrated (12, 17, 36, 37). The main problems, which this study also encountered, were the low frequency of follow-up eye examinations and the variable time interval between them (17, 28). These examinations are important for three reasons.
Firstly, they serve to determine whether a patient undergoing the treatment has stable VA in the long-term, or the VA deteriorates in the course of the treatment. In all of the patients in this study, before the commencement of the therapeutic procedure, the results of VA from two or more examinations were available only rarely. As a result it was not possible to determine whether deterioration of VA was also taking place at the time of commencement of treatment. In the literature it is stated that an improvement of sight following treatment is rather the exception (0-32%), which is in congruence with this study (16%) (17, 25). It is necessary to consider whether chemotherapy represents an unnecessary burden in the case of patients with stable VA. From the literature it ensues that in certain cases it may not be possible to prevent deterioration of VA by means of chemotherapy (9). It is in the general interest to identify these cases and not to commence treatment if the natural development of the pathology would have the same consequence for VA as chemotherapy. Because at present genetic analysis does not enable us to predict the severity of OPG, it is necessary to ensure regular, standardised ophthalmological observation of NF1 patients and patients with diagnosed OPG (5). This study furthermore documents that in the group with NF1 an improvement of VA most often occurred spontaneously (32%). However, for patients without NF1 these statistics are unavailable, because all 10 patients underwent treatment and improvement of VA took place in only one of them (10%). A confirmed characteristic finding in the NF1 - group was nystagmus (36). The results further confirm that sporadic form of OPG has a far more rapid and serious course, which as a rule requires immediate treatment (36, 37).
The second aim of regular eye examinations is to evaluate the influence of the tumour on VA. The poor correlation of the progression of the tumour and VA, which is well documented in the literature, excludes radiological finding as the sole criterion for commencement of treatment, because it cannot counter the risks in connection therewith (6, 9, 11, 17). Factors considered clinically significant are 1) deterioration of VA by 0.2 logMAR (Logarithm of the Minimum Angle of Resolution) or more, 2) confirmation of deteriorating VA or 3) a new finding (10). Due to insufficient data this correlation was not investigated in this study. Regular eye checks further serve for identification of patients with risk factors. The first risk factor is VA worse than 6/24 before the commencement of treatment, because the result is generally a further deterioration (25). The second factor is age of less than 2 years or more than 5 years. In a study of 115 patients there was a deterioration following chemotherapy in 50% of patients younger than 2 years and also in 36% older than 5 years (17). The majority of children with OPG are treated before reaching 1 year of age (10, 35). Baseline VA worse than 6/24 and low age were risk factors also in this cohort of patients. In NF1 - patients the median age was 2 years and 9 months, and median VA 6/24, in the NF1+ patients 4 years and 3 months with VA of 6/6. The results confirmed not only the predictive capability of these factors, but also document the more aggressive course of sporadic form of OPG. The third risk factor is bilateral affliction or localisation of the tumour in the chiasm/post-chiasmal region or the hypothalamus, in which long-term deterioration of VA occurs in 32% of patients (11, 36). The influence of localisation of the tumour on resulting VA, which is currently the subject of examination, could not be evaluated in our study (38). With the exception of intraconal, chiasmal and hypothalamic tumours, after the first chemotherapy VA has long-term predictive capability, and it is therefore necessary to consider secondary treatment if the first results is unsatisfactory (11). The third argument for more frequent ophthalmological examination is to evaluate the treatment and long-term prognosis, which although worse in the short term for the NF1 - group, in the long term may be the same as in the NF1+ group (34). Frequent problems, to which attention has been drawn also by other authors of retrospective studies, are non-standard and insufficient evaluation of VA. In a number of patients treated with chemotherapy, who were transferred into our care from other centres, the baseline value of VA was not recorded and it was therefore not possible to confirm the effectiveness of treatment. In this case it is important to ensure co-operation of the patient with the doctor and respecting of the appointments for follow-up examinations. If it is not possible to evaluate VA objectively due to lack of co-operation on the part of the patient, the VA examination must be repeated within 2 weeks, and if the examination fails to produce results a second time, it is necessary to document this (16). None of the cohort of patients in this study was observed for a sufficiently long period to enable an objective evaluation of the effectiveness of treatment and a comparison of the long-term survival prognosis between the NF1 - and NF1+ groups. Because the chiasmal and post-chiasmal region is linked with a worse prognosis, it is important to differentiate individual localisations according to the modified Dodge classification. However, some studies do not use this classification, and differences in interpretation exist among individual radiologists. It is possible that the poor correlation between the volume of the tumour and its influence on VA is caused by imprecision in the estimation of the size of the tumour from 2D MRI images. Volumetric Magnetic Imaging (VMI) is an alternative method for objective evaluation, but is limited by problems with measurement of tumours in the post-chiasmal region (10). Since neither genetic analysis nor biomarkers are reliably able to predict the development of OPG, it is necessary to ensure regular standardised observation. An annual ophthalmological examination is recommended for children with NF1 (4). This should be performed every 3 months if OPG is confirmed on MRI (5, 15), see table 3.
