-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Low-intensity cognitive-behaviour therapy interventions for obsessive-compulsive disorder compared to waiting list for therapist-led cognitive-behaviour therapy: 3-arm randomised controlled trial of clinical effectiveness
Karina Lovell and colleagues reveal that low-intensity computerised cognitive behaviour therapy provides no clinical benefit to OCD patients.
Published in the journal: . PLoS Med 14(6): e32767. doi:10.1371/journal.pmed.1002337
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002337Summary
Karina Lovell and colleagues reveal that low-intensity computerised cognitive behaviour therapy provides no clinical benefit to OCD patients.
Introduction
Obsessive-compulsive disorder (OCD) has an estimated lifetime prevalence of 2%–3% and is rated among the top 10 causes of disability worldwide, with an estimated US$8.4 billion attributable to OCD in the United States [1]. Providing accessible and effective care for OCD is a priority worldwide.
However, there is evidence that people with OCD struggle to access treatment, with consistent reports of a marked delay between OCD onset and management. One study found a 10-year gap between onset and seeking help and 17 years between onset and receiving effective help [2].
In OCD, both medication and psychological therapy are effective, with the “gold standard” psychological therapy intervention being therapist-led cognitive-behaviour therapy (CBT) [3], with 1-hour weekly sessions delivered predominantly face-to-face over 12–16 weeks. However, it is relatively costly, and the limited availability of specialist therapists means that access can be poor, with long waiting times. Additionally, OCD is characterised by intrusive, unwanted, recurrent, and distressing thoughts, images, or impulses (i.e., obsessions) and repetitive actions or rituals (compulsions). These obsessions and compulsions can make it more difficult for patients to engage with treatment because of fears of contamination or causing harm to others.
Conventional ways of delivering psychological therapy are being challenged. Health systems under financial pressure need to manage demand more effectively through new methods of delivery [4], and innovation is needed to meet the needs of diverse patient populations with complex needs. Research has shown how conventional therapist-led CBT can be delivered effectively in low-intensity forms including guided self-help (written CBT materials with limited telephone or face-to-face support) or computerised CBT (cCBT; web-based CBT materials and limited telephone support). Both forms are potentially cheaper and more accessible than conventional therapist-led CBT and demonstrate evidence of effectiveness in a range of disorders [5,6]. Low-intensity CBT interventions for OCD may provide more rapid relief of symptoms, reduce the need for more expensive therapist-led CBT, and encourage more efficient use of healthcare resources when delivered as part of a stepped care system [7].
At the time this study was commissioned, the evidence base for low-intensity interventions in OCD was far from definitive. Much of the evidence for guided self-help was based on small open or uncontrolled studies [8,9] or comparisons of nonguided self-help with guided self-help [10]. A systematic review of cCBT for OCD found only 4 studies [11]. cCBT reduced rituals and obsessions and improved functioning and was more effective than attention control [12], but not as effective as therapist-led CBT. Clearly, the potential benefits of both guided self-help and cCBT need to be demonstrated in large-scale trials.
We conducted a randomised trial for patients with OCD, allocating patients to either (a) guided self-help prior to therapist-led CBT, (b) cCBT prior to therapist-led CBT, or (c) a waiting list for therapist-led CBT only. We aimed to provide a definitive answer to the following questions:
Does access to guided self-help or cCBT provide more rapid improvement in OCD symptoms at 3 months compared to a waiting list for therapist-led CBT?
Does access to guided self-help or cCBT improve patient satisfaction at 3 months compared to a waiting list for therapist-led CBT?
Does access to guided self-help or cCBT prior to therapist-led CBT provide longer-term improvement in OCD symptoms at 12 months compared to therapist-led CBT alone?
Does access to guided self-help or cCBT reduce uptake of therapist-led CBT over 12 months?
Methods
This study was approved by the National Research Ethics Service Committee North West–Lancaster (reference number 11/NW/0276). All participants provided informed consent to take part in the trial.
Study design and participants
We conducted a pragmatic trial, delivered in routine service settings, to provide a balance between internal and external validity and maximise relevance to clinical guidelines [13,14]. The study protocol has been published [15]. The study is reported as per Consolidated Standards of Reporting Trials (CONSORT) guidelines, as described in the CONSORT checklist (S2 Text). Potential participants were most frequently identified by administrative and clinical staff in primary-and secondary-care screening waiting lists, although self-referral was used at 1 site to increase recruitment (via adverts in newspapers, community facilities, and social media). Potential participants were provided with an information pack. Those who provided consent to contact took part in a telephone eligibility screen to determine if they were over 18 years old and not currently receiving a psychological therapy for OCD or experiencing severe and distressing psychotic symptoms. Participants passing the initial screen were offered a face-to-face eligibility appointment.
We included patients who were (1) aged 18+ years, (2) able to read English, (3) currently waiting for access to therapist-led CBT, (4) meeting DSM-IV criteria for OCD (assessed using the Mini-International Neuropsychiatric Interview [16]), and (5) scoring 16+ on the Yale-Brown Obsessive Compulsive Checklist–Self-Report (Y-BOCS-SR [17]).
We excluded patients (1) experiencing active suicidal or psychotic thoughts, (2) meeting DSM-IV alcohol or substance dependence criteria, (3) receiving psychological treatment for OCD, or (4) with language difficulties that would preclude participation.
Randomisation and masking
Patients were randomised (ratio 1 : 1:1) via a secure web system independently administered by a trials unit to ensure concealment of allocation. Allocation was minimised on OCD severity, OCD duration, antidepressant use, and depression severity. It was not possible to mask participants or clinical staff to treatment allocation. Research staff undertaking assessments were masked to allocation: unmasking was reported in 30%, 22%, and 26% of the 3, 6, and 12 month interviews, respectively. When this occurred, subsequent assessments were done by another researcher to limit bias.
Procedures
cCBT was delivered using OCFighter (www.ccbt.co.uk), a commercial OCD program. OCFighter involves 9 steps (focussed on exposure and response prevention) to help people with OCD carry out treatment and monitor progress. Participants received a secure login and were advised to use cCBT at least 6 times over 12 weeks. Participants received six 10-minute telephone calls, for risk assessment, progress review, and problem solving.
Guided self-help was delivered using the book Obsessive Compulsive Disorder: A Self-Help Book [18], which is focussed on exposure and response prevention. Participants received weekly guidance, with an initial session (60 minutes face to face or by telephone, dependent on patient preference) followed by 10 30-minute sessions over 12 weeks. The support involved an explanation of the workbook, help devising goals, risk assessment, support for conducting CBT homework, progress review, and problem solving.
