-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Death Be Not Proud—Cell Death Control in Plant Fungal Interactions
article has not abstract
Published in the journal: . PLoS Pathog 9(9): e32767. doi:10.1371/journal.ppat.1003542
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003542Summary
article has not abstract
While the concept of programmed cell death (PCD) or its morphological equivalent, apoptosis, was recognized in pockets of research prior to the 1970s, it was not until 1972 that Kerr, Wyllie, and Currie [1] first promulgated the phenomenon. It took nearly 20 years for the original idea to gain acceptance, thanks in large part to seminal studies conducted with Caenorhabditus elegans, which provided a solid genetic basis for these observations [2]. These studies also brought forth the idea that cell suicide is central to the life and well-being of multicellular organisms, and is neither uncommon nor normally detrimental to the organism.
Despite the simplicity and finality of the concept of death, the manifold processes by which cells die are not necessarily equivalent. While the role of cell death is firmly established in mammalian disease, it has not been as deeply characterized in other systems. In this Pearl article, we highlight several major ways in which cells die in the context of the high-stakes arms race between fungal pathogens and their plant hosts (Fig. 1).
Fig. 1. Cell death outcomes following fungal pathogen attack. 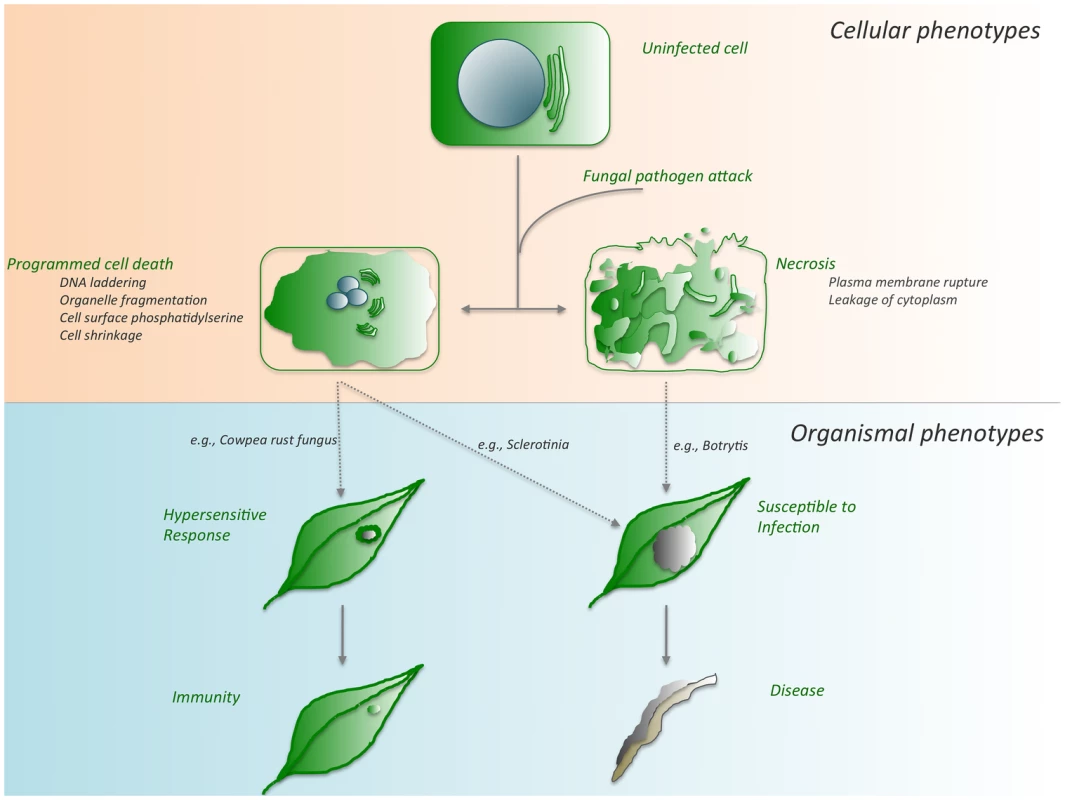
Depending on the genotypes of both the plant host and the fungal invader, any one of several cell death pathways can be activated. Although these pathways intersect in cell death, they culminate in disparate outcomes, immunity, or disease as detailed in the text. During the recognition of fungal challenge by the plant, host-controlled HR-PCD leads to a restricted cell death phenotype and ultimately immunity. Conversely, pathogen-mediated PCD suppresses this host recognition, leading to unrestricted spread of the pathogen accompanied by PCD, susceptibility, and disease. Some Have Called Thee Mighty and Dreadful [3]
The material reality of death can sometimes involve the transition of a mighty living system into dreadful chaos. The process of necrotic cell death exemplifies this notion, and is associated with organelle swelling, a complete disorganization and de-compartmentalization of cellular contents, cell lysis via rupture of the plasma membrane, and leakage of cellular debris into the environment. During necrosis, the dying cell has little to no genetic control of these events. In contrast, apoptotic PCD events unfold in a genetically controlled, highly orchestrated process that includes cell shrinkage, fragmentation and “laddering” of DNA, plasma membrane blebbing, and phosphatidylserine externalization. A hybrid phenomenon, termed “programmed necrosis” (necroptosis) has also been recently described [4]. In this physiologically and pathologically relevant process, first documented in mammalian systems [5], the observed necrotic cell death functions as a back-up system that is activated when the PCD machinery is impaired. In many cases, necroptosis is induced following the initial activation of the extrinsic (receptor-mediated) PCD pathway [6]. Elevations in reactive oxygen species (ROS) are typical of necroptotic cells [7]. As functional parallels exist between plant and animal PCD (see below), it is plausible that necroptosis may constitute a novel hub of plant PCD systems.
