-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Single center experience with the treatment of older patients with myelodysplastic syndrome, not eligible for intensive therapy and its financial consequences
Liečba starších pacientov s myelodysplastickým syndrómom, nevhodných na intenzívnu terapiu a jej finančné dôsledky – skúsenosti jedného centra
Myelodysplastický syndróm (MDS) je heterogénna skupina myeloidných neoplázií, charakterizovaná cytopéniou periférnej krvi a zvýšeným rizikom prechodu do akútnej leukémie. Väčšina pacientov s myelodysplastickým syndrómom sú starší pacienti. Vyšší vek a komorbidity pacientov často neumožňujú podanie intenzívnej alebo kuratívnej terapie. V týchto prípadoch je vhodná podporná terapia. V súbore pacientov sme retrospektívne hodnotili klinické a hematologické charakteristiky v čase diagnózy, liečbu a prežívanie 19 pacientov s myelodysplastickým syndrómom, ktorí boli diagnostikovaní na Klinike hematológie a transfuziológie, Univerzitnej nemocnice v Bratislave (Slovenská Republika) od novembra 2007 do októbra 2014. Vek v súbore sa pohyboval od 60 do 90 rokov, s prevahou ženského pohlavia. Podľa WHO 2008 kritérií, bola u 9 pacientov diagnostikovaná refraktérna anémia (RA), u 2 RA s prstencovými sideroblastmi, u 2 RA s excesom blastov -1, u 2 RA s excesom blastov -2 a u 4 refraktérna cytopénia s mnoholíniovou dyspláziou. Podľa IPSS (Medzinárodný prognostický skórovací systém) bolo 12 pacientov s nízkym a 7 pacientov s vysokým rizikom. Všetci pacienti boli v čase diagnózy anemickí, s hladinou erytropoetínu menej ako 500 U/l (30.8-363 U/l, s mediánom 109 U/l) a boli liečení rastovými faktormi: 19 samotným erytropoetínom alfa, u 3 bol súčasne podávaný faktor stimulujúci granulocytové kolónie. Žiadny z pacientov neodpovedal na terapiu faktormi a všetci zostali transfúzne závislí. Závislosť od pravidelného podávania transfúzií sa objavila u všetkých pacientov, zatiaľ čo potreba pravidelného podávania trombocytových koncentrátov sa objavila u 5 pacientov. Chelačná terapia bola zahájená u 13 železom preťažených pacientov, keď hladina sérového feritínu stúpla nad 1000 ng/ml. Hladina sérového železa sa pri zahájení terapie pohybovala od 1019–4241 ng/ml, s mediánom 1205 ng/ml. Pacienti boli liečení podávaním deferasiroxu perorálne, v dávke od 10 mg do 30 mg/kg za deň. V piatich prípadoch sa počas 5–32 týždňov, s mediánom 15 týždňov podarilo znížiť hladinu sérového feritínu pod 1000 ng/ml. Traja pacienti ešte nedosiahli uspokojivú hladinu a naďalej užívajú medikáciu, 5 pacientov zomrelo pred dosiahnutím uspokojivej hladiny sérového feritínu. Všetci pacienti zahájili terapiu deferasiroxom po opakovanom podaní transfúzií. Dokázali sme, že pacienti preťažení železom sú vo vyššom riziku vzniku infekcie, ako pacienti s fyziologickými hladinami sérového feritínu. Náklady na liečbu boli nasledovné: transfúzie 226.56–13 140.48 €, s mediánom 1 283.84 € na 1 pacienta; trombocytové koncentráty 3 216.66–11 794.42 €,s mediánom 4 824.99 € na 1 pacienta; podanie subkutánnych G-CSF injekcií 6 567.04–52 536.32 €, s mediánom 34 476.96 € na 1 pacienta a podanie subkutánnych erytropoetínových injekcií 3 096–154 800 €, s mediánom 55 728 € na 1 patienta. Priemerné výdavky na jedného pacienta v skupine transfúzne závislých pacientov boli 2 794.24 €, v porovnaní s priemernými výdavkami na jedného pacienta v skupine transfúzne závislých pacientov s chelačnou terapiou, ktoré boli 22 611.28 €. Náklady liečby infekčných komplikácií počas doby sledovania boli od 2.81–5 074.87 € na pacienta, s mediánom 830 €. Podporná terapia pacientov s MDS ovplyvňuje kvalitu života chorých. Pacienti vyžadujú časté kontroly zdravotného stavu, trpia rôznymi infekčnými a krvácavými komplikáciami, musia užívať lieky. Bohužiaľ, je to jediná cesta, ako zmierniť symptómy tohto ochorenia.
KĽÚČOVÉ SLOVÁ:
myelodysplastický syndróm – podporná terapia – rastové faktory – transfúzie – chelačná terapia
Authors: L. Masákova 1; S. Fliegová 1; I. Bizíkova 1; J. Lukáš 1; I. Simančíková 1; M. Masáková 2; A. Hatalová 1; M. Mistrík 1; A. Bátorová 1
Authors place of work: Clinic of Hematology and Transfusiology, Faculty of Medicine, University Hospital, Comenius University 1; Pediatric ENT Department, Faculty of Medicine, Comenius University Bratislava, Slovakia 2
Published in the journal: Transfuze Hematol. dnes,22, 2016, No. 2, p. 106-114.
