-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Studies of the properties of tablets from directly compressible isomalt
Studium vlastností tablet z přímo lisovatelného isomaltu
Práce se zabývá studiem vlastností tablet ze dvou typů přímo lisovatelného isomaltu – galenuIQTM 720 a galenuIQTM 721. Studovala se pevnost tablet v tahu a doba rozpadu tablet, obojí v závislosti na lisovací síle, přídavku stearanu hořečnatého a stearylfumarátu sodného (Pruvu) jako mazadel v koncentracích 0,5% a 1% a 50% přídavku modelových léčivých látek kyseliny askorbové a kyseliny acetylsalicylové. Tablety byly lisovány lisovacími silami 6, 8 a 10 kN, výlisky s léčivy pouze lisovací sílou 10 kN. Pevnost tablet z obou látek rostla s lisovací silou, vlivem mazadel se nesnížila. Doba rozpadu byla delší s látkou galenIQTM 720, u obou látek byla prodloužena mazadly a rostla s lisovací silou. Přítomnost léčiv snížila pevnost tablet, pevnější byly výlisky s kyselinou acetylsalicylovou. U obou léčiv nebyl výrazný rozdíl v rámci typu použitého isomaltu ani mazadla. Doba rozpadu byla kratší u tablet s kyselinou askorbovou, kdy byla nejkratší s látkou galenIQTM 721 s Pruvem, kdežto v případě kyseliny acetylsalicylové byla nejkratší s látkou galenIQTM 721 se stearanem hořečnatým.
Klíčová slova:
přímo lisovatelný isomalt – mazadla – pevnost tablet v tahu – doba rozpadu tablet
Authors: J. Mužíková; V. Pavlasová
Authors place of work: Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmaceutical Technology, Czech Republic
Published in the journal: Čes. slov. Farm., 2009; 58, 172-178
Category: Původní práce
Summary
The paper deals with the study of the properties of tablets from two types of directly compressible isomalt – namely galenIQTM 720 and galenIQTM 721. The study focuses on the dependence of tensile strength and disintegration time of the tablets, on both the compression force, the addition of lubricants, namely magnesium stearate and sodium stearyl fumarate (Pruv) with the concentrations of 0.5% and 1%, and a 50% addition of model active ingredients, namely ascorbic acid and acetylsalicylic acid. The used compression forces were 6, 8 and 10 kN, tablets containing drugs were compressed only with the force of 10 kN. The tensile strength of tablets from both substances increased with increasing compression force, the presence of lubricants did not result in any decrease in strength. The disintegration time of tablets was longer with the use of substance galenIQTM 720, it was prolonged by lubricants and increased with increasing compression force for both substances. The presence of drugs decreased tensile strength, and the tablets containing acetylsalicylic acid showed higher tensile strength. For both drugs, differences within the type of isomalt or lubricants were not significant. The disintegration time of tablets was shorter when ascorbic acid was used; it was the shortest in the case of galenIQTM 721 with Pruv, but in the case of acetylsalicylic acid the disintegration time was the shortest with galenIQTM 721 with magnesium stearate.
Key words:
directly compressible isomalt – lubricants – tensile strength of tablets – disintegration time of tabletsIntroduction
The interest in the use of sugar alcohols in pharmaceutical formulations has recently increased due to their sweetening capacity, a lower content of calories, and a non-cariogenic character. In addition, they can be administered to diabetic patients. Sugar alcohols may be used as fillers in normal as well as chewing tablets. After a suitable adjustment of tablets, e.g. by granulation or spray drying, they can serve as dry binders in directly compressible tablets 1). The sugar alcohols employed for these purposes include also isomalt. Isomalt is a mixture of hydrogenated monosaccharides and disaccharides and its principal components are the disaccharidic alcohols 1-O-α-D-glucopyranosyl-D-mannitol dihydrate (GPM) and 6-O-α-D-glucopyranosyl-D-sorbitol (GPS). Isomalt possesses a half of the sweetening capacity of saccharose and low negative heat of dissolution, it is non-cariogenic and a small source of energy, as it is not absorbed in the small intestine, but fermented in the large intestine.1) Due to stable glycosidic bonds, it is resistant to chemical degradations and it does not produce a cooling effect in the mouth. Its agglomerated product, composed of small primary particles, was developed for direct compression. Isomalt adjusted in this way possesses excellent flowability, a high dilution potential, and low hygroscopicity 2). Ndindayno et al.3) have demonstrated that the directly compressible properties of isomalt can be improved by melting. After melting, the crystalline form is converted into the amorphous one. The disintegration time of the tablet compressed from molten isomalt and the release of the active ingredient from the tablet are very quick. A problem is agglomeration caused by recrystallization, where the amorphous phase passes to the stable crystalline phase in the presence of aerial humidity. This problem must be solved in further research.
