-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Exprese galektinu-3, cytokeratinu 19, neural cell adhesion molecule a E-cadherinu ve variantách papilárního karcinomu štítné žlázy
Expression of Galectin-3, Cytokeratin 19, Neural Cell Adhesion Molecule and E-cadherin in Certain Variants of Papillary Thyroid Carcinoma
The immunohistochemical expression of galectin-3 (Gal3), cytokeratin 19 (CK19), neural cell adhesion molecule (NCAM), and E-cadherin (Ecad) was evaluated to assess their use in diagnostics of papillary thyroid carcinoma (PTC). A total of 84 PTCs - 36 classical variants (cPTCs), 26 follicular variants (fPTCs), and 22 papillary microcarcinomas (mPTCs) were studied. Expression of Gal3 was found in 36/36 (100%) cPTCs, 24/26 (92%) fPTCs, and 19/22 (86%) mPTCs. CK19 expression was detected in 34/36 (94%) cPTCs, 17/26 (65%) fPTCs, and 13/22 (59%) mPTCs. Expression of NCAM was seen in 5/36 (14%) cPTCs, 7/26 (27%) fPTCs, and 9/22 (41%) mPTCs. Ecad expression was found in 23/36 (64%) cPTCs, 17/26 (65%) fPTCs, and 18/22 (82%) mPTCs. A significant difference in CK19 expression was observed between cPTC and both fPTC and mPTC (p < 0.001). Furthermore, extrathyroid tumor spread significantly correlated with both level of CK19 expression and loss of Ecad expression (p = 0.001, p = 0.04). Our findings suggest that Gal3 and CK19 are useful markers for PTC, although decreased CK19 expression in mPTC and fPTC must be considered. Furthermore, CK19 and Ecad may play a role in extrathyroid tumor spread.
Key words:
papillary thyroid carcinoma – galectin-3 – cytokeratin 19 – neural cell adhesion molecule – E-cadherin
Autoři: J. Laco 1; A. Ryška 1; J. Čáp 2; P. Čelakovský 3
Působiště autorů: The Fingerland Department of Pathology, 2Second Department of Internal Medicine, and 3Department of Otorhinolaryngology, Charles University Faculty of Medicine and Faculty Hospital in Hradec Králové 1
Vyšlo v časopise: Čes.-slov. Patol., 44, 2008, No. 4, p. 103-107
Kategorie: Původní práce
Souhrn
Cílem studie bylo pomocí nepřímé imunohistochemie zjistit expresi galektinu-3 (Gal3), cytokeratinu 19 (CK19), neural cell adhesion molecule (NCAM) a E-cadherinu (Ecad) ve variantách papilárního karcinomu štítné žlázy (PTC) s ohledem na možné využití těchto markerů v bioptické diagnostice. Soubor tvořilo 84 případů – 36 klasických variant PTC (cPTC), 26 folikulárních variant PTC (fPTC) a 22 papilárních mikrokarcinomů (mPTC). Gal3 byl exprimován ve 36/36 (100 %) cPTC, ve 24/26 (92 %) fPTC a v 19/22 (86 %) mPTC. Exprese CK19 byla zastižena ve 34/36 (94 %) cPTC, v 17/26 (65 %) fPTC a ve 13/22 (59 %) mPTC. Exprese NCAM byla prokázána v 5/36 (14 %) cPTC, v 7/26 (27 %) fPTC a v 9/22 (41 %) mPTC. Ecad byl exprimován ve 23/36 (64 %) cPTC, v 17/26 (65 %) fPTC a v 18/22 (82 %) mPTC. V expresi CK19 byl zjištěn signifikantní rozdíl mezi cPTC versus fPTC a mPTC (p < 0,001). Dále byla prokázána signifikantní korelace mezi expresí CK19 a ztrátou exprese Ecad ve vztahu k šíření nádoru mimo štítnou žlázu (p = 0,001, p = 0,04). Detekci exprese Gal3 a CK19 lze tedy doporučit jako pomocné kritérium v diagnostice PTC, nicméně je třeba upozornit na nižší expresi CK19 ve fPTC a v mPTC. K detailnímu posouzení úlohy CK19 a Ecad při šíření PTC mimo štítnou žlázu je třeba dalších studií.
Klíčová slova:
štítná žláza – papilární karcinom – galektin-3 – cytokeratin 19 – neural cell adhesion molecule – E-cadherinPapillary thyroid carcinoma (PTC) is the most frequent endocrine malignancy with worldwide increasing incidence (9). In the last WHO classification (2004), at least fifteen variants of PTC differing not only by their microscopical appearance but also by their biological behaviour and prognosis are recognized (9). The classical variant of PTC (cPTC) is characterized by overall papillary architecture of the tumor; on the contrary, the follicular variant of PTC (fPTC) displays a follicular growth pattern making sometimes the differentiation from follicular adenoma/carcinoma a challenge. The papillary microcarcinoma (mPTC) is defined as an incidentally found PTC measuring 1 cm, irrespective of its growth pattern; its differential diagnosis includes mainly a fibrous scar with entrapped normal follicles - a condition known as fibrosing thyroiditis (26).
Galectin-3 (Gal3), a member of a family of ß-galactoside binding lectins, has been proved to be implicated in various biological processes, incl. regulation of cell growth, cell-cell and cell-matrix interactions, as well as apoptosis and neoplastic transformation and metastatic spread (17). The alterations in Gal3 expression in various malignant tumors, e.g. gastrointestinal carcinomas (29), breast carcinoma (6), and prostatic carcinoma (32), have been observed. Most previous studies focused on Gal3 expression in thyroid gland tumors have reported Gal3 presence in malignant tumors, whereas it was absent in benign lesions as well as in normal gland tissue (2, 3, 5, 7, 10, 14, 19, 24) .