Pathways for apoptotic programmed cell death are relatively well characterized in mammals. This is in contrast to plants and phytopathogenic fungi where core regulatory elements of apoptosis pathways have not been found [8]. However, expression of mammalian apoptosis regulators confers phenotypes in plants and fungi that are consistent with their known functions [9]. In addition, numerous studies with baker's yeast (Saccharomyces cerevisiae) have shown that this organism exhibits several, but not all, of the hallmarks of apoptosis [10]. Production of ROS, chromatin condensation, DNA fragmentation, and externalization of phosphatidylserine have been shown in yeast, but there is not complete agreement as to whether “true” apoptosis occurs. As is the case in plants, yeast do not possess bona fide caspases that possess the precise structural characteristics that define these proteases. However, both yeast and plants harbor distantly related meta-caspases, identified computationally (for a lively discussion of the pros and cons of yeast PCD vs. mammalian PCD, see [10], [11]).
Finally, there have been several comparative studies that examined plant apoptotic-like PCD in the context of biotic and abiotic stress responses, senescence, or other aspects of plant growth and development [12]. Plant and animal cell death regimes clearly displayed differences. For example, plant cells have a rigid cell wall and lack caspases or phagocytic machinery. However, plant and animal PCD share important similarities, including chromatin condensation, DNA laddering, the generation of ROS, and the externalization of phosphatidylserine. Importantly, the underlying conceptual framework for PCD, ranging from development to pathogen attack to abiotic stress, is remarkably conserved for all eukaryotes. Thus, an analysis of cell suicide in plant and animal systems suggests that these processes are observed across kingdoms. For a more detailed discussion of this topic, see [13].
With Poison, War, and Sickness Dwell [3]
Many necrotrophic plant pathogenic fungi produce phytotoxic metabolites and peptides that play a central role in their pathogenic programs. These “poisons” are used by pathogens to attack their susceptible plant hosts. Phytotoxins can be non-host-specific and thus target a broad range of host plants, or host-specific and thus target a single plant species or even a particular cultivar within a given species [14]. However, while fungal toxins can be simply toxic, increasing evidence suggests that some toxins induce signaling that directs host pathways towards PCD, which exclusively benefits the fungus. For example, the filamentous ascomycete Cochliobolus victoriae is a necrotrophic fungal pathogen of Arabidopsis and oats and the causative agent of Victoria blight [7], a disease which decimated U.S. oat production in the 1940s. The fungal host–selective toxin victorin is a chlorinated cyclic pentapeptide that elicits several defense responses, PCD, and disease. In Arabidopsis, victorin targets a Nod-like NB-LRR-type resistance (R) protein, designated LOV1 [15]. This R protein must be present for fungal susceptibility. The fungus exploits R gene – mediated resistance to incite disease by inducing plant defense responses. Thus, the host R gene, which triggers cell death as a presumable defense, can function as both a resistance and a susceptibility factor. Taken together, the evidence indicates that death-inducing “poisons” can display subtlety in controlling the outcome of fungal pathogen–host interactions.