Category: Souhrnné práce, původní práce, kazuistiky
Summary
Myelodysplastic syndrome (MDS) represents a heterogeneous group of myeloid neoplasms characterized by peripheral blood cytopenias and increased risk of leukemic evolution. Most patients with myelodysplastic syndrome are elderly. Higher age and comorbidities often do not permit intensive or curative treatment. Therefore, supportive care is a legitimate approach. Nineteen patients who were diagnosed as having myelodysplastic syndrome at the Clinic of Hematology and Transfusiology, University Hospital Bratislava (Slovak Republic), between November 2007 and October 2014, were retrospectively evaluated for clinical and hematologic features at diagnosis, treatment and survival. The patients were aged from 60 to 90 years, with a female majority. According to the WHO 2008 criteria, 9 patients were classified as having refractory anemia (RA), 2 as RA with ringed sideroblasts, 2 as RA with excess of blasts-1, 2 as RA with excess of blasts-2 and 4 as refractory cytopenia with multilineage dysplasia. According to the IPSS (International Prognostic Scoring System), 12 were classified as low risk and 7 as high risk. All patients were anemic, with erythropoietin levels less than 500 U/l, (30.8-363 U/l, median 109 U/l) and all were treated with growth factors: 19 with erythropoietin alfa alone and 3 concomitantly with granulocyte-colony stimulating factor. None of the patients responded to the combination of growth factors and all remained transfusion dependent. In all patients, regular need for red blood cell transfusions developed, whereas platelet transfusion dependence occurred only in the case of 5 patients. Iron chelation therapy was started in 13 iron overloaded patients, when their serum ferritin level exceeded the limit of 1000 ng/mL. The serum ferritin level was found to be between 1019–4241 ng/mL, median 1205 ng/mL. The patients were treated with deferasirox, administered orally at a dose between 10 mg to 30 mg/kg per day. In 5 cases, the serum ferritin level decreased below 1000 ng/mL during weeks 5 to 32, median 15 weeks. Three patients in this group have as yet not attained a satisfactory level and are still receiving medication. Five patients died before attaining satisfactory serum ferritin levels. All patients started iron chelation therapy after having received multiple RBC transfusions. We demonstrated that iron overloaded patients are at higher risk of infectious complications compared to patients with normal ferritin levels. Treatment costs were as follows: red blood transfusions 226.56–13 140.48 €, median 1 283.84 € per patient; platelet transfusions 3 216.66–11 794.42 €, median 4 824.99 € per patient; administration of subcutaneous G-CSF injections 6 567.04–52 536.32 €, median 34 476.96 € per patient and administration of subcutaneous erythropoietin injections 3 096–154 800 €, median 55 728 € per patient. The average expenditure in the group of transfusion dependent patients was 2 794.24 €/per patient, compared to an average expenditure in the group of transfusion dependent patients with chelation therapy of 22 611.28 €/per patient. The estimated cost of infectious complications during the period of analysis was between 2.81–5074.87 € per patient, median 830 €. Supportive care of MDS patients affects quality of life, as these patients require frequent follow-up, suffer from infections, bleeding, and must take medication. Unfortunately, this is the only means of alleviating their disease symptoms.
KEY WORDS:
myelodysplastic syndrome – supportive care – growth factors – transfusions – chelation therapyINTRODUCTION
Myelodysplastic syndrome (MDS) represents a heterogeneous group of myeloid neoplasms characterized by peripheral blood cytopenias and increased risk of leukemic evolution [1]. Most patients with myelodysplastic syndrome are elderly (median age 70 years) and consequently the incidence and prevalence of MDS rise as the population ages [2]. Higher age and comorbidities often do not permit intensive or curative treatment (chemotherapy, hypomethylating agents or stem cell transplantation), even in the group of higher risk MDS patients, thus shortening patient survival and leading to poor quality of life. Thus in such cases, supportive care is recommended, namely red blood cell and platelet transfusions, administration of hematopoietic growth factors, anti-infectives and iron chelation therapy.
The International Prognostic Scoring System (IPSS) is the tool most widely used to assess the risk of transformation to leukemia and to guide treatment decisions. This categorizes patients according to the amount of marrow blasts, cytopenias and cytogenetics into 4 risk groups (IPSS low, intermediate-1, intermediate-2 and high risk MDS). 2-year overall survival in older patients with advanced MDS (IPSS intermediate 2 and high risk) is only 20% (3). We report our experience with best supportive care in patients with MDS not eligible for intensive treatment.