The agglomerated isomalt product is manufactured by the German firm Palatinit GmbH under the name of galenIQ™, types 720 and 721 being intended for direct compression. The individual types differ in the ratios of basic disaccharidic alcohols; in type 720 the ratio of GPM and GPS is 1 : 1 and in type 721 this ratio is 1 : 3, and due to this fact it is more soluble in water 2). The present paper studies the properties of tablets, in particular the tensile strength and disintegration time in the above-mentioned types of directly compressible isomalt in dependence on compression force, addition of two types of lubricants in two concentrations, and two types of active ingredients in one concentration.
EXPERIMENTAL PART
Materials
galenIQ™ 720 – agglomerated isomalt (1-O-α-D--glucopyranosyl-D-mannitol dihydrate and 6-O-α-D-glucopyranosyl-D-sorbitol in the ratio of 1 : 1) (Palatinit GmbH, Germany); galenIQ™ 721 -agglomerated isomalt (1-O-α-D-glucopyranosyl-D-mannitol dihydrate and 6 O-α-D-glucopyranosyl-D-sorbitol in the ratio of 1 : 3) (Palatinit GmbH, Germany); Pruv® – sodium stearyl fumarate (J. Rettenmaier & Söhne GmbH + Co, Rosenberg, Germany); magnesium stearate (Acros organics, New Jersey, USA); ascorbic acid (Northeast General Pharmaceutical Factory, China); acetylsalicylic acid (Merck KgaA, Darmstadt, Germany).
Preparation of tableting materials and tablets
The tests included tableting materials from dry binders without lubricants, mixtures with lubricants, and mixtures with active ingredients. Altogether 18 tableting materials of the following composition were used (Table 1 and 2).
Tab. 1. Tableting materials without active ingredients 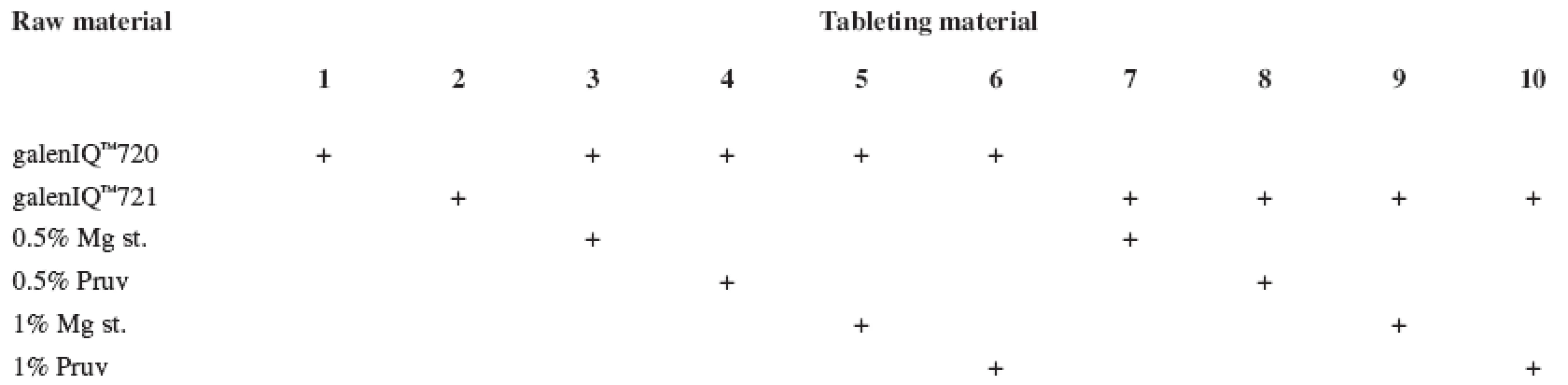
Mg st. – magnesium stearate Tab. 2. Tableting materials with active ingredients 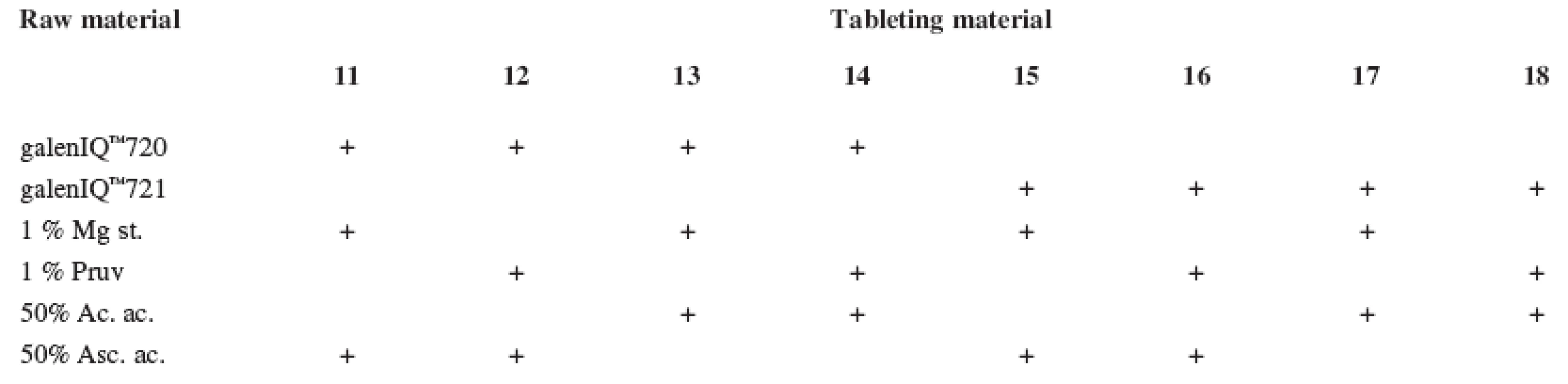
Mg st. – magnesium stearate, Ac. ac. – acetylsalicylic acid, Asc. ac. – ascorbic acid Dry binders with lubricants were mixed for 5 minutes in a stainless steel cube KB 15S (Erweka GmbH, Hausenstamm, Germany). Mixtures with active ingredients were prepared by mixing the dry binder and the active ingredient for 5 minutes, then the lubricant was added and the material was mixed for 5 minutes again. The rotation rate of the mixing cube was always 17 revolutions/minute. The amount of prepared tableting materials without active ingredients was always 30 g, with active ingredients, 20 g.