Support for both cCBT and guided self-help was provided by “psychological well-being practitioners.” These are graduates with no prior clinical qualifications who receive 12 months training and who are responsible for delivering guided self-help CBT and general education for anxiety and depression in England. Most have limited OCD-specific training. They were trained in both interventions over 3 days by the research team (with additional support from the company supplying cCBT). All staff received telephone supervision every fortnight from the research team or from experienced therapists within routine services. In total, 93 psychological well-being practitioners managed patients in the trial (range 1–18 patients), with 46 practitioners allocated patients in both interventions. Psychological well-practitioner characteristics are reported in Table A in S1 Appendix.
Psychological well-being practitioners recorded dates, length, and mode of contact for all sessions and were asked to record sessions to examine fidelity. We also received automated recordings of cCBT use. Fidelity was evaluated by an independent rater blind to outcome. We defined tasks to be carried out in both interventions, which were coded from recordings as “implicit,” “explicit,” or “absent,” and an overall rating was generated using a 5-point scale (“unacceptable” to “excellent”).
The comparator was waiting list for therapist-led CBT. As the trial was a pragmatic design within routine services, we were unable to mandate a waiting period for therapist-led CBT. We expected that most patients would start therapist-led CBT 3–6 months following allocation, after receiving their low-intensity interventions (where allocated). Therapist-led CBT was typically 8–20 face-to-face, 45–60-minute weekly sessions.
In this pragmatic trial, we placed no restrictions on treatment after randomisation. Before seeing a therapist, patients on waiting lists sometimes received interventions other than our low-intensity interventions, including education, medication, or nonspecific interventions (such as “stress management”). All additional care outside the trial protocol was recorded as part of the economic evaluation.
Clinical outcomes
We conducted follow-up assessments at 3 months (primary outcome timepoint), 6 months, and 12 months following randomisation. The primary outcome measure, Yale-Brown Obsessive Compulsive Scale–Observer Rated (Y-BOCS-OR) [17], was collected face to face at baseline. At follow-up time points where face-to-face collection was not possible following a highly structured standardised operating procedure, telephone or postal assessment using the Y-BOCS-SR was attempted.
The Y-BOCS-OR is an interview-administered structured assessment that provides an indication of OCD symptom severity. It consists of 2 comprehensive symptom checklists exploring current and past symptoms of obsession and compulsion (over the past week and past symptoms) and a 10-item severity scale exploring current obsessive and compulsive symptoms. Impairment over 5 clinical domains is identified: time consumed, functional impairment, psychological distress, efforts to resist, and perceived sense of control on a 5-point Likert scale from 0 (none) to 4 (extreme). Scores from the 10 items are summed to identify level of severity (0–7 subclinical, 8–15 mild, 16–23 moderate, 24–31 severe, and 32–40 extreme).
Secondary outcomes were collected at baseline and at the 3, 6, and 12-month follow-up. Outcomes included the Y-BOCS-SR, a self-report version of the Y-BOCS-OR scale. When it was not possible to complete the Y-BOCS-OR, the Y-BOCS-SR was used as a proxy.
Other secondary outcomes (all self-report) included the Short Form-36 (SF-36) [19] for health-related quality of life; Clinical Outcomes in Routine Evaluation (CORE-OM) [20] for distress; Patient Health Questionnaire (PHQ-9) [21] for depression; Generalized Anxiety Disorder 7-item (GAD-7) scale [22] for generalised anxiety disorder; Work and Social Adjustment Scale (WSAS) [23] for functional impairment; IAPT Employment Status Questions (A13-A14) [24] for employment rates and receipt of statutory sick pay; and the Client Satisfaction Questionnaire (CSQ-8-UK) [25] for satisfaction. Comorbidities (Clinical Interview Schedule-Revised; CIS-R) [26] and demographics were collected at baseline only.
Statistical analysis
With 3 pair-wise comparisons, alpha was set at 1.67%. We assumed a standard deviation for the primary outcome of 7.3, a correlation between baseline and follow up of 0.43 [27], and a therapist intracluster correlation (ICC) between therapists (0.06) and within therapist (0.015). Assuming 85% retention, 432 patients were required. Trial monitoring suggested a lower follow-up rate, and thus, the sample size was increased to 473 to retain power. In total, 366 patients at follow-up provided power greater than 80% to detect a clinically significant difference of 3 Y-BOCS points for each comparison.
Preliminary modelling determined methods for handling missing data (full details provided in the statistical analysis plan: https://dx.doi.org/10.6084/m9.figshare.3503885). There was no deviation from the prespecified plan, all analyses were conducted, and those not present in this manuscript are reported in the Health Technology Assessment (HTA) report [28]. Missing baseline covariates were imputed by single imputation [29] using other covariates. Analyses of the primary outcome were based on a linear mixed model with random effects for psychological well-being practitioners. As practitioners were crossed with treatment, correlated random effects were included for each treatment, enabling estimation of the ICC for cCBT and guided self-help. We included the following covariates: OCD duration and severity; anxiety, depression, antidepressant use; and gender. Binary outcomes (e.g., uptake of therapist-led CBT) used logistic regression to estimate adjusted odds ratios (ORs), with the same baseline covariates. Analysis used intention-to-treat subject to the availability of data. Distributional assumptions of the models were checked. All outcomes included in the Obsessive Compulsive Treatment Efficacy Trial (OCTET) protocol are detailed.
Patient involvement
Patients and members of the public were involved throughout the trial, including the design, management, and conduct of the trial. From the outset, the chief executive of a national user-led organisation (Anxiety UK) was involved as a coapplicant and collaborator. Members of an OCD self-help group assisted with the development of the guided self-help manual and adaptations to one of the trial outcome measures. A service user with OCD was a member of the trial steering committee, while another conducted some of the patient acceptability interviews. The findings have been disseminated to trial participants, and the results presented at a national user conference.
Results
Recruitment, retention, and baseline characteristics
We opened recruitment in 15 clinical sites in England between February 2011 and May 2014, with the last follow-up in May 2015. There were 2 postrandomisation exclusions: one aged under 18 years and one at risk of suicide. In total, 473 eligible patients were randomised (see Fig 1). Baseline sociodemographic characteristics are presented in Table 1, with baseline clinical data presented in Table 2. Data were indicative of severe OCD, mild-to-moderate depression, and moderate anxiety. Just over half reported previous professional help with OCD, and around half were using antidepressants. More than half reported OCD for more than 10 years. There were no substantial differences at baseline.
