-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Estimated Glomerular Filtration Rate in Oncology Patients before Cisplatin Chemotherapy
Odhadovaná glomerulární filtrace u onkologických pacientů před chemoterapií cisplatinou
Východiska:
Cílem práce je porovnat glomerulární filtraci měřenou izotopovou metodu (measured glomarular filtration rate – mGFR DTPA) a odhadovanou glomerulární filtraci (estimated GFR – eGFR). Glomerulární filtrace (GFR) je odhadovaná ze sérového kreatininu (eGFRcreatinine), sérového cystatinu C (eGFRcystatin C) a pomocí kombinované rovnice (eGFRcreatinine + cystatin C). Studie se zaměřuje na onkologické pacienty zvažované pro léčbu cis‑diamindichlorplatinou (cisplatin). Hodnotili jsme dopad různých GFR metod na redukci dávky cisplatiny.Materiál a metody:
Studovaná populace byla tvořena 112 po sobě jdoucími pacienty z onkologického centra ve Zlíně v České republice, kteří byli zvažováni pro léčbu cisplatinou. mGFR DTPA byla provedena dynamickou renální scintigrafií využívající kyselinu diethyltriaminopentaaoctovou (DTPA) značenou izotopem technecia 99mTc. Kreatinin a cystatin C byly stanoveny nově standardizovanými metodami. Odhad GFR byl počítán podle The Chronic Kidney Disease Epidemiology (CKD ‑ EPI) rovnic, které byly vytvořeny v letech 2009 a 2012.Výsledky:
Medián (mezikvartilové rozpětí) mGFR DTPA byl 1,335 ml/ s/ 1,73 m2 (1,070 – 1,725). Medián eGFRcystatin C 1,195 ml/ s/ 1,73 m2 (0,885 – 1,625) byl nižší než medián mGFR DTPA (p < 0,05). Medián eGFRcreatinine 1,460 ml/ s/ 1,73 m2 (1,210 – 1,660) byl vyšší než mGFR DTPA (p < 0,05). Korelační analýza a Bland ‑ Altmanův rozdílový graf ukazují velké individuální rozdíly mezi mGFR DTPA a všemi eGFR. Závěr: Onkologičtí pacienti jsou specifická skupina pacientů, která se liší od všeobecné populace. Byly nalezeny významné individuální rozdíly mezi mGFR DTPA a všemi eGFR. To má velký dopad na detekci pacientů s CKD a potenciální úpravu dávky léků.Klíčová slova:
kreatinin – cystatin C – chronické selhání ledvin – glomerulární filtrace – nemoci ledvin – technecium Tc 99m pentetát – cisplatina – vyšetření funkce ledvin
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Obdrženo:
16. 2. 2015Přijato:
26. 5. 2015
Authors: T. Salek 1,2; P. Veselý 3; J. Bernatek 4
Authors place of work: Department of Clinical Biochemistry, Tomas Bata Hospital in Zlin a. s., Czech Republic 1; Department of Biomedical Sciences, Faculty of Medicine, University of Ostrava, Czech Republic 2; Oncology Centre, Tomas Bata Hospital in Zlin a. s., Czech Republic 3; Department of Nuclear Medicine, Tomas Bata Hospital in Zlin a. s., Czech Republic 4
Published in the journal: Klin Onkol 2015; 28(4): 273-277
Category: Původní práce
doi: https://doi.org/10.14735/amko2015273Summary
Background:
The aim of the study is to compare measured glomerular filtration rate by technetium radiolabled diethylene tiamine pentaacetic acid (mGFR DTPA) to estimated GFR (eGFR). Glomerular filtration rate (GFR) is estimated from serum creatinine (eGFRcreatinine), serum cystatin C (eGFRcystatin C) and by combined equation (eGFRcreatinine + cystatin C). This study focuses on oncology patients considered for treatment with cis‑diamminedichloroplatinum (cisplatin). We evaluated the impact of different GFR methods on the reduction of cisplatin dose.Patients and Methods:
The study population consisted of 112 consecutive oncology patients from oncology center treated in the town of Zlin in the Czech Republic, who were considered for cisplatin treatment. mGFR DTPA was performed by dynamic renal 99mTc scintigraphy method using diethyltriaminepentaacetic acid. Creatinine and cystatin C were determined by newly standardized tests. Estimation of GFR was calculated using The Chronic Kidney Disease Epidemiology (CKD ‑ EPI) equations which were established in 2009 and 2012.Results:
The median (interquartile range) of mGFR DTPA was 1.335 ml/ s/ 1.73 m2 (1.070 – 1.725). The median of eGFRcystatin C 1.195 ml/ s/ 1.73 m2 (0.885 – 1.625) was lower than mGFR DTPA (p < 0.05). The median of eGFRcreatinine 1.460 ml/ s/ 1.73 m2 (1.210 – 1.660) was higher than mGFR DTPA (p < 0.05). Correlation analysis and Bland ‑ Altman plots show high individual differences between mGFR DTPA and all eGFR‘s. Conclusions: Oncology patients are a very special group of patients who differ from general population. There are significant individual differences between mGFR DTPA and all eGFR‘s, impacting detection rate of CKD and potential drug dosage adjustment.Key words:
creatinine – cystatin C – renal insufficiency, chronic – glomerular filtration rate – kidney diseases – technetium Tc 99m pentetate – cisplatin – Kidney function testsBackground
Therapeutic doses of drugs excreted by kidneys must be adjusted according to the glomerular filtration rate (GFR). The GFR is the most important parameter of kidney function. The decision GFR point of 1.0 ml/ s/ 1.73 m2 is used for reduction of dose of drugs excreted by kidneys. The state of GFR below 1.0 ml/ s/ 1.73 m2 for more than three consecutive months is also defined as chronic kidney disease (CKD) according to Kidney Disease Improving Global Outcomes (KDIGO) guidelines [1]. Cisplatin is a well‑established chemotherapeutic agent for many solid tumors. This drug has multiple nephrotoxic side effects [2]. The dose of cisplatin must be decreased in correlation with the decline of GFR [3].
Reduction of drug doses is a very important reason why we need a reliable method for the assessment of GFR.
Isotopic methods are also clinically available methods for the determination of GFR but are available only in specialized centers, are invasive, time consuming, have radiation burden and are not feasible for all patients.
Calculating clearance of some substances with measuring urine output is also difficult.
Estimating GFR from a serum endogenous substance without urine collection is another way to determine GFR. The serum creatinine and cystatin C are the most commonly established serum markers to the estimation of GFR. Creatinine is the waste product of muscle energy metabolism. It is produced at a constant rate. Cystatin C is produced by all nucleated cells at a constant rate [4]. Both creatinine and cystatin C methods have been standardized and CKD ‑ EPI equations for estimation of GFR have been established [5].
Oncology patients are a specific subgroup of patients. They are characterized by the burden of tumor mass and by often reduced muscle mass.
We compared mGFR DTPA and eGFR from serum creatinine and cystatin C using new CKD ‑ EPI equations in oncology patients considered for treatment with cisplatin.
Oncologists reduce the dose of cisplatin when the GFR is below 1.0 ml/ s/ 1.73 m2. We also evaluated impact of different GFR methods on this therapeutic dose decision making.
Patients and methods
Patients
The study population consisted of 112 consecutive oncology patients from Oncology center of Tomas Bata regional hospital in the town of Zlin, who were considered for treatment with cisplatin.
Majority of patients (pts) had head and neck cancer (46 pts). One patient with metastatic bladder cancer was included (palliative chemotherapy), and five other patients with urothelial carcinoma were treated in adjuvant setting (one patient treated with chemotherapy after nephrectomy, four pts with concomitant chemo ‑ radiotherapy). The frequency of other tumors in descending order was as follows: 16 pts with cervical cancer, 11 pts with esophageal cancer, 9 pts with gastric cancer, 7 pts with testicular tumors (all of them in adjuvant setting), 5 pts with endometrial uterine cancer, 3 pts with cancer of biliary tract, 3 pts with occult primary cancer, 2 pts with anal cancer, 2 pts with malignant melanoma, 1 patient with lung cancer (non‑small cell lung cancer), and 1 patient with squamous‑cell gynecological cancer. Fortyseven of all patients were treated in palliative setting.