All tableting materials were used to produce 16 tablets compressed with the use of a special die with an upper and a lower punch on a material testing equipment T1 – FRO 50 TH.A1K Zwick/Roell (Zwick GmbH & Co, Ulm, Germany). Proper compaction took place by applying the pressure on the upper punch. The tablets were of a cylindrical shape without facets with a diameter of 13 mm and weight of 0.5 ± 0.0010 g. Compression velocity was 1 mm/s and compression forces 6, 8 and 10 kN, in the case of pure dry binders, 8 kN. Mixtures including active ingredients were compressed using only a compression force of 10 kN.
Measurement of tensile strength of tablets and evaluation of the lubricant sensitivity of tableting materials
Tensile strength was always evaluated in 10 tablets, first no sooner than 24 hours after compaction. Measurements were performed on a Schleuniger apparatus (Dr. Schleuniger Pharmatron AG, Solothurn, Switzerland), which measured tablet sizes accurate to 0.01 mm and destruction force in N. Tensile strength of tablets was calculated according to Eq. [1]:
where P is the tensile strength of tablets (MPa), F is the destruction force (N), d is the tablet diameter [mm], and h is the thickness of the tablet (mm) 4).
LSR (lubricant sensitivity ratio) values, which make it possible to quantify and mutually compare the lubricant sensitivity of tableting materials, were calculated according to Eq. [2]:
where Csu is the crushing strength of tablets without an added lubricant and Csl is the crushing strength with a lubricant. The more this value approaches 1, the more the dry binder is sensitive to an added lubricant from the viewpoint of decreased strength of tablets 5). In the present paper, the values of tensile strength, not those of crushing strength, are used in the equation.
Measurement of disintegration time of tablets
Disintegration times of tablets were evaluated earliest 24 hours after compaction always in 6 tablets. Measurements were performed on an apparatus for the determination of disintegration time of tablets Erweka ZT 301 (Erweka GmbH, Hausenstamm, Germany) following the method described in the chapter Pharmaceutical Technical Procedures in the Ph. Eur. 2005. The test was carried out without discs in the medium of purified water tempered to 37 °C ± 1 °C. The tablet was considered disintegrated at the moment when there was no remainder on the net.
