-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Antimicrobial effect of novel hydrogel matrix based on natural polysaccharide Sterculia urens
Authors: B. Lipový 1,2; J. Holoubek 1,2; L. Vacek 1,3; F. Růžička 1,3; E. Nedomová 4; H. Poštulková 4; L. Vojtová 4
Authors place of work: Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Department of Burns and Plastic Surgery, University Hospital Brno, Czech Republic 2; Department of Microbiology, St. Anna University Hospital, Brno, Czech Republic 3; CEITEC – Central European Institute of Technology, Brno University of Technology, Czech Republic 4
Published in the journal: Epidemiol. Mikrobiol. Imunol. 67, 2018, č. 4, s. 166-174
Category: Původní práce
Summary
Introduction:
Materials for modern wound-management are a very broad and heterogeneous group. One of the most important representatives is natural materials, or more precisely polysaccharides isolated from various plants and animals. With the increasing resistance of pathogens to established antimicrobial agents, there is also an attempt to discover new mechanisms of the effects of these materials. Gum karaya (GK) is a very promising representative of the natural polysaccharides group and, since it is obtained from Sterculia urens as resin, it is also possible to assume its certain antimicrobial activity.
Material and methodology:
The antimicrobial potential of GK and chitosan (Ch) has been tested on several preselected strains to match the real epidemiological situation of the agents of infectious complications in the field of burned wounds. Tested strains included representatives of gram-positive and gram-negative bacteria as well as selected yeasts. Methicillin susceptible Staphylococcus aureus CCM 4223 (ATCC 29213), methicillin resistant Staphylococcus aureus CCM 4750 (ATCC 43300), Klebsiella pneumoniae CCM 4985 (ATCC 700603), Candida albicans CCM 8261 (ATCC 90028), Pseudomonas aeruginosa CCM 3955 (ATCC 27853) were obtained from the Czech Collection of Microorganisms. Pseudomonas aeruginosa FF 1, Pseudomonas aeruginosa FF 2 and Pseudomonas aeruginosa FF 3 (all multi-resistant clinical strains), Staphylococcus epidermidis A 013, Staphylococcus epidermidis A 117, and Candida parapsilosis BC 11 were obtained from the Collection of Microorganisms at the St. Anne’s University Hospital, Brno. Antimicrobial tests were performed using the disk diffusion test methodology.
Another set of antimicrobial tests was obtained by measuring the growth curves.
Results:
Bacteriostatic activity testing showed 1% GK concentration and both 1% and 0.5% chitosan concentration effective against all pathogens tested. The combination of GK50/Ch50 in concentrations of 1% and 0.5% had similar or better effect. Lower concentrations of the combined material are poorly effective against tested strains. Bactericidal activity testing has not produced positive results, except for Candida spp., where only a partial effect of GK50/Ch50 was observed at 1% concentration.
In the growth curve test, the efficiency of both GK alone and chitosan was found to be significantly higher in gram-positive bacteria compared to gram-negative ones. In the case of this experiment, only a one-tenth concentration was used compared to the disk diffusion test concentration. This results correspond with the data from the bacteriostatic activity testing.
Conclusion:
This is the first publication that attempts to comprehensively define the potential for GK antimicrobial activity and also the possible potentiation of this activity with the use of chitosan. Further experiments are needed to extend the antimicrobial efficiency to gram-negative bacteria.
Keywords:
Gum Karaya – hydrogel – antimicrobial activity – wound healing – burn wound
INTRODUCTION
Today’s modern times are a great challenge for the advanced application of biopolymer materials in the clinical medicine field. The demand for new technological solutions is increasing along with higher demands on treatment quality. The vision of current clinical practice in the treatment of chronic skin defects and acute wounds caused by high energy, such as mechanical trauma or burn trauma, is not only a matter of patien's survival but also an improvement in the quality of their life [1].
There are a number of natural polymers which are well researched and improved (hyaluronic acid, cellulose, starch, chitosan). On the other hand, there are polysaccharides that are not well researched because they have only been discovered and described recently. Gum Karaya (GK) is an example of not so well-known natural polysaccharide with a wide spectrum of utilisation mainly as food supplement and recently as wound healing coverings [2].
Surgical infection is a major player in delayed wound healing and belongs among the most frequent complications in daily clinical practice. In the modern concept of wound healing, emphasis is now placed on the maximum possible antiseptic efficiency across the spectrum of potential pathogens, and not only from bacterial representatives [3].
In this paper, results of in vitro testing exhibit potential antimicrobial effect of novel hydrogels based on natural polysaccharide GK are presented.
Theoretical background
The present study is an attempt to prepare the no-vel Gum Karaya-chitosan (GK-Ch) hydrogels (natural component) based on polyvinyl alcohol (PVA) polymer matrix (synthetic component) crosslinked by citric acid (CA).
GK is a natural gum exudate of Sterculia urens, a tree native to India and belongs to the Sterculiaceae family. The wider applications of GK are due to its unique features such as high swelling and water retention capacity, high viscosity properties, inherent nature of anti-microbial activity, abundant availability and lower price [4]. It is also evidenced from literature that GK was used as a laxative due to its high swelling ability and formation of discontinuous mucilage. GK has found many applications in pharmaceutical formulations such as tablets, emulsions, ointments or any other sustained released or controlled released formulations [5].