Thou Art Slave to Fate [3]
Host-controlled PCD is often required for defense against pathogens. Indeed, the well-studied plant PCD commonly known as the hypersensitive response (HR) is correlated with host defenses that serve to restrict pathogen growth. In the plant host, HR-PCD limits the spread of the pathogen, which is particularly relevant in the case of interactions between biotrophic pathogens and their plant hosts, where host-controlled cell death is correlated with resistance. Conversely, several fungal pathogens appear to promote host cell death, thereby subverting plant cell death pathways for fungal nutrient acquisition. Thus, programmed cell death is a common readout observed during both susceptible and resistant interactions. The eventual victor is decided by which side is in control of cell death, cellular context, and the activities of the combatants. Therefore, the way host cells die provides insight into the eventual fate of the fungal host interaction.
Poppies and Charms Can Make Us Sleep as Well [3]
The cellular mechanisms that can drive cell death are manifold. Two such “poppies and charms” that can lead to cell death are pyroptosis and autophagy. In animal systems, apoptosis can provide for the “clean” removal of dying cells, with limited induction of inflammatory responses. However, in pyroptosis, cell death is accompanied by the activation of inflammatory signaling. First noted in macrophages [16] and more recently reported in mammalian cells infected with viral or bacterial pathogens [17], pyroptosis typically engages the host inflammasome, a macromolecular complex that senses pathogen-associated molecular patterns (PAMPS) or danger-associated molecular patterns (DAMPS) to drive caspase-1 (and possibly caspase-11) dependent pro-inflammatory cascades [18]. Recently, these ideas have been extended to interactions between fungal pathogens and their susceptible plant hosts [19]. For example, while there are no bona fide caspases in plants, plant vacuolar processing enzyme gamma (VPEg) exhibits caspase 1-like activity in plant systems [20], [21]. Plant VPE protease activity is suppressed by caspase-1 specific inhibitors (xVAD-fmk) and is necessary for cell death mediation from a wide range of plant pathogens. The Fusarium verticilliodes toxin Fumonisin B1 requires the vacuolar processing enzyme (VPE) for PCD, as VPE mutants prevent mycotoxin-triggered death [22]. Similarly, the model oomycete pathogen, Hyaloperonospora arabidopsidis, induces VPE activity during infection of Arabidopsis [23]. This finding is somewhat surprising, as Hpa is a biotroph. It was proposed that increased VPE activity may benefit the pathogen by mediating protein turnover and nutrient release. The plant and animal enzymes, while having similar model substrate specificity, clearly display differences. Unlike mammalian caspase 1 which is localized in the cytosol, VPE is localized in the plant vacuole. Therefore, activation of VPE signaling and proteolysis contribute to vacuolar breakdown and apoptosis.
Autophagy can also promote cell death during interactions between fungal pathogens and their susceptible plant hosts. Autophagy was originally understood to be a process whereby starved cells cannibalize their organelles and cytosolic components to promote their survival. However, autophagy as a means for cell survival and homeostasis is now also appreciated as a mechanism to control interactions between fungal pathogens and plants.
The role of plant autophagy in response to fungal pathogens has been investigated in several studies; however, the mechanistic details are incomplete. Autophagy-defective Arabidopsis plants were more resistant to the biotrophic fungus Golovinomyces cichoracearum in a salicylic acid (SA)-dependent manner [24]. Increased resistance to the virulent biotrophic bacterial pathogen Pseudomonas syringae pv. tomato was also observed in plants harboring mutations in autophagy proteins [25], [26]. These studies suggest a negative effect of plant autophagy in resistance responses toward biotrophic pathogens and are in contrast to N-mediated resistance to tobacco mosaic virus (TMV), which requires functional autophagic machinery for an effective (HR-PCD) defense response [27]. Thus, in various host pathogen interactions, the consequences of host autophagy-mediated PCD can be diverse.
Although the effect of autophagy on biotrophic interactions is complex and perhaps indirect in its relationship to disease, necrotrophic organisms feed off of dead cells and thus it might be expected that conditions favorable to cell death would promote nectrotrophic growth. Indeed, Arabidopsis ATG5, ATG10, and ATG18a mutants developed spreading necrosis and enhanced disease susceptibility upon infection with necrotrophic fungus Alternaria brassicicola [25], [26]. This includes elevated ROS, which appeared in noninfected regions as well. Similar runaway cell death symptoms were observed in the autophagy mutants after application of fumonisin B1. In contrast, no difference in growth characteristics was observed for the obligate biotroph H. arabidopsidis [25]. Thus, runaway (unrestricted) cell death in the mutants indicates that autophagy plays an anti-death role, perhaps by limiting ROS effects and restricting pathogen spread. Indeed, elevated spontaneous ROS accumulation is readily observed in autophagy mutants [28]. Further support for susceptibility to nectrotrophic lifestyles was shown by enhanced rates of infection by the necrotrophic pathogen Botrytis cinerea in AtATG18a mutants [29]. Not unexpectedly, the necrotrophic pathogen B. cinerea has been reported to benefit from PCD [30]. Thus, cell death induced by autophagy might be expected to result in enhanced fungal growth. It is reasonable that a necrotroph would benefit from a local environment of dead tissue. However, this is clearly not the case, as B. cinerea triggers autophagous cell death, resulting in a restricted growth phenotype (Dickman, unpublished observations). We interpret these observations to indicate that the process by which host cells die (i.e., the means by which death occurs) is key for determining the ultimate outcome of interactions between host and pathogen [31].