PATIENTS AND METHODS
Patient Characteristics and Clinical Characteristics
All patients who were diagnosed as having MDS at the Clinic of Hematology and Transfusiology, Faculty of Medicine, University Hospital, Comenius University, Bratislava, Slovakia, between November 2007 and October 2014, were retrospectively evaluated for clinical and hematologic features at diagnosis, treatment and survival. The cytological diagnosis of MDS was made according to the WHO criteria - see the characteristics of the group, Table 1.
Tab. 1. The characteristics of the group of patients 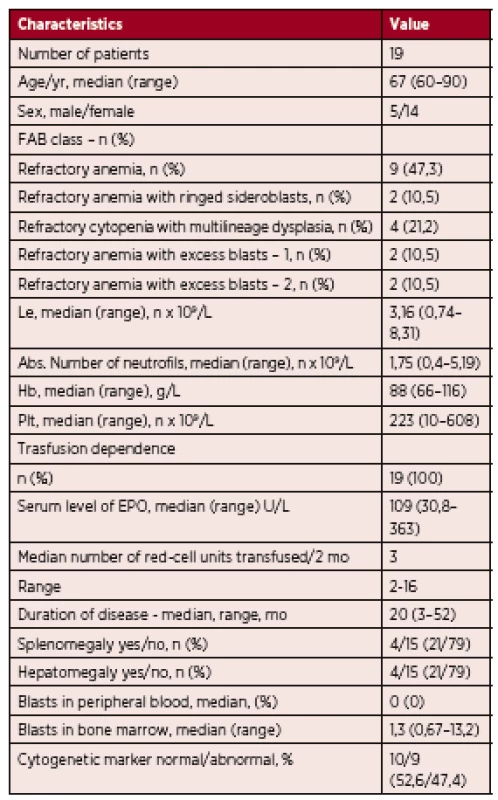
n – number of patients, Le – leukocytes, Hb – hemoglobin, Plt – platelets, mo – month, EPO – epoetin Nineteen patients were included in the analysis. Five were male and fourteen were female. The median age at diagnosis was 67 years (range 60 to 90 years). According to the WHO 2008 criteria, 9 patients were classified as having refractory anemia (RA; 47.3%), 2 were classified as having RA with ringed sideroblasts (RARS; 10.5%), 2 were classified as having RA with excess of blasts 1 (RAEB 1; 10.5%), 2 were classified as having (RAEB 2; 10.5%), and 4 were classified as having refractory cytopenia with multilineage dysplasia (RCMD; 21.2%).
All 19 patients were treated with the best supportive care. Despite the fact that 7 patients were categorized as IPSS high risk MDS, the hypomethylating treatment was not eligible for them, due to very high age and severe comorbidities.
All patients were anemic (male hemoglobin < 135 g/L, female hemoglobin < 120 g/L), their serum level of erythropoietin before starting therapy was below 500 U/l, (30,8-363 U/L, median 109 U/L) and all 19 patients were transfusion dependent. 3 patients were neutropenic (absolute neutrophil count < 1.0 x 109/L) and 7 patients had thrombocytopenia (platelets < 150 x109/L).
Cytogenetic analysis was performed at diagnosis. 11 patients were classified as having low risk cytogenetic abnormalities, 5 as having intermediate and 3 as having poor cytogenetic abnormalities. The most common cytogenetic abnormalities were 5q - and 7q-.
The IPSS was calculated according to Greenberg et al. [4]: 10 patients were classified as low risk (52.6%), 2 were classified as intermediate 1 (10.5%), 6 were classified as intermediate 2 (31.5%) and 1 was classified as high risk (5.2%) – Figure 1.
Fig. 1. Patients categorized by IPSS scoring system into 4 groups 
A total of 18 patients developed the disease de novo. In one case was diagnosed a secondary MDS, after the treatment of a breast cancer.
The Kaplan Meier method was used to estimate overall survival. The Pearson correlation test was applied to estimate the correlation between ferritin level and incidence of infectious complications. The difference was considered to be statistically significant if the p value was less than 0.05.
RESULTS
At the time of diagnosis, all patients except one were symptomatic. The most common symptoms were paleness, fatigue (weakness) and shortness of breath. Other less frequent symptoms included joint pain, fever and hemorrhagic diathesis (Figure 2). As all the patients were anemic and their serum erythropoietin level was below 500 U/L before starting therapy, they were all started on erythropoietin alfa 30 000 U twice a week, administered as a single subcutaneous injection at the time of diagnosis, with treatment duration between 82-298 days, median 112 days. Despite treatment with growth factors (erythropoietin alone, or in three cases together with G-CSF), all patients required leukocyte-depleted red cell blood transfusions. The need for transfusions occurred between days 0–1380, median 24.5 days after diagnosis. Transfusions were administered when hemoglobin levels reached < 90 g/L, or when symptoms of anemia were present. Blood transfusions were administered at a frequency ranging from 2 to 16, median 3 units per patient, over a period of 8 weeks. Each patient received from 3 to 174, median 17 units of RBC transfusions during the period of this analysis, with no complications during or after administration – see Table 2.