The chemical composition of GK (Sterculia gum) varies a little between the exploited tree species. The gum is calcium and magnesium salt, with a central chain of D-galactose, L-rhamnose and D-galacturonic acid units with some side chains containing D-glucuronic acid. It contains uronic acid residues and acetyl groups. Due to the presence of these acetyl groups, natural GK is insoluble and only swells. The solubility of GK increased after alkali treatment due to the elimination of acetyl groups, when multivalent ions (Ca2+, Mg2+) were exchanged by monovalent ions (Na+, K+) [2].
Chitosan is a cationic heteropolysaccharide and is obtained by alkaline deacetylation of chitin. Chitosan is a copolymer of N-acetyl-D-glucosamine and D-glucosamine consisting of linear β-1,4-linked units. Both the content and sequence of these units will determine the physico-chemical and biological properties of the polymer [6].
Our initial in vitro tests demonstrated absolute cell non-toxicity of GK on mouse 3T3 fibroblasts and very low material adhesion to newly created neoepithel (Figure 1).
Fig. 1. Viability of mouse 3T3 fibroblasts under a fluorescence microscope and a peel-off test of hydrogel adhesion to keratinocytes 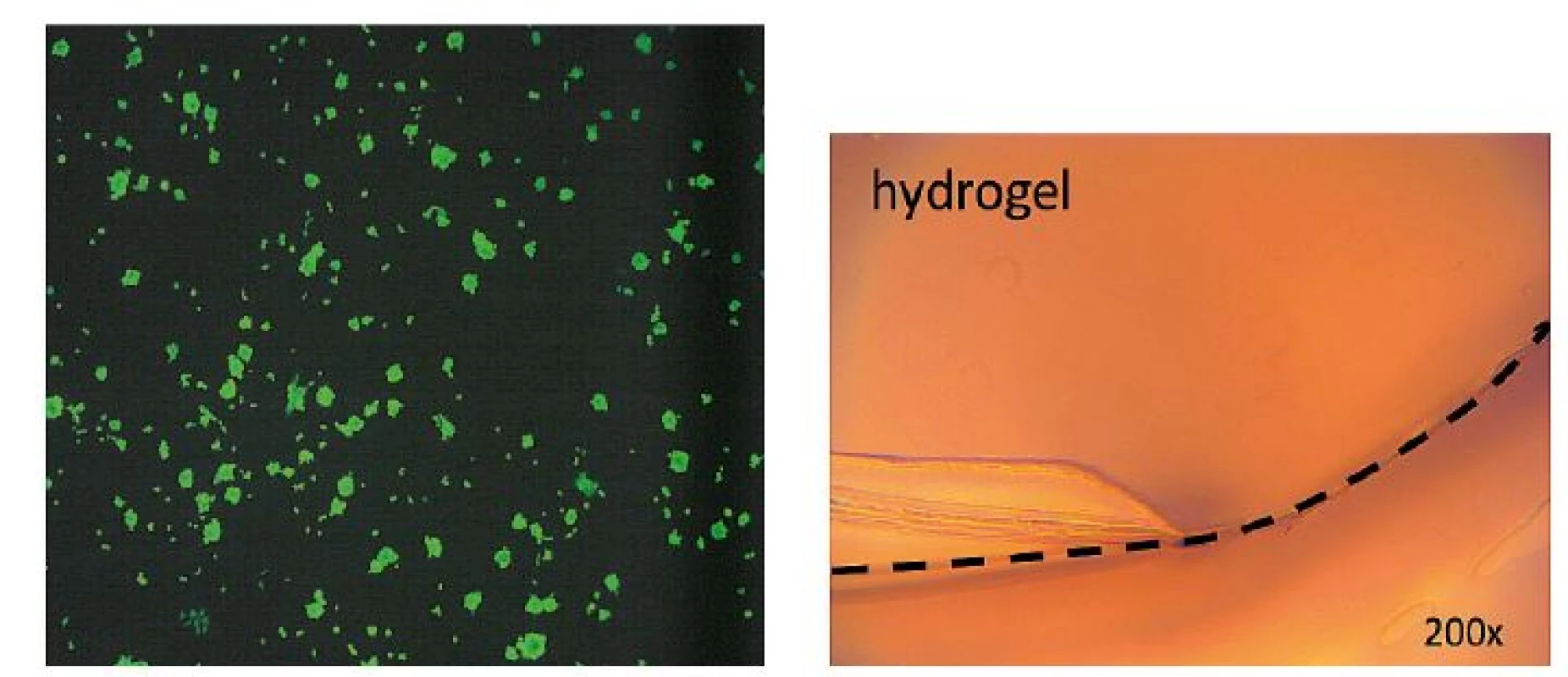
ANTIMICROBIAL ACTIVITY TESTING
Strains, media and antimicrobial substances
Methicillin susceptible Staphylococcus aureus CCM 4223 (ATCC 29213), methicillin resistant Staphylococcus aureus CCM 4750 (ATCC 43300), Klebsiella pneumoniae CCM 4985 (ATCC 700603), Candida albicans CCM 8261 (ATCC 90028), Pseudomonas aeruginosa CCM 3955 (ATCC 27853) were obtained from the Czech Collection of Microorganisms. Pseudomonas aeruginosa FF 1, Pseudomonas aeruginosa FF 2 and Pseudomonas aeruginosa FF 3 (all multi-resistant clinical strains), Staphylococcus epidermidis A 013, Staphylococcus epidermidis A 117, and Candida parapsilosis BC 11 were obtained from the Collection of Microorganisms at St. Anne’s University Hospital, Brno.