And Death Shall Be No More; Death, Thou Shalt Die [3]
The struggle between plant hosts and their fungal pathogens to control the mechanisms by which host cells die can have profound effects on the ultimate outcome of the interaction. With this idea in mind, it is notable that, to date, very few examples of fungal anti-death factors have been described. It has been shown that B. cinerea undergoes massive PCD following penetration of the host plant and establishment of a primary necrotic lesion [32]. The host defense molecule camalexin was shown to be capable of triggering PCD in B. cinerea. In response, the fungus suppresses this host-induced PCD to establish infection. Despite these intriguing observations, the anti-death factors that mediate the suppression of host PCD in this system have yet to be identified. The identification of factors that suppress host cell death in necrotrophic fungal pathosystems (e.g., see [33]) constitutes an important line of future investigation.
Zdroje
1. KerrJF, WyllieAH, CurrieAR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26 (4) 239–257.
2. EllisRE, YuanJY, HorvitzHR (1991) Mechanisms and functions of cell death. Annu Rev Cell Biol 7 : 663–698.
3. Donne J (1633) “Death Be Not Proud” (Holy Sonnet XI of XIX).
4. ChristoffersonDE, YuanJ (2010) Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol 22 (2) 263–268.
5. KaczmarekA, VandenabeeleP, KryskoDV (2013) Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38 (2) 209–223.
6. ChaabaneW, et al. (2013) Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp (Warsz) 61 (1) 43–58.
7. ZhouZ, HanV, HanJ (2012) New components of the necroptotic pathway. Protein Cell 3 (11) 811–817.
8. Dickman MB, Reed JC (2003) Paradigms for programmed cell death in animals and plants. In: Programmed Cell Death in Plants, ed. J Gray, pp.26–43. Blackwell, Oxford.
9. DickmanMB, ParkYK, OltersdorfT, LiW, ClementeT, FrenchR (2001) Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc Natl Acad Sci U S A 98 : 6957–6962.
10. Carmona-GutierrezD, FrohlichKU, KroemerG, MadeoF (2010) Metacaspases are caspases. Doubt no more. Cell Death Differ 17 : 377–78.
11. EnokssonM, SalvesenGS (2010) Metacaspases are not caspases–always doubt. Cell Death Differ 17 : 1221.
12. WilliamsB, DickmanM (2008) Plant programmed cell death: can't live with it; can't live without it. Mol Plant Pathol 9 : 531–544.
13. DickmanMB, FluhrR (2013) Centrality of host cell death in plant-microbe interactions. Annu Rev Phytopathol 51 : 25.1–25.28.
14. WolpertTJ, DunkleLD, CiuffettiLM (2002) Host-selective toxins and avirulence determinants: what's in a name? Annu Rev Phytopathol 40 : 251–285.
15. LorangJ, LorangJ, KidarsaT, BradfordCS, GilbertB, et al. (2012) Tricking the guard: exploiting plant defense for disease susceptibility. Science 338 (6107) 659–662.
16. BergsbakenT, FinkSL, CooksonBT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7 (2) 99–109.
17. Rosales-ReyesR, Pérez-LópezA, Sánchez-GómezC, Hernández-MoteRR, Castro-EguiluzD, et al. (2012) Salmonella infects B cells by macropinocytosis and formation of spacious phagosomes but does not induce pyroptosis in favor of its survival. Microb Pathog 52 (6) 367–374.
18. BrozP, RubyT, BelhocineK, BouleyDM, KayagakiN, et al. (2012) Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490 (7419) 288–291.
19. Hara-NishimuraI, HatsugaiN (2011) The role of vacuole in plant cell death. Cell Death Differ 18 (8) 1298–1304.
20. HatsugaiN, KuroyanagiM, YamadaK, MeshiT, TsudaS, et al. (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305 (5685) 855–858.
21. RojoE, MartínR, CarterC, ZouharJ, PanS, et al. (2004) VPEgamma exhibits a caspase-like activity that contributes to defense against pathogens. Curr Biol 14 (21) 1897–1906.