Cytokeratin 19 (CK19) is expressed in various types of normal epithelial cells as well as in wide range of malignant epithelial tumors, e.g. gastrointestinal carcinomas, breast carcinoma etc. (21). In thyroid gland, CK19 is reported to be expressed namely in PTC, thus making its detection useful in differential diagnosis between fPTC versus follicular adenoma (FA) and/or follicular carcinoma (FC) and between PTC versus papillary hyperplasia in goiters (3, 13, 16, 20, 24, 25).
Neural cell adhesion molecule (NCAM), a transmembrane glycoprotein, plays an important role in cell-cell interactions (8). There are only few studies focused on NCAM expression in thyroid gland and its tumors; NCAM was shown to be expressed mainly in the normal gland follicles and benign thyroid tumors (33, 35).
E-cadherin (Ecad), a transmembrane glycoprotein, plays a crucial role in cell-cell adhesion and in intercellular signaling (18). Decreased or absent Ecad expression has been observed in various malignant tumors, including lobular breast carcinoma, diffuse gastric adenocarcinoma, etc. (31). In thyroid gland, Ecad expression has been found to be preserved in normal tissue and benign tumors, and frequently absent in malignant tumors (4, 12, 22, 28).
The aim of the study was to analyze the expression of the four above mentioned markers in cPTC, fPTC and mPTC and to assess their potential use in diagnostics and prognosis of these particular PTC variants.
Materials and methods
A total of 84 cases of PTCs (36 cPTCs, 26 fPTCs, and 22 mPTCs) were retrieved from the archive files. All patients underwent lobectomy or subtotal/total thyreoidectomy in years 1998 - 2006. In each case, gender and age of the patient, size and extrathyroid spread of the tumor, vascular invasion (i.e. invasion of tumor cells in blood and/or lymphatic vessels), and presence of metastases were noted.
In all cases, the tissue was fixed in 10% formalin, routinely processed, embedded in paraffin and stained with hematoxylin-eosin.
In addition, representative sections were used for immunohistochemical detection of the studied markers. The antigen retrieval was performed in a microwave oven (Panasonic) for Gal3 and NCAM, in Microwave Vacuum Histoprocessor RHS 1 (Milestone) for Ecad, and using 0.0033% ficin for CK19, respectively. Further steps were performed in Dako Autostainer (DakoCytomation). Endogenous peroxidase activity was inhibited by immersing the sections in 3% H2 O2. The sections were then treated with monoclonal antibodies to Gal3 (1 : 100, clone 9C4, Novocastra), to CK19 (1 : 100, clone RCK 108, DakoCytomation), to NCAM (1 : 100, clone 1B6, Novocastra), and to Ecad (1 : 100, clone M3612, DakoCytomation), respectively. A section from each case was treated with PBS instead of the primary antibody to provide a negative control. Finally, the sections were incubated with EnVision+ Dual Link System Peroxidase (DakoCytomation). The reaction was visualized using diaminobenzidine. In addition to the negative controls, sections of anaplastic large cell lymphoma, phyllodes tumor of breast, appendix, and tonsil provided positive controls for Gal3, CK19, NCAM, and Ecad, respectively.
Brown staining of cell cytoplasm (Gal3, CK19) and cell membrane (NCAM, Ecad) was interpreted as positive. The immunostaining was scored as follows: 0: absent; +: 1-10% tumor cells stained; ++: 11-25% tumor cells stained; +++: 26-50% tumor cells stained; ++++: 51-75% tumor cells stained; +++++: 76-100% tumor cells stained. The staining intensity was interpreted as weak, moderate, or strong.
For statistical analysis, NCSS 2004 programm, including „@@“ test and Fisheręs exact test, and Kruskal-Wallis analysis were used. Differences were accepted as statistically significant at p < 0.05.
Results
The detailed clinico-pathological data of the patients are shown in Table 1. There were 69 (82%) females and 15 (18%) males aged 15 – 80 years (mean: 53 Ī 17 years; median: 55 years). The tumor size ranged from 1 to 105 mm (mean: 21 Ī 18 mm; median: 15 mm); extrathyroid spread was detected in 17/84 (20%) cases. Vascular invasion was present in 11/84 (13%) cases, while metastases (detected in cervical lymphatic nodes only) were found in 10/84 (12%) patients. The follow-up period ranged from 8 to 94 months (median: 65 months).