The results of strengths and disintegration times were statistically processed by means of the computer programmes Excel and Qcexpert. Elementary data analysis yielded the mean values with standard deviations, which were plotted into dependences on compression force. In the cases of unclear significance of differences in the values, unpaired t-test at a level of significance of 0.05 was employed.
RESULTS AND DISCUSSION
The paper examined the properties of tablets, tensile strength and disintegration time, from the directly compressible isomalt galenIQ 720 and 721 in dependence on compression force. The impact factors were additions of two types of lubricants in two concentrations and two types of active ingredients in one concentration. The compression forces were 6, 8 and 10 kN and they were selected in such a way to make the tensile strength of tablets made of isomalt with lubricants oscillate within the range of the optimal strength of tablets (0.56–1.11 MPa) 6). The mixtures with active ingredients were compressed using only the compression force of 10 kN. The lubricants used were magnesium stearate and sodium stearyl fumarate in the concentrations of 0.5 and 1%. The model active ingredients were ascorbic acid and acetylsalicylic acid.
Figure 1 shows the dependence of tensile strength of tablets on compression force for galenIQ 720. In the compression force of 8 kN, it also states the value of strength for pure substance, which with the use of this compression force could be still compacted without a lubricant, though there was a problem of its sticking to the matrix. This value was intended to serve for the evaluation of the influence of lubricants on the strength of compacts, which should be theoretically decreased due to the plastic behaviour of isomalt 1). However, there was no decrease in strength due to the lubricants. The tablets with a 0.5% addition of lubricants were stronger than those with a 1% addition. The lowest strength was found in the tablets containing 1% of Pruv, the value of strength for the compression force of 8 kN not being statistically significantly different from the value for the pure substance. The values of strength of tablets increased with compression force. Luhn 7) examined also the influence of the concentration of magnesium stearate and the length of the period of mixing on tablet strength in the substance galenIQ 720. He found that the length of the period of mixing longer than 15 minutes resulted in lower tablet strength, but with increased concentration of the stearate from 0.5 to 2% there was no influence on the strength.
Fig. 1. Tensile strength of tablets in function of compression force: galen IQ 720 g 720 – galenIQ 720, Mg stearas – magnesii stearas 
Figure 2 represents the identical dependence for galenIQ 721. Neither in this case the strength of tablets was influenced by lubricants, only the value for the mixture with 0.5% of Pruv was statistically significantly lower. In the compression forces of 6 and 10 kN, there is a statistically significant difference only between the strengths of compacts with 0.5% of lubricants, the compacts containing 0.5% of magnesium stearate being stronger. In tablets containing 1% of lubricants, there is no statistically significant difference in the values of strength within the type of the lubricant used. The strength of tablets was increased with compression force.
Fig. 2. Tensile strength of tablets in function of compression force: galen IQ 721 g 721 – galenIQ 721, Mg stearas – magnesii stearas 
As follows from the comparison of both graphs, there is no marked difference between the strengths of tablets made from both types of isomalt. No effect of the lubricant on tablet strength is confirmed by the LSR (lubricant sensitivity ratio) values presented in Table 3. The more the value approaches 1, the higher sensitivity of the dry binder to the lubricant is observed. In the substance galenIQ 720, the LSR values were minus, the strength of tablets with lubricants was even marginally higher.