Blood agar was prepared from Columbia blood agar base (Oxoid, UK) and 5% v/v sterile defibrinated sheep blood and used for disk diffusion tests. Brain Heart Infusion (Oxoid, UK) was used for growth curves measurement. Normal saline was used to dilute bacterial inoculum and antimicrobial substances.
Gum Karaya (commercial grade) deacetylated according to [7] and low viscosity chitosan (degree of deacetylation ≥ 75%) were purchased from Sigma-Aldrich. Deacetylated GK (1% w/v), chitosan (1% w/v) and mixture GK50/Ch50 (1% + 1% w/v) were tested for their antimicrobial properties. GK (1% w/v) and chitosan (1% w/v) were prepared by diluting the original GK (2% w/v in 0.1 M HCl) and chitosan (2% w/v in 0.1 M HCl) samples with saline. A concentration gradient of antimicrobial substances was obtained by the serial dilution with saline. The final concentration gradient used: 1%, 0.5%, 0.25%, 0.125% and 0.0625% (all samples % w/v). Saline was used as a growth control.
Disk diffusion tests
Antimicrobial test were performed using disk diffusion test methodology. Briefly, a bacterial inoculum was prepared in saline to the density of a McFarland 0.5 turbidity standard from overnight culture on blood agar. Blood agar plates were inoculated with the bacterial inoculum by swabbing.
When bacteriostatic activity was tested, a 10 µl drop of the antimicrobial substance was placed on the blood agar surface 15 minutes after inoculation and then the plates were placed in a temperature of 37 °C for 24 hours. When bactericidal activity had been tested, the inoculated blood agar plates were first placed in a temperature of 37 °C for 24 hours. Then a 10 µl drop of antimicrobial substance was placed on the surface and the plates were placed in a temperature of 37 °C for a further 24 hours.
The samples were evaluated on a scale of 0 to 3 (0 for total inhibition, no colonies inside the inhibition zone; 1 for substantial inhibition, < 10 colonies inside the inhibition zone; 2 for weak inhibition, > 10 colonies inside the inhibition zone; 3 for no visible inhibition zone).
Growth curves
Another set of antimicrobial tests were obtained by measuring growth curves at OD600 nm. Briefly, a bacterial inoculum was prepared in saline to the density of a McFarland 0.5 turbidity standard from overnight culture on blood agar. Working inoculum was prepared by diluting bacterial inoculum ten times in brain heart infusion (Oxoid, UK). 90 µl of the working inoculum and 10 µl of the antimicrobial substance were pipetted into the 384-well plate with non-treated surface (Nunc, Denmark) and sealed with a sealing tape (Nunc, Denmark). Therefore, the concentration gradient of the antimicrobial substance is ten times lower than the one used in the disk diffusion test. Then 20-hours cultivation at 37 °C with shaking took place. Optical Density (OD) was measured every five minutes at OD600 nm with Infinite® M200 PRO Reader (Tecan, Switzerland). The samples were evaluated on a scale of 0 to 3 (0 for total inhibition, i.e. no changes in optical density; 1 for substantial inhibition, i.e. optical density rises after 12 hours or more; 2 for weak inhibition, i.e. optical density rises more than two hours later than the control samples; 3 for no visible inhibition, i.e. optical density corresponds with the control samples).
All tests were performed at least three times in triplicate.
RESULTS
Antimicrobial activity of GK, chitosan and GK/Ch
The bacteriostatic activity results using the disk diffusion test are summarised in Table 1. GK at 1% concentration completely or substantially inhibited the growth of all tested microbial strains. GK at 0.5% concentration substantially or weakly inhibited the growth of all tested microbial strains. Lower GK concentrations failed to inhibit the growth. Chitosan at 1 % and 0.5 % concentrations completely or substantially inhibited the growth of all tested microbial strains. Chitosan at 0.25% substantially or weakly inhibited the growth of all tested microbial strains. Lower chitosan concentrations did not inhi-bit growth, except from staphylococcal and candida strains, which showed weak growth inhibition. GK50/Ch50 mixture at 1% and 0.5% concentration completely or substantially inhibited the growth of all tested microbial strains. GK50/Ch50 mixture at 0.25% substantially or weakly inhibited the growth of all tested microbial strains. Lower GK50/Ch50 mixture concentrations affected mostly the candida growth. The rest of the tested microbial strains were inhibited weakly or not at all (see Table 1).