Tab. 1. Clinicopathological data of patients 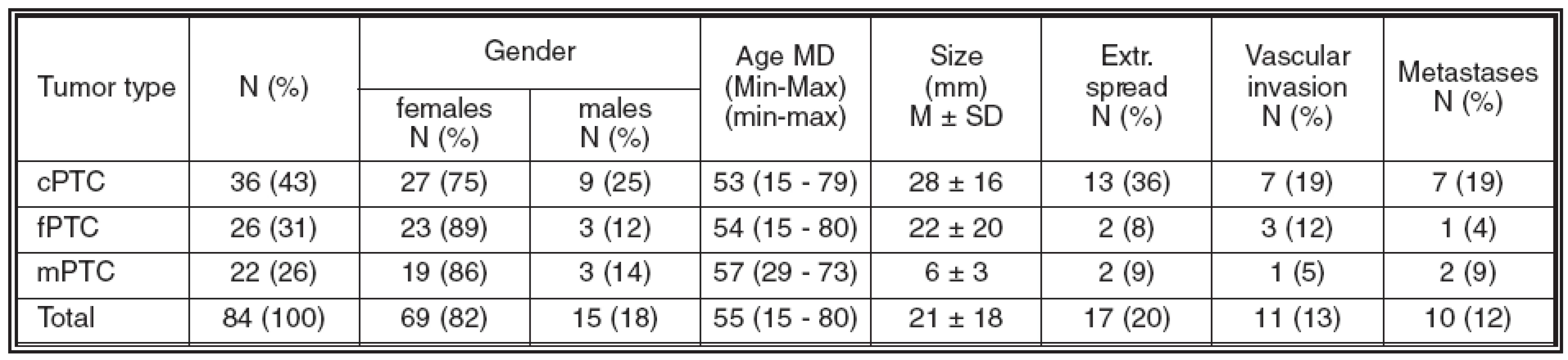
Abbrev.: cPTC = classical variant of papillary thyroid carcinoma; fPTC = follicular variant of papillary thyroid carcinoma; mPTC = papillary microcarcinoma of thyroid; MD = median; M = mean; SD = standard deviation; extr. = extrathyroid; Min-Max = range In the normal thyroid gland, Gal3 expression was detected in the cytoplasm of fibroblasts and endothelial cells, as well as in the nuclei of intrafollicular macrophages. Furthermore, solid cell nests were Gal3 positive in both cases where these were observed. Normal follicular cells were either negative or showed only focal and usually weak Gal3 expression, predominantly in close vicinity of a tumor displaying strong Gal3 expression. To the contrary, no expression of CK19 in normal thyroid tissue was detected. As a rule, NCAM and Ecad expression was present in the basolateral part of cell membrane of normal follicular cells.
The expression of the studied markers in the PTC variants (Fig. 1-3) is summarized in Table 2. Gal3 expression was detected in 79/84 (94%) of PTCs. In all PTC variants, a predominantly strong expression was seen in more than 75% of tumor cells. In fPTC, the Gal3 expression was also present in the colloid within neoplastic follicles. CK19 was expressed in 64/84 (76%) of PTCs. The staining intensity was mainly strong, while the percentage of positive tumor cells varried among the PTC variants. In both cPTC and mPTC, CK19 was usually expressed in more than 75% of tumor cells, while in fPTC its expression was limited mainly to less than 50% of tumor cells. A moderate to strong expression of NCAM was detected in 19/84 (23%) of PTCs; it was limited usually to less than 50% of tumor cells. Finally, weak to strong expression of Ecad was detected in 58/84 (69%) of PTCs. In both cPTC and fPTC it was limited to less than 50% of tumor cells, contrary to mPTC, where Ecad was expressed mostly in more than 75% of tumor cells.
Obr. 1. Papillary growth pattern of cPTC; ground glass nuclei of tumor cells are evident (HE, original magnification 200x). Upper right: Diffuse strong Gal3 expression in cPTC (original magnification 100x). Lower right: Diffuse focally strong expression of CK19 in cPTC (original magnification 100x) 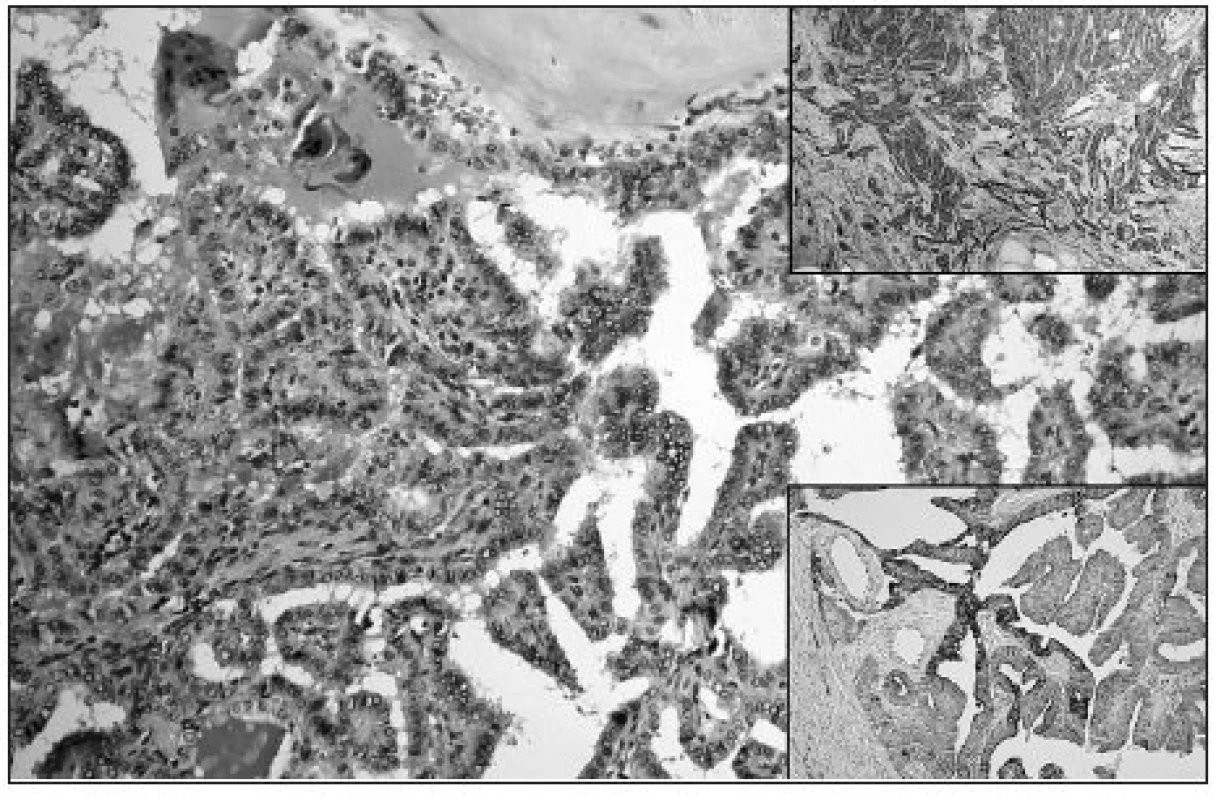