The study lasted from April 2012 to June 2013 and was approved by the Ethics Committee of Tomas Bata regional hospital.
All patients had mGFR DTPA imaging, serum creatinine and serum cystatin C tests performed.
As for co morbid conditions, 53 pts had also arterial hypertension and 17 pts had diabetes mellitus. Nine patients had preexisting nephropathy (3 with hyperuricemia, 2 were after unilateral nephrectomy, 2 with hydronephrosis solved with stenting or nephrostomia, 1 with chronic pyelonephritis, and 1 with nephrolithiasis).
mGFR DTPA
mGFR DTPA was performed at the department of nuclear medicine. The radioactive agent of 99mTc DTPA was applied to patients in a single bolus injection without urine collection [6].
Creatinine
Serum creatinine was determined by enzymatic photometric method standardized against certified reference material named NIST SRM 967 [7,8]. The Chronic Kidney Disease Epidemiology (CKD ‑ EPI) Collaboration research group developed equation for estimation of GFR from serum creatinine in 2009. It is named CKD ‑ EPI equation (eGFRcreatinine) [9].
Cystatin C
Cystatin C was measured by immunoturbidimetric standardized method [10]. In 2012 the CKD ‑ EPI Collaboration research group developed an equation for estimation of GFR from serum cystatin C (eGFRcystatin C) and a combined equation for estimation from both serum creatinine and cystatin C (eGFRcreatinine + cystatin C) [5].
Statistical methods
The D’Agostino ‑ Pearson test was used to assess normal distribution of GFR results.
The GFR results did not have a normal distribution, so we used nonparametric tests for data analysis.
The Friedman test was used for comparison of four medians.
The Spearman correlation analysis was performed for correlation among GFR methods.
The Bland ‑ Altman plots were used for comparison of two GFR methods.
Results
mGFR DTPA and eGFR results are compared in Tab. 1.
Tab. 1. Results of mGFR DTPA and eGFR methods. 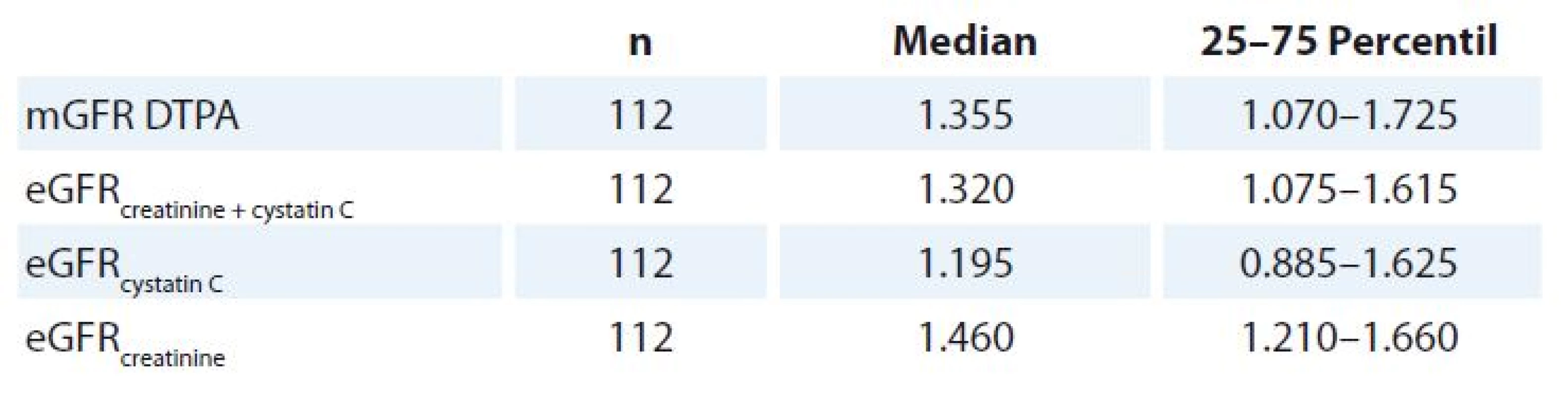
The median of eGFRcystatin C 1.195 ml/ s/ 1.73 m2 (0.885 – 1.625) was lower than mGFR DTPA 1.335 ml/ s/ 1.73 m2 (1.070 – 1.725) (p < 0.05).
The median of eGFRcreatinine 1.460 ml/ s/ 1.73 m2 (1.210 – 1.660) was higher than mGFR DTPA (p < 0.05).
mGFR DTPA results would detect CKD and reduce cisplatin dose in 20 patients, eGFRcystatin C in 31 patients, eGFRcreatinine in 10 patients and eGFRcreatinine + cystatin C in 22 patients.
Correlations among GFR methods are shown in Tab. 2. Correlation analysis found no difference among correlation coefficients of mGFR DTPA and all eGFR‘s (p > 0.05).
Tab. 2. Results of Spearman correlation among GFR methods. 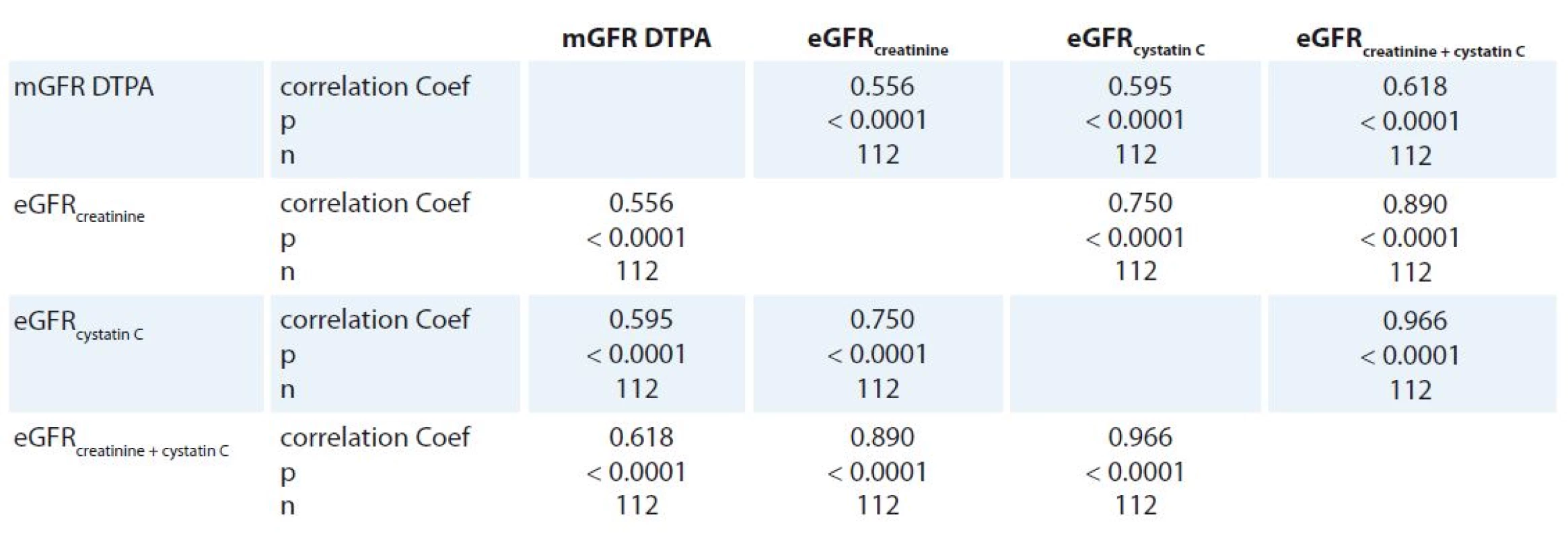
eGFR – estimated GFR, mGFR DTPA – compare measured glomerular filtration rate The individual differences between mGFR and three estimated GFR‘s are displayed in Graphs 1 – 3.
Graph 1 shows high degree of individual differences between methods and the trend that in the range of GFR bellow 1.0 ml/ s/ 1.73 m2 eGFRcreatinine is more apparently higher than mGFR DTPA.
Graph 1. Bland-Altman difference plot between mGFR DTPA and eGFR<sub>creatinine*</sub> 
Graphs 2 and 3 show high degree of individual differences between methods and no trend.
Graph 2. Bland-Altman difference plot between mGFR DTPA and eGFR<sub>cystatin C*</sub>. 
Graph 3. Bland-Altman difference plot between mGFR DTPA and eGFR<sub>creatinine + cystatin C*</sub>. 
Discussion
Population of 112 oncology patients with diagnoses which require treatment with cisplatin was involved in this study. Because cisplatin is nephrotoxic, hydration and reduction of dose of cisplatin according to GFR are key stones of nephrotoxicity prevention.
The dose reduction of nephrotoxic drugs was historically calculated from serum creatinine according to the Cockcroft & Gault formula [11].
Later, it was recommended to reduce the dose of cisplatin in patients with creatinine clearance 50 – 60 ml/ min. The cisplatin is not given to patients with clearance of creatinine below 40 ml/ min. But clearance of creatinine overestimates the true GFR [12]. Further, urine collections are cumbersome, and incomplete collections are frequent in clinical practice. All methods which we compare in this study do not require urine collection.
Today the KDIGO guidelines recommend the decrease of cisplatin dose when GFR is below 1.0 ml/ s/ 1.73 m2. eGFRcreatinine and eGFRcystatin C are recommended only for clinically stable patients. The guidelines recommend using reference method in clinical situation where eGFRcreatinine or eGFRcystatin C are inaccurate or biased [1]. This may be the case in many oncology patients and our results confirm it.
Performance of reference methods, such as inulin clearance, is impractical in clinical practice. One of the methods for determining GFR is mGFR DTPA. Today, single bolus mGFR DTPA is regarded unsuitable as a reference method by some investigators [13]. Limitations of the mGFR DTPA method were discussed also earlier, before standardization of creatinine measurement [14]. It is recommended that all GFR studies at a given center should use the same radiopharmacum [15]. Current protocol for prevention of nephrotoxicity of cisplatin is available. This protocol takes into account hydration and renal function. Patients should be advised to drink 2 liters of fluid over the 24 hours following the therapy. Ensured urine output is over 100 ml/ hour prior to the cisplatin dose [16]. If cisplatin is changed for carboplatin, Calvert formula for drug dosing can be used [17].
The highest median of GFRs was the median of eGFRcreatinine. Oncology patients tend to have reduced muscle mass [18]. Falsely reduced serum creatinine causes falsely increased estimation of GFR. It highlights the importance of screening for malnutrition in oncology patients [19].
The Bland ‑ Altman plot comparing mGFR DTPA and eGFRcreatinine indicates that in the range of GFR bellow 1.0 ml/ s/ 1.73 m2, eGFRcreatinine gives more apparently higher values than mGFR DTPA. Similar results were also found in other cohorts of patients [20 – 22].
The lowest median of GFRs was that of eGFRcystatin C. Cystatin C may be produced by some tumor cells. It was shown that oncology patients have increased serum level of cystatin C [23,24]. It may explain our results.
GFR usually decreases with age. Age, gender, ethnicity and serum level of creatinine and cystatin C are included in CKD ‑ EPI equations [1].
All three Bland ‑ Altman plots and correlation coefficients show high individual differences between mGFR DTPA and any eGFR. It indicates that estimations of GFR from serum creatinine and/ or cystatin C are not reliable methods for determination of GFR in oncology patients. We compared eGFRcreatinine and eGFRcystatin C in 352 consecutive stable patients with CKD and found the Spearman correlation coefficient of 0.912 (p < 0.001). We used the same methods and equations as in this oncology patients study [25]. The Spearman correlation coefficient between eGFRcreatinine and eGFRcystatin C in this oncology patient cohort was 0.750 (p < 0.0001). It shows that correlation between these two methods of GFR is much better in stable patients with CKD than in oncology patients.
The limitation of this study is the absence of inulin reference method for determination of GFR and the limited number of patients. We also did not measure muscle mass in our patients.
Conclusions
Oncology patients are a very special group of patients who differ from general population.
There are significant individual differences between mGFR DTPA and all eGFR‘s. It has an important impact on the detection rate of CKD and a potential drug dosage adjustment.
The median of eGFRcystatin C was lower than mGFR DTPA.
The median of eGFRcreatinine was higher than mGFR DTPA.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Submitted: 16. 2. 2015
Accepted: 26. 5. 2015
MUDr. Tomas Salek, Ph.D.
Department of Clinical Biochemistry
Tomas Bata Hospital in Zlin a. s.
Havlickovo nabrezi 600
762 75 Zlin
Czech Republic
e-mail: tsalek@seznam.cz
Zdroje
1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3 : 1 – 150.
2. Miller RP, Tadagavadi RK, Ramesh G et al. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010; 2(11): 2490 – 2518. doi: 10.3390/ toxins2112490.
3. Reed E, Jacob J, Brawley O. Measures of renal function in patients with cisplatin‑related chronic renal disease. J Natl Med Assoc 1991; 83(6): 522 – 526.
4. Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem 2012; 58(4): 680 – 689. doi: 10.1373/ clinchem.2011.167494.
5. Inker LA, Schmid CH, Tighiouart H et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367(1): 20 – 29. doi: 10.1056/ NEJMoa1114248.
6. Vížďa J, Lepej J, Křížová H et al. Atlas scintigrafie ledvin = Atlas of renal scintigraphy. 1. vyd. Praha: Agentura Pankrác 2002 : 72.
7. Dodder NG, Tai SS, Sniegoski LT et al. Certification of creatinine in a human serum reference material by GC ‑ MS and LC ‑ MS. Clin Chem 2007; 53(9): 1694 – 1699.
8. Drion I, Cobbaert C, Groenier KH et al. Clinical evaluation of analytical variations in serum creatinine measurements: why laboratories should abandon Jaffe techniques. BMC Nephrol 2012; 13 : 133. doi: 10.1186/ 1471 ‑ 2369 ‑ 13 ‑ 133.
9. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150(9): 604 – 612.
10. Grubb A, Blirup ‑ Jensen S, Lindström V et al. First certified reference material for cystatin C in human serum ERM ‑ DA471/ IFCC. Clin Chem Lab Med 2010; 48(11): 1619 – 1622. doi: 10.1515/ CCLM.2010.318.
11. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16(1): 31 – 41.
12. Jabor A, Hornová L, Fantová L et al. Vyšetření funkce ledvin: možnosti biochemické laboratoře. Postgrad Med 2006; 9 : 18 – 23.
13. Xie P, Huang JM, Liu XM et al. (99m)Tc ‑ DTPA renal dynamic imaging method may be unsuitable to be used as the reference method in investigating the validity of CDK ‑ EPI equation for determining glomerular filtration rate. PLoS One 2013; 8(5): e62328. doi:10.1371/ journal.pone.0062328.
14. Itoh K. Comparison of methods for determination of glomerular filtration rate: Tc ‑ 99m ‑ DTPA renography, predicted creatinine clearance method and plasma sample method. Ann Nucl Med 2003; 17(7): 561 – 565.
15. Fleming JS, Zivanovic MA, Blake GM et al. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun 2004; 25(8): 759 – 769.
16. National health service. ASWCS Cisplatin Hydration Protocol [homepage on the Internet]. [accessed 13 June 2014]. Available from: http:/ / www.avon.nhs.uk/ aswcs ‑ chemo/ NetworkPolicies/ .
17. Calvert AH, Newell DR, Gumbrell LA et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 1989; 7(11): 1748 – 1756.
18. Šefr R, Ondrák M, Krsička P et al. Liposarcoma retroperitonei permagnum. Klin Onkol 2012; 25(4): 306.
19. Illa P, Tomíšková M, Skřičková J. Screening rizika malnutrice versus ukazatelé nutričního stavu a systémové zánětlivé odpovědi u pacientů s nově diagnostikovaným karcinomem plic. Klin Onkol 2014; 27(4): 261 – 268. doi: 10.14735/ amko2014261.
20. Šálek T, Ponížil P. Estimated glomerular filtration rate in diabetic patients. Klin Biochem Metab 2014; 22(43): 4 – 7.
21. Jabor A, Franeková J, Kubíček Z et al. eGFR a problémy interpretace rovnic CKD ‑ EPI. Klin Biochem Metab 2014; 22(43): 8 – 10.
22. Delanaye P, Cavalier E, Moranne O et al. Creatinine ‑ or cystatin C‑based equations to estimate glomerular filtration in the general population: impact on the epidemiology of chronic kidney disease. BMC Nephrol 2013; 14 : 57. doi: 10.1186/ 1471 ‑ 2369 ‑ 14 ‑ 57.
23. Kos J, ˘Stabuc B, Cimerman N et al. Serum cystatin C, a new marker of glomerular filtration rate, is increased during malignant progression. Clin Chem 1998; 44(12): 2556 – 2557.
24. Zhang X, Hou Y, Niu Z et al. Clinical significance of detection of cathepsin X and cystatin C in the sera of patients with lung cancer. Zhongguo Fei Ai Za Zhi 2013; 16(8): 411 – 416. doi: 10.3779/ j.issn.1009 ‑ 3419.2013.08.04.
25. Šálek T, Palička V. Comparison of creatinine clearance and estimated glomerular filtration rate in patiens with chronic kidney disease. Klin Biochem Metab 2014; 22(43): 121–12 4.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2015 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Nejasný stín na plicích – kazuistika
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- Editorial
- Modern Nanomedicine in Treatment of Lung Carcinomas
- Potential of Cell‑free Circulating DNA in Diagnosis of Cancer
- The Possibility of Epidermal Growth Factor Receptor Inhibition in Anal Cancer
- Cost‑effectiveness Analysis of Panitumumab Plus mFOLFOX6 Compared to Bevacizumab Plus mFOLFOX6 for First‑line Treatment of Patients with Wild‑type RAS Metastatic Colorectal Cancer – Czech Republic Model Adaptation
- Estimated Glomerular Filtration Rate in Oncology Patients before Cisplatin Chemotherapy
- Incidence and Prognostic Value of Known Genetic Aberrations in Patients with Acute Myeloid Leukemia – a Two Year Study
- Extraoseus Ewing‘s Sarcoma, Primary Affection of Uterine Cervix – Case Report
- Embryonal Tumors with Multilayer Rosettes – Rare Central Nervous System Tumors in Infants
- Anticoagulation and Thrombembolism During Bevacizumab Treatment – To Be Careful or Fearful?
- SOUTĚŽ O NEJLEPŠÍ PRÁCI
- Domácí parenterální výživa v onkologii
- Aktuality z odborného tisku
- Plicní fibróza po oxaliplatině
- SOUTĚŽ NA PODPORU AUTORSKÝCH TÝMŮ PUBLIKUJÍCÍCH V ZAHRANIČNÍCH ODBORNÝCH TITULECH
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Extraoseus Ewing‘s Sarcoma, Primary Affection of Uterine Cervix – Case Report
- Domácí parenterální výživa v onkologii
- Embryonal Tumors with Multilayer Rosettes – Rare Central Nervous System Tumors in Infants
- Potential of Cell‑free Circulating DNA in Diagnosis of Cancer
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



