-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Research Agenda for Malaria Eradication: Vaccines
Vaccines could be a crucial component of efforts to eradicate malaria. Current attempts to develop malaria vaccines are primarily focused on Plasmodium falciparum and are directed towards reducing morbidity and mortality. Continued support for these efforts is essential, but if malaria vaccines are to be used as part of a repertoire of tools for elimination or eradication of malaria, they will need to have an impact on malaria transmission. We introduce the concept of “vaccines that interrupt malaria transmission” (VIMT), which includes not only “classical” transmission-blocking vaccines that target the sexual and mosquito stages but also pre-erythrocytic and asexual stage vaccines that have an effect on transmission. VIMT may also include vaccines that target the vector to disrupt parasite development in the mosquito. Importantly, if eradication is to be achieved, malaria vaccine development efforts will need to target other malaria parasite species, especially Plasmodium vivax, where novel therapeutic vaccines against hypnozoites or preventive vaccines with effect against multiple stages could have enormous impact. A target product profile (TPP) for VIMT is proposed and a research agenda to address current knowledge gaps and develop tools necessary for design and development of VIMT is presented.
Published in the journal: . PLoS Med 8(1): e32767. doi:10.1371/journal.pmed.1000398
Category: Review
doi: https://doi.org/10.1371/journal.pmed.1000398Summary
Vaccines could be a crucial component of efforts to eradicate malaria. Current attempts to develop malaria vaccines are primarily focused on Plasmodium falciparum and are directed towards reducing morbidity and mortality. Continued support for these efforts is essential, but if malaria vaccines are to be used as part of a repertoire of tools for elimination or eradication of malaria, they will need to have an impact on malaria transmission. We introduce the concept of “vaccines that interrupt malaria transmission” (VIMT), which includes not only “classical” transmission-blocking vaccines that target the sexual and mosquito stages but also pre-erythrocytic and asexual stage vaccines that have an effect on transmission. VIMT may also include vaccines that target the vector to disrupt parasite development in the mosquito. Importantly, if eradication is to be achieved, malaria vaccine development efforts will need to target other malaria parasite species, especially Plasmodium vivax, where novel therapeutic vaccines against hypnozoites or preventive vaccines with effect against multiple stages could have enormous impact. A target product profile (TPP) for VIMT is proposed and a research agenda to address current knowledge gaps and develop tools necessary for design and development of VIMT is presented.
Summary Points
-
Vaccines for malaria eradication need to have an impact on transmission rather than focusing on mortality and morbidity reduction alone
-
Vaccines that interrupt malaria transmission (VIMT) may target many stages of the parasite’s life cycle, not just the sexual and mosquito stages as in classical blocking vaccines and multiple plasmodium species, in particular Plasmodium vivax
-
Novel vaccine delivery approaches and adjuvants need to be developed
-
Other priority areas for research and development include the development of tools to measure transmission rates and the development of robust assays of functional immune responses in individuals, which could inform vaccine development
-
A better understanding of the dynamics between the multiplication of parasites, gametocytogenesis, and malaria transmission rates in populations is also needed
Introduction
Vaccines are the most cost-effective tools for public health and have been instrumental in previous elimination campaigns against smallpox [1], polio [2], and measles [3],[4]. Vaccines have also been useful for sustained control of diseases such as neonatal tetanus [5], and vaccines such as Haemophilus influenzae type b conjugate vaccine have the potential to lead to elimination in some settings [6].
Here, we discuss the research and development agenda for the development of vaccines that can serve as key components of a future arsenal of tools to eradicate malaria. Current efforts to develop malaria vaccines are primarily directed towards reducing the morbidity and mortality that are associated with malaria and focus on P. falciparum. For example, the Malaria Vaccine Roadmap [7] has a strategic goal of developing a vaccine with 80% protective efficacy against P. falciparum by 2020. However, if malaria vaccines are to contribute to programs for malaria elimination, they will need to have an impact on malaria transmission. The scientific and ethical basis for the development of vaccines referred to as transmission-blocking vaccines (TBVs) that specifically target malaria sexual stage antigens with the goal of having an impact on transmission has been described previously [8],[9]. Here, we refocus attention on the development of vaccines that can be used in concert with other malaria control interventions to interrupt malaria transmission and eventually contribute to the eradication of this disease. We also recommend that vaccine development efforts need to pay attention to Plasmodium species other than P. falciparum, especially Plasmodium vivax, if malaria eradication is to be achieved.
Rationale of the Proposed malERA Approach to Development of Malaria Vaccines
First, we introduce the broad concept of VIMT. VIMT may be composed of one or more of the following components: classical TBVs that target sexual and mosquito stage parasite antigens; highly effective pre-erythrocytic vaccines that reduce asexual and sexual stage parasite prevalence rates; highly effective asexual erythrocytic stage vaccines that inhibit multiplication of asexual stage parasites efficiently to reduce blood-stage parasite densities and have an impact on malaria transmission; and vaccines that target vector antigens to disrupt parasite development in the vector. It seems obvious that a highly effective pre-erythrocytic vaccine that prevents erythrocytic stage infection will reduce transmission, but the effect of partially effective pre-erythrocytic or asexual blood-stage vaccines on individual infectivity needs investigation. A successful VIMT must primarily reduce malaria transmission. However, VIMTs that include pre-erythrocytic and/or asexual blood-stage vaccine components may also provide individuals with protection against malaria. Such VIMT would also protect the population against epidemic spread following reintroduction of malaria after elimination, an important characteristic given that the gains accrued through many years of elimination can be rapidly reversed if malaria is reintroduced to a population with no antimalarial immunity [10].
Second, the observed impact of concerted nonvaccine malaria control efforts on transmission dynamics in several malaria-endemic regions has shown that high-intensity transmission settings (entomological inoculation rate, EIR >50) can be converted to low-to-moderate intensity transmission settings (EIR <10) [11],[12]. Implementation of VIMT together with such control efforts may successfully drive down transmission rates to reduce the effective reproduction rate (Reffective) to below 1.0.
Third, the consultative group introduces the concept of a detailed TPP for this class of vaccines and urges that novel clinical development methods and approaches be considered to shorten the time to VIMT registration and implementation.