Tab. 3. Recommended observation intervals for patients with NF1. Source: de Blank P.M.K. et al., 2017 (10) 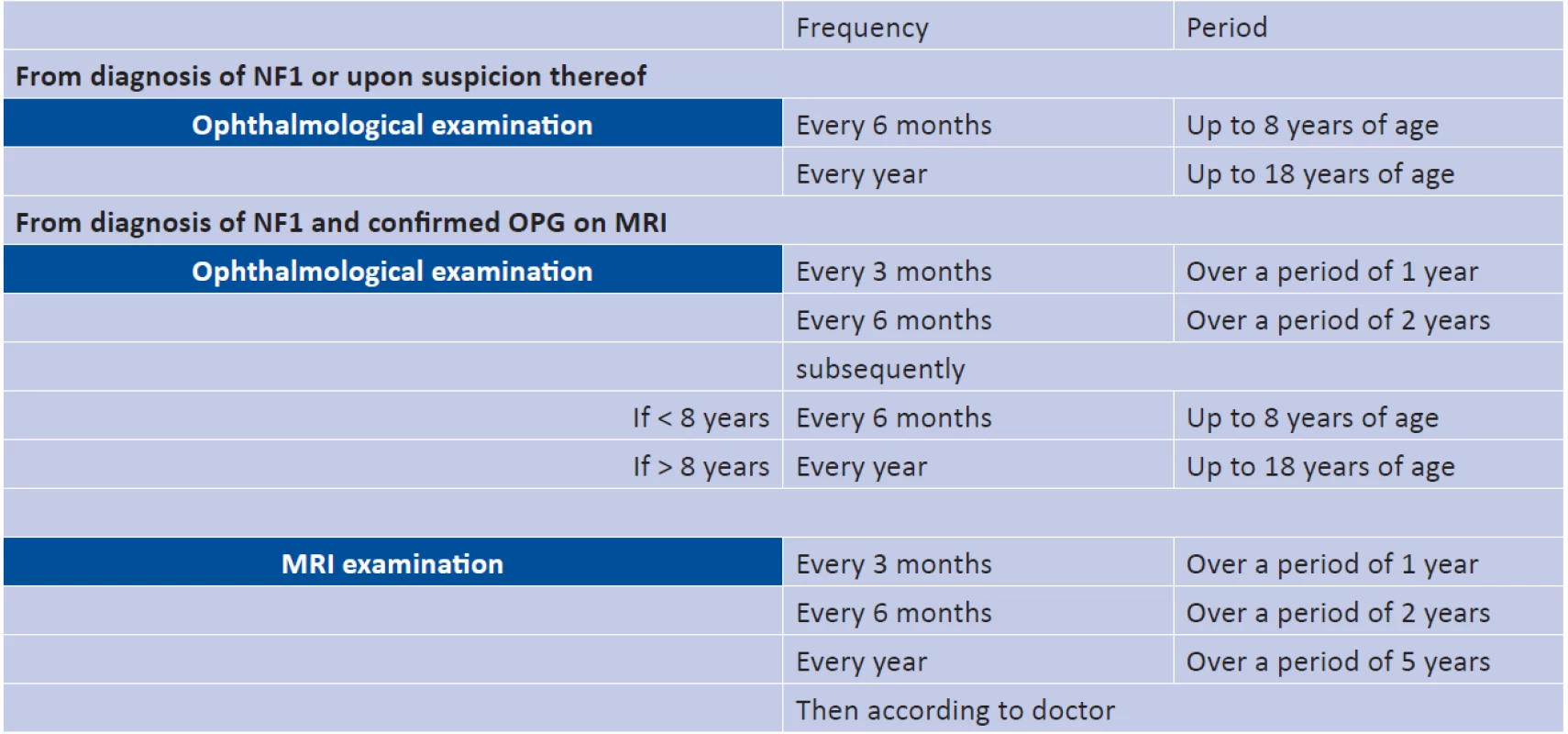
At each examination it is necessary to quantitatively evaluate VA and the papilla of the optic nerve on the ocular fundus (16, 35). In small children, VA is evaluated with the use of Teller Cards (Cardiff Test, Single Book), in older children with HOTV optotypes. Examination of VA and the visual field should be conducted at every visit to an ophthalmologist. However, in the case of perimetric examination, patient co-operation is necessary, which is limited by age and the length of examination (5-7 minutes per one eye) and complicated by the increased incidence of attention disorders in patients with NF1 (13, 35, 39). If nystagmus, strabismus, loss of colour perception, edema of the optic nerve or discolouration of the papilla is recorded, the patient should be monitored with increased attention, similarly as in the case of asymptomatic growth of a tumour (10). More examinations for statistical purposes are not recommended, although other methods exist which have the potential as a biomarker of VA. Optical coherence tomography (OCT), measuring the thickness of the retinal nerve fibre layer (RNFL) shows promising results. However, the use of OCT as a biomarker of VA is not recommended until a correlation has been demonstrated between a reduction of the RNFL and future loss of VA. Although loss of VA often correlates with a reduction of the RNFL, it appears that shrinkage of the RNFL occurs over the long term, and in certain cases continues also after the stabilisation of visual acuity (10). Visual evoked potentials (VEP) have high sensitivity (90-100%), and as a result may be used for timely identification of a tumour. At the same time, however, they have low specificity (60-69%) and correlate with visual acuity in only 50% of cases (10, 16). Some patients with NF1 manifest abnormal results, even though OPG does not occur in them (24). Furthermore, VEPs are not capable of determining which OPG requires treatment, or of monitoring change of VA in dependency upon treatment. As a result they cannot serve as a clinical biomarker, because this must be capable of identifying and predicting loss of VA before the commencement of the therapeutic procedure (10, 16).