Fig. 2. Symptoms presented at the time of diagnosis 
Tab. 2. Results of analysis 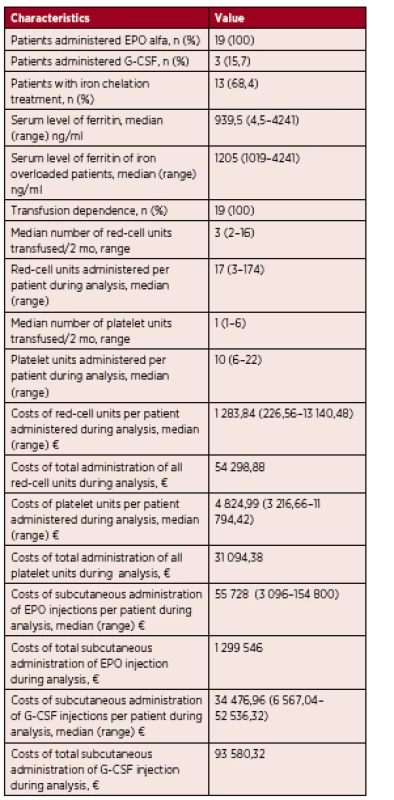
n – number of patients, EPO – epoetin, EPO alfa – epoetin alfa, G-CSF – granulocyte – colony stimulating factor, mo – month, € – euro Patients with a platelet count lower than 10 x 109/l or those with a platelet count lower than 20 x 109/L and at risk of bleeding (fever, infection) received prophylactic leukocyte-depleted platelet transfusions. Only 5 patients in the analysis required platelet transfusions. The frequency of platelet transfusions ranged from 1 to 6, median 1 unit, over a period of 8 weeks. Each patient received from 6 to 22, median 10, platelet transfusions during the period of this analysis. In one case, short term unconsciousness occurred during platelet administration.
At the time of diagnosis, three patients were also neutropenic. These patients received 48 000 U of granulocyte-colony stimulating factor (G-CSF) twice a week and this treatment started at the same time as erythropoietin.
Iron chelation therapy was started in 13 iron overloaded patients from our cohort, when serum ferritin levels exceeded 1000 ng/mL. Serum ferritin levels ranged from 1019–4241 ng/mL, median 1205 ng/mL at the start of treatment. These patients were treated with deferasirox administered orally at a dose from 10 mg to 30 mg/kg/day. In 5 cases, the serum ferritin level decreased below 1000 ng/mL during weeks 5 to 32, median 15. Three patients in this group have not as yet achieved a satisfactory level and are still on this medication. Five patients treated with deferasirox died before achieving a satisfactory serum ferritin level. All patients started iron chelation therapy after receiving multiple RBC transfusions. In five cases, we were able to determine the exact number of the transfusions administered 10 to 50, median of 37, before starting iron chelating therapy. Four patients (21%) experienced drug-related adverse events, namely gastrointestinal symptoms (diarrhea) in 2 cases and a rise in creatinine levels in 2 cases, which led to dose reduction in all the cases.
The ferritin level in our cohort ranged from 4.5–4241 ng/L, median 939.5 ng/L. Infectious complications occurred in 8 cases (42.1%), 1 to 6 episodes, median 1 episode per patient during the study. The most common complications were pneumonia, urogenital tract infection or inflammation of the soft tissues of the hallux. We found that in those cases, where infections occurred, the median ferritin level was 1803 ng/L, as opposed to those cases with no recorded infection, where the median ferritin level was 280 ng/l (p = 0.0157) – Figure 3. The most common antibiotics used to treat infections were ciprofloxacin and cefixime.
Fig. 3. Correlation between level of ferritine and appearance of infectious complications 
According to IWG criteria [5], the disease remained stable in three cases; disease progression between the day 89 and 425 day, median 318, was observed in 8 patients and in 8 cases disease progressed to secondary acute myeloid leukemia between day 144 and 422, median 264. Overall survival for all 19 patients ranged from 99–1606 days, median 746 days – see Kaplan-Meier survival curve, Figure 4. Six patients remain alive: 3 with stable disease, 3 with disease progression. One patient progressed from RCDM to RAEB 1 and 2 patients progressed from RAEB 1 to RAEB 2.
Fig. 4. Kaplan-Meier survival curve for overall survival 
On the final day of the study, 13 patients were no longer alive. According to the IPSS, 7 were classified as low risk, 1 as intermediate-1 risk, 4 as intermediate-2 risk and 1 as high risk. The causes of death were: secondary leukemia in 7 cases, intracranial hemorrhage associated with secondary leukemia in 1 case, infectious complications in 4 cases, unknown cause of death in one case.