Fourth, the consultative group lays out a detailed research agenda that must be developed, funded, and implemented in parallel with VIMT development efforts. This agenda includes development of critical tools that will be required to register and implement such a vaccine. In particular, we identify the need to develop robust assays to measure biologically relevant transmission-blocking activities at the individual level that are validated as surrogates of reductions in transmission rates at the population level. If this goal is achieved, such assays could become the key tool for measurement of primary vaccine efficacy endpoints in conditional registration trials, thereby simplifying the clinical development program.
Finally, the consultative group considers that interested industrial partners should be identified early on in development, because expertise in applied immunology, vaccinology, product development, manufacturing, and regulatory activities is concentrated within industry and will play an essential role in the successful development of VIMT. In addition, it will be important to engage with regulatory agencies to define efficient yet sound regulatory strategies to develop and register new tools that can meet the needs of global malaria elimination and eradication efforts.
TPP for VIMT
A TPP is an industry-standard tool that gives clear guidance on the critical characteristics of a candidate product under development. TPPs are developed early in the development process and ensure that research and development efforts are focused on those activities that are necessary to develop a product that will meet the needs of end users. Table 1 presents a TPP for VIMT. For each characteristic in this TPP, we propose a “desired target” (aspirational) and a “minimally acceptable target” (must achieve). A vaccine candidate that does not meet or exceed most, if not all, of the minimally acceptable targets is likely to have a significantly reduced likelihood of successful introduction and uptake.
Tab. 1. TPP VIMT. 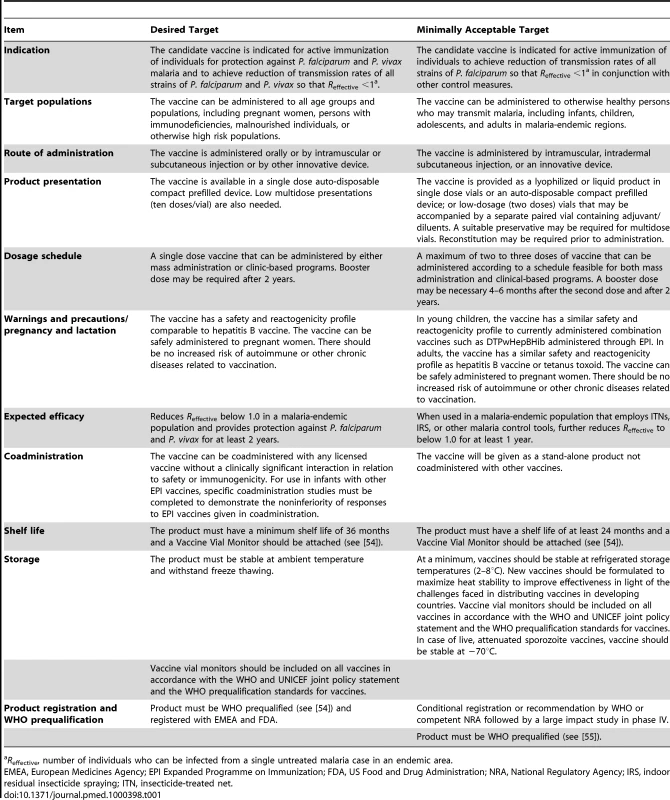
Reffective, number of individuals who can be infected from a single untreated malaria case in an endemic area. P. falciparum and P. vivax are the two most common Plasmodium species that cause human malaria. P. falciparum is responsible for most malaria-related deaths. As a result, previous efforts to develop vaccines for malaria have focused on P. falciparum, which causes ∼500 million cases of malaria annually and is critically important for Africa. However, P. vivax causes significant morbidity in other regions of the world including South and Southeast Asia and Latin America with around 75–90 million cases of P. vivax malaria reported annually [13]. Recent clinical epidemiology studies have confirmed that P. vivax can cause severe disease and may also contribute to malaria-associated mortality [14]–[17]. Efforts to eliminate malaria outside Africa must therefore address both parasite species. Ideally, VIMT should reduce transmission rates so that Reffective for both P. falciparum and P. vivax is driven to less than 1 and should provide protection against clinical malaria caused by both parasite species. At a minimum (and possibly more realistically), VIMT should achieve reduction of transmission rates (Reffective <1) of at least all P. falciparum strains leading to elimination of P. falciparum when used in conjunction with other control measures in elimination/eradication campaigns.
As better control is achieved, exposure to malaria parasites will decrease and “naturally acquired” immunity may play a diminished role. The mechanisms of clinical immunity observed in populations under high exposure may have little relevance as, increasingly, most infections will occur in people with little previous exposure. Therefore, our TPP specifies that a vaccine intended to interrupt transmission should not presume an age-specific risk or preexisting state of immunity against malaria disease or transmission. It is likely that VIMT may need to be implemented in the entire population.
Other ideal as well as minimally acceptable parameters for VIMT include product presentation, dosage, storage, and coadministration with other immunizations. These parameters are detailed in Table 1.
Research in Support of Development of VIMT
Much of the ongoing work on malaria vaccine development has focused on the development of interventions that address disease manifestations and the work has been primarily focused on P. falciparum. To support the development of vaccines and other tools necessary for malaria eradication new dimensions need to be added to the fundamental research portfolio (see [18] also). For example, P. vivax needs to be added, and efforts need to be refocused on the development of vaccines that target sexual and mosquito stages of malaria parasites, which should interrupt transmission. The expanded portfolio also needs to include more research on vaccine delivery systems and adjuvants, the transmission dynamics and population biology of malaria parasites, and measurements of transmission rates.
Human Malaria Parasites beyond P. falciparum
VIMT that target P. falciparum alone are likely to be deployed only in regions where P. falciparum is the species predominantly responsible for malaria. Regions where P. vivax is responsible for a significant proportion of the malaria burden will require VIMT that target both species.
Control efforts in regions where P. falciparum and P. vivax both occur indicate that it is more difficult to reduce transmission of P. vivax than of P. falciparum This increased difficulty is attributed in part to the development of gametocytes earlier during blood-stage infections with P. vivax than is the case for P. falciparum, which allows transmission before clinical symptoms are apparent. Other factors contributing to the difficulty of reducing P. vivax transmission include: the development of hypnozoites that remain latent in hepatocytes and lead to blood-stage infections months or even years later; transmission by outdoor biting mosquitoes; and the ability of P. vivax to complete its life cycle in a wider range of climatic and ecological conditions than P. falciparum. Because of these unique features of P. vivax, traditional malaria control efforts such as vector control, bednets, and early detection and treatment often fail to control P. vivax transmission. Vaccines that elicit long-lasting immune responses that prevent infection or inhibit gametocyte development or transmission of sexual stages are likely to be more effective tools for control of P. vivax. Given that latent hypnozoites can lead to blood-stage infections years after an infective bite, it may be necessary to continue deployment of VIMT that target P. vivax after elimination is achieved. An alternative would be to develop vaccine components that can target and eliminate hypnozoites. Design of such vaccines will require better understanding of the unique aspects of the biology of P. vivax hypnozoites at the molecular level.