CONCLUSION
At present, the standard treatment is chemotherapy, which is indicated on the basis of the clinical and radiological progression of the tumour. However, the influence of chemotherapy on VA is debatable, since its most frequent result is stabilisation of VA, and in certain cases it is most probably not possible to avoid loss of VA with the aid of chemotherapy. For these patients chemotherapy may represent and unnecessary burden. Unfortunately no clinical biomarker exists which could reliably predict subsequent loss of VA. Future studies will need to compare the results of radiological findings and ophthalmological examinations with potential clinical biomarkers of VA, observe changes thereof within the same time intervals and in dependency on treatment. It is also necessary to ensure long-term observation of patients, in order to differentiate short-term improvement from the long-term trend of development of the pathology. However, this shall be possible only in the case of co-ordination of examinations between individual specialists.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
Received: 18. 2. 2019
Accepted: 8. 7. 2019
Available on-line: 6.1.2020
MUDr. Aneta Siwá
Dětská oční klinika LF MU a FN Brno
Černopolní 9,
613 00 Brno
Zdroje
1. Aihara, Y., Chiba, K., Eguchi, S. et al.: Pediatric Optic Pathway/Hypothalamic Glioma. Neurol Med Chir, 58(1); 2018 : 1–9.
2. Autrata, R.: Choroby víček a orbity. In Autrata, R. (Ed), Dětská oftalmologie I. část. Sborník prací lékařské fakulty Masarykovy univerzity č. 122. Masarykova univerzita, Brno, 2008, s. 16–17.
3. Awdeh, RM., Keihna, EN., Drewry, RD. et al.: Visual outcomes in pediatric optic pathway glioma after conformal radiation therapy. Int J Radiat Oncol Biol Phys, 84(1); 2012 : 46–51.
4. Bakker, AC., La Rosa, S., Sherman, LS. et al.: Neurofibromatosis as a gateway to better treatment for a variety of malignancies. Prog Neurobiol, 2017(152): 149–165.
5. Balcer, LJ., Liu, GT., Heller, G. et al.: Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol, 131(4); 2001 : 442–445.
6. Cameron, F., Parsa, R.: Neurogenic tumors. In Pediatric Ophthalmology and Strabismus Fourth Edition. Eds Hoyt, C.S., Taylor, D. Edinburgh: Elsevier Ltd., 2012, s. 206–215.