Obr. 2. Follicular architecture of fPTC; note intranuclear grooves in some of tumor cells (HE, original magnification 400x). Upper right: Moderate membranous expression of NCAM in fPTC (original magnification 200x). Lower right: Moderate membranous Ecad expression in fPTC (original magnification 200x) 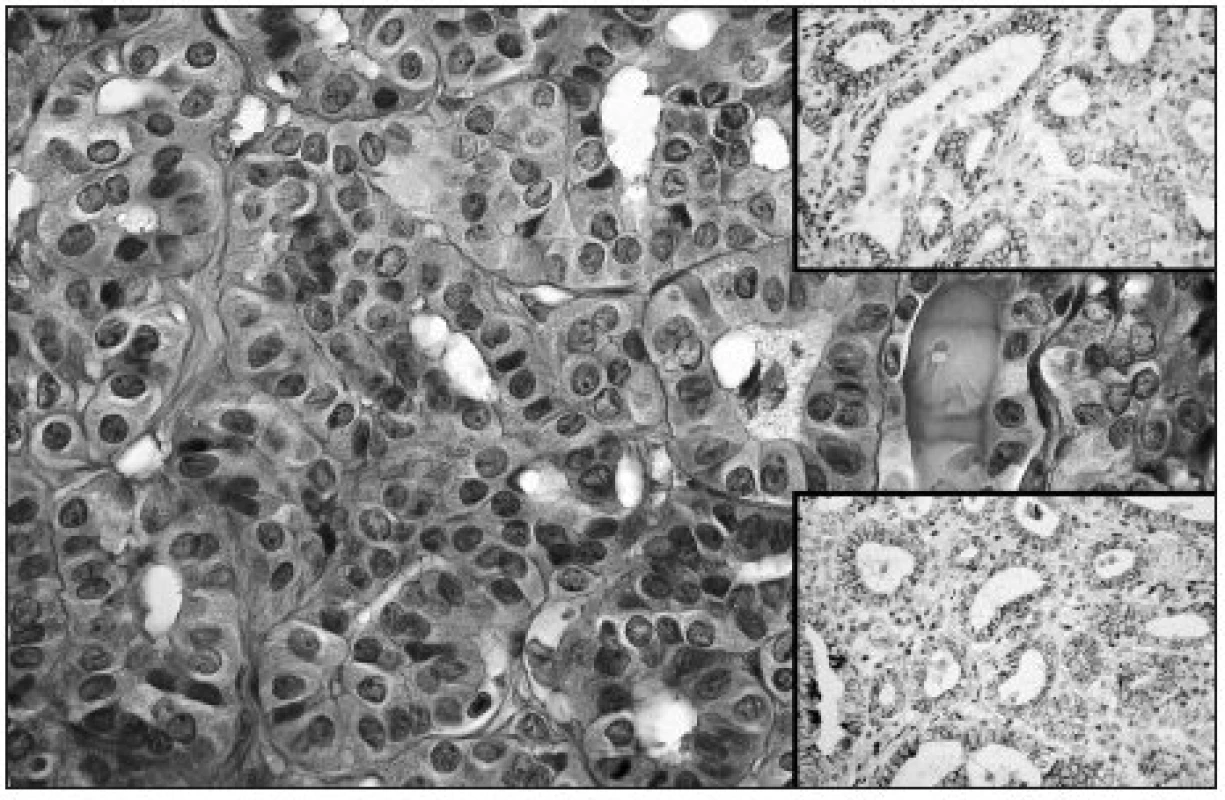
Obr. 3. mPTC with follicular growth pattern resembling fibrosing thyroiditis (HE, original magnification 40x). Upper left: Diffuse strong Gal3 expression in mPTC (original magnification 100x). Upper right: Diffuse strong expression of CK19 in mPTC (original magnification 100x). Lower left: Diffuse strong membranous NCAM expression in mPTC (original magnification 200x). Lower right: Diffuse strong expression of Ecad in mPTC (original magnification 100x) 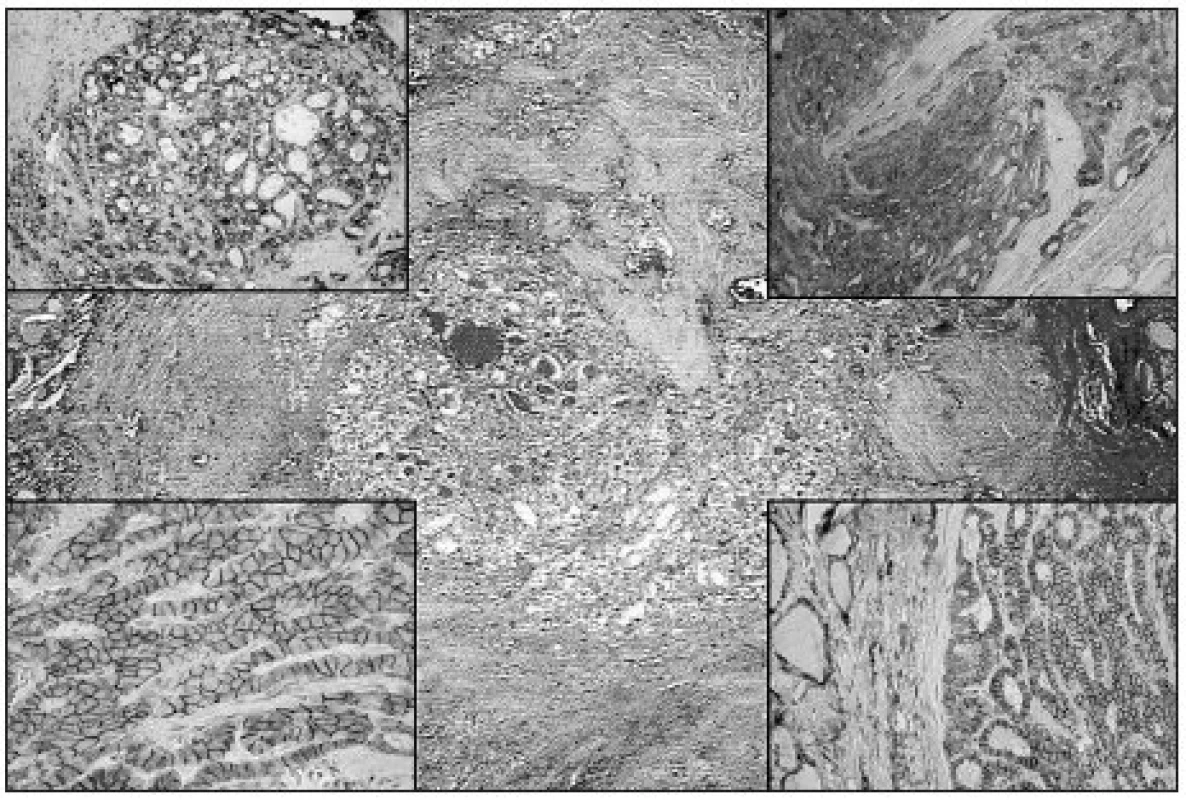
A statistically significant difference in CK19 expression was observed between cPTC and both fPTC and mPTC (p < 0.001). On the other hand, there was no significant difference in Gal3, NCAM, and Ecad expression between PTC variants, respectively (p > 0.05).
Regarding extrathyroid tumor spread, there was statistically significant positive correlation with CK19 expression (p = 0.001) and negative correlation with Ecad expression (p = 0.04); on the other hand, there was no correlation with both Gal3 and NCAM expression (p > 0.05, p > 0.05). Finally, no correlations between expression of all the studied markers and the presence of metastases were observed (p > 0.05).
Discussion
The spectrum of primary thyroid neoplasms is quite limited (9). However, as the diagnostic criteria may be relatively subjective and their evaluation expert-dependent, the diagnostics of thyroid lesions still represents a challenge. The most frequent diagnostic pitfalls include differentiation of FA from minimally invasive FC, differentiation of follicular neoplasms from fPTC, and distinction between fibrosing thyroiditis and mPTC (26).
Increasing knowledge of Gal3 shows, that this lectin is overexpressed in thyroid gland malignancies, particularly in PTC, with sensitivity and specifity varying between 86-94% and 36-98%, respectively (2, 19, 23, 24). However, only a limited number of studies have dealt with Gal3 expression in the individual variants of PTC. We believe that Gal3 expression in fPTC is of particular interest. Gal3 has been reported to be expressed in 33%, in 83%, and in 100% of fPTCs (7, 11, 23); in our study it was detected in 24/26 (92%) of fPTCs. Although in herein presented study the Gal3 expression in fPTC was not significantly lower in comparison with cPTC, the possibility of decreased Gal3 expression in fPTC (see above) must be considered in the differential diagnosis between follicular neoplasms versus fPTC. In a recent study (15) the sensitivity and specificity of Gal3 for the diagnosis of fPTC versus FA and FC were 92% and 63%, respectively. On the contrary, we (15), as well as other investigators (10, 24) have reported a significantly higher Gal3 expression in fPTC in comparison with FA and FC, respectively. Therefore, with knowledge of the above data, immunohistochemical identification of Gal3 may be a useful adjunct to the histopathological diagnosis of thyroid nodules with follicular growth pattern. In contrast to Kawachi et al. (14), who found a positive correlation between Gal3 expression and presence of both synchronous and metachronous metastases of PTC, we were unable to prove such a relationship, probably because of a limited follow-up period in our series and a lower number of PTCs studied.
The studies focused on the use of Gal3 in differential diagnosis between FA versus FC have shown inconsistent results; Gal3 expression was detected in 0-63% of FAs compared to 50-94% of FCs (2, 3, 10, 11, 14, 23, 24) . Similarly, some investigators have demonstrated a significant difference of Gal3 expression between FA and FC (2, 3, 10, 14, 23, 24), whereas the others have failed to confirm this finding (11, 15) relying the differential diagnosis on demonstration of complete transcapsular invasion and/or angioinvasion of tumor cells. The main cause of these varying results may be probably in the variations of immunohistochemical demonstration of Gal3. Nowadays, the biotin-free detection systems are strongly recommended (34).