Economic consequences
One red blood cell transfusion (from apheresis, deleucotized) costs 75.52 €. The overall number of transfusions for these 19 patients during the period of analysis was 719 units, corresponding to a total of 54 298.88 €, with an average expenditure per patient between 226.56 € and 13 140.48 €, median 1 283.84 € per patient. One platelet transfusion (from apheresis, deleucotized) costs 536.11 €. The overall number of all platelet transfusions for 5 of 19 the patients in our group during the period of analysis was 58 units, corresponding to a total of 31 094.38 €, with an average expenditure per patient between 3 216.66 € and 11 794.42 €, median 4 824.99 €. One subcutaneous injection of G-CSF costs 205.22 €. The overall number of subcutaneous G-CSF injections for 3 of the19 patients in our group during the period of analysis was 456, corresponding to 93 580.32 €, with an average expenditure per patient between 6 567.04 € and 52 536.32 €, median 34 476.96 €. One subcutaneous injection of erythropoietin costs 774.0 €. The overall number of subcutaneous EPO injections for the 19 patients during the period of analysis was 1 679, corresponding to 1 299 546 €, with an average expenditure per patient between 3 096 € and 154 800 €, median 55 728 €. Chelation therapy is also very expensive. One package of deferasirox (28 tbl) costs 659.16 €. The average expenditure in our transfusion dependent patients was 2 794.24 €/per patient, compared to an average expenditure for the group of transfusion dependent patients with chelation therapy of 22 611.28 €/per patient. It is clear that chelation therapy increases the costs of medical care significantly. Infectious complication costs refer to the costs of a single infectious episode or the costs of all infectious episodes per patient. The outpatient treatment of an infectious episode with antibiotics costs on average 11.25 €, compared to infectious complications treated during hospitalization that are on average 830 € in the case of an internal medicine department and 5000 € in the case of a department of hematology. The cost of infectious complications during the period of analysis were estimated to range from 2.81 € to 5074.87 € per patient, median 830 €.
DISCUSSION
The supportive care approach is justifiable in the case of patients with low risk MDS, higher age and those with concomitant diseases. As Malcovati et al. demonstrated, the life expectancy of patients without therapy (no transfusion dependency, mild cytopenias) aged 70 years or older suffering from refractory anemia or MDS with isolated del (5q) was not significantly shorter than that of the general population [6]. On the other hand, List et al. studied the effect of azacitidine (AZA) on overall survival in higher-risk myelodysplastic syndromes without complete remission and found that AZA maintained a significant survival benefit vs conventional care regimens (CCR) when patients achieving complete remission were excluded from the analysis (hazard ratio for OS = 0.65, 95% CI: 0.48, 0.88). The 1-year survival rates were significantly higher in AZA-treated patients than in CCR-treated patients: 68.2% vs 55.6%, respectively (p=0.015) [7]. Michael Lübbert et al. compared low-dose decitabine (cytidine analog) with best supportive care in elderly patients with intermediate or high-risk MDS ineligible for intensive chemotherapy. They found that overall survival prolongation with decitabine versus best supportive care was not statistically significant, but progression-free survival was significantly longer with decitabine versus best supportive care (6.6 v 3.0 months). AML transformation was significantly (p = 0.036) reduced at 1 year (from 33% with best supportive care to 22% with decitabine) [8].
In cases where intensive chemotherapy is not possible, patients may benefit from the administration of growth factors – erythropoietin given according to the predictive model for erythropoietin treatment. Predicted response: 0 points 74%, 1 point 23%, 2 points 7% (Table 3). All patients in our cohort scored 1 point according to this predictive model and all remained transfusion dependent.
Tab. 3. Predictive model of therapy with epoetin [9] ![Predictive model of therapy with epoetin [9]](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/2ac4701b160afe5c06652ae0425d9fca.png)
TU/month – tranfusion unit, S-Epo – level of serum epoetin Predicted answer: 0 points 74%, 1 point 23%, 2 points 7% Sometimes, the administration of G-CSF is needed. One randomized study comparing treatment with erythropoietin and G-CSF vs supportive care in low-grade anemic MDS showed that the active approach significantly improved red blood cell count [10]. Another randomized trial comparing the effect of erythropoietin vs erythropoietin and G-CSF given for 8 weeks in low risk MDS showed a significantly higher response rate in patients receiving the combination of growth factors [11]. According to published guidelines, EPO should be administered 8 weeks at a dose between 30 000-40 000 IU/week or at a higher dose of 60 000-80 000 IU/week. If there is no response, EPO should be combined with G-CSF for the next 8 weeks at a dose of 150–300 µg/week. If still no response occurs, the treatment should be terminated (according to the National Comprehensive Cancer Network, NCCN). In our cohort, patients failed to respond to a combination of growth factors. We postulate that this was due to the comorbidities of these patients that decreased the efficacy of erythropoietin therapy such as a second malignancy, diabetes, thyroid disease as well as to the short duration of erythropoietin administration.
Patients receiving regular transfusions invariably develop secondary iron overload. Whole blood contains about 0.47 mg/mL of iron, while pure RBC concentrates contain 1.16 mg/mL of iron. Thus, one unit of blood contains 200 to 250 mg of iron and overload can occur after 20 to 25 transfusions [1]. Retrospective studies have shown that elevated serum ferritin significantly worsens the survival of transfusion-dependent MDS patients [12]. Metzgeroth G. et al. demonstrated the efficiency of deferasirox administered in a once-daily dose of 20–30 mg/kg for 12 months. This therapy was effective in reducing median ferritin concentration from 1.515 ng/ml (range 665–6.900) to 413 ng/ml (range 105–3.052) [13]. In our cohort, in 5 patients the level of serum ferritin decreased below 1000 ng/ml in 5 to 32 weeks, median 15. Three patients have not as yet achieved a satisfactory level and are still taking the medication. Five patients treated with deferasirox died without achieving satisfactory ferritin levels.