22. ZhangH, DongS, WangM, WangW, SongW, et al. (2010) The role of vacuolar processing enzyme (VPE) from Nicotiana benthamiana in the elicitor-triggered hypersensitive response and stomatal closure. J Exp Bot 61 (13) 3799–3812.
23. Misas-VillamilJC, ToengesG, KolodziejekI, SadaghianiAM, KaschaniF, et al. (2013) Activity profiling of vacuolar processing enzymes reveals a role for VPE during oomycete infection. Plant J 73 (4) 689–700.
24. WangY, NishimuraMT, ZhaoT, TangD (2011) ATG2, an autophagy-related protein, negatively affects powdery mildew resistance and mildew-induced cell death in Arabidopsis. Plant J 68 (1) 74–87.
25. LenzHD, HallerE, MelzerE, GustAA, NurnbergerT (2011) Autophagy controls plant basal immunity in a pathogenic lifestyle-dependent manner. Autophagy 7 (7) 773–774.
26. LenzHD, HallerE, MelzerE, KoberK, WursterK, et al. (2011) Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J 66 (5) 818–830.
27. LiuY, SchiffM, CzymmekK, TalloczyZ, LevineB, Dinesh-KumarSP (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell 20;121 (4) 567–77.
28. YoshimotoK, JikumaruY, KamiyaY, KusanoM, ConsonniC, et al. (2009) Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21 (9) 2914–2927.
29. LaiZ, WangF, ZhengZ, FanB, ChenZ (2011) A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J 66 (6) 953–968.
30. GovrinEM, LevineA (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10 (13) 751–757.
31. KabbageM, WilliamsB, DickmanM (2013) Cell death control: The interplay of apoptosis and autophagy in the pathogenicity of Sclerotinia sclerotiorum. PLoS Pathog 9: e100328 doi:10.1371/journal.ppat.1003287
32. ShlezingerN, MinzA, GurY, HatamI, DagdasYF, et al. (2011) Anti-apoptotic machinery protects the necrotrophic fungus Botrytis cinerea from host-induced apoptotic-like cell death during plant infection. PLoS Pathog 7 (8) e1002185 doi:10.1371/journal.ppat.1002185
33. ZhuW, WeiW, FuY, ChengJ, XieJ, et al. (2013) A secretory protein of necrotrophic fungus Sclerotinia sclerotiorum that suppresses host resistance. PLoS ONE 8 (1) e53901 doi:10.1371/journal.pone.0053901
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- První vakcína proti klíšťové encefalitidě: vakcína FSME-IMMUN
-
Všechny články tohoto čísla
- Cross-Serotype Immunity Induced by Immunization with a Conserved Rhinovirus Capsid Protein
- The CLIP-Domain Serine Protease Homolog SPCLIP1 Regulates Complement Recruitment to Microbial Surfaces in the Malaria Mosquito
- Aggressive Chemotherapy and the Selection of Drug Resistant Pathogens
- Host Adaptation Is Contingent upon the Infection Route Taken by Pathogens
- Acute Neonatal Infections ‘Lock-In’ a Suboptimal CD8+ T Cell Repertoire with Impaired Recall Responses
- Lymph Node Colonization Dynamics after Oral Typhimurium Infection in Mice
- Highly Significant Antiviral Activity of HIV-1 LTR-Specific Tre-Recombinase in Humanized Mice
- Emerging and Emerged Pathogenic Species: Beyond the Paradigm
- Cross-Seeding of Misfolded Proteins: Implications for Etiology and Pathogenesis of Protein Misfolding Diseases
- Emergence of the Middle East Respiratory Syndrome Coronavirus
- Memory of Infections: An Emerging Role for Natural Killer Cells
- Death Be Not Proud—Cell Death Control in Plant Fungal Interactions
- Self and Non-self Discrimination of Intracellular Membranes by the Innate Immune System
- Innate Immune Sensing of Flaviviruses
- Bringing Culture to the Uncultured: and Lessons for Obligate Intracellular Bacterial Pathogens
- Atomic Force Microscopy: A New Look at Pathogens
- Methionine Biosynthesis in Is Tightly Controlled by a Hierarchical Network Involving an Initiator tRNA-Specific T-box Riboswitch
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Memory of Infections: An Emerging Role for Natural Killer Cells
- Emergence of the Middle East Respiratory Syndrome Coronavirus
- Emerging and Emerged Pathogenic Species: Beyond the Paradigm
- Death Be Not Proud—Cell Death Control in Plant Fungal Interactions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání