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flow chart illustrating recruitment. 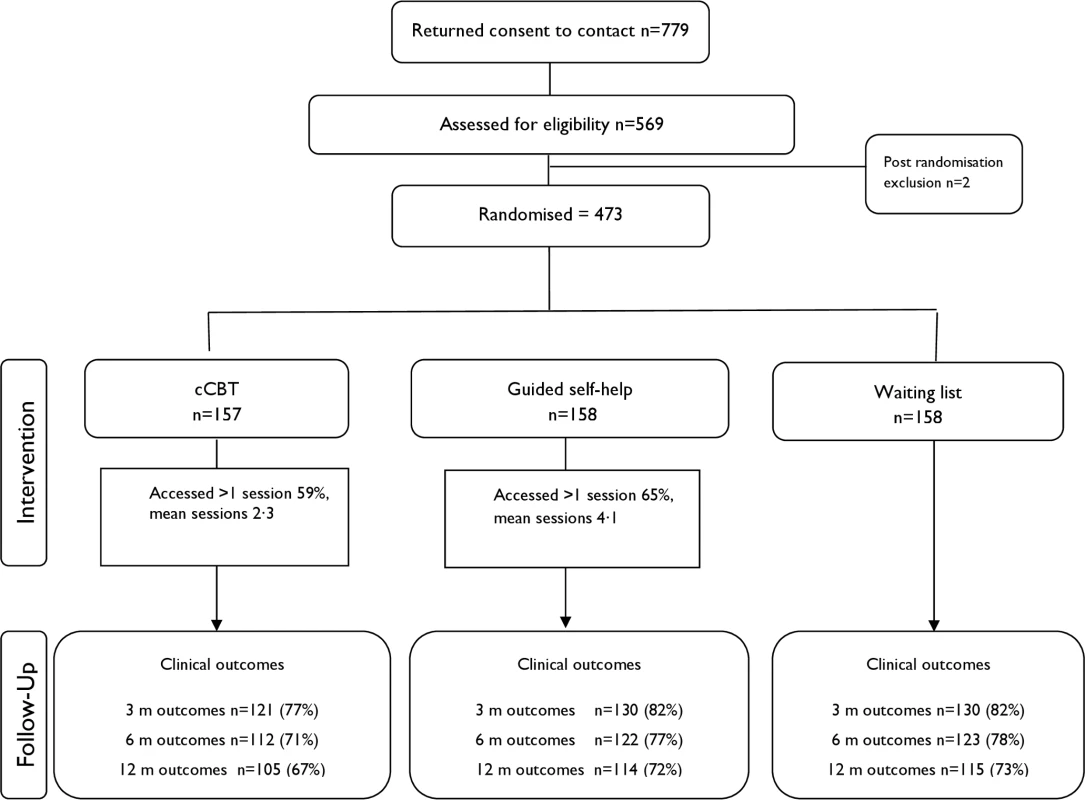
Abbreviations: cCBT, computerised cognitive-behaviour therapy; m, month; QALY, quality-adjusted life year. Tab. 1. Baseline sociodemographic variables. 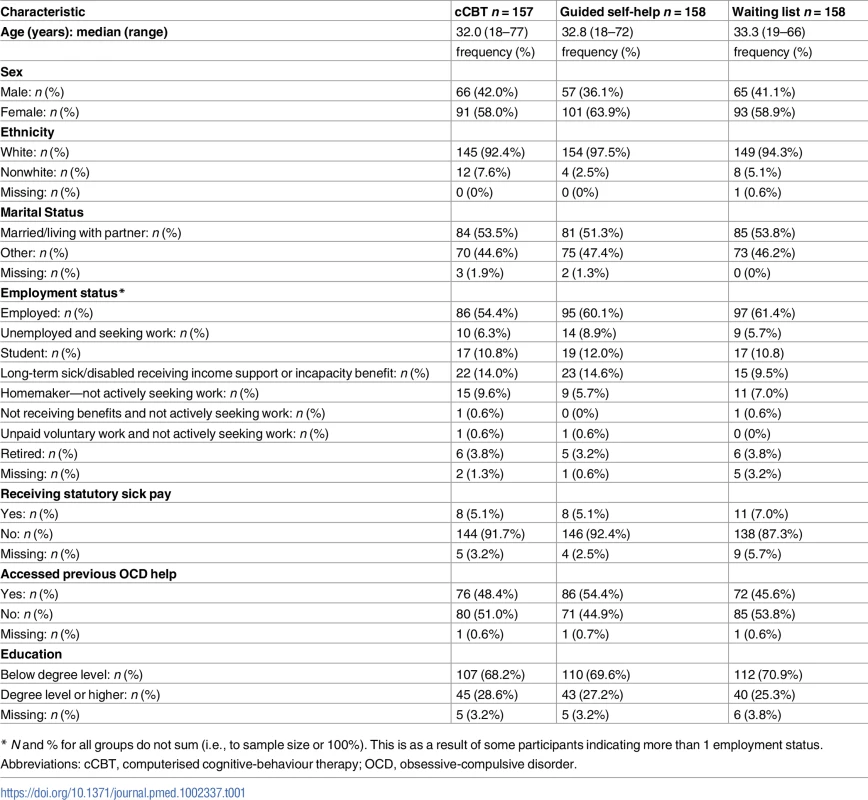
* N and % for all groups do not sum (i.e., to sample size or 100%). This is as a result of some participants indicating more than 1 employment status. Tab. 2. Baseline clinical characteristics. 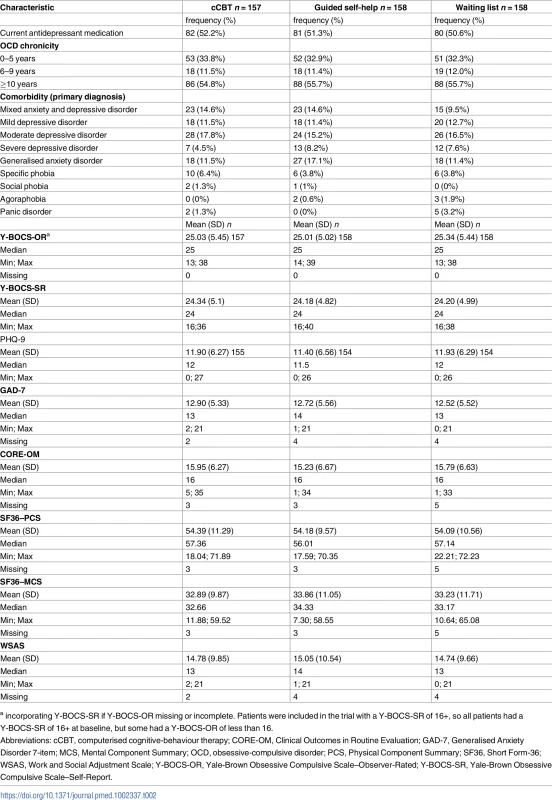
a incorporating Y-BOCS-SR if Y-BOCS-OR missing or incomplete. Patients were included in the trial with a Y-BOCS-SR of 16+, so all patients had a Y-BOCS-SR of 16+ at baseline, but some had a Y-BOCS-OR of less than 16. Treatment fidelity and adherence
Patient flow is shown in Figs 1 and 2. Retention rates were 81% at 3 months, 75% at 6 months, and 71% at 12 months and were broadly similar across arms (Fig 1). Contrary to expectation, approximately 29% of patients started to access therapist-led CBT prior to the 3-month assessment (Fig 2). More detailed data on CBT uptake and predictors of uptake are detailed separately (Tables B and C in S1 Appendix).
Fig. 2. Flow chart illustrating therapist-led CBT uptake. 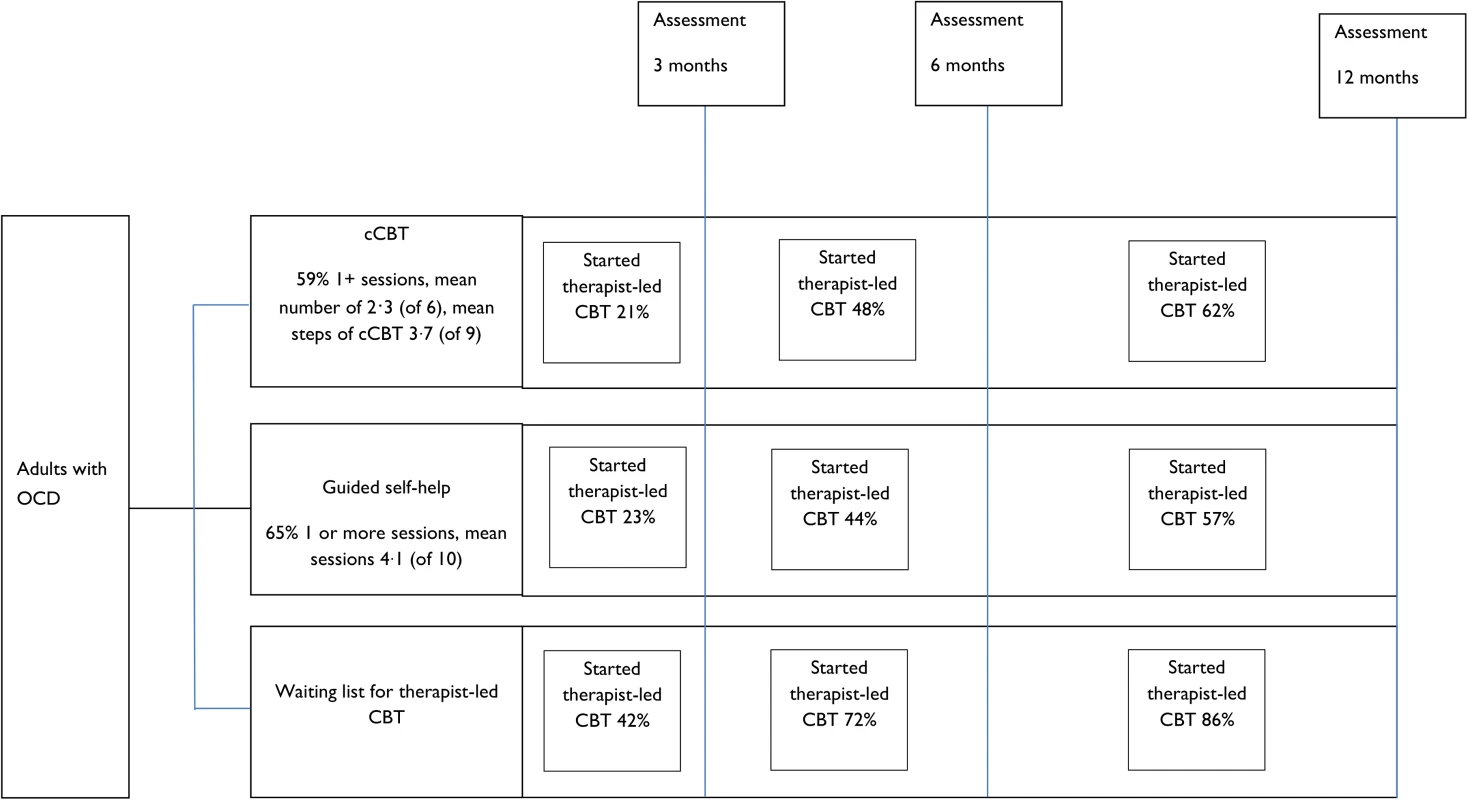
Abbreviations: cCBT, computerised cognitive-behaviour therapy; OCD, obsessive-compulsive disorder. Fifty-nine percent of participants had at least 1 session with a psychological well-being practitioner in cCBT. The mean number of sessions was 2.3 (SD 2.5), and the average length was 13.4 minutes (93% by telephone). Of the 9 cCBT steps, the mean number completed was 3.7 (SD 3.2). Sixty-five percent of participants had at least 1 session with a psychological well-being practitioner in guided self-help. The mean number of sessions was 4.1 (SD 4.3) over 57 minutes for session 1 and 31 minutes for sessions 2–11 (48% face-to-face, 26% telephone, 22% both, 4% missing). Rates of recording for fidelity assessment were low (26% guided self-help, 17% cCBT). Of the sessions recorded in cCBT, 11 (65%) were rated “good” and 6 (35%) as “excellent.” Of the sessions in guided self-help, 9 (21%) were rated “satisfactory,” 24 (56%) rated “good,” and 10 (23%) “excellent.”
Table 3 gives the summary statistics for the primary (Y-BOCS-OR) and selected secondary outcomes (Y-BOCS-SR, CSQ-8, and EuroQol five dimensions questionnaire [EQ-5D]). Complete outcomes are reported separately (Table D in S1 Appendix).
Tab. 3. Outcome measure summary statistics and intervention effects at 3, 6, and 12 months. 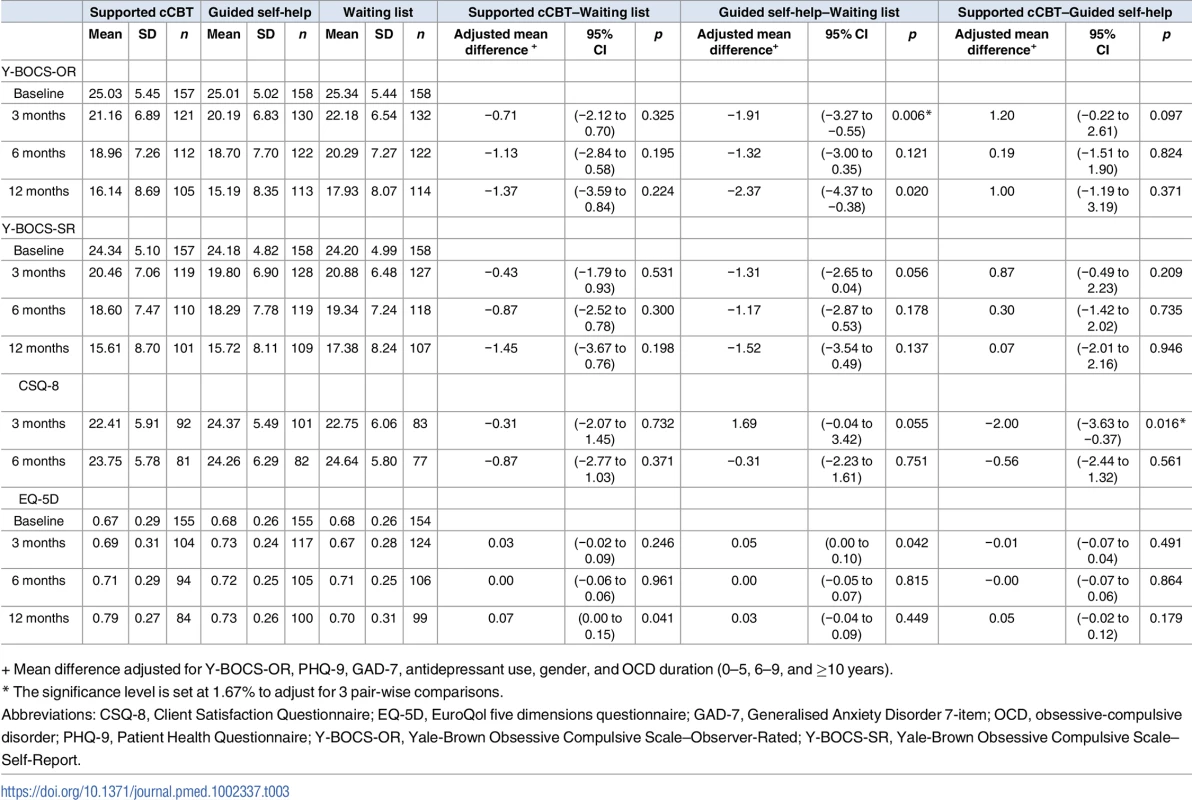
+ Mean difference adjusted for Y-BOCS-OR, PHQ-9, GAD-7, antidepressant use, gender, and OCD duration (0–5, 6–9, and ≥10 years). Does access to guided self-help or cCBT provide more rapid improvement in OCD symptoms at 3 months compared to a waiting list for therapist-led CBT?
There was no significant benefit of access to cCBT (adjusted mean difference = −0.71, 95% CI −2.12 to 0.70, p = 0.325). There was statistically significant benefit of guided self-help (adjusted mean difference = −1.91, 95% CI −3.27 to −0.55, p = 0.006), although the effect was less than the prespecified “clinically important difference” of 3 points.
Analyses of secondary outcomes (Tables E, F, and G in S1 Appendix) showed only 1 significant difference, an effect of cCBT on anxiety (adjusted mean difference = −1.50, 95% CI −2.67 to −0.33, p = 0.012).
Does access to guided self-help or cCBT improve patient satisfaction at 3 months compared to a waiting list for therapist-led CBT?
Satisfaction data are shown in Table 3. There were no differences in patient satisfaction among patients receiving cCBT compared to those allocated to a waiting list (adjusted mean difference = −0.31, 95% CI −2.07 to 1.45, p = 0.732). Patients receiving guided self-help tended to be more satisfied than those allocated to a waiting list for therapist-led CBT (adjusted mean difference = 1.69, 95% CI −0.04 to 3.42, p = 0.055), although the estimate did not reach significance according to the corrected significance level. Patients receiving cCBT were less satisfied than those receiving guided self-help (adjusted mean difference = −2.00, 95% CI −3.63 to −0.37, p = 0.016).
Does access to guided self-help or cCBT prior to therapist-led CBT provide longer-term improvement in OCD symptoms at 12 months compared to therapist-led CBT alone?
There was no significant long-term benefit from access to either guided self-help or cCBT (cCBT adjusted mean difference = −1.37, 95% CI −3.59 to 0.84, p = 0.224; guided self-help adjusted mean difference −2.37, 95% CI −4.37 to −0.38, p = 0.02; Table 2).
As a post hoc analysis, we tested whether low-intensity interventions were formally noninferior to waiting list for therapist-led CBT at 12 months. A 98.33% confidence interval corresponds to a 1.67% significance level that we have used for hypothesis testing. For the comparison of cCBT against waiting list, the 98.33% confidence interval is −4.07 to 1.33, and for guided self-help against waiting list, it is −4.81 to 0.06. Given that the upper limits are substantially smaller than the prespecified criterion for a clinically important difference (3 points), we conclude that both interventions are noninferior to waiting list at 12 months.
Does access to guided self-help or cCBT reduce uptake of therapist-led CBT over 12 months?
Therapist-led CBT uptake is shown in Fig 2. Both interventions were associated with significantly lower uptake of therapist-led CBT at 12 months (cCBT adjusted OR = 0.34, 95% CI 0.15 to 0.79 p = 0.011; guided self-help adjusted OR = 0.27, 95% CI 0.12 to 0.60 p = 0.001) (Table 4).
Tab. 4. Logistic regression model for CBT uptake at 6 and 12 months. 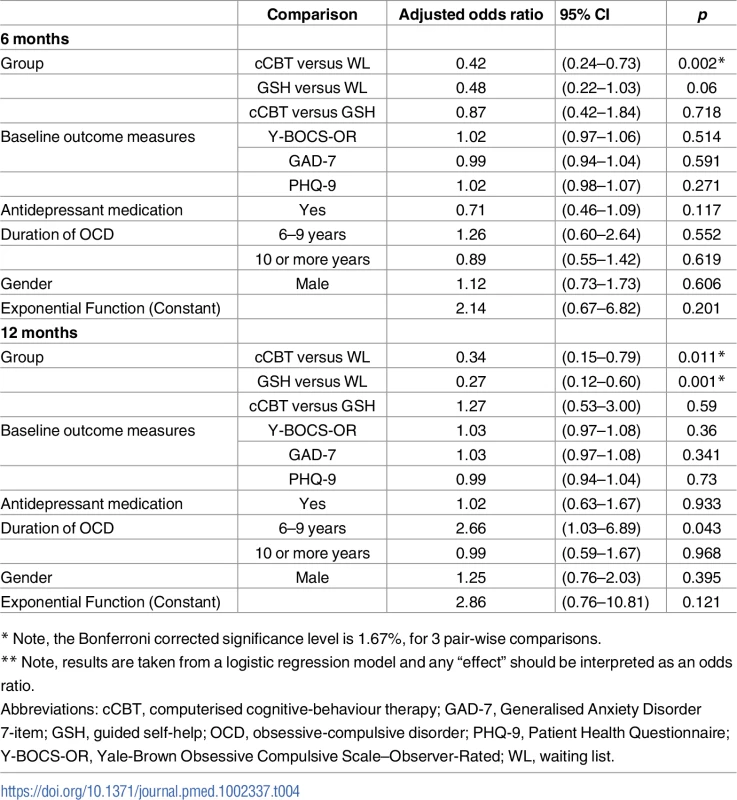
* Note, the Bonferroni corrected significance level is 1.67%, for 3 pair-wise comparisons. Post hoc, we compared intervention use and 12-month OCD outcomes among guided self-help and cCBT patients who did and did not access therapist-led CBT (Table H in S1 Appendix). Although lacking randomisation, the data do not suggest that those who accessed only guided self-help or cCBT demonstrated markedly worse outcomes than those who accessed both a low-intensity intervention and therapist-led CBT (Table I in S1 Appendix).