7. Campen, CJ., Gutmann, DH.: Optic Pathway Gliomas in Neurofibromatosis Type 1. J Child Neurol, 33(1); 2017 : 73–81.
8. Ceballos-Quintal, JM., Pinto-Escalante, D., Castillo-Zapata, I. et al.: A new case of Klippel-Trenaunay-Weber (KTW) syndrome: Evidence of autosomal dominant inheritance. Am J Med Genet, 63(3); 1996 : 426–427.
9. Dalla Via, P., Opocher E., Pinello, ML. et al.: Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neuro Oncol, 9(4); 2007 : 430–437.
10. de Blank, PMK., Fisher, MJ., Liu, GT. et al.: Optic Pathway Gliomas in Neurofibromatosis Type 1: An Update: Surveillance, Treatment Indications, and Biomarkers of Vision. J Neuroophthalmol, 37(Suppl); 2017 : 23–32.
11. Dodgshun, AJ., Elder, JE., Hansford, JR. et al.: Long‐term visual outcome after chemotherapy for optic pathway glioma in children: Site and age are strongly predictive. Cancer, 121(23); 2015 : 4190–4196.
12. Driever, PH., von Hornstein, S., Pietsch, T. et al.: Natural history and management of low-grade glioma in NF-1 children. J Neurooncol, 100(2); 2010 : 199–207.
13. Erdoğan-Bakar, E., Cinbis, M., Ozyürek, H. et al.: Cognitive functions in neurofibromatosis type 1 patients and unaffected siblings. Turk J Pediatr, 51(6); 2009 : 565–71.
14. Evans, DGR., Baser, ME., McGaughran, J. et al: Malignant peripheral nerve sheath tumors in neurofibromatosis. J Med Genet, 39(5); 2002 : 311–314.
15. Ferner, RE., Huson, SM., Thomas, N. et al.: Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet, 44(2); 2007 : 81–88.
16. Fisher, MJ., Avery, RA., Allen, JC. et al.: Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology, 81(21 Suppl 1); 2013 : 15–24.
17. Fisher, MJ., Loguidice, M., Gutmann, DH. et al.: Visual outcomes in children with neurofibromatosis type 1–associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro Oncol, 14(6); 2012 : 790–797.
18. Friedrich, RE., Nuding, MA.: Optic Pathway Glioma and Cerebral Focal Abnormal Signal Intensity in Patients with Neurofibromatosis Type 1: Characteristics, Treatment Choices and Follow-up in 134 Affected Individuals and a Brief Review of the Literature. Anticancer Res, 36(8); 2016 : 4095–4121.
19. Gerinec, A.: Fakomatózy. In Gerinec, A. (Ed), Detská oftalmológia. Martin, Osveta, 2005, s. 517–520.
20. Gnekow, AK., Falkenstein, F., von Horstein, S. et al.: Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. J Neurooncol, 14(10); 2012 : 1265–1284.
21. Grigg, J., Jamieson, R.: Phakomatoses. In Pediatric Ophthalmology and Strabismus Fourth Edition. Eds Hoyt, C.S., Taylor, D. Edinburgh: Elsevier Ltd., 2012, s. 675–689.
22. Happle, R.: Klippel-Trenaunay syndrome: is it a paradominant trait? Br J Dermatol, 128; 1993 : 465.
23. Hudson, S., Beck, L.: Ophthalmic manifestations of neurofibromatosis. Br J Dermatol, 71(3); 1987 : 235–238.
24. Iannaccone, A., McCluney, RA., Brewer, VR. et al.: Visual evoked potentials in children with neurofibromatosis type 1. Doc Ophthalmol 105; 2002 : 63–81.
25. Kalin-Hajdu, E., Décarie, JC., Marzouki, M. et al.: Visual acuity of children treated with chemotherapy for optic pathway gliomas. Pediatr Blood Cancer, 61(2); 2013 : 223–227.
26. Kissil, J., Blakeley, J., Ferner, R. et al.: What’s New in Neurofibromatosis? Proceedings from The 2009 NF Conference: New Frontiers. Am J Med Genet A, 152 A(2); 2010 : 269–283.
27. Korf, BR.: Clinical features and pathobiology of neurofibromatosis. J Child Neurol, 17(8); 2002 : 573–577.
28. Moreno, L., Bautista F., Ashley S. et al.: Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer, 46(12); 2010 : 2253–2259.
29. Otradovec, J.: Ložiskové příznaky nitrolebních nádorů. In Otradovec, J. (Ed), Klinická neurooftalmologie. Praha, Grada, 2003, s. 364–367.