Tab. 3. Values of LSR for galen IQ 720 and 721 at the compression force of 8 kN 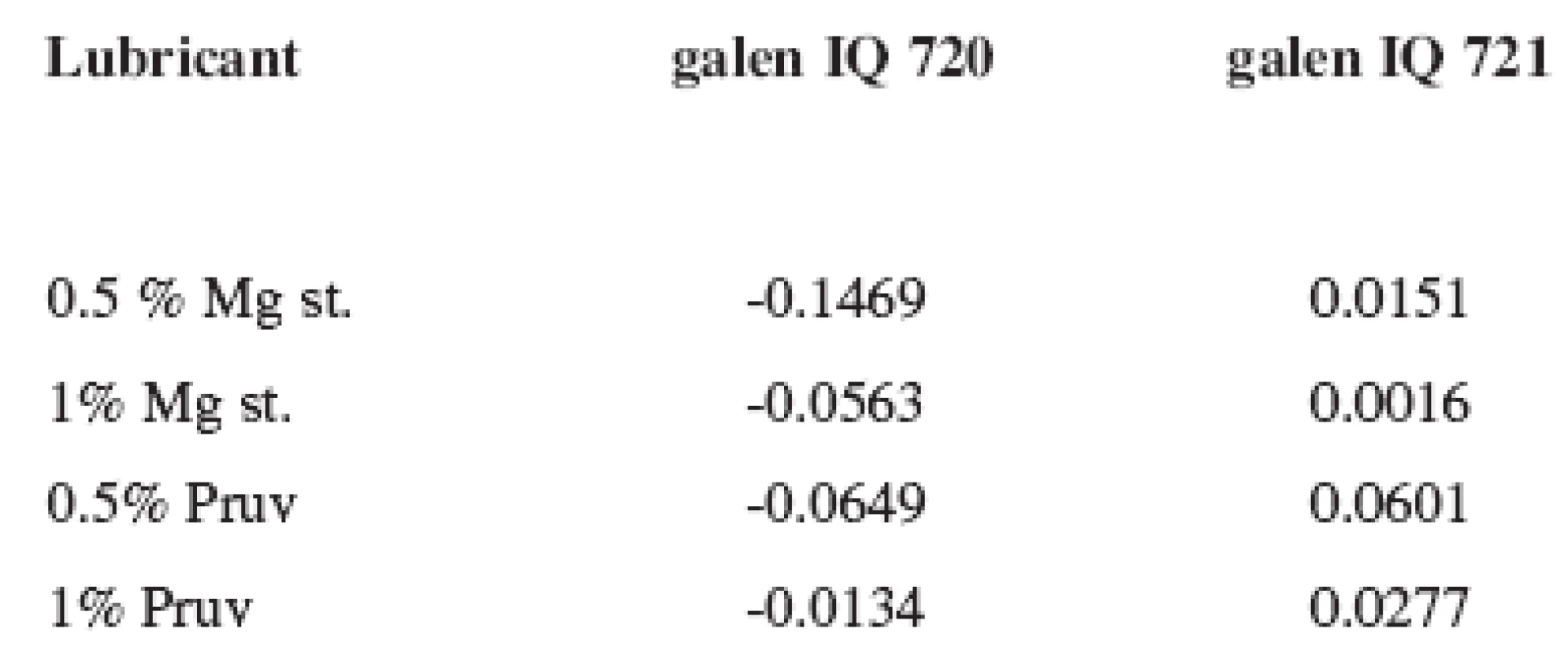
Mg st. – magnesium stearate Figure 3 shows the dependence of disintegration times of tablets on compression force for galenIQ 720. A longer disintegration time was observed in the tablets with 1% of lubricants, the shortest disintegration time was found in the compacts with 0.5% of magnesium stearate, the value in the compression force of 8 kN not being statistically significantly different from the value for the pure substance.
Fig. 3. Disintegration time in function of compression force: galen IQ 720 g 720 – galenIQ 720, g 721 – galenIQ 721, Mg stearas – magnesii stearas 
Figure 4 describes the identical dependence for galenIQ 721. Disintegration time was prolonged by the action of lubricants.
The comparison of the values of disintegration times presented in the Graphs shows a marked difference in the disintegration times of the compacts made from both substances. The compacts containing the substance galenIQ 721 unambiguously possessed a shorter disintegration time due to a higher content of GPS, which is more soluble in water than GPM 1).
Fig. 4. Disintegration time in function of compression force: galen IQ 721 g 720 – galenIQ 720, Mg stearas – magnesii stearas 
Figure 5 lists the values of the strength of compacts containing active ingredients at the compression force of 10 kN. The presence of active ingredients decreased the strength of tablets. The strength of tablets containing acetylsalicylic acid was higher, which could be theoretically assumed, due to better compressibility of the active ingredient resulting from the prevailing mechanism of compaction by plastic deformation. Ascorbic acid is compressed by fragmentation of its particles and this mechanism does not guarantee good compressibility and strength of compacts 8). Within the framework of the employed dry binder and lubricant, there were no marked differences between the values of strength of tablets.