The results of studies focused on CK19 expression in thyroid gland neoplasms have come to a consistent result, that PTC, irrespective of its histological variant, is characterized by strong and diffuse expression of CK19 (3, 5, 10, 16, 24) . However, most studies did not concentrate on detailed examination of CK19 expression in the particular PTC variants. There is an exception in the study of Cheung et al. (13), who reported CK19 expression in only 57% of fPTCs compared to 80% of positive cases of cPTCs, and the study of Baloch et al. (1), who found CK19 expression in all fPTCs and cPTCs in their series. In our current study, we have proved a significantly lower CK19 expression not only in fPTC but also in mPTC compared to cPTC. Although the exact cause of this phenomenon is unclear, the possible explanation of the lower CK19 expression in fPTC in contrast to cPTC might be the presence of different RET/PTC rearrangements (i.e. RET/PTC3 in fPTC versus RET/PTC1 in cPTC) (30). Another reason could be the presence of PAX8/PPAR@ translocation in fPTC. This molecular change is typical for FC; it has been described in fPTC, but not in cPTC (30). Recently, we have reported (15) a significantly higher CK19 expression in fPTC versus both FA and FC, making CK19 with Gal3 (see above) a possible useful marker in differential diagnosis in this field of thyroid pathology. The significantly lower CK19 expression in mPTC may be explained by a hypothesis, that CK19 is aberrantly expressed by the tumor cells in advanced stages of tumorigenesis. This explanation is furthermore supported by our finding of positive correlation between CK19 expression and extrathyroid spread of the tumor. In addition, this finding is of important diagnostic value limiting the use of CK19 in differential diagnosis between fibrosing thyroiditis and mPTC.
There is only a limited number of studies dealing with expression of NCAM in thyroid gland lesions; the NCAM expression is decreasing in the sequence normal thyroid gland – benign tumors – malignant tumors (33, 35). Recently, the NCAM expression in various types of PTC was analyzed by Scorpino et al. (27), who have demonstrated its decreased or absent expression in all the PTCs from their series. In the herein presented study, we were unable to prove any difference in NCAM expression between all the PTC variants, although decrease in NCAM expression in both cPTC and fPTC in comparison with mPTC is noticeable (see Table 2). This finding may be explained by the alteration in the adhesive and migratory properties of tumor cells. Thus, although NCAM may play certain biological role in thyroid gland embryonic development and follicle formation, its precise role in thyroid gland tumorigenesis remains to be elucidated by further studies and the use of NCAM as a diagnostic marker in daily routine practice cannot be recommended.
There is an increasing evidence of a potential role of Ecad in tumorigenesis of thyroid neoplasms. Ecad expression is, similarly to NCAM, decreasing in the sequence normal thyroid gland – benign tumors – malignant tumors, which indicates similar biological function of these two proteins (4, 12, 22). Naito et al. (22) found absent Ecad expression in 21% of PTCs in their series; in the herein presented study, 26/84 (31%) of PTCs were Ecad negative. In addition, there was a negative correlation between Ecad expression and extrathyroid spread of PTC, indicating an impaired function of Ecad in advanced stages of PTC. In contrary to Naito et al. (22), who demonstrated a negative correlation between Ecad expression in PTC and the presence of synchronous metastases, we have not found this relationship, probably because of a higher metastatic rate in PTCs in the former series (metastases detected in 30/53 (57%) of PTCs) in contrast to our series (12%). Although we have found Ecad expression to be decreased in fPTC and cPTC versus mPTC (see Table 2), there was no statistically significant difference detected. Like NCAM, Ecad may play an important role in thyroid tumorigenesis, but its exact function remains to be elucidated and at this time its use in differential diagnosis cannot be recommended.
Tab. 2. Expression of the studied markers in individual PTC variants 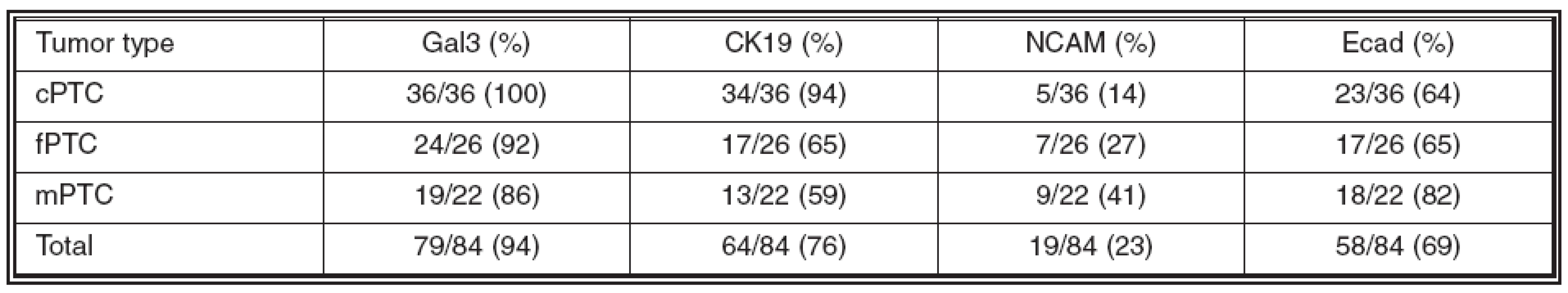
Abbrev.: cPTC = classical variant of papillary thyroid carcinoma; fPTC = follicular variant of papillary thyroid carcinoma; mPTC = papillary microcarcinoma of thyroid; Gal3 = galectin-3; CK19 = cytokeratin 19; NCAM = neural cell adhesion molecule; Ecad = Ecadherin In conclusion, our findings suggest that Gal3 and CK19 are useful markers for PTC, although not definitely solving the classical diagnostic pitfalls. Particularly, decreased CK19 expression in mPTC and fPTC must be considered. To the best of our knowledge, the significantly lower CK19 expression in mPTC in comparison to cPTC has not been previously reported. On the other hand, NCAM and Ecad, although probably playing important biological roles in thyroid gland embryonic development as well as in thyroid tumorigenesis, are not recommended for daily practice. In addition, CK19 and Ecad may play a role in extrathyroid spread of PTC; however, the exact explanation of this finding as well as the potential use of CK19 and Ecad as predictive markers must be elucidated by further studies.
Acknowledgements
The authors thank Mrs. E. Šišková, Mrs. J. Herelová, Mrs. B. Špicarová and Mrs. M. Žáková for their excellent technical support, and RNDr. E. Čermáková for performing statistical analysis.
Supported by Research project of Ministry of Health of Czech Republic 00179906.
Corresponding author:
Jan Laco, M.D., Ph.D.
The Fingerland Department of Pathology, Faculty Hospital
Sokolská 581
500 05 Hradec Králové, Czech Republic
Telephone number: 00420-495 832 548
Fax number: 00420-495 832 004
E-mail: lacoj@lfhk.cuni.cz
Zdroje
1. Baloch, Z.W., Abraham, S., Roberts, S. et al.: Differential expression of cytokeratins in follicular variant of papillary carcinoma: an immunohistochemical study and its diagnostic utility. Hum. Pathol., 30, 1999, s. 1166-1171.
2. Bartolazzi, A., Gasbarri, A., Papotti, M. et al.: Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet, 357, 2001, s. 1644-1650.
3. Beesley, M.F., McLaren, K.M.: Cytokeratin 19 and galectin-3 immunohistochemistry in the differential diagnosis of solitary thyroid nodules. Histopathology, 41, 2002, s. 236-243.
4. Brabant, G., Hoang-Vu, C., Cetin, Y. et al.: E-cadherin: a differentiation marker in thyroid malignancies. Cancer Res., 53, 1993, s. 4987-4993.
5. Casey, M.B., Lohse, C.M., Lloyd, R.V.: Distinction between papillary thyroid hyperplasia and papillary thyroid carcinoma by immunohistochemical staining for cytokeratin 19, galectin-3, and HBME-1. Endocr. Pathol., 14, 2003, s. 55-60.
6. Castronovo, V., Van Den Brule, F.A., Jackers, P. et al.: Decreased expression of galectin-3 is associated with progression of human breast cancer. J. Pathol., 179, 1996, s. 43-48.
7. Coli, A., Bigotti, G., Zucchetti, F. et al.: Galectin-3, a marker of well-differentiated thyroid carcinoma, is expressed in thyroid nodules with cytological atypia. Histopathology, 40, 2002, s. 80-87.
8. Crossin, K.L., Krushel, L.A.: Cellular signaling by neural cell adhesion molecules of the immunoglobulin superfamily. Dev. Dyn., 218, 2000, s. 260-279.
9. DeLellis, R.A., Lloyd, R.V., Heitz, P.H., Eng, Ch.: WHO Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARCPress, 2004, s. 57-66.
10. de Matos, P.S., Ferreira, A.P., de Oliveira Facuri, F. et al.: Usefulness of HBME-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology, 47, 2005, s. 391-401.
11. Herrmann, M.E., LiVolsi, V.A., Pasha, T.L. et al.: Immunohistochemical expression of galectin-3 in benign and malignant thyroid lesions. Arch. Pathol. Lab. Med., 126, 2002, s. 710-713.
12. Huang, S.H., Wu, J.C., Chang, K.J. et al.: Expression of the cadherin-catenin complex in well-differentiated human thyroid neoplastic tissue. Thyroid, 9, 1999, s. 1095-1103.
13. Cheung, C.C., Ezzat, S., Freeman, J.L. et al.: Immunohistochemical diagnosis of papillary thyroid carcinoma. Mod. Pathol., 14, 2001, s. 338-342.
14. Kawachi, K., Matsushita, Y., Yonezawa, S. et al.: Galectin-3 expression in various thyroid neoplasms and its possible role in metastasis formation. Hum. Pathol., 31, 2000, s. 428-433.
15. Laco, J., Ryška, A.: The use of immunohistochemistry in the differential diagnosis of thyroid gland tumors with follicular growth pattern. Čes.-slov. Patol., 42, 2006, s. 120-124 (in Czech).
16. Lam, K.Y., Lui, M.C., Lo, C.Y.: Cytokeratin expression profiles in thyroid carcinomas. Eur. J. Surg. Oncol., 27, 2001, s. 631-635.
17. Liu, F.T., Patterson, R.J., Wang, J.L.: Intracellular functions of galectins. Biochim. Biophys. Acta, 1572, 2002, s. 263-273.
18. Martins, L., Matsuo, S.E., Ebina, K.N. et al.: Galectin-3 messenger ribonucleic acid and protein are expressed in benign thyroid tumors. J. Clin. Endocrinol. Metab., 87, 2002, s. 4806-4810.