Tab. 1. Bacteriostatic activity results using the disk diffusion test 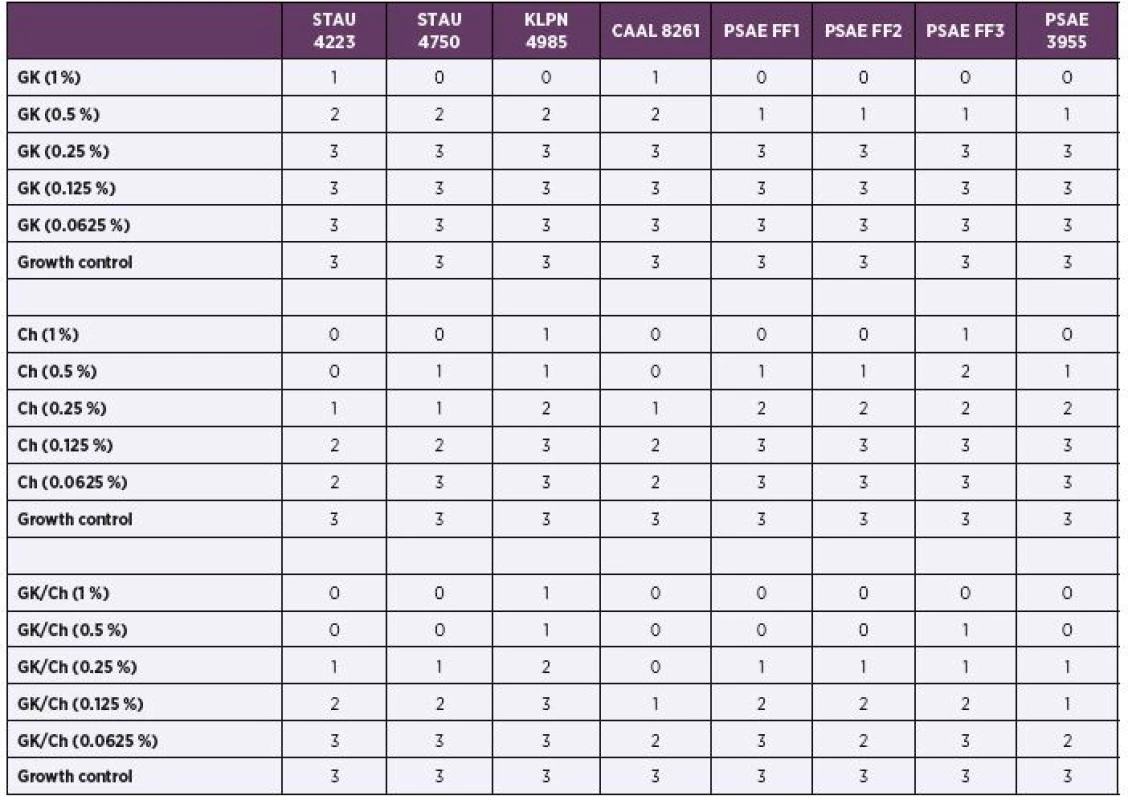
GK – Gum Karaya; Ch – chitosan; GK/Ch – Gum Karaya and chitosan mixture. STAU 4223 – Staphylococcus aureus CCM 4223, STAU 4750 – Staphylococcus aureus CCM 4750, KLPN 4985 – Klebsiella pneumoniae CCM 4985, CAAL 8261 – Candida albicans CCM 8261, PSAE 3955 – Pseudomonas aeruginosa CCM 3955, PSAE FF 1 – Pseudomonas aeruginosa FF 1, PSAE FF 2 – Pseudomonas aeruginosa FF 2, PSAE FF 3 – Pseudomonas aeruginosa FF 3. 0 – total inhibition, no colonies inside the inhibition zone; 1 – substantial inhibition, <10 colonies inside the inhibition zone; 2 – weak inhibition, > 10 colonies inside the inhibition zone; 3 – no visible inhibition zone. The bactericidal activity results using disk diffusion tests have not shown any positive results, with the exception of the candida strain, which showed weak growth inhibition in GK50/Ch50 mixture at 1 % concentration (data not shown).
The growth inhibition was also tested using growth curves (final antimicrobial concentrations were ten times lower than in the disk diffusion test).
In the case of gram-positive bacteria (Staphylococcus aureus, Staphylococcus epidermidis), a mild inhibitory growth effect of GK alone was demonstrated, which was defined not only with a flatter growth curve compared to the control, but also compared to different GK concentrations. The best values were obtained with mixture of Gum Karaya and chitosan (Figures 2, 3).