Fig. 5. Tensile strength of tablets at the compression force of 10kN: galen IQ 720 and 721 with active ingredients g 721 – galenIQ 721, Mg stearas – magnesii stearas 
Figures 6 and 7 show the values of disintegration times of compacts with ingredients. The Graphs are divided into two, as the values of disintegration times of compacts containing ascorbic acid are markedly lower due to its good solubility in water. In the compacts with ascorbic acid, the tablets containing the substance galen IQ 720 possessed a longer disintegration time due to its lower solubility. In both cases, the compacts with stearate disintegrated longer than those with Pruv. The tablets containing acetylsalicylic acid possessed long disintegration times, high above the pharmacopoeial limit of classic tablets (15 min) because of bad solubility of acetylsalicylic acid. A shorter disintegration time was observed in the compacts containing galen IQ 721 with the use of the same lubricant. The disintegration time was longer in the case of both substances with less hydrophobic Pruv, though there were no marked differences in the strengths of tablets. A longer disintegration time would be theoretically assumed in the tablets containing magnesium stearate, which is more hydrophobic.
Fig. 6. Disintegration time of tablets at the compression force of 10 kN: galen IQ 720 and 721 with ascorbic acid g 720 – galenIQ 720, g 721 – galenIQ 721, Mg stearas – magnesii stearas 
Fig. 7. Disintegration time of tablets at the compression force of 10 kN: galen IQ 720 and 721 with acetylsalicylic acid g 720 – galenIQ 720, g 721 – galenIQ 721, Mg stearas – magnesii stearas 
The compressibility of both types of directly compressible isomalt is the same, but when we need prepare the tablets with a shorter disintegration time, then dry binder galen IQ 721 is more suitable use.
The study was supported by the grant MSM 0021620822 and by the firm PALATINIT GmbH Germany, which supplied the samples of the dry binders tested.
Address for correspondence:
PharmDr. Jitka Mužíková, Ph.D.
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmaceutical Technology, Czech Republic
Heyrovského 1203, 500 05 Hradec Králové
e-mail: muzikova@faf.cuni.cz
Received 22 July 2009
Accepted 17 August 2009
Zdroje
1. Bolhuis, G. K., Armstrong, N. A.: Pharm. Dev. Technol., 2006; 11, 111–124.
2. PALATINIT GmbH: galenIQTM – The smart excipient, Fir. Lit., 2007, Web: htpp://www.beneo-palatinit.com [1.11.2007].
3. Ndindayino, F., Henrist, D., Kiekens, F., Van den Mooter, G., Vervaet, C., Remon, J. P.: Int. J. Pharm., 2002; 235, 1–2, 149–157.
4. Fell, J. T., Newton, J. M.: J. Pharm. Sci., 1970; 59, 5, 688–691.
5. Bos, C. E., H. Bolhuis, H., Van Doorne, Lerk, C. F.: Pharm. Weekbl., 1987; Sci. Ed. 9, 274–282.
6. Belousov, V. A.: Khim. Farm. Zh., 1976; 10, 105–111.
7. Luhn, O.: Manuf. Chem., 2006; June: 36–38.
8. Bolhuis, G. K., Chowhan, Z. T.: Materials for direct compaction. In: Alderborn, G, Nystrőm, Ch. eds. Pharmaceutical Powder Compaction Technology. Inc.: New York: Marcel Dekker 1996; 486–500.
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2009 Číslo 4- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Přerušovaný půst může mít významná zdravotní rizika
-
Všechny články tohoto čísla
- Standard prescriptions for the formulation of medicinal preparations in pharmacies III Some possibilities of using isopropyl alcohol
- Cardioprotective effect of 2’,3,4’--trihydroxychalcone in preclinical experiment
- Determination of the coating thickness of HPMC hard capsules by near-infrared reflectance spectroscopy
- The role of flavonoid osajin in renal ischemia-reperfusion model
- Effects of combined hormonal deprivation and fungal elicitation on the production of coumarins in cell suspension cultures of Angelica archangelica L.
- Studies of the properties of tablets from directly compressible isomalt
- XLIX. sympozium z historie farmacie a veterinární medicíny
- Determination of the constituents of propolis of different geographical origin
- Z činnosti farmaceutických společností
- Na památku prof. RNDr. PhMr. Karla Paláta, CSc.
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Standard prescriptions for the formulation of medicinal preparations in pharmacies III Some possibilities of using isopropyl alcohol
- Determination of the constituents of propolis of different geographical origin
- Studies of the properties of tablets from directly compressible isomalt
- Determination of the coating thickness of HPMC hard capsules by near-infrared reflectance spectroscopy
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