Other Plasmodium species such as Plasmodium ovale and Plasmodium malariae account for less than 5% of malaria cases worldwide. Natural infection of humans by Plasmodium knowlesi has recently been reported [19],[20]. Thus, we need to be prepared for the emergence of new Plasmodium species that can cause human malaria. It remains to be seen whether these parasite species will survive once efforts to eliminate P. falciparum and P. vivax are successful. For now, then, efforts should be focused on developing VIMT for P. falciparum and P. vivax malaria, but it will be important to monitor the epidemiology of P. ovale, P. malariae, and P. knowlesi as elimination of P. falciparum and P. vivax progresses. Decisions to support development of vaccines that block transmission of these parasite species may need to be made in the future.
Discovery Research
Malaria parasites have a complex life cycle during which they infect humans and are transmitted by Anopheline mosquitoes. The successful completion of the parasite life cycle requires specific molecular interactions between the parasite and various host and vector tissues. A clear understanding of the molecular interactions that mediate invasion of hepatocytes by Plasmodium sporozoites, invasion of erythrocytes by Plasmodium merozoites, and traversal of mosquito midgut epithelium by Plasmodium ookinetes may allow the development of strategies to target these key interactions and disrupt the parasite life cycle thereby reducing malaria transmission rates. It may be necessary to combine components that target different stages of malaria parasites to achieve synergistic effects that provide protection and reduce malaria transmission rates. For example, partially effective pre-erythrocytic and blood-stage components may not have any effect on transmission but the addition of such partially effective components to classical TBVs might allow the development of a multicomponent VIMT that can reduce malaria transmission as well as provide protection against malaria.
Targeting the Sexual and Mosquito Stages
Gametocytes are the source of the epidemiologically important transmission of all malaria parasites. In P. falciparum, recent work has demonstrated that the developmental switch from asexual replication to sexual stage development occurs at the ring stage and that all schizonts from that ring parasite are committed to form gametocytes upon invasion of new red blood cells [21]. P. falciparum then undergoes sequential development through five distinct morphological stages to form mature male and female gametocytes. Within the mosquito midgut, mature male and female gametes are released and fertilization occurs to form a zygote. The resultant motile ookinete passes through the midgut wall, undergoes reduction division, and forms an oocyst. Each step in this developmental pathway involves unique processes, including the transcription of specific genes, the expression of specific proteins, the upregulation of specific biochemical pathways, and the formation of new morphological structures. Understanding the regulation of this developmental process could be the key to developing new interventions that target sexual and mosquito stages to interrupt transmission. For example, direct targeting of the developing gametocyte has the potential advantage of targeting a small subset of infected red blood cells that express proteins or pathways specific to parasite sexual development. A drug or a vaccine that could inhibit the initial switch to sexual development, coupled with a vaccine that targets gamete antigens might provide a powerful combinatorial approach to reduce transmission (also see [22]).
There is a large body of work on the key antigens on the surface of gametes of both P. falciparum and P. vivax [9]. Several of these antigens have been tested in animal models as transmission-blocking vaccines, at least two which have been tested in humans [23],[24]. A phase I trial of the P. vivax ookinete surface antigen Pvs25 formulated with Alhydrogel demonstrated acceptable safety and reactogenicity with induction of anti-Pvs25 immunoglobulin G (IgG) with functional transmission-blocking activity in a membrane-feeding assay. However, these data suggest that a more immunogenic formulation would be desirable to achieve higher transmission-blocking activity [23]. More recently, a trial of ISA51 formulations of Pvs25 and Pfs25 was terminated because of unacceptable reactogenicity [24]. The expression of correctly folded Pfs48/45 gametocyte surface antigen has recently resulted in a demonstration of transmission-reducing activity in sera from immunized animals [25],[26].
Targeting Pre-erythrocytic and Asexual Stages
Highly effective pre-erythrocytic stage vaccines can, in principle, reduce the prevalence of blood-stage parasites, including both the asexual stages and the gametocytes. Such vaccines can provide protection against malaria and reduce malaria transmission. Immunization with irradiated sporozoites has elicited complete protection against sporozoite challenge in experimental animal models and in humans. Thus, in principle, it should be possible to target pre-erythrocyte stage antigens to elicit complete protection against parasite infection. Protective immune mechanisms elicited by irradiated sporozoites are not well understood but are thought to include antibody responses against sporozoite antigens that prevent hepatocyte infection, and cellular responses that clear infected hepatocytes. Better understanding of the correlates of immunity elicited by immunization with irradiated sporozoites could guide the development of highly effective pre-erythrocytic subunit vaccines that both provide protection and reduce parasite transmission. A recombinant vaccine based on the circumsporozoite protein, RTS,S has been shown to elicit partial protection against P. falciparum infection [27],[28]. It seems unlikely, however, that RTS,S will have significant impact on gametocyte prevalence or affect malaria transmission.
Other vaccines based on irradiated sporozoites or genetically modified attenuated sporozoites have provided protection in challenge models [29],[30]. Such whole organism attenuated vaccines may provide effective protection against malaria and significantly reduce parasite transmission. However, considerable technological challenges in terms of manufacturing, formulation, and delivery of such attenuated sporozoite vaccines need to be overcome.
During P. vivax infections, some infected hepatocytes differentiate into latent hypnozoite stages that can yield merozoites after a long latency period. The biology of hypnozoites is very poorly understood but the development of drugs or vaccines that can clear hypnozoites is critical for success of efforts to eradicate P. vivax [22]. The development of methods for in vitro culture of hypnozoites could greatly help improve our understanding of this latent stage. In vitro culture of hypnozoites would allow the application of whole genome approaches such as transcriptomics and proteomics to the identification of parasite proteins expressed in hypnozoites. It may be possible to elicit cellular immune responses against such hypnozoite specific proteins to clear these latent stages. Vaccines against pre-erythrocytic stages of P. vivax that are effective against both developing and resident hypnozoites would be of inestimable benefit in efforts to eliminate P. vivax.