Fig. 2. Optical sensity (OD) measurement dynamics within 20 hours in Staphylococcus aureus CCM 4223 strain Curves (C+ control, GK/2 Gum Karaya in double dilution, Ch/2 chitosan in double dilution, GK+Ch Gum Karaya and chitosan mixture). 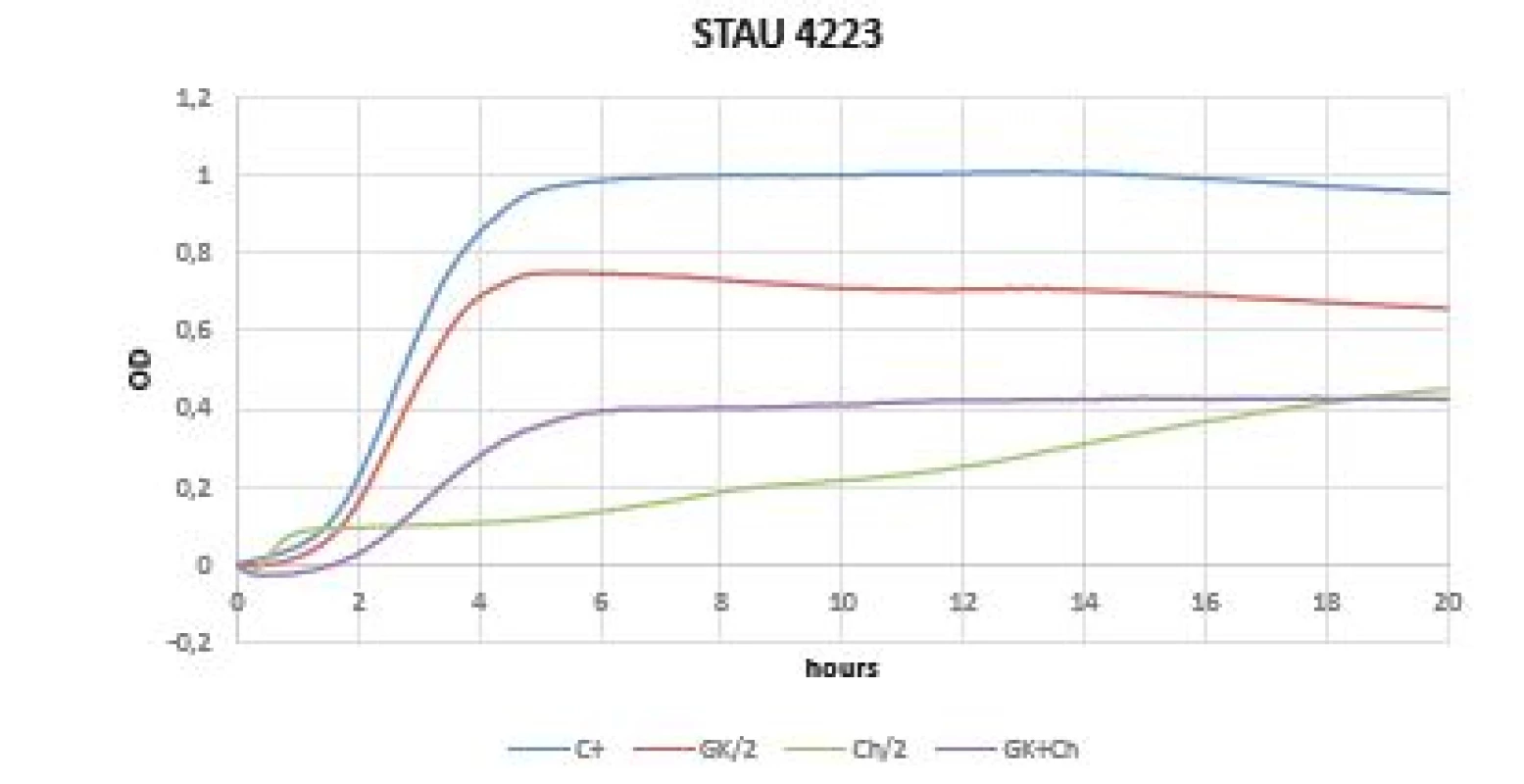
Fig. 3. Optical density (OD) measurement dynamics within 20 hours in Staphylococcus epidermidis A 013 strain Curves (C+ control, GK/2 Gum Karaya in double dilution, Ch/2 chitosan in double dilution, GK+Ch Gum Karaya and chitosan mixture). 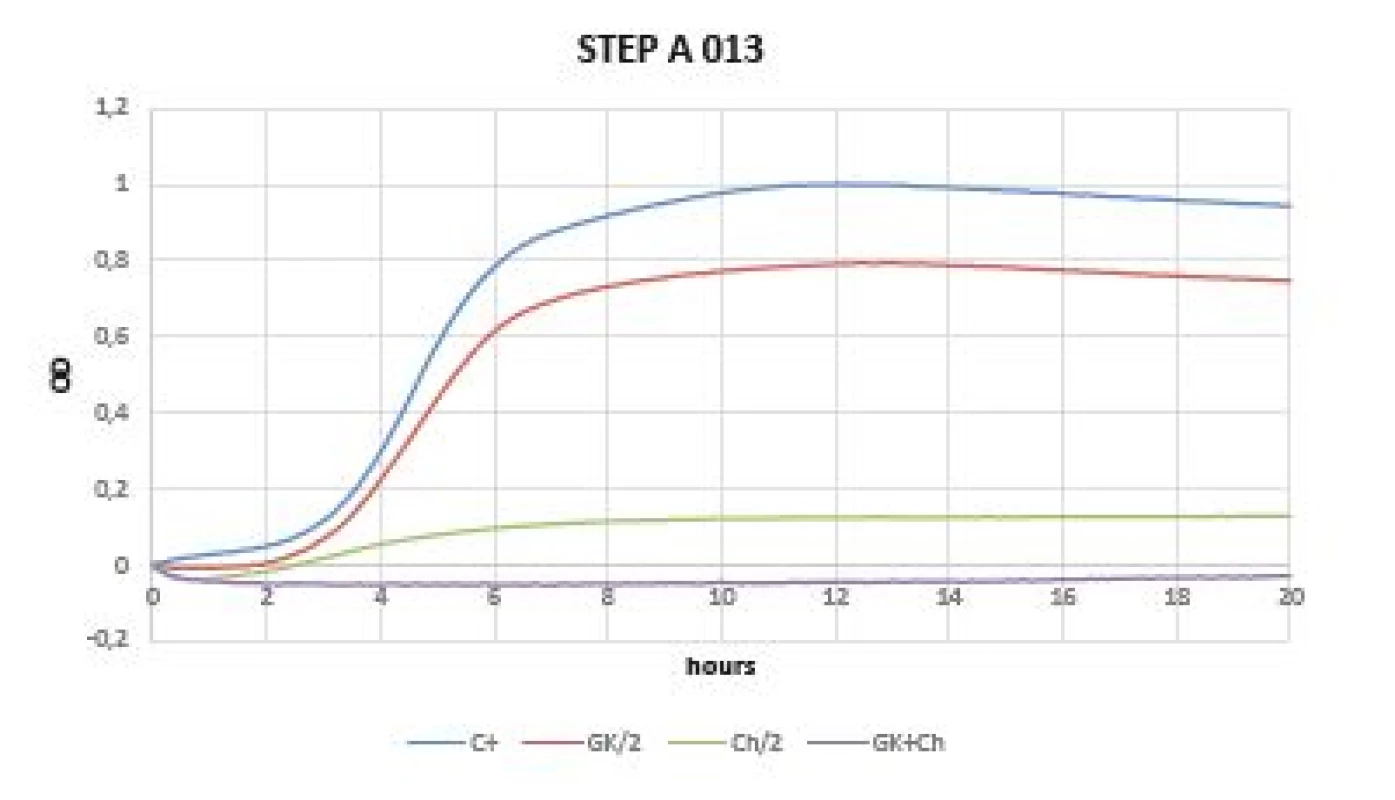
Virtually no inhibition was observed in gram-negative bacteria when comparing the OD change dynamics within 20 hours (Figures 4, 5). OD was increased immediately after time 0 of experiment (especially in Klebsiella pneumoniae CCM 4985 strain). Better dynamics of OD was observed in all tested Pseudomonas aeruginosa strains (FF1, FF2, FF3, 3955). Within 5 hours of starting the OD measurement, the effect on inhibition of pseudomonas growth, the GK-Ch combination in particular, was noticeable; however, after this time an abrupt increase in OD occurred, which continued until the end of the experiment, i.e. up to 20 hours (Figure 4).
Fig. 4. Optical density (OD) measurement dynamics within 20 hours in Klebsiella pneumoniae CCM 4985 strain Curves (C+ control, GK/2 Gum Karaya in double dilution, Ch/2 chitosan in double dilution, GK+Ch Gum Karaya and chitosan mixture). 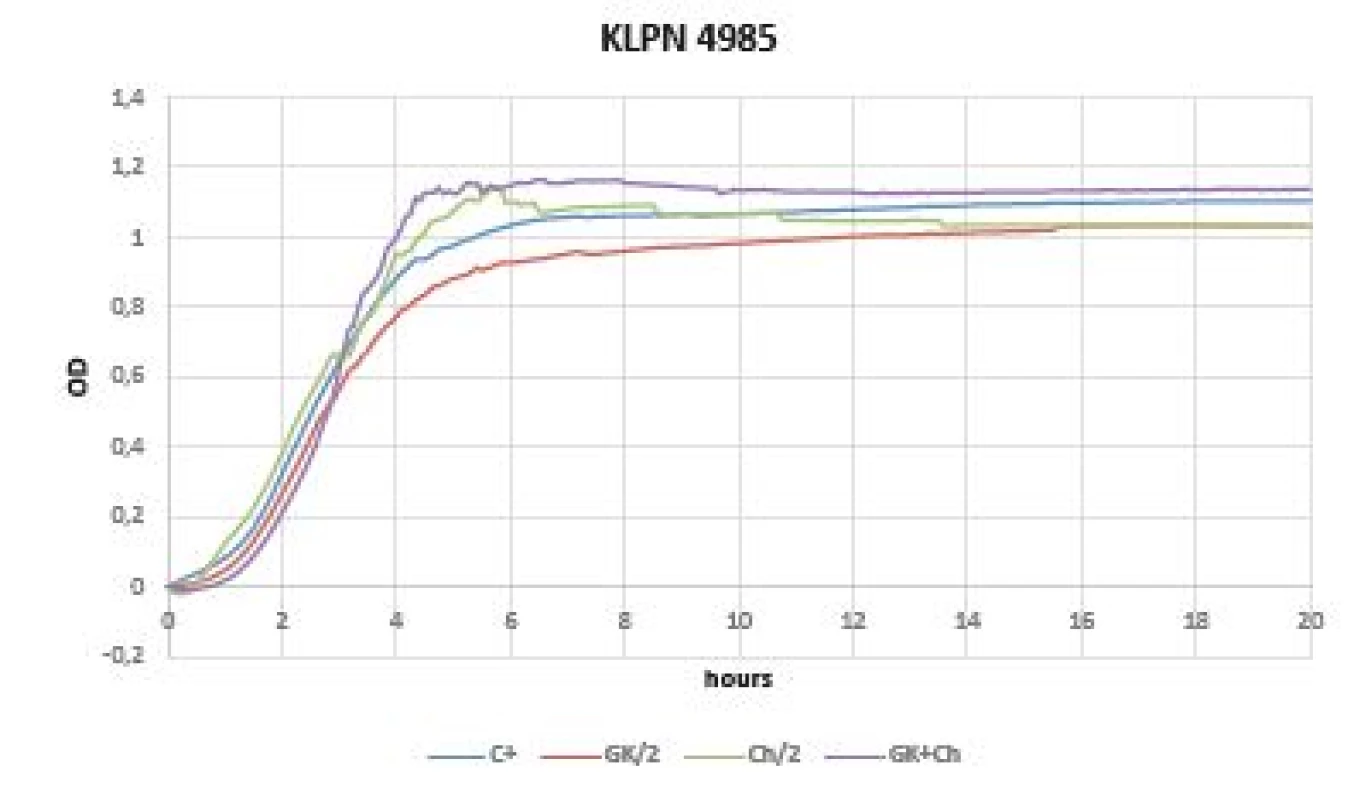
Fig. 5. Optical density (OD) measurement dynamics within 20 hours in Pseudomonas aeruginosa FF1 strain Curves (C+ control, GK/2 Gum Karaya in double dilution, Ch/2 chitosan in double dilution, GK+Ch Gum Karaya and chitosan mixture). 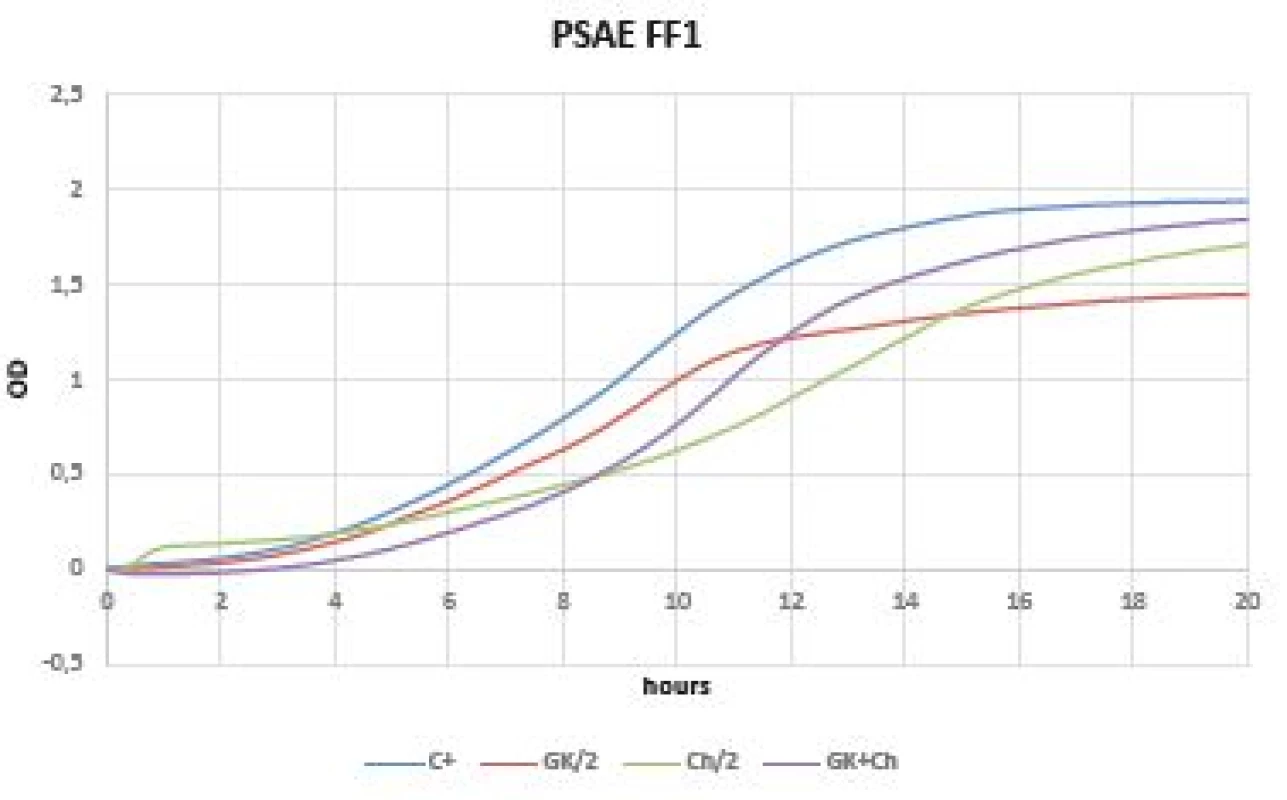
Very promising results were observed within a test of yeasts (Candida albicans CCM 8261, Candida parapsilosis BC 11). The initial drop of OD values are caused by the chitosan solubility in the cultivation medium and should not be mistaken with an antimicrobial effect. The growth inhibition was observed in GK - mixture in both pathogens (Figures 6, 7).
