-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Peculiarities of Prion Diseases
article has not abstract
Published in the journal: . PLoS Pathog 10(11): e32767. doi:10.1371/journal.ppat.1004451
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004451Summary
article has not abstract
Introduction
Prion diseases (PrDs) are transmissible and fatal neurodegenerative diseases naturally occurring in humans and animals, “mad cow” disease being the most infamous. Their development and propagation requires endogenous prion protein (PrP) and derives from the conversion of PrP to a misfolded form, which combines with other misfolded PrP molecules to form small nuclei (seeds). The seeds can then result in an exponential increase in additional misfolded PrP molecules, eventually accumulating into large aggregates. However, the physiological roles of normal and misfolded PrP, mechanisms of the conformational transition, and the associated nature of the infectious and neurotoxic agents still remain enigmatic. In this review, we address five questions regarding PrDs that we are frequently asked by laypeople and scientists new to the field.
What Causes PrDs?
Understanding the mechanisms of PrDs will likely lead to interventions, but researchers and physicians are challenged by three fundamentally different forms—acquired, sporadic and familial—comprising a wide range of highly heterogeneous subtypes and phenotypes with various clinical and histopathological features [1]. Sadly, the terminology in this field is frustratingly confusing and, for example, the distinctions between infectious and acquired or spontaneous and sporadic PrDs are sometimes blurred. Infectious means that an individual's disease could be horizontally transmitted to another individual. Acquired means an individual became diseased because of infection. The distinction seems subtle but is important because one term refers to the cause of the disease while the other refers to a feature of the mainfested disease. Moreover, all PrDs are infectious, but not all PrDs are acquired. For example, familial PrDs are caused by a mutation, not by being infected, but are nonetheless infectious. Spontaneous means the disease was not triggered by an infection, whereas sporadic means there is no evidence that the diseased individual was infected or had a mutation. Familial PrDs begin spontaneously and the same is likely true for sporadic cases.
Although acquired PrDs have immense notoriety, they account for less than 1% of human cases and are on the decline [2]. Since recent reviews have explored important questions of how prions enter the body and invade the brain [3], and mechanisms of familial prion diseases and the fascinating issue of selective vulnerability [4], [5], we focus here on sporadic human PrDs, representing the most common and yet least understood form. Surprisingly, even though they all involve PrP, cases can be phenotypically classified as sporadic Creutzfeldt-Jakob disease (sCJD), fatal insomnia, or protease-sensitive prionopathy [6]. While triggers for acquired and familial forms are clear (infections and mutations, respectively), the trigger for sporadic forms is murky, though there are some ideas. Considering the low incidence and the typically advanced age at onset, it seems likely that a certain threshold of disease-causing prions has to be exceeded before pathogenesis begins [7]. Since sporadic PrDs occasionally occur in adolescents [8], factors other than age, such as diet, environment, or genetics, may be involved. A transient perturbation of a microenvironment (inflammation, stress from dysregulated neural activity, deprivation of critical nutrients, impairment of waste removal, etc.) could induce wild-type PrP to spontaneously misfold into a prion conformation or impair its degradation, or permit the nucleation of prion seeds. Alternatively, a surprisingly large number of adult human neurons are aneuploid [9], and since increased expression of wild-type PrP can induce PrDs [10], increased PrP gene copies (and thus expression) could trigger the disease process. It is also conceivable that somatic mutations induce PrP to spontaneously misfold into low (non-toxic) levels of prions, and sometimes this process spirals out of control. These initial events then trigger the beginning of PrDs that propagate through the brain when accompanied by age-related changes of proteostasis, regardless of the initial cause. Notably, this is likely true for familial PrDs, too, which is remarkable since the disease-inducing mutant proteins are present throughout life, and yet emerge only after decades of expression. This implies that prions [7], and PrP itself [11], are effectively cleared from the brain by a mechanism that diminishes with aging. Just as the body usually removes cancers quite efficiently, the brain might routinely eliminate low levels of prions until adverse conditions give rise to a critical mass of prions, causing a lethal chain reaction (Figure 1). Regardless of how it begins, once a PrD is established it will progress rapidly, analogous to the instantaneous crystallization of a supersaturated salt solution upon addition of a minuscule crystal (seed).