19. Mehrotra, P., Okpokam, A., Bouhaidar, R. et al.: Galectin-3 does not reliably distinguish benign from malignant thyroid neoplasms. Histopathology, 45, 2004, s. 493-500.
20. Miettinen, M., Kovatich, A.J., Karkkainen, P.: Keratin subsets in papillary and follicular thyroid lesions. A paraffin section analysis with diagnostic implications. Virchows Arch., 431, 1997, s. 407-413.
21. Moll, R., Franke, W.W., Schiller, D.L. et al.: The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell, 31, 1982, s. 11-24.
22. Naito, A., Iwase, H., Kuzushima, T. et al.: Clinical significance of E-cadherin expression in thyroid neoplasms. J. Surg. Oncol., 76, 2001, s. 176-180.
23. Oestreicher-Kedem, Y., Halpern, M., Roizman, P. et al.: Diagnostic value of galectin-3 as a marker for malignancy in follicular patterned thyroid lesions. Head Neck, 26, 2004, s. 960-966.
24. Prasad, M.L., Pellegata, N.S., Huang, Y. et al.: Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod. Pathol., 18, 2005, s. 48-57.
25. Raphael, S.J., McKeown-Eyssen, G., Asa, S.L.: High-molecular-weight cytokeratin and cytokeratin-19 in the diagnosis of thyroid tumors. Mod. Pathol., 7, 1994, s. 295-300.
26. Rosai, J., Kuhn, E., Carcangiu, M.L.: Pitfalls in thyroid tumour pathology. Histopathology, 49, 2006, s. 107-120.
27. Scarpino, S., Di Napoli, A., Melotti, F. et al.: Papillary carcinoma of the thyroid: low expression of NCAM (CD56) is associated with downregulation of VEGF-D production by tumour cells. J. Pathol., 212, 2007, s. 411-419.
28. Scheumman, G.F., Hoang-Vu, C., Cetin, Y. et al.: Clinical significance of E-cadherin as a prognostic marker in thyroid carcinomas. J. Clin. Endocrinol. Metab., 80, 1995, s. 2168-2172.
29. Schoeppner, H.L., Raz, A., Ho, S.B. et al.: Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer, 75, 1995, s. 2818-2826.
30. Sobrinho-Simoes, M., Preto, A., Rocha, A.S. et al.: Molecular pathology of well-differentiated thyroid carcinomas. Virchows Arch., 447, 2005, s. 787-793.
31. Van Aken, E., De Wever, O., Correia da Rocha, A.S. et al.: Defective E-cadherin/catenin complexes in human cancer. Virchows Arch., 439, 2001, s. 725-751.
32. van den Brule, F.A., Waltregny, D., Liu, F.T. et al.: Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int. J. Cancer, 89, 2000, s. 361-367.
33. Vargas, F., Tolosa, E., Sospedra, M. et al.: Characterization of neural cell adhesion molecule (NCAM) expression in thyroid follicular cells: induction by cytokines and over-expression in autoimmune glands. Clin. Exp. Immunol., 98, 1994, s. 478-488.
34. Volante, M., Bozzalla-Cassione, F., Orlandi, F. et al.: Diagnostic role of galectin-3 in follicular thyroid tumors. Virchows Arch., 444, 2004, s. 309-312.
35. Zeromski, J., Biczysko, M., Stajgis, P. et al.: CD56(NCAM) antigen in glandular epithelium of human thyroid: light microscopic and ultrastructural study. Folia Histochem. Cytobiol., 37, 1999, s. 11-17.
Štítky
Patologie Soudní lékařství Toxikologie
Článek ÚVODNÍKČlánek OSOBNÍ ZPRÁVYČlánek Heřman Šikl (1888–1955)Článek Jaká je vaše diagnóza?Článek Jiří Kudrmann osmdesátiletýČlánek PoděkováníČlánek Plán akcí – jaro 2009Článek Jaká je vaše diagnóza?Článek JAK SE VÁM LÍBÍ?
Článek vyšel v časopiseČesko-slovenská patologie

2008 Číslo 4-
Všechny články tohoto čísla
- Dlaždicobuněčný karcinom kůže různého stupně – rozdíly v expresi CD10
- Historky ze života Heřmana Šikla
- Exprese galektinu-3, cytokeratinu 19, neural cell adhesion molecule a E-cadherinu ve variantách papilárního karcinomu štítné žlázy
- OSOBNÍ ZPRÁVY
- Heřman Šikl (1888–1955)
- Historky ze života Heřmana šikla
- Jaká je vaše diagnóza?
- Významné životní výročí prof. MUDr. Aleny Linhartové, DrSc.
- Jiří Kudrmann osmdesátiletý
- Poděkování
- MUDr. Vladimír Zeman, CSc., emeritní primář, osmdesátníkem
- Plán akcí – jaro 2009
- Jaká je vaše diagnóza?
- JAK SE VÁM LÍBÍ?
- ÚVODNÍK
- Co je nového v patologii štítné žlázy
- Na raftu a na membráně. Jedna zkušenost z biologie lymfoproliferativních onemocnění
- Historky ze života Heřmana Šikla
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dlaždicobuněčný karcinom kůže různého stupně – rozdíly v expresi CD10
- Co je nového v patologii štítné žlázy
- Heřman Šikl (1888–1955)
- Historky ze života Heřmana Šikla
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