Vaccines based on asexual blood-stage antigens may be effective at reducing parasite densities and provide protection against clinical disease but it is not clear whether such vaccines can reduce malaria transmission rates effectively. Basic research is needed to understand the dynamics of the relationship between asexual stage parasite growth, sexual stage parasite densities in blood, and individual infectivity or transmission efficiency. Recombinant vaccines based on asexual blood-stage antigens tested in human clinical trials have not yielded high rates of growth inhibition thus far and are unlikely to have significant impact on gametocyte prevalence or infectivity of individuals. Irrespective of whether vaccines based on asexual blood-stage antigens can reduce sexual stage parasite densities and reduce transmission, combinations of asexual blood-stage vaccines with classical TBVs will enable development of VIMT that provide direct benefit to vaccine recipients by providing protection against clinical disease in addition to reducing transmission.
Targeting the Vector to Reduce Malaria Transmission
As described earlier, Plasmodium parasites have an obligatory development stage in the mosquito during which zygotes transform into ookinetes that traverse the midgut epithelium to establish oocysts on the outer wall of the midgut. Attachment and invasion of the midgut epithelium requires specific interactions between ookinete surface proteins and midgut receptors. A set of conserved “invasion receptors” on the midgut of diverse Anopheline species are used by Plasmodium ookinetes to attach to the midgut epithelium [31]. Antibodies directed against such receptors have been shown to block development of oocysts in membrane-feeding transmission-blocking assays [31]. A vaccine based on such conserved vector antigens should be effective against all species of Plasmodium and obviate the need to develop separate vaccines for different Plasmodium species. Moreover, since such vaccines target vector antigens, parasite strain diversity, which has been a major problem for malaria vaccine development, will be overcome. Such novel strategies will require significant fundamental research to understand vector-parasite interactions [32].
Host-Parasite and Vector-Parasite Interactions
Plasmodium sporozoites invade human hepatocytes in a two-step process. In the first step, sporozoites pass through multiple hepatocytes by rupturing the plasma membrane of target hepatocytes [33]. After traversing multiple hepatocytes, sporozoites finally invade target hepatocytes by forming a parasitophorous vacuole where they multiply and differentiate into merozoites. Identification of key parasite proteins that mediate the two-step invasion process could provide functional targets for intervention. Sporozoite surface proteins such as the circumsporozoite protein (CSP) and thrombospondin-related protein (TRAP) have been shown to play a role in hepatocyte binding and invasion [34]–[37]. Both proteins contain functional cysteine-rich regions that share homology with thrombospondin and that mediate attachment to hepatocyte receptors. Antibodies targeting such functional regions can block hepatocyte invasion. Vaccines that elicit high-titer long-lasting antibodies against such functional domains might reduce the prevalence of blood-stage infection effectively. Similarly, antibodies targeting merozoite antigens such as the 175-kD erythrocyte binding antigen (EBA175) [38]–[41], Duffy binding protein [42], or PfRH proteins [43], which mediate critical interactions with erythrocyte receptors, can inhibit multiplication of blood-stage parasites. Ookinete antigens that interact with the midgut wall to mediate traversal may also be useful as recombinant malaria vaccine candidates that block parasite transmission by mosquitoes.
Because processes such as host cell invasion involve multiple steps, some of the processes highlighted above may be mediated by multiple pathways that are redundant. As a result, effective inhibition of host invasion by parasites may require targeting of a combination of receptor-ligand interactions that mediate invasion. A clear understanding of the sequence of events and functional roles of different receptor-ligand interactions will be critical for the development of vaccines that target multiple steps to provide synergistic inhibition of invasion and parasite multiplication at different stages of the parasite life cycle.
It will also be important to develop functional assays that can be used to evaluate antibody responses against the parasite antigens that mediate host cell invasion and transmission to mosquitoes. These functional assays may directly test the inhibitory activity of antibodies elicited by vaccine candidates against the biological processes themselves or may be reduced to biophysical or biochemical assays in which antibodies are tested for inhibition of functions such as receptor binding or proteolytic cleavage that are known to mediate the biological processes. Harmonization of such assays is important so that results from different research groups are comparable and to facilitate decision making for down-selection of vaccine candidates during preclinical and clinical development. Currently, there are no clear correlates of immunity against pre-erythrocytic and blood-stage parasites. Immuno-assays can be validated only once a vaccine demonstrates efficacy in a clinical trial. Once an immune correlate for protection is identified, it can be used for decision making in clinical development.
Vaccine Delivery Systems and Adjuvants
The development of subunit vaccines will require the use of potent adjuvants and/or efficient vaccine delivery systems to elicit robust and sustainable immune responses. The unavailability of a wide range of potent adjuvants with a proven safety record in humans has been a bottleneck in the development of recombinant protein–based vaccines for malaria. Better understanding of mechanisms that activate the innate immune system might enable the design of adjuvants that elicit potent immune responses. Alternative methods to deliver antigens such as the use of virus-like particles or prime-boost strategies that use combinations of different viral vectors (e.g., recombinant adenovirus and modified vaccine virus–based vectors) or viral vectors and recombinant proteins have provided effective means to elicit potent immune responses [44], but further research on vaccine delivery systems is urgently required for development of effective malaria vaccines.
When the VIMT include multiple components, it will be important to develop formulations or delivery systems that are compatible with each component. A clear understanding of the correlates of protective immunity elicited by each component may allow the identification and development of a compatible delivery system or adjuvant formulation for the combination vaccine. Analysis of candidate vaccine–elicited immune responses in functional assays will allow optimization of compatible formulations. Importantly, development of multicomponent VIMT may require collaboration between researchers who have developed the individual components. It will be important to develop innovative licensing arrangements that ensure accessibility of each component for commercial development of such multicomponent VIMT.
Understanding Transmission Dynamics and Population Biology of Malaria Parasites
As campaigns to reduce transmission of malaria are successful, it will be necessary to understand the changes in parasite population dynamics and population structure. In particular, it will be desirable to determine whether specific parasite strains dominate as the transmission pattern changes and whether this has implications with regard to antigenic diversity or parasite virulence. Field trials with P. falciparum blood-stage vaccines have provided evidence for allele-specific protection, which suggests that large-scale immunization may lead to the selection of “vaccine-resistant” parasites that can escape immune responses elicited by the vaccine [45]. A second important question is to determine whether reemergent parasites have been introduced from an outside source or whether they are parasites that have escaped control measures. These two options have very different implications for intervention strategies during the pre-elimination stage. Tools to track such parasites will be useful for surveillance as control efforts move towards eradication.