Myelodysplastic syndromes are associated with the risk of severe infections. While neutropenia is likely to be the main predisposing factor, several other immune defects have been reported (impaired neutrophil function, B-, T - and NK-cell defects) and the possible consequences of iron overload cannot be ruled out either [14]. Iron overload has an adverse impact on the risk of bacterial and fungal infections. It is known, that iron overload enhances bacterial growth and virulence, alters phagocytosis and that free iron impairs the natural resistance to infection. No prophylactic measures against infections have been shown to be effective in MDS [15]. We also demonstrated in our cohort that increased ferritin levels are associated with a higher risk of infectious complications. It is very important to monitor the level of serum ferritin in patients receiving RBC transfusions regularly.
The treatment of patients with MDS has an impact on their quality of life (QOL). They need to be examined regularly; they suffer from recurrent infectious complications, anemic symptoms and bleeding. Regular administration of subcutaneous injections, RBC or platelet transfusions is required. This is associated with many complication, alloimmunisation, iron overload, etc. The results of a study conducted by the Myelodysplastic Syndromes Foundation using data from 29 patient forums in the US and Europe showed fatigue to be the symptom that most affected patient QOL, although another important factor was the time required to manage the disease (doctor appointments, diagnostic tests, transfusions and management of adverse events) [16]. The QLQ-C30 (Quality of Life Questionnaire-Core 30) was devised by the European Organization for Research and Treatment of Cancer (EORTC) as a multidimensional questionnaire (i.e. covering the various physical, emotive, social, etc. dimensions of the patient) to assess the quality of life a patient assigns him/herself (17). All the scores determined by the patient are reported on a scale of 0–100 (an increasing quality scale) and can be aggregated using a single mean. By using the QLQ-C30 questionnaire in 32 Italian patients, Caocci et al. [18] revealed a significant (p < 0.001) difference between the QOL of non-transfused patients (66.7) and the QOL of transfused patients (32.9). There was a similar difference (65 vs. 31.8, respectively, p < 0.001) also for fatigue.
Goldberg S. et al. calculated the economic impact of a newly diagnosed myelodysplastic syndrome associated with blood transfusion requirements. They found that over 3-year period of patient treatment, Medicare costs for MDS patients were 156. Transfused patients had a higher use of hospital inpatient and outpatient services and incurred significantly higher mean costs than non-transfused patients ( 824 vs. 519, p < 0.001) (19). Another study by Greenberg P. et al. also studied the financial consequences of MDS treatment. The results estimated an average annual cost for potentially anemia-altering drugs of 577 per patient, ranging from 000 to 000, depending on the specific therapy. In patients who failed such therapies, annual costs for iron chelation plus red blood cell transfusion were estimated to average 412 [20]. Brereton et al. [21] provided an estimate of the social cost of intermediate-2 and high risk MDS in the United Kingdom. With an estimated incidence of 761 new patients annually, the overall cost range was put at between 12 and 16 million pounds (with a mean cost per patient of approximately £ 18 400). Santini et al. [22] evaluated the social cost of MDS in Italy. The analysis conducted on 225 patients (of which 88% were low and intermediate-1 risk) estimated an overall cost related to the consumption of healthcare and non-healthcare resources and productivity loss for patients and caregivers of 27 980 € per patient/year. The identified cost-drivers were anti-anemia drugs in low - and intermediate 1-risk patients and anticancer agents in intermediate-2 and high-risk patients. Transfusion dependence proved to be a statistically significant predictor of cost increase (P = 0.006). A retrospective study conducted by Frytak et al. [23] estimated the financial burden of MDS in the US for transfusion independent (TI) and transfusion dependent (TD) patients separately. 336 TD patients and 2 864 TI patients were identified. The costs of admissions (to hospital wards and emergency department visits), outpatient clinics and doctors‘offices as well as of medications and transfusions were recorded. The overall mean annual cost was $ 19 811 (of which 85% for medical care and 15% for medication) for TI patients and $ 51 066 (92% for medical care and 8% for medication) for TD patients. For the latter sum ($ 51 066), costs of $ 16 586 were due to transfusions (equal to half the difference in cost between TI and TD), whereas medication costs had a marginal impact on the total: 8%-15%. In our cohort, the cost of red blood transfusion administration was 226.56-13 140.48 €, median 1 283.84 € per patient; the cost of platelet transfusion administration was 3 216.66–11 794.42 €, median 4 824.99 € per patient; the cost of subcutaneous G-CSF injections of was 6 567.04–52 536.32 €, median 34 476.96 € per patient and the cost of erythropoietin subcutaneous injections was 3 096-154 800 €, median 55 728 € per patient. The average expenditure in the group of transfusion dependent patients was 2 794.24 €/per patient, compared to an average expenditure in the group of transfusion dependent patients with chelation therapy of 22 611.28 €/per patient. The cost of infectious complications during the period of analysis was estimated to be between 2.81–5074.87 € per patient, median 830 €.
Cost effectiveness analysis
It is very hard to decrease the costs of patients with MDS receiving supportive care. When the growth factors appear during the treatment, EPO and G-CSF should not be further administrated if they fail to induce a response. There are also no established indications for the primary or secondary prophylactic use of G-CSF in order to avoid febrile neutropenia. Neutropenia does not warrant the administration of prophylactic anti-infectives. The main reason is that the duration of neutropenia would necessitate the continuous use of antibiotics or antifungals for months or years. This would probably lead to an unacceptable risk of induced resistance, well-illustrated in the case of long-term quinolone [24, 25] and antifungal triazole treatment [26, 27] as well as the risk of drug-induced adverse effects. On the other hand, it is very important to identify the onset of infection, because outpatient treatment is much cheaper than inpatient treatment. Finally, careful selection of patients eligible for hypomethylating therapy vs supportive care is very important for decreasing treatment costs.
CONCLUSION
All patients from our cohort were treated with growth factors. The patients failed to respond and all remained transfusion dependent. The need for regular red cell transfusion occurred in all the patients, whereas platelet transfusions were required only by 5 patients. Iron chelation therapy was started in 13 iron overloaded patients. And involved the oral administration of deferasirox. In 5 cases, serum ferritin levels fell below 1000 ng/ml. We also demonstrated that increased ferritin levels are associated with a higher risk of infectious complications. Supportive care is expensive, but medical costs can be decreased by administering growth factors if indicated, by attempting to manage infectious complications in the outpatient setting and by carefully selecting patients eligible for hypomethylating therapy vs supportive care.
Supportive care in MDS patients affects patient quality of life. Patients need to be examined regularly and suffer from recurrent infectious complications, anemic symptoms and bleeding. Regular administration of subcutaneous injections, RBC or platelet transfusions is required. This is associated with many complications. Unfortunately, this is the only means of alleviating disease symptoms, albeit a very expensive one.
The role of the authors in preparing the article
All the authors participated in this study.
ML, FS, BI, LJ., SI, HA, BA – clinical management of patients
MM – supervision and revision of the manuscript
MM – data processing, statistics
Statement about conflict of interest
The authors declare no conflict of interest associated with the topic and publication of this article. This work was not supported by any pharmaceutical company.
Doručeno do redakce dne 15. 2. 2016.
Přijato po recenzi dne 5. 4. 2016.
MUDr. Lenka Masáková
Oddelenia hematológie a transfúziológie
Univerzitná nemocnicaAntolská 11
851 07 Bratislava
Slovenská republika
e-mail: lenka@wnet.sk
Zdroje
1. Malcovati L, Hellström-Lindberg E, Bowen D, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendation from the European LeukemiaNet. Blood 2013; 122(17): 2943–2958.
2. Ria R, Moschetta M, Reale A, et al. Managing myelodysplastic symptoms in elderly patients. Clin Interv Aging 2009; 4 : 413–423.
3. Mistrík M, Kubalová S, Masáková L, Bátorová A. Transplantácia krvotvorných buniek pre myelodysplastický syndróm. Onkológia 2014; 9(5): 320–322.
4. Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997; 89(6): 2079–2088.
5. Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International working group (IWG) response criteria in myelodysplasia. Blood 2006; 108(2): 419–425.
6. Malcovati L, Germing U, Kuendgen A, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007; 25(23): 3503–3510.
7. List AF, Fenaux P, Mufti GJ, et al. Effect of azacitidine (AZA) on overall survival in higher-risk myelodysplastic syndromes (MDS) without complete remission. J Clin Oncol 2008; 26(15): suppl 7006.
8. Lübbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate - or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol 2011; 29(15): 1987–1996.
9. Hellström-Lindberg E, Gulbrandsen N, Lindberg G, et al. A validated decision model for treating the anaemia of myelodysplastic syndromes with erythropoietin + granulocyte colony-stimulating factor: significant effects on quality of life. Br J Haematol 2003; 120(6): 1037–1046.
10. Casadevall N, Durieux P, Dubois S, et al. Health, economic, and quality-of-life effects of erythropoietin and granulocyte colonystimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood 2004;104(2): 321–327.
11. Balleari E, Rossi E, Clavio M, et al. Erythropoietin plus granulocyte colonystimulating factor is better than erythropoietin alone to treat anemia in low-risk myelodysplastic syndromes: results from a randomized singlecentre study. Ann Hematol 2006;85(3): 174–180.
12. Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol 2005; 23(30): 7594–7603.
13. Metzgeroth G, Dinter D, Schultheis B, et al. Deferasirox in MDS patients with transfusion-caused iron overload a-phase-II study. Ann Hematol 2009; 88(4): 301–310.
14. Pullarkat V. Objectives of iron chelation therapy in myelodysplastic syndromes: more than meets the eye? Blood 2009; 114(26): 5251–5255.
15. Bulen JJ, Rogers HJ, Spalding PB, et al. Natural resistance, iron and infection: a challenge for clinical medicine. J Med Microbiol 2006; 55(3): 251–258.
16. Lucioni C, Finelli C, Mazzi S, et al. Costs and quality of life in patients with myelodysplastic syndromes. Am J Blood Res 2013; 3(3): 246–259.
17. EORTC QLQ-C30. Available online: http://groups.eortc.be/qol/eortc-qlq-c30.
18. Caocci G, Baccoli R, Ledda A, et al. A mathematical model for the evaluation of amplitude of hemoglobin fluctuations in elderly anemic patients affected by myelodysplastic syndromes: correlation with quality of life and fatigue. Leuk Res 2007; 31 : 249–252.
19. Goldberg SL, Chen E, Sasane M, Paley C, Guo A, Laouri M. Economic impact on US Medicare of a new diagnosis of myelodysplastic syndromes and the incremental costs associated with blood transfusion need. Tranfusion 2012; 52(10): 2131–2138.
20. Greenberg PL, Cosler LE, Ferro SA, Lyman GH. The costs of drugs used to treat myelodysplastic syndromes following National Comprehensive Cancer Network Guidelines. J Natl Compr Canc Netw 2008;6 : 942–953.
21. Brereton N, Nicklasson L, Mufti GJ. UK estimate of burden of disease associated with management of intermediate-2 or higher-risk myelodysplastic syndromes. Blood 2011; 118(21): Abstract 4749.
22. Santini V, Sanna A, Bosi A, et al. An observational multicenter study to assess the cost of illness and quality of life in patients with myelodysplastic syndromes in Italy. Blood 2011; 118(21): Abstract 1023.
23. Frytak JR, Henk HJ, De Castro CM, et al. Estimation of economic costs associated with transfusion dependence in adults with MDS. Curr Med Res Opin 2009; 25 : 1941–1951.
24. Almyroudis N, Segal BH. Antibacterial prophylaxis in patients with cancer and neutropenia. N Engl J Med 2006;354(1): 90–94.
25. Baden L. Prophylactic antimicrobial agents and the importance of fitness. N Engl J Med 2005; 353(10): 1052–1054.
26. Alanio A, Cordonnier C, Bretagne S. Azole resistance in Aspergillus fumigatus – current epidemiology and future perspectives. Curr Fungal Infect Rep 2011; 5 : 168–178.
27. Howard S, Cerar D, Anderson M, et al. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis 2009; 15(7): 1068–1076.
Štítky
Hematologie a transfuzní lékařství Interní lékařství Onkologie
Článek vyšel v časopiseTransfuze a hematologie dnes
Nejčtenější tento týden
2016 Číslo 2- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Nejasný stín na plicích – kazuistika
-
Všechny články tohoto čísla
- Single center experience with the treatment of older patients with myelodysplastic syndrome, not eligible for intensive therapy and its financial consequences
- Recommendations for the diagnosis and treatment of chronic lymphocytic leukaemia (CLL) Czech Group for Chronic Lymphocytic Leukaemia, section of the Czech Society of Haematology, CzMA
- Indications for allogeneic and autologous haematopoietic cell transplantations in the Czech Republic in 2016: Recommendations of the Transplant Section of the Czech Society of Haematology and the Czech Society of Oncology of the J. E. Purkyně Medical Association
- Cena České hematologické společnosti za nejlepší původní vědeckou práci v oboru hematologie v roce 2015
- Výběr z tisku a zprávy o knihách
- Analysis of the relationship of cytogenetic results with serum free light chain ratio κ/λ(FLC-r, FreeliteTM), heavy/light chain pairs of immunoglobulin ratio (HLC-r, HevyliteTM), and selected prognostic factors assessed at diagnosis of multiple myeloma
- Využití interfázní fluorescenční in situ hybridizace pro analýzu CD34+ buněk v periferní krvi u nemocných s myelodysplastickými syndromy
- Prophylaxis versus on demand treatment in adult patients with severe haemophilia A – FN Brno experience
- Transfuze a hematologie dnes
- Archiv čísel
- Aktuální číslo
- Pouze online
- Informace o časopisu
Nejčtenější v tomto čísle- Indications for allogeneic and autologous haematopoietic cell transplantations in the Czech Republic in 2016: Recommendations of the Transplant Section of the Czech Society of Haematology and the Czech Society of Oncology of the J. E. Purkyně Medical Association
- Single center experience with the treatment of older patients with myelodysplastic syndrome, not eligible for intensive therapy and its financial consequences
- Recommendations for the diagnosis and treatment of chronic lymphocytic leukaemia (CLL) Czech Group for Chronic Lymphocytic Leukaemia, section of the Czech Society of Haematology, CzMA
- Prophylaxis versus on demand treatment in adult patients with severe haemophilia A – FN Brno experience
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