30. Otradovec, J.: Fakomatózy. In Otradovec, J. (Ed), Klinická neurooftalmologie. Praha, Grada, 2003, s. 396–403.
31. Otradovec, J.: Choroby zrakového nervu. In Otradovec, J. (Ed), Klinická neurooftalmologie. Praha, Grada, 2003, s. 193–196.
32. Petrák, B., Plevová, P., Novotný, J. et al.: Neurofibromatosis von Recklinghausen. Klin Onkol, 22(Suppl); 2009 : 38–44.
33. Petrák, B., Bendová, Š., Lisý, J. et al.: Neurofibromatosis von Recklinghausen typ 1 (NF1) – klinický obraz a molekulárně-genetická diagnostika. Cesk Patol, 51(1); 2015 : 34–40.
34. Rakotonjanahary, J., De Carli, E., Delion, M. et al.: Mortality in Children with Optic Pathway Glioma Treated with Up-Front BB-SFOP Chemotherapy. PLoS ONE [online], 10(6); 22. června 2015. [cit. 15. prosince 2018]. Dostupné na WWW: < https://doi.org/10.1371/journal.pone.0127676 >.
35. Rasool, N., Odel, JG., Kazim, M.: Optic pathway glioma of childhood. Curr Opin Ophthalmol, 28(3); 2017 : 289–295.
36. Robert-Boire, V., Rosca, L., Samson, Y. et al.: Clinical Presentation and Outcome of Patients With Optic Pathway Glioma. Pediatr Neurol, 75; 2017 : 55–60.
37. Singhal, S., Birch, JM., Kerr, B. et al.: Neurofibromatosis type 1 and sporadic optic gliomas. Arch Dis Child, 87(1); 2002 : 65–70.
38. Taylor, T., Jaspan, T., Milano, G. et al.: Radiological classification of optic pathway gliomas: experience of a modified functional classification system. Br J Radiol, 81(970); 2008 : 761–766.
39. Torres Nupan, MM., Van Meerbeke, AV., López Cabra, CA. et al.: Cognitive and Behavioral Disorders in Children with Neurofibromatosis Type 1. Front Pediatr [online]. Frontiers Media S.A, 5(227); 2017. [cit. 15. prosince 2018]. Dostupné na WWW:<https://www.frontiersin.org/articles/10.3389/fped.2017.00227/full>
40. Vivarelli, R., Grosso, S., Calabrese, F. et al.: Epilepsy in neurofibromatosis 1. J Child Neurol, 18(5); 2003 : 338–42.
41. Walker, DA., Liu, J., Kieran, M. et al.: A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol, 15(4); 2013 : 462–468.
42. National Institute of Neurological Disorders and Stroke: Neurofibromatosis Fact Sheet. National Institute of Neurological Disorders and Stroke [online]. Květen 2011. National Institute of Health, 11-2126.; 6. července 2018. [cit. 15. prosince 2018]. Dostupné na WWW: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Neurofibromatosis-Fact-Sheet
Štítky
Oftalmologie
Článek vyšel v časopiseČeská a slovenská oftalmologie
Nejčtenější tento týden
2019 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Léčba chronické blefaritidy vyžaduje dlouhodobou péči
- První schválený léčivý přípravek pro terapii Leberovy hereditární optické neuropatie dostupný rovněž v ČR
- Familiární středomořská horečka
- Kontaktní dermatitida očních víček
-
Všechny články tohoto čísla
- Slovo vedoucího redaktora
- Pozdní opacifikace hydrofobní nitrooční čočky AcryNovaTMPC 610Y
- Léčba vitreomakulární trakce intravitreální aplikací perfluoropropanu
- Ranibizumab v léčbě makulárního edému při větvové sítnicové žilní okluzi – dvouleté výsledky léčby
- NEUROFIBROMATÓZA 1. TYPU A GLIOM OPTIKU
- Bilaterální současně probíhající akutní glaukom uzavřeného úhlu u Miller Fisherova syndromu
- Lichen planus jako možná vzácná příčina očního onemocnění
- Výročie narodenia prof. MUDr. Milana Izáka, PhD, FEBO
- Panu docentovi s láskou...
- Doc. MUDr. Vladimír Krásnik, PhD oslavuje 60-tku
- Česká a slovenská oftalmologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- NEUROFIBROMATÓZA 1. TYPU A GLIOM OPTIKU
- Léčba vitreomakulární trakce intravitreální aplikací perfluoropropanu
- Lichen planus jako možná vzácná příčina očního onemocnění
- Doc. MUDr. Vladimír Krásnik, PhD oslavuje 60-tku
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