Measuring Malaria Transmission Rates
A key to the evaluation of vaccines that block transmission will be the measurement of transmission. The anticipated clinical outcome of vaccination will be the reduction of transmission in the community. It is therefore necessary to develop robust and readily usable tools to evaluate transmission levels in various epidemiological settings ranging from high transmission areas to areas of very low prevalence and transmission. In particular, as various malaria control measures are introduced, the transmission dynamics will change and robust evaluation of transmission will be challenging. Harmonization of existing tools for measurement of transmission rates is a high priority [46],[47].
It is particularly important to be able to measure the effect on infectivity of an individual after vaccination with either a pre-erythrocytic or a blood-stage vaccine, and to understand the relation of this result to an effect on transmission in the community. Clinical efficacy trials of such vaccines have tended to focus on their impact on blood-stage infection or clinical disease; the impact of such vaccines on transmission remains to be determined. An important aspect of strategic thinking around malaria vaccines in years to come will be a greater emphasis on the evaluation of the impact of all classes of vaccines on transmission.
A second priority is the development of markers that define the infectivity of an individual for mosquitoes. These markers could include bioassays, serological parameters, or molecular markers. There is a need for robust models that predict the relationship of rates of individual infectivity to transmission at the community level in different epidemiological settings. Once this relationship is established, such markers could be used as surrogates of vaccine efficacy on transmission at the population level.
Strategies for Product and Clinical Development of VIMT
Product Development Based on TPP
Once TPPs are defined, they should be used to guide product development and evaluate the project in terms of achieving desired goals set for the vaccine candidates. It is important to understand where the project stands in terms of development. Terminology should be used appropriately and be in line with the development phase of the product (Figure 1).
Fig. 1. Classification of programs. 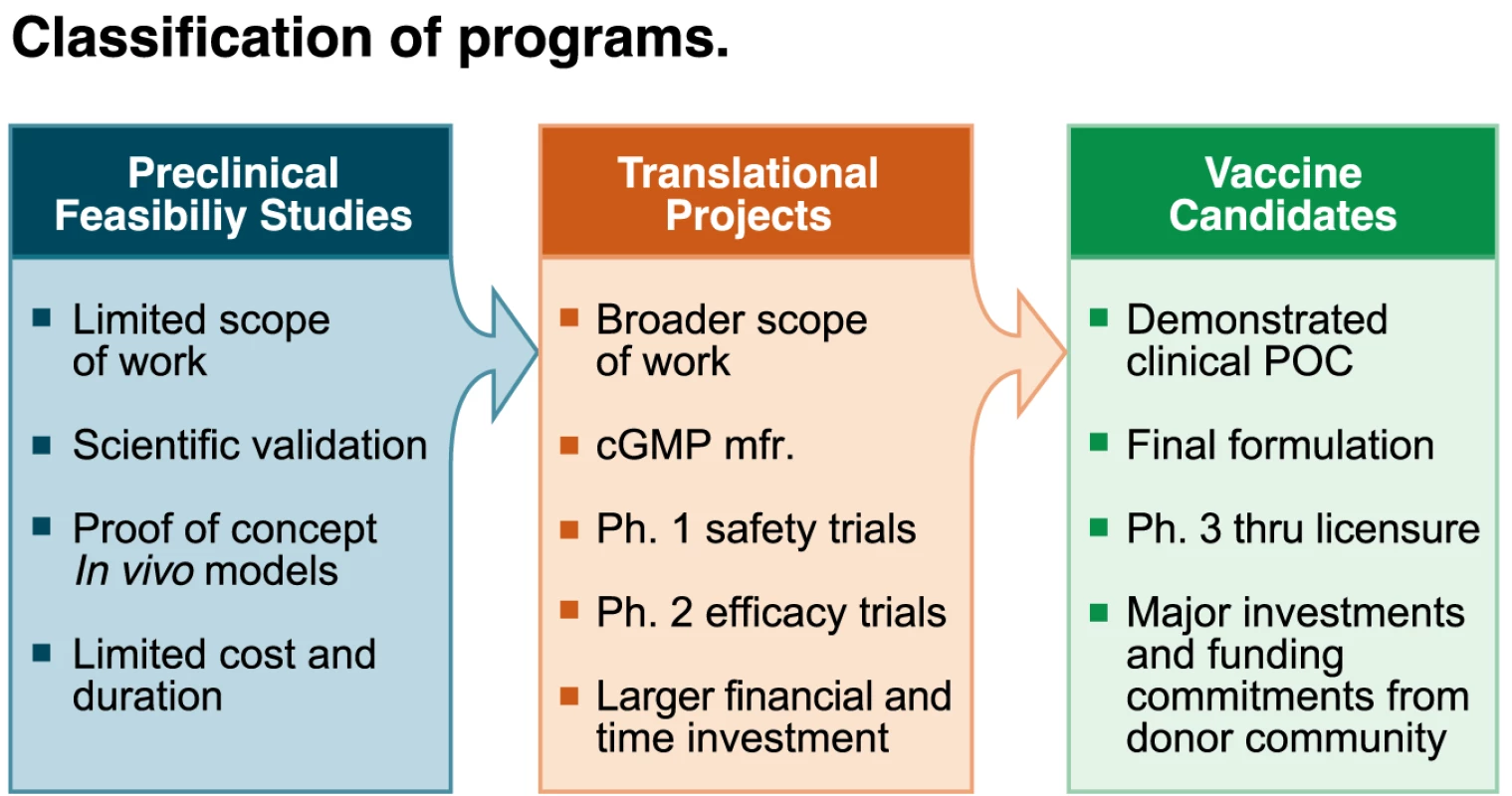
Image credit: Fusión Creativa. Preclinical feasibility studies are conducted first to validate the scientific rationale for vaccine design. At this stage of the project, questions have to be asked that address issues such as whether the project is likely to achieve the final desired TPP. Numerous preclinical feasibility studies may be undertaken to assess a variety of antigens, adjuvants, and delivery systems. Importantly, immune responses with the experimental vaccine produced at pilot scale need to be evaluated in animal models, preferably using functional assays, to validate the concept and progress it to a translational project stage.