Discussion
Principal outcomes
We assessed the role of 2 low-intensity interventions (guided self-help and cCBT) in OCD. Prior to access to therapist-led CBT, guided self-help demonstrated statistically significant benefits over the waiting list, but the difference did not meet the prespecified criterion for clinical significance. cCBT did not demonstrate significant benefit at the 3 - or 12-month follow-up. Access to low-intensity interventions does not provide more rapid symptom relief.
Over 12 months, access to low-intensity interventions prior to therapist-led CBT did not significantly augment the effects of therapist-led CBT on OCD symptoms in the longer term. Rapid access to low-intensity interventions did lead to significant reductions in uptake of therapist-led CBT, which did not compromise patient outcomes at 12 months.
Strengths and limitations
To our knowledge, we conducted the largest trial of psychological therapy for OCD worldwide. We achieved acceptable levels of retention. When patients were not able to provide the primary clinical outcome using the observer-reported version, we used self-report as a proxy. These different measures show high associations [30,31], with some evidence of lower scores in the self-reported version, but proxy measures were only used in 8% and 11% of cases at 3 and 12 months, with minimal differences in rates of use between arms. In this pragmatic trial, recruitment was over multiple sites and involved a large number of psychological well-being practitioners. This enhances external validity, as delivery was not restricted to a small number of specialised sites or highly selected professionals. However, many psychological well-being practitioners only saw a few patients, which restricted the opportunity to practice their skills. Uptake of the interventions was reasonable (65% guided self-help and 59% cCBT). Collecting detailed data on fidelity proved difficult, but analysis of the data provided some evidence that delivery of guided self-help and cCBT was in line with protocols.
Several issues are worth noting in this pragmatic design. First, we did not mandate a defined waiting time for therapist-led CBT, although the expectation was 3–6 months. In practice, around 40% of patients allocated to a waiting list for therapist-led CBT started to receive some contact with their therapist before 3 months, compared with just over 20% in the guided self-help and cCBT groups. This would reduce differences in outcomes between guided self-help, cCBT, and the waiting list comparator at 3 months, leading to conservative estimates of effect. Still, these data refer to patients receiving any contact with therapist-led CBT, which in most cases would involve an initial session or two, rather than a full “dose” of treatment. Nevertheless, relatively quick access to CBT in the waiting list arm would have reduced short-term benefit associated with the low-intensity interventions. The effects of low-intensity interventions may have been more pronounced at 3 months if CBT was less accessible than in the current trial. The longer-term analyses are less affected, as all patients were expected to receive both a low-intensity intervention (where allocated) and therapist-led CBT over 12 months.
We have shown that uptake of therapist-led CBT was lower in the groups allocated to low-intensity treatment. This could reflect positive outcomes from some aspects of the low-intensity interventions, although our analysis showed that this was largely restricted to patient satisfaction rather than clinical benefits. Even in the absence of significant clinical benefits, providing low-intensity treatments may give patients a sense of support and progress. When combined with natural improvement in symptoms over time (as found in all groups), this may mean that patients do not feel a need for further intensive support or no longer wish to engage with services. However, the numbers of patients attending therapist-led CBT increased in all groups over time. It is possible that, had our follow-up been longer than 12 months, eventual uptake of therapist-led CBT across all groups would be the same.
Secondly, we placed no restrictions on medication use, and in line with most psychological therapy trials in OCD, a proportion of patients were taking medication. Baseline self-reported use of antidepressant treatment is provided in Table 1, showing that about half the patients reported using antidepressants, with no differences between groups. Data on antidepressant use after allocation showed that, over the 12-month period of the trial, self-reported use of antidepressants decreased (cCBT 26%, guided self-help [GSH] 32%, and waiting list 27%) [28]. We do not have details of the nature or quality of antidepressant prescription. Although antidepressants are effective in OCD [3,32], it seems unlikely that such small differences between arms would be a major driver of study outcomes.
Thirdly, there was no attempt to match the level or type of clinician contact across the 2 low-intensity interventions: indeed, the study was specifically designed to test the relative value of 2 different forms of low-intensity intervention. GSH involved both a different delivery format and more clinician contact, so our trial is not a strict comparison of paper and digital interventions. Although increased clinician contact may well enhance acceptability and effectiveness, its additional costs were accounted for in the economic analysis.
Fourth, we did not undertake quality assurance of the therapist-led CBT provided to all patients. As noted above, therapist-led CBT was provided by a range of practitioners in a range of areas and is likely to be reasonably representative of the treatment provided in the National Health Service (NHS) in England, which remains optimal for a pragmatic trial. Formal measurement of quality would have been preferable but logistically complex.
The trial adopted aspects of a stepped care model, whereby patients are offered a low-intensity intervention first, with a proportion progressing to therapist-led CBT. However, unlike true stepped care, there was no regular assessment of outcome, and access to therapist-led CBT was available to all, rather than as part of a defined “stepping” mechanism following nonresponse to treatment or deterioration. Therefore, our analysis does not assess the benefits of low-intensity treatment in a full stepped care model.
Interpretation of the results in the context of the wider literature
At the initiation of this trial, the evidence base was very limited [11]. While the current trial was being delivered, a number of additional studies were published. One small trial (n = 56) used similar interventions to the present study (GSH and supported cCBT) and compared their effects in a group of volunteers recruited through a website. Very large effects were found post-treatment [33]. A second trial (n = 86) exploring a minimally supported cCBT intervention again found very large effects in a sample recruited online through a research centre [34]. A third trial randomised 34 patients with OCD to a supported internet-based writing therapy and again found very large effects among a sample recruited via public notices. A long-term follow-up [35] of a previous trial [12] showed enduring effects for cCBT and that “booster” treatments were effective in maintaining gains. Finally, one trial (n = 128) explored the effects of unsupported written material about metacognitive therapy in patients with OCD from internet groups, self-help organisations, and clinical facilities and found small benefits [36].
The picture from these trials is more positive about the clinical benefits of “low-intensity” treatments, especially supported cCBT, with most effect sizes over 0.5 and some exceeding 1.0. This contrasts markedly with the modest clinical impacts observed in the current study. There are a number of reasons that could account for these differences. The interventions do vary, although it is not clear that the variation is large enough to account for the large variation in effects. The current sample of patients have more severe symptoms at baseline (Y-BOCS scores of 25, compared to 20–21 in the other studies), although again it is not clear that such modest differences would be expected to lead to such profound variation in impact. Our current study is far larger than the other trials, and some report quite large differences at baseline, which can occur when small numbers of patients are randomised [33,36]. A potentially important issue is the method of recruitment. The vast majority of the patients in the current study were recruited through routine clinical services, whereas a number of the other trials used recruitment through the internet; this may produce a sample with different clinical features and one that is much more amenable to online cCBT interventions. Similar differences in effects between large pragmatic trials in routine services and smaller trials recruiting through the internet have been recently reported in depression [37].
Implications for service delivery
The trial demonstrated that neither form of low-intensity CBT was responsible for clinically significant improvements in OCD symptoms among patients on the waiting list.
In the absence of any significant clinical benefit over waiting list only, readers may have concerns about reductions in the use of therapist-led CBT at 12 months, as this might reflect the substitution, or delay, of an evidence-based treatment. It may be that access to GSH or cCBT means that patients are put off from engaging in subsequent therapist-led CBT. We found no evidence that lower uptake of therapist-led CBT was associated with worse outcome over 12 months. Concerns that GSH or cCBT inappropriately discourages patients from engaging in subsequent therapist-led CBT are not supported by the wider literature, which shows an increased likelihood of help seeking and greater healthcare use following self-help [38,39]. It is possible that some patients who are offered GSH or cCBT improve so that they do not need subsequent therapist-led CBT or make an informed choice to discontinue therapy sooner rather than later. However, as noted earlier, differences in uptake between arms may have reduced if a longer follow-up had been possible.
Our results raise questions about the targeting of low-intensity interventions. Recruiting from waiting lists identified a sample with severe symptoms and a relatively long history of treatment. Although most showed significant improvements over time (around 8–9 points on the Y-BOCS across groups), the means at 12 months still showed significant symptoms (around 16 points), meaning many would continue to be eligible for the trial. Routine provision of CBT within the NHS in line with clinical guidelines clearly leaves many patients with clinically significant residual symptoms. Low-intensity treatments may be better targeted at a less severely ill group, closer to the onset of their OCD. However, as this patient group is characterised by late presentation to services, the viability of this is unclear.
Neither cCBT nor GSH showed clinically significant benefits at 3 months. Further development of more effective low-intensity interventions may be required. Uptake of the interventions was relatively low, although not abnormally so for a pragmatic trial. Both interventions may benefit from enhancements that might improve motivation or adherence, which might translate to greater clinical benefit.
The clinical results alone do not support an important role for low-intensity interventions in the care pathway for OCD. However, full interpretation of the benefits of low-intensity interventions for OCD also demands consideration of cost-effectivness, using comprehensive assessments of costs, as well as appropriate measures of the impact of these interventions on health-related quality of life and associated utility. We report the results of this analysis separately [28].
Supporting Information
Zdroje
1. National Institute for Health and Clinical Excellence. Obsessive-compulsive disorder: core interventions in the treatment of obsessive-compulsive disorder and body dysmorphic disorder (Clinical guideline 31). London: NICE; 2005.
2. Hollander E, Broatch J, Himelein C, Rowland C, Stein D, Kwon J. Psychosocial function and economic costs of obsessive-compulsive disorder. CNS Spect 1997; 2 : 16–25.
3. Skapinakis P, Caldwell D, Hollingworth W, Bryden P, Finberg N, Salkolvskis P, Welton N, Baxter H, Kessler D, Churchill R and Lewis G. A systematic review of the clinical effectiveness and cost-effectiveness of pharmacological and psychological interventions for the management of obsessive-compulsive disorder in children/adolescents and adults. Health Technol Assess 2016; 20 (43). doi: 10.3310/hta20430 27306503
4. Richards DA, Ekers D, McMillan D, Taylor RS, Byford S, Warren FC, et al. Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression (COBRA): a randomised, controlled, non-inferiority trial. Lancet Published Online July 22, 2016; http://dx.doi.org/10.1016/S0140-6736(16)31140-0.
5. Andrews G, Cuijpers P, Craske M, McEvoy P, Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLoS ONE 2010;5(10): e13196. doi: 10.1371/journal.pone.0013196 20967242
6. Cuijpers P, Donker T, Van Straten A, Li J, Andersson G. Is guided self-help as effective as face to face psychotherapy for depression and anxiety disorders? A systematic review and meta-analysis of comparative outcome studies. Psychol Med 2010; 40 : 1943–57. doi: 10.1017/S0033291710000772 20406528
7. Bower P, Gilbody S. Stepped care in psychological therapies: access, effectiveness and efficiency: narrative literature review. Brit J Psychiat 2005; 186 : 11–17. doi: 10.1192/bjp.186.1.11 15630118
8. Lovell K, Ekers D, Fullford J, Baguley C, Bradshaw T. A pilot study of a self-help manual with minimal therapist contact in the treatment of obsessive-compulsive disorder. Clin Eff Nurs 2005; 8(2):122–127.
9. Tolin DF, Diefenbach GJ, Maltby N, Hannan SE. Stepped care for obsessive-compulsive disorder: A pilot study. Cogn Behav Pract 2005; 12(4):403–414.
10. Tolin KL, Hannan DF, Maltby S, Diefenbach N, Worhunsky P, Brady RE. A RCT of self-directed versus therapist-directed CBT for OCD patients with prior medication trials. Behav Ther 2007; 38 : 179–191. doi: 10.1016/j.beth.2006.07.001 17499084
11. Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al. Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation. Health Technol Assess 2006; 10 : 1–168.
12. Andersson E, Enander J, Andren P, Hedman E, Ljotsson B, Bergstrom J, et al. Internet-based cognitive behaviour therapy for obsessive-compulsive disorder: a randomized controlled trial. Psychol Med 2012;42(10):2193–203. doi: 10.1017/S0033291712000244 22348650
13. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. Brit Med J 2015; 350: h2147. doi: 10.1136/bmj.h2147 25956159
14. Sox HC, Lewis RJ. Pragmatic Trials: Practical Answers to “Real World” Questions. JAMA 2016; 316(11):1205–1206. doi: 10.1001/jama.2016.11409 27654606
15. Gellatly J, Bower P, McMillan D, Roberts C, Byford S, Bee P, et al. Obsessive Compulsive Treatment Efficacy Trial (OCTET) comparing the clinical and cost effectiveness of self-managed therapies: study protocol for a randomised controlled trial. Trials 2014; 15(1):278.
16. Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59 Suppl 20 : 22–33.
17. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale: 1. Development, use and reliability. Arch Gen Psychiatry1989; 46 : 1006–1011. 2684084
18. Lovell K, Gega L. Obsessive Compulsive Disorder: A Self-Help Book. The University of Manchester; 2011.
19. Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care 1992; 30 : 473–483. 1593914
20. Evans C, Connell J, Barkham M, Margison F, McGrath G, Mellor-Clark J, et al. Towards a standardised brief outcome measure: psychometric properties and utility of the CORE-OM. Br J Psychiatry 2002; 180(1):51–60.
21. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282 : 1737–44. 10568646
22. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166 : 1092–1097. doi: 10.1001/archinte.166.10.1092 16717171
23. Mundt JC, Marks IM, Greist JH, Shear K. The work and social adjustment scale: a simple accurate measure of impairment in functioning. Br J Psychiatry 2002; 180 : 461–4. 11983645
24. The IAPT Data Handbook. Guidance on recording and monitoring outcomes to support local evidence-based practice. 2015. URL: www.iapt.nhs.uk/silo/files/the-iapt-data-handbook.pdf (accessed 3 November 2011).
25. Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979; 2(3): 197–207. 10245370
26. Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorders in the community: a standardised assessment for use by lay interviewers. Psychol Med 1992; 22 : 465–486. 1615114
27. Lovell K, Cox D, Haddock G, Jones C, Raines D, Garvey R. Telephone administered cognitive behaviour therapy for treatment of obsessive compulsive disorder: Randomised controlled non-inferiority trial. Brit Med J 2006; 333(883).
28. Lovell K, Bower P, Gellatly J, Byford S, Bee P, McMillan D, et al. Clinical, cost-effectiveness and acceptability of low intensity interventions in the management of OCD: the OCTET randomised controlled trial. Health Technology Assessment [Online]. Available at: https://www.journalslibrary.nihr.ac.uk/programmes/hta/098101/#/
29. White RI, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med 2005; 24(7): 993–1007. doi: 10.1002/sim.1981 15570623
30. Federici A, Summerfeldt LJ, Harrington JL, McCabe RE, Purdon CL, Rowa K, et al. Consistency between self-report and clinician-administered versions of the Yale-Brown Obsessive-Compulsive Scale. J Anxiety Disord 2010; 24(7): 729–33. doi: 10.1016/j.janxdis.2010.05.005 20561767
31. Steketee G., Frost R., & Bogart K. The Yale-Brown obsessive compulsive scale: interview versus self-report. Behav Res Ther 1996; 34 : 675–684. 8870295
32. Soomro GM, Altman D, Rajagopal S, Oakley-Browne M. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database of Systematic Reviews 2008; Issue 1, CD00176.
33. Wootton BM, Dear BF, Johnston L, Terides MD, Titov N. Remote treatment of obsessive-compulsive disorder: A randomized controlled trial. J Obsess-Compul Rel Disord 2013, 2(4), 375–384. http://doi.org/10.1016/j.jocrd.2013.07.002
34. Mahoney AEJ, Mackenzie A, Williams AD, Smith J, Andrews G. Internet cognitive behavioural treatment for obsessive compulsive disorder: A randomised controlled trial. Behav Res Ther 2014; 63 : 99–106 doi: 10.1016/j.brat.2014.09.012 25461784
35. Andersson E, Steneby S, Karlsson K, Ljótsson B, Hedman E, Enander, et al. Long-term efficacy of Internet-based cognitive behavior therapy for obsessive–compulsive disorder with or without booster: a randomized controlled trial. Psychol Med 2014; 44(13):2877–87. doi: 10.1017/S0033291714000543 25066102
36. Hauschildt M, Schröder J, Moritz S. Randomized-controlled trial on a novel (meta-) cognitive self-help approach for obsessive-compulsive disorder (“myMCT”). J Obsess-Compul Rel Disord 2016;10 : 26–34.
37. Gilbody S, Littlewood E, Hewtii C, Brierley G, Tharmanathan P, Araya R, et al. Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial Brit Med J 2015; 351:h5627 doi: 10.1136/bmj.h5627 26559241
38. Christensen H, Griffiths KM, Mackinnon AJ, Brittliffe K. Online randomized controlled trial of brief and full cognitive behavior therapy for depression. Psychol Med 2006; 36, 1737e1746.
39. Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The Effectiveness of Web-Based vs. Non-Web-Based Interventions: A Meta-Analysis of Behavioral Change Outcomes. J Med Internet Res 2004; 6(4):e40. doi: 10.2196/jmir.6.4.e40 15631964
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 6- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Vaccination to prevent human papillomavirus infections: From promise to practice
- Reducing US cardiovascular disease burden and disparities through national and targeted dietary policies: A modelling study
- Contribution of cognitive performance and cognitive decline to associations between socioeconomic factors and dementia: A cohort study
- Modelled health benefits of a sugar-sweetened beverage tax across different socioeconomic groups in Australia: A cost-effectiveness and equity analysis
- Risk factors and short-term projections for serotype-1 poliomyelitis incidence in Pakistan: A spatiotemporal analysis
- The US President’s Malaria Initiative and under-5 child mortality in sub-Saharan Africa: A difference-in-differences analysis
- Estimating the causal influence of body mass index on risk of Parkinson disease: A Mendelian randomisation study
- Low-intensity cognitive-behaviour therapy interventions for obsessive-compulsive disorder compared to waiting list for therapist-led cognitive-behaviour therapy: 3-arm randomised controlled trial of clinical effectiveness
- Population-level impact of an accelerated HIV response plan to reach the UNAIDS 90-90-90 target in Côte d’Ivoire: Insights from mathematical modeling
- Validity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: An observational study
- Malaria control adds to the evidence for health aid effectiveness
- Effectiveness and equity of sugar-sweetened beverage taxation
- A Collection on the prevention, diagnosis, and treatment of sexually transmitted infections: Call for research papers
- Pathways and progress to enhanced global sexually transmitted infection surveillance
- Elimination of mother-to-child transmission of HIV and Syphilis (EMTCT): Process, progress, and program integration
- Assessing process, content, and politics in developing the global health sector strategy on sexually transmitted infections 2016–2021: Implementation opportunities for policymakers
- Validity of a minimally invasive autopsy tool for cause of death determination in pediatric deaths in Mozambique: An observational study
- Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide
- Vaccination to prevent human papillomavirus infections: From promise to practice
- A Collection on the prevention, diagnosis, and treatment of sexually transmitted infections: Call for research papers
- Elimination of mother-to-child transmission of HIV and Syphilis (EMTCT): Process, progress, and program integration
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