Fig. 1. PrP expression, prions and disease. 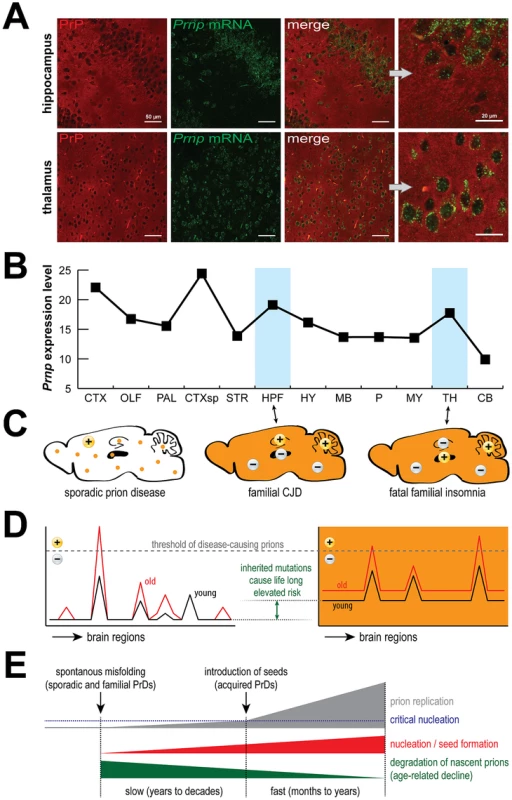
PrP is expressed by most cells (A) and is rather evenly distributed across the mouse brain (B). Surprisingly, PrDs may develop sporadically or be induced by mutations (C, orange color), but despite widespread expression, different regions are targeted in different familial PrDs (+) and the same is true for sporadic PrDs. Age-related effects likely permit disease-inducing prions to accumulate beyond a threshold that can be reversed in young but not old brains (D), which eventually leads to critical nucleation, followed by rapid replication and pathological changes (E). Data in (B) were obtained from the Allen Brain Atlas website (www.brain-map.org). Abbreviations: CTX, cortex; OLF, olfactory bulb; HPF, hippocampal formation; CTXsp, cortical subplate; STR, striatum; PAL, pallidum; TH, thalamus; HY, hypothalamus; MB, midbrain; P, pons; MY, medulla; CB, cerebellum. HPF is targeted in CJD mice, TH is targeted in FFI mice, and CB is targeted in both models. How Is It Known That Sporadic Prion Diseases Are Not Actually Acquired?
Two lines of reasoning support this conclusion. First, there are straightforward explanations of how this can happen. Recent experiments demonstrate that mutant forms of PrP do not require an infection to trigger the disease process [4], [5]. We speculate this is because the mutations either induce PrP to misfold into a prion conformation or impair its degradation, and the same probably happens for sporadic forms except that perturbation of the microenvironment orchestrates PrP's misfolding (see above).
Second, there are subtle but important differences between PrDs that can provide additional clues of an infection. Clinically acquired (iatrogenic) PrDs are typically identified by the correlation of a neuropathologically confirmed PrD following certain medical procedures, sometimes experimentally proven to be the cause [12]. Variant sCJD, caused by consumption of cattle with PrD (the infamous “mad cow” disease), was highly clustered geographically, often affecting young adults, and disease-related PrP aggregates were present in lymphoid tissue, a feature generally absent from other human PrDs [13]. In contrast, sporadic PrDs occur rather evenly (and sparsely) distributed across the globe, typically in people older than 50 years, without any telling epidemiological signals of localized clusters that would be indicative of infection. It is impossible to know with complete certainty that a sporadic PrD case is not actually caused by infection, but there is good reason to think not.
How Can Pathological Processes of Sporadic PrDs Be Studied?
Human PrDs are intensively studied with respect to etiological, clinical, neuropathological, biochemical, molecular biological, and many other features. Nonetheless, this knowledge can be greatly enhanced using creative experimental models to reveal initiating processes. In addition to the widely used mouse models of acquired PrDs, which represent the human forms extraordinarily well, numerous transgenic mouse lines were generated to model familial PrDs [4], [5], [14]. Though they are not perfect, these models are well defined in terms of disease onset and progression, neuropathological and clinical features, making them quite useful to study familial PrDs. In contrast, modeling sporadic PrDs is extremely difficult without knowledge and use of a disease-initiating trigger. Nevertheless, two approaches are thought to reproduce aspects of these very rare diseases.
The first used a highly sensitive amplification technique to detect prions in brain extracts of uninfected, healthy wild-type mice [15]. However, it was concluded that the prions were generated in the in vitro reaction, rather than the brain, and therefore, in a sense, model an acquired disease when introduced back into an intact brain. In the second, mice genetically engineered to over-express wild-type PrP (from bank voles) spontaneously developed prion infectivity and disease amazingly reminiscent of conventional (acquired) prion models [10]. Is this a model of sporadic PrDs? The ideal model would include a population of seemingly similar individuals, where most do not but some do get sick, and none are infected or carry abnormal Prnp gene dosages or sequences. Such a model does not exist, though it would be invaluable for studying disease triggers. Therefore, despite enormous efforts and much progress, additional models of all forms of prion diseases, especially sporadic forms, are needed to reveal basic and specific mechanisms in humans.
What Does “Prion-Like” Mean?
The term prion originally defined disease-inducing proteins that are infectious, but the definition is evolving in at least two new directions. In one direction, prion relates to proteins that naturally assemble into nuclear or cytoplasmic foci, often via prion domains. Prion assemblies were reported to provide positive biological functions in yeast [16]. Long used in the yeast prion field, the term “prion-like” caught the attention of an even wider audience with the report that a sea-slug protein carrying a domain similar to that in a yeast prion could assemble into a “prion-like” state that was beneficial for memory [17]. These and other works inspired the development of an algorithm to identify additional “prion-like” yeast proteins that performed new functions in a self-templating aggregated state [18]. The usage continued to spread (pun intended!) when this algorithm was applied to the mammalian genome and amino-acid stretches similar to those found in yeast prions were identified in self-assembling proteins that also tend to accumulate into pathological aggregates [19]. However, it should be recognized that the parameters of this algorithm were based on known yeast prion domains, which tend to have low complexity and a strong enrichment for glutamines and asparagines. Although mammalian PrP can be contorted into highly infectious prions, and it bears a low complexity region (i.e., repeated sequence), it lacks enrichment of glutamines and asparagines and, indeed, does not score highly as a prion with this algorithm. Importantly, other mammalian proteins appear to behave as prions in cancer [20] and inflammation [21], [22], suggesting there may be more “prion-like” mammalian proteins to discover.
The term “prion-like” is also evolving to propose mechanisms for spreading of neurodegenerative disease-related protein aggregates (such as those observed in Alzheimer, Huntington, and Parkinson [PD] diseases, to name a few) across the brain as diseases progress. Some quite provocative experiments have demonstrated that aggregated forms of neurodegenerative disease-related proteins, when extracted from brains of diseased humans or transgenic mice and injected into the brains of otherwise healthy mice, induced the formation of similar protein aggregates [23], [24], and sometimes similar processes were associated with toxicity [25]. This concept was invigorated by results of an experimental therapy for PD that involved grafting of fetal tissue into patients' brains. Several years later, the brains were examined histologically and the observation of PD-related aggregates in the grafted tissues [26] led many authors to suggest that the aggregates are spreading within the brain via a “prion-like” mechanism. These and similar observations have been extrapolated to suggest that the diseases might spread between individuals in a “prion-like” mechanism, though arguments against this have been articulated [27]. Therefore, the term “prion-like” is rather loosely applied and, though it is functionally useful to aid in scientific discussions, molecules receiving this label should not be assumed to be readily infectious between individuals.
Are There Any Cures for Prion Diseases?
Unfortunately, there are currently no cures for any neurodegenerative disease. But a number of groups have worked tirelessly to uncover therapies targeted for PrDs and some recent reports offer hope. One approach is to eliminate PrP, the substrate necessary to maintain prion diseases. Suppression of PrP expression using transgenic tools was quite effective [28], and with the ongoing development of new technologies for gene delivery, there is hope this will be a viable strategy in the future.
An alternative, perhaps complementary approach would be to chemically interfere with disease progression. Many compounds have been identified that show great promise, but for brevity we describe just one example, anle138b [29]. When administered to mice 120 days after prion infection, a time when mice are clinically affected, it extended survival from approximately 160 days to approximately 220 days, representing approximately 7% of normal lifespan. The question then becomes if the treatment was applied to humans at a similar stage of disease and was equally effective, would it extend lifespan 60 days or 7% of expected lifespan (5 to 6 years for developed nations)? Amazingly, anle138b also modified disease progression in a model of familial PD and two models of chemically induced PD [29]. It is tempting to speculate that both diseases involve a common, prion-like mechanism that was targeted by anle138b. The truly remarkable point is that this molecule was discovered from studies of PrDs, and it highlights the fact that, although these diseases are rare in humans, studies of PrD mechanisms and interventions are certainly worthwhile.
Zdroje
1. GambettiP, KongQ, ZouW, ParchiP, ChenSG (2003) Sporadic and familial CJD: classification and characterisation. Br Med Bull 66 : 213–239.
2. BrownP, BrandelJP, SatoT, NakamuraY, MacKenzieJ, et al. (2012) Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis 18 : 901–907.
3. AguzziA, ZhuC (2012) Five questions on prion diseases. PLoS Pathog 8: e1002651.
4. JacksonWS (2014) Selective vulnerability to neurodegenerative disease: the curious case of Prion Protein. Dis Model Mech 7 : 21–29.
5. WattsJC, PrusinerSB (2014) Mouse Models for Studying the Formation and Propagation of Prions. J Biol Chem 289 : 19841–19849.
6. PuotiG, BizziA, ForloniG, SafarJG, TagliaviniF, et al. (2012) Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 11 : 618–628.
7. PrusinerSB (2013) Biology and genetics of prions causing neurodegeneration. Annu Rev Genet 47 : 601–623.
8. MurrayK, RitchieDL, BruceM, YoungCA, DoranM, et al. (2008) Sporadic Creutzfeldt-Jakob disease in two adolescents. J Neurol Neurosurg Psychiatry 79 : 14–18.
9. MoschB, MorawskiM, MittagA, LenzD, TarnokA, et al. (2007) Aneuploidy and DNA replication in the normal human brain and Alzheimer's disease. J Neurosci 27 : 6859–6867.
10. WattsJC, GilesK, StohrJ, OehlerA, BhardwajS, et al. (2012) Spontaneous generation of rapidly transmissible prions in transgenic mice expressing wild-type bank vole prion protein. Proc Natl Acad Sci U S A 109 : 3498–3503.
11. AgostiniF, DottiCG, Perez-CanamasA, LedesmaMD, BenettiF, et al. (2013) Prion protein accumulation in lipid rafts of mouse aging brain. PLoS ONE 8: e74244.
12. GibbsCJJr, AsherDM, KobrineA, AmyxHL, SulimaMP, et al. (1994) Transmission of Creutzfeldt-Jakob disease to a chimpanzee by electrodes contaminated during neurosurgery. J Neurol Neurosurg Psychiatry 57 : 757–758.
13. HillAF, ButterworthRJ, JoinerS, JacksonG, RossorMN, et al. (1999) Investigation of variant Creutzfeldt-Jakob disease and other human prion diseases with tonsil biopsy samples. Lancet 353 : 183–189.
14. JacksonWS, BorkowskiAW, WatsonNE, KingOD, FaasH, et al. (2013) Profoundly different prion diseases in knock-in mice carrying single PrP codon substitutions associated with human diseases. Proc Natl Acad Sci U S A 110 : 14759–14764.
15. BarriaMA, MukherjeeA, Gonzalez-RomeroD, MoralesR, SotoC (2009) De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog 5: e1000421.
16. TrueHL, LindquistSL (2000) A yeast prion provides a mechanism for genetic variation and phenotypic diversity. Nature 407 : 477–483.
17. SiK, LindquistS, KandelER (2003) A neuronal isoform of the aplysia CPEB has prion-like properties. Cell 115 : 879–891.
18. AlbertiS, HalfmannR, KingO, KapilaA, LindquistS (2009) A systematic survey identifies prions and illuminates sequence features of prionogenic proteins. Cell 137 : 146–158.
19. KingOD, GitlerAD, ShorterJ (2012) The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res 1462 : 61–80.
20. SilvaJL, RangelLP, CostaDC, CordeiroY, De Moura GalloCV (2013) Expanding the prion concept to cancer biology: dominant-negative effect of aggregates of mutant p53 tumour suppressor. Biosci Rep 33: e00054.
21. CaiX, ChenJ, XuH, LiuS, JiangQX, et al. (2014) Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156 : 1207–1222.
22. FranklinBS, BossallerL, De NardoD, RatterJM, StutzA, et al. (2014) The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 15 : 727–737.
23. KaneMD, LipinskiWJ, CallahanMJ, BianF, DurhamRA, et al. (2000) Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. J Neurosci 20 : 3606–3611.
24. Meyer-LuehmannM, CoomaraswamyJ, BolmontT, KaeserS, SchaeferC, et al. (2006) Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 313 : 1781–1784.
25. LukKC, KehmVM, ZhangB, O'BrienP, TrojanowskiJQ, et al. (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209 : 975–986.
26. LiJY, EnglundE, HoltonJL, SouletD, HagellP, et al. (2008) Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med 14 : 501–503.
27. AsheKH, AguzziA (2013) Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion 7 : 55–59.
28. MallucciG, DickinsonA, LinehanJ, KlohnPC, BrandnerS, et al. (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302 : 871–874.
29. WagnerJ, RyazanovS, LeonovA, LevinJ, ShiS, et al. (2013) Anle138b: a novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson's disease. Acta Neuropathol 125 : 795–813.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Acidification Activates Motility and Egress by Enhancing Protein Secretion and Cytolytic ActivityČlánek Plasticity between MyoC- and MyoA-Glideosomes: An Example of Functional Compensation in InvasionČlánek Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent MannerČlánek NK Cell Activation in Human Hantavirus Infection Explained by Virus-Induced IL-15/IL15Rα Expression
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
-
Všechny články tohoto čísla
- Peculiarities of Prion Diseases
- Inhibitors of Peptidyl Proline Isomerases As Antivirals in Hepatitis C and Other Viruses
- War and Infectious Diseases: Challenges of the Syrian Civil War
- Microbial Contamination in Next Generation Sequencing: Implications for Sequence-Based Analysis of Clinical Samples
- Acidification Activates Motility and Egress by Enhancing Protein Secretion and Cytolytic Activity
- Co-dependence of HTLV-1 p12 and p8 Functions in Virus Persistence
- Shed GP of Ebola Virus Triggers Immune Activation and Increased Vascular Permeability
- Plasticity between MyoC- and MyoA-Glideosomes: An Example of Functional Compensation in Invasion
- The Type III Translocon Is Required for Biofilm Formation at the Epithelial Barrier
- Retromer Regulates HIV-1 Envelope Glycoprotein Trafficking and Incorporation into Virions
- IFI16 Restricts HSV-1 Replication by Accumulating on the HSV-1 Genome, Repressing HSV-1 Gene Expression, and Directly or Indirectly Modulating Histone Modifications
- Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent Manner
- Silencing by H-NS Potentiated the Evolution of
- Crystal Structure of Cytomegalovirus IE1 Protein Reveals Targeting of TRIM Family Member PML via Coiled-Coil Interactions
- GAPDH-A Recruits a Plant Virus Movement Protein to Cortical Virus Replication Complexes to Facilitate Viral Cell-to-Cell Movement
- Genomic Insights into the Fungal Pathogens of the Genus : Obligate Biotrophs of Humans and Other Mammals
- Unravelling Human Trypanotolerance: IL8 is Associated with Infection Control whereas IL10 and TNFα Are Associated with Subsequent Disease Development
- The Skin Microbiome: A Focus on Pathogens and Their Association with Skin Disease
- Human Cytomegalovirus Vaccine Based on the Envelope gH/gL Pentamer Complex
- IL-37 Inhibits Inflammasome Activation and Disease Severity in Murine Aspergillosis
- Host-Specific Parvovirus Evolution in Nature Is Recapitulated by Adaptation to Different Carnivore Species
- Activation of HIV Transcription with Short-Course Vorinostat in HIV-Infected Patients on Suppressive Antiretroviral Therapy
- PUL21a-Cyclin A2 Interaction is Required to Protect Human Cytomegalovirus-Infected Cells from the Deleterious Consequences of Mitotic Entry
- Programmed Ribosomal Frameshift Alters Expression of West Nile Virus Genes and Facilitates Virus Replication in Birds and Mosquitoes
- Aminoterminal Amphipathic α-Helix AH1 of Hepatitis C Virus Nonstructural Protein 4B Possesses a Dual Role in RNA Replication and Virus Production
- NK Cell Activation in Human Hantavirus Infection Explained by Virus-Induced IL-15/IL15Rα Expression
- Structure and Specificity of the Bacterial Cysteine Methyltransferase Effector NleE Suggests a Novel Substrate in Human DNA Repair Pathway
- Genetics, Receptor Binding Property, and Transmissibility in Mammals of Naturally Isolated H9N2 Avian Influenza Viruses
- A Gatekeeper Chaperone Complex Directs Translocator Secretion during Type Three Secretion
- A Conserved Peptide Pattern from a Widespread Microbial Virulence Factor Triggers Pattern-Induced Immunity in
- Succinate Dehydrogenase is the Regulator of Respiration in
- The Plasmodesmal Protein PDLP1 Localises to Haustoria-Associated Membranes during Downy Mildew Infection and Regulates Callose Deposition
- Dysregulated B Cell Expression of RANKL and OPG Correlates with Loss of Bone Mineral Density in HIV Infection
- Restriction of Genetic Diversity during Infection of the Vector Midgut
- The Epithelial αvβ3-Integrin Boosts the MYD88-Dependent TLR2 Signaling in Response to Viral and Bacterial Components
- The Relationship between Host Lifespan and Pathogen Reservoir Potential: An Analysis in the System
- Multiple Roles of the Cytoskeleton in Bacterial Autophagy
- The Evolution and Genetics of Virus Host Shifts
- ChIP-seq and In Vivo Transcriptome Analyses of the SREBP SrbA Reveals a New Regulator of the Fungal Hypoxia Response and Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Coronavirus Cell Entry Occurs through the Endo-/Lysosomal Pathway in a Proteolysis-Dependent Manner
- Peculiarities of Prion Diseases
- Host-Specific Parvovirus Evolution in Nature Is Recapitulated by Adaptation to Different Carnivore Species
- War and Infectious Diseases: Challenges of the Syrian Civil War
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