For the translational stage, a significant investment of resources is necessary, not least because the prototype vaccine must be produced under current good manufacturing practices. Thus, only the most promising approaches can be moved into this and later stages of development. The translational project, which will have a set of precise go/no-go milestones, drives a research program of relevance to public health from the preclinical phase, through phase I trials to evaluate safety, and into phase II trials to evaluate efficacy. A successful translational project will deliver a vaccine that should be ready for phase III trials.
A product can be considered as a vaccine candidate once its manufacturability has been established and it has undergone a successful proof-of-concept phase II efficacy trial (Figure 1). For “classical” pre-erythrocytic or asexual stage vaccines, this typically requires either a phase IIa challenge trial or an efficacy trial in an endemic country. For VIMT, proof of concept may not need to be established in a malaria-endemic setting, provided that a robust read-out measurable at the level of the individual vaccinees has been shown to predict an effect on transmission at the population level. By this stage the product is fully characterized and will not change substantially. Major investments will be required, however, to complete the development program to deliver a viable vaccine for use in public health programs. Other considerations for a successful vaccine include the requirement for WHO prequalification of the vaccine for use in developing countries, an understanding in the affected communities of the ethical and practical issues associated with a long program of testing, and a significant commitment of the donor community to provide funds to support country-wide vaccine launches.
Clinical Development and Regulatory Strategy
A vaccine that has an effect on transmission alone may not provide direct benefit to the individual. Registration pathways for such a vaccine are therefore likely to be complex, and the licensure endpoints will require careful consideration and discussion with regulatory agencies early in the development program. If the vaccine also provides individual benefit, the regulatory pathway could well be simpler.
One approach to registration for VIMT is for phase I/II programs to focus on identification of well-tolerated and immunogenic vaccine doses and schedules across a wide age range of vaccine recipients using standard safety assessments and immunologic readouts tailored for the vaccine candidate being evaluated. Randomized, controlled phase IIb proof-of-concept studies should be designed to permit the identification of a suitable vaccine efficacy endpoint at the individual level that can be validated for use in phase III trials. This endpoint must be identified and agreed in advance with regulatory agencies. The possible endpoints might include: percent reduction in parasite prevalence, especially gametocyte prevalence; percent reduction in individual infectivity as measured by percent reduction in oocyst and sporozoite counts in membrane-feeding assays; and percent reduction in infected mosquitoes fed on vaccinated volunteers that can transmit malaria to susceptible volunteers. We recognize that such efficacy endpoints at the individual level will only be surrogates for effects on malaria transmission rates at the population level. Thus, a necessary stage after conditional registration based on surrogate efficacy data will be definitive community-scale phase IV trials, which will measure reductions in effective reproduction rate (Reffective) as a postmarketing commitment.
Alternatively, some experts have argued that it should be possible to design and conduct cluster-randomized trials to evaluate the efficacy of VIMT in terms of reductions in transmission rates in malaria-endemic settings. Measurement of surrogate efficacy parameters at the individual level using robust assays in such trials may allow the identification of correlates of efficacy at the population level. Such an approach would follow the more traditional route of registering a vaccine after collecting evidence for efficacy in phase IIb/III trials. Ultimately, it will be important to study the efficacy of combination of vaccines with other interventions aimed at reducing transmission.
Decision Making in Development of VIMT
Existing methods for measurement of transmission intensity need to be harmonized and optimized to ensure that good baseline estimates are available prior to introduction of a package of interventions such as drugs and vaccines. Thus, an essential step will be a consultation process that decides on the relative utility of assays that assess the infectiousness of individuals [48], that measure transmission-blocking activity of sera [49] raised against sexual stage or mosquito antigens, and that consider trial designs to measure the impact of vaccines targeting any life cycle stage on malaria transmission [50].
Possible trial designs include community-randomized trials that use measurement of the reduction in the proportion of gametocyte carriers, the reduction in the infectiousness of humans to mosquitoes in individually randomized controlled trials, and the reduction in infection of humans as endpoints. However, the development of an assay or trial design that could provide robust, reproducible data on vaccine impact on transmission without performing large-scale community-randomized trials would be a major step forward in increasing efficiencies and timelines.
Many questions will need to be addressed to aid decision making during development of VIMT. For example, can assays such as the membrane-feeding assay be validated to meet the requirements of the International Conference of Harmonization? If so, what level of reduced infectivity as demonstrated by this assay is likely to provide community-level reduction in infection? Questions like these need to be answered so that decisions can be made about the packages of interventions required to bring the Reffective below 1 during elimination campaigns. An assessment of existing modeling work may provide information on this sort of issue [51],[52]. Other questions that will need answering include: what population coverage and level of transmission-blocking efficacy should we require from a vaccine intervention before it is transitioned into elimination campaigns and are there assays other than the membrane-feeding assay that will be useful in measurement of infectiousness of humans (for example, nucleic acid amplification-based assays for gametocytaemia)? Ways will also need to be found to optimize mosquito-feeding experiments linked to clinical vaccine trials for decision-making purposes (see also [53]).
Importantly, every step of the vaccine development, clinical evaluation, regulatory, and implementation process for VIMT needs to focus on using the TPP for vaccines and targeting transmission rather than morbidity during decision making. In addition, it will be essential to make decisions about the need to include packages of interventions when evaluating vaccines that reduce transmission (see also [52]). Decisions will also have to be made about who should receive VIMT. In endemic regions, VIMT would be delivered to infants, preferably through the routine expanded program of immunization and through periodic campaigns to the rest of the population. In regions of low malaria transmission, it may not be necessary to immunize the entire population. Instead it may be more effective to identify and immunize individuals who are responsible for the majority of the transmission in the community.
Assessment of interruption of transmission presents novel challenges and large costs, hence every effort must be made to find and adopt the most efficient mechanism for assessing efficacy. For example, could a competent regulatory authority be provided with sufficiently compelling evidence of the biological interruption of transmission activity of a vaccine (either prevention of gamtetocyte production or effects of antisera on transmission to mosquitoes) to allow registration of a vaccine with an indication for interruption of transmission at the community level, without the requirement for large-scale community randomized trial data? As mentioned earlier, phase IV studies could then follow to provide the required safety database, and measures of community effects on transmission for implementation. Industry involvement may be critical to successfully drive such a development pathway for VIMT. It will therefore be important to engage leaders of key vaccine industries as well as regulatory agencies and ethicists from affected countries in discussions early in the development pathway.
Conclusions
Vaccines can play a key role in multisectoral efforts to eliminate and eventually eradicate malaria. Current efforts to develop malaria vaccines are primarily focused on reducing infection rates, blocking replication of the parasite in the bloodstream, and the pathologic effects of the parasite in individuals, thereby reducing malaria morbidity and mortality in vaccinated individuals. Some of these vaccines, if highly effective, may also reduce transmission. These efforts need continued support.
For elimination, it is important to view vaccines for their potential contribution to reduction of transmission, and to support additional novel approaches to vaccines that directly target sexual and mosquito stages for use in malaria control programs. In this context, we propose the broader concept of VIMT and present an actionable research and development agenda to develop such vaccines (Box 1). We also propose that novel product development and regulatory strategies that reduce the time to market should be investigated to develop, license, and implement such vaccines.
Box 1. Summary of the Research and Development Agenda for Vaccines
A prioritized research and development agenda to enable the development of VIMT for use as critical components in malaria elimination efforts includes:
-
Development and application of novel vaccine delivery approaches and/or adjuvants to elicit long-lasting protective efficacy that makes significant impact on malaria transmission rates under diverse epidemiological settings.
-
Expansion of vaccine development efforts to cover Plasmodium species other than P. falciparum, especially P. vivax (including hypnozoites).
-
Understanding the dynamics between multiplication of asexual stage parasites, gametocytogenesis, and malaria transmission rates at the population level.
-
Development of robust assays to study functional immune responses at the individual level that can predict effect on malaria transmission at the population level and allow decision making in product development.
-
Development of tools to measure malaria transmission rates, thereby facilitating clinical development of vaccines that reduce malaria transmission.
Zdroje
1. HendersonDA
1987 Principles and lessons from the smallpox eradication programme. Bull World Health Organ 65 535 546
2. JohnJ
2009 Role of injectable and oral polio vaccines in polio eradication. Expert Rev Vaccines Jan 8 5 8
3. MossWJ
GriffinDE
2006 Global measles elimination. Nat Rev Microbiol 12 900 908
4. BremanJG
de QuadrosCA
DowdleWR
FoegeWH
HendersonDA
2011 The role of research in viral disease eradication and elimination programs: Lessons for malaria eradication. PLoS Med 8 e1000405 doi:10.1371/journal.pmed.1000405
5. RoperMH
VandelaerJH
GasseFL
2007 Maternal and neonatal tetanus. Lancet 370 1947 1959
6. AdegbolaRA
SeckaO
LahaiG
Lloyd-EvansN
NjieA
2005 Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: A prospective study. Lancet 366 144 150
7. Malaria Vaccine Technology Roadmap Final Report 2006 Available: http://www.malariavaccineroadmap.net
8. WHO 2000 Malaria transmission blocking vaccines: An ideal public good. Geneva: World Health Organization. Available: http://www.who.int/vaccine_research/feuille_1_4-1pdf
9. CarterR
2001 Transmission blocking malaria vaccines. Vaccine 19 2309 2314
10. RobertsDR
ManguinS
MoudhetJ
2000 DDT house spraying and re-emerging malaria. Lancet 356 330 332
11. O'MearaWP
BejonP
MwangiTW
OkiroEA
PeshuN
2008 Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 372 1555 1562
12. CeesaySJ
Casals-PascualC
ErskineJ
AnyaSE
DuahNO
2008 Changes in malaria indices between 1999 and 2007 in The Gambia: A retrospective analysis. Lancet 372 1545 1554
13. MendisK
SinaBJ
MarchesiniP
CarterR
2001 The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg 64 97 106
14. KocharDK
DasA
KocharSK
SaxenaV
SirohiP
2009 Severe Plasmodium vivax malaria: A report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg Feb 80 194 198
15. KocharDK
SaxenaV
SinghN
KocharSK
KumarSV
2005 Plasmodium vivax malaria. Emerg Infect Dis Jan 11 132 134
16. PriceRN
TjitraE
GuerraCA
YeungS
WhiteNJ
2007 Vivax malaria: Neglected and not benign. Am J Trop Med Hyg 77 79 87
17. PriceRN
DouglasNM
AnsteyNM
2009 New developments in Plasmodium vivax malaria: Severe disease and the rise of chloroquine resistance. Curr Opin Infect Dis 22 430 435
18. The malERA Consultative Group on Basic Science and Enabling Technologies 2011 A research agenda for malaria eradication: Basic science and enabling technologies. PLoS Med 8 e1000399 doi:10.1371/journal.pmed.1000399
19. LeeKS
Cox-SinghJ
BrookeG
MatusopA
SinghB
2009 Plasmodium knowlesi from archival blood films: Further evidence that human infections are widely distributed and not newly emergent in Malaysian Borneo. Int J Parasitol 9 1125 1128
20. Cox-SinghJ
DavisTM
LeeKS
ShamsulSS
MatusopA
2008 Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis 46 165 171
21. DixonMW
ThompsonJ
GardinerDL
TrenholmeKR
2008 Sex in Plasmodium: A sign of commitment. Trends Parasitol 24 168 175
22. The malERA Consultative Group on Drugs 2011 A research agenda for malaria eradication: Drugs. PLoS Med 8 e1000402 doi:10.1371/journal.pmed.1000402
23. MalkinEM
DurbinAP
DiemertDJ
SattabongkotJ
WuY
2005 Phase 1 vaccine trial of Pvs25H: A transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 23 3131 3138
24. WuY
EllisRD
ShafferD
FontesE
MalkinEM
2008 Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One 3 e2636 doi:10.1371/journal.pone.0002636
25. OutchkourovNS
RoeffenW
KaanA
JansenJ
LutyA
2008 Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci U S A 105 4301 4305
26. ChowdhuryDR
AngovE
KariukiT
KumarN
2009 A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS One 4 e6352 doi:10.1371/journal.pone.0006352
27. AlonsoPL
SacarlalJ
AponteJJ
LeachA
MaceteE
2004 Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: Randomised controlled trial. Lancet 364 1411 1420
28. AlonsoPL
SacarlalJ
AponteJJ
LeachA
MaceteE
2005 Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: Single-blind extended follow-up of a randomised controlled trial. Lancet 366 2012 2018
29. HoffmanSL
BillingsleyPF
JamesE
RichmanA
LoyevskyM
2010 Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin 6 97 106
30. PinderM
MoorthyVS
AkanmoriBD
GentonB
BrownGV
2010 Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Vaccine 6 97 106
31. DinglasanRR
KalumeDE
KanzokSM
GhoshAK
MuratovaO
2007 Disruption of Plasmodium falciparum development by antibodies against a conserved mosquito midgut antigen. Proc Natl Acad Sci U S A 104 13461 13466
32. The malERA Consultative Group on Vector Control 2011 A research agenda for malaria eradication: Vector control. PLoS 8 e1000401 doi:10.1371/journal.pmed.1000401
33. MotaMM
HafallaJC
RodriguezA
2002 Migration through host cells activates Plasmodium sporozoites for infection. Nat Med 8 1318 1322
34. SultanAA
ThathyV
FrevertU
RobsonKJ
CrisantiA
1997 TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell 90 511 522
35. CeramiC
FrevertU
SinnisP
TakacsB
ClavijoP
1992 The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 70 1021 1033
36. TewariR
SpaccapeloR
BistoniF
HolderAA
Crisanti A. 2002 Function of region I and II adhesive motifs of Plasmodium falciparum circumsporozoite protein in sporozoite motility and infectivity. J Biol Chem 277 47613 47618
37. WengelnikK
SpaccapeloR
NaitzaS
RobsonKJ
JanseCJ
1999 The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J 18 5195 5204
38. NarumDL
HaynesJD
FuhrmannS
MochK
LiangH
2000 Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acids. Infect Immun 68 1964 1966
39. PandeyKC
SinghS
PattnaikP
PillaiCR
PillaiU
2002 Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol Biochem Parasitol 123 23 33
40. PattnaikP
ShakriAR
SinghS
GoelS
MukherjeeP
2007 Immunogenicity of a recombinant malaria vaccine based on receptor binding domain of Plasmodium falciparum EBA-175. Vaccine 25 806 813
41. SimBK
ChitnisCE
WasniowskaK
HadleyTJ
MillerLH
1994 Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264 1941 1944
42. GrimbergBT
UdomsangpetchR
XainliJ
McHenryA
PanichakulT
2007 Plasmodium vivax invasion of human erythrocytes inhibited by antibodies directed against the Duffy binding protein. PLoS Med 4 e337 doi:10.1371/journal.pmed.0040337
43. GaoX
YeoKP
AwSS
KussC
IyerJK
2008 Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS Pathog 4 e1000104 doi:10.1371/journal.ppat.1000104
44. DraperSJ
MooreAC
GoodmanAL
LongCA
HolderAA
2008 Effective induction of high-titer antibodies by viral vector vaccines. Nat Med 14 819 821
45. TakalaSL
PloweCV
2009 Genetic diversity and malaria vaccine design, testing and efficacy: preventing and overcoming ‘vaccine resistant malaria’. Parasite Immunol 31 560 573
46. The malERA Consultative Group on Cross Cutting Issues for Eradication 2011 A research agenda for malaria eradication: Cross cutting issues for eradication. PLoS 8 e1000404 doi:10.1371/journal.pmed.1000404
47. The malERA Consultative Group on Basic Monitoring, Evaluation, and Surveillance 2011 A research agenda for malaria eradication: Monitoring, evaluation, and surveillance. PLoS 8 e1000400 doi:10.1371/journal.pmed.1000400
48. BousemaJT
SchneiderP
GouagnaLC
DrakeleyCJ
TostmannA
2006 Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis 193 1151 1159
49. van der KolkM
De VlasSJ
SaulA
van de Vegte-BolmerM
ElingWM
2005 Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission-reducing activity using empirical data. Parasitology 130 13 22
50. WHO 1997 Guidelines for the evaluation of Plasmodium falciparum vaccines in populations exposed to natural infection. Geneva WHO
51. PennyMA
MaireN
StuderA
SchapiraA
SmithTA
2008 What should vaccine developers ask? Simulation of the effectiveness of malaria vaccines. PLoS One 3 e3193 doi:10.1371/journal.pone.0003193
52. The malERA Consultative Group on Modeling 2011 A research agenda for malaria eradication: Modeling. PLoS 8 e1000403 doi:10.1371/journal.pmed.1000403
53. The malERA Consultative Group on Diagnoses and Diagnostics 2011 A research agenda for malaria eradication: Diagnoses and diagnostics. PLoS 8 e1000396 doi:10.1371/journal.pmed.1000396
54. World Health Organization 2000 Making use of vaccine vial monitors. Flexible vaccine management for polio. Geneva: World Health Organization Available: www.who.int/vaccines-documents/DocsPDF00/www516.pdf
55. World Health Organization 2006 Procedures for assessing the acceptability, in principle, of vaccines for purchase by United Nations agencies. Geneva: World Health Organization Available: www.who.int/vaccines-documents/DocsPDF06/812.pdf
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 1- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- A Simple Novel Method for Determining Mortality Rates in HIV Treatment Programs Worldwide
- Setting Implementation Research Priorities to Reduce Preterm Births and Stillbirths at the Community Level
- A Research Agenda for Malaria Eradication: Monitoring, Evaluation, and Surveillance
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- A Research Agenda to Underpin Malaria Eradication
- Correcting Mortality for Loss to Follow-Up: A Nomogram Applied to Antiretroviral Treatment Programmes in Sub-Saharan Africa
- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- Setting Research Priorities to Reduce Almost One Million Deaths from Birth Asphyxia by 2015
- Predicting Live Birth, Preterm Delivery, and Low Birth Weight in Infants Born from In Vitro Fertilisation: A Prospective Study of 144,018 Treatment Cycles
- Some Lessons for the Future from the Global Malaria Eradication Programme (1955–1969)
- A Research Agenda for Malaria Eradication: Basic Science and Enabling Technologies
- A Research Agenda for Malaria Eradication: Vector Control
- The Role of Research in Viral Disease Eradication and Elimination Programs: Lessons for Malaria Eradication
- The Influence of Distance and Level of Care on Delivery Place in Rural Zambia: A Study of Linked National Data in a Geographic Information System
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
- Development of a Standardized Screening Rule for Tuberculosis in People Living with HIV in Resource-Constrained Settings: Individual Participant Data Meta-analysis of Observational Studies
- WHO/PLoS Collection “No Health Without Research”: A Call for Papers
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- A Research Agenda for Malaria Eradication: Vaccines
- A Research Agenda for Malaria Eradication: Health Systems and Operational Research
- A Research Agenda for Malaria Eradication: Diagnoses and Diagnostics
- A Research Agenda for Malaria Eradication: Drugs
- A Research Agenda for Malaria Eradication: Modeling
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