Fig. 6. Optical density (OD) measurement dynamics within 20 hours in Candida albicans CCM 8261 strain Curves (C+ control, GK/2 Gum Karaya in double dilution, Ch/2 chitosan in double dilution, GK+Ch Gum Karaya and chitosan mixture). 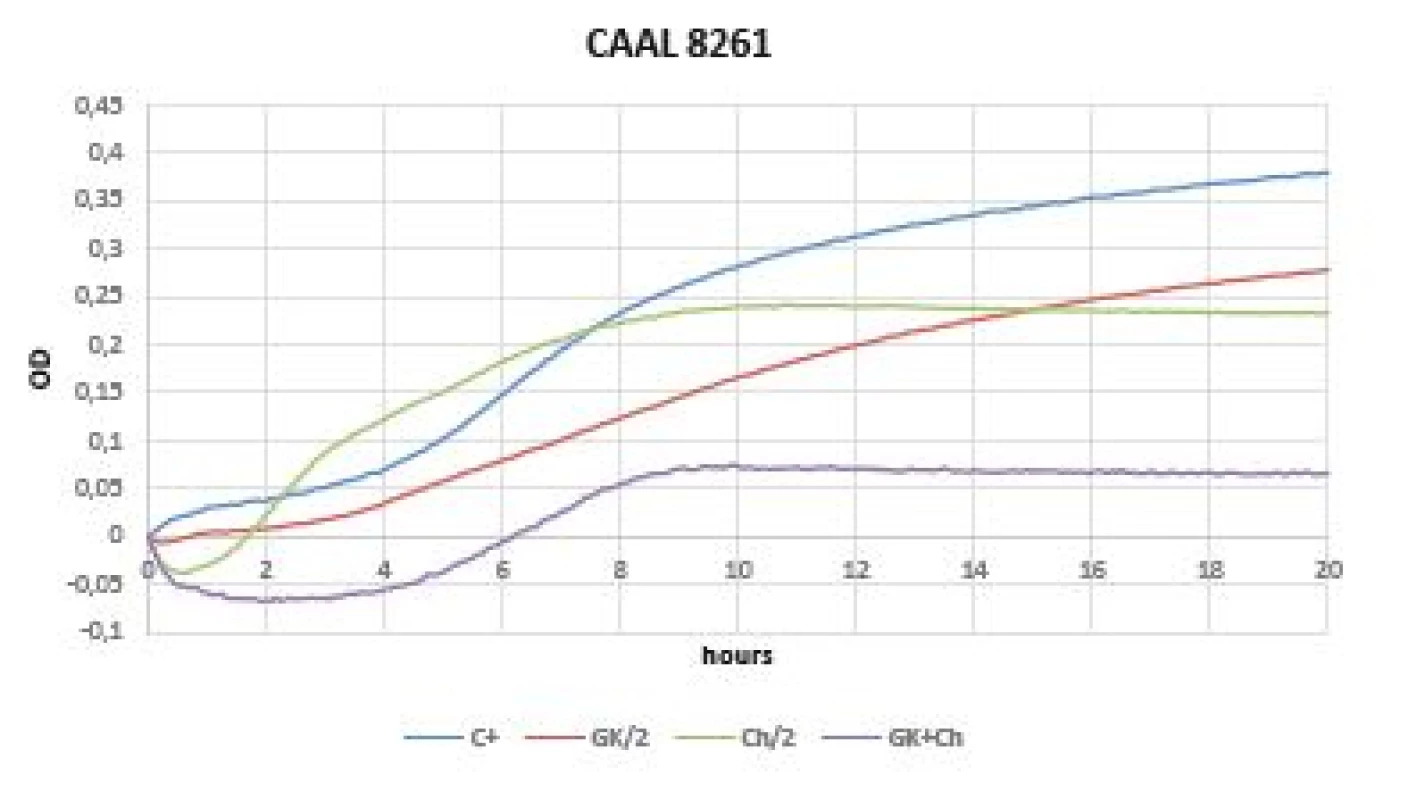
Fig. 7. Optical (OD) measurement dynamics within 20 hours in Candida parapsilosis BC 11 strain Curves (C+ control, GK/2 Gum Karaya in double dilution, Ch/2 chitosan in double dilution, GK+Ch Gum Karaya and chitosan mixture) 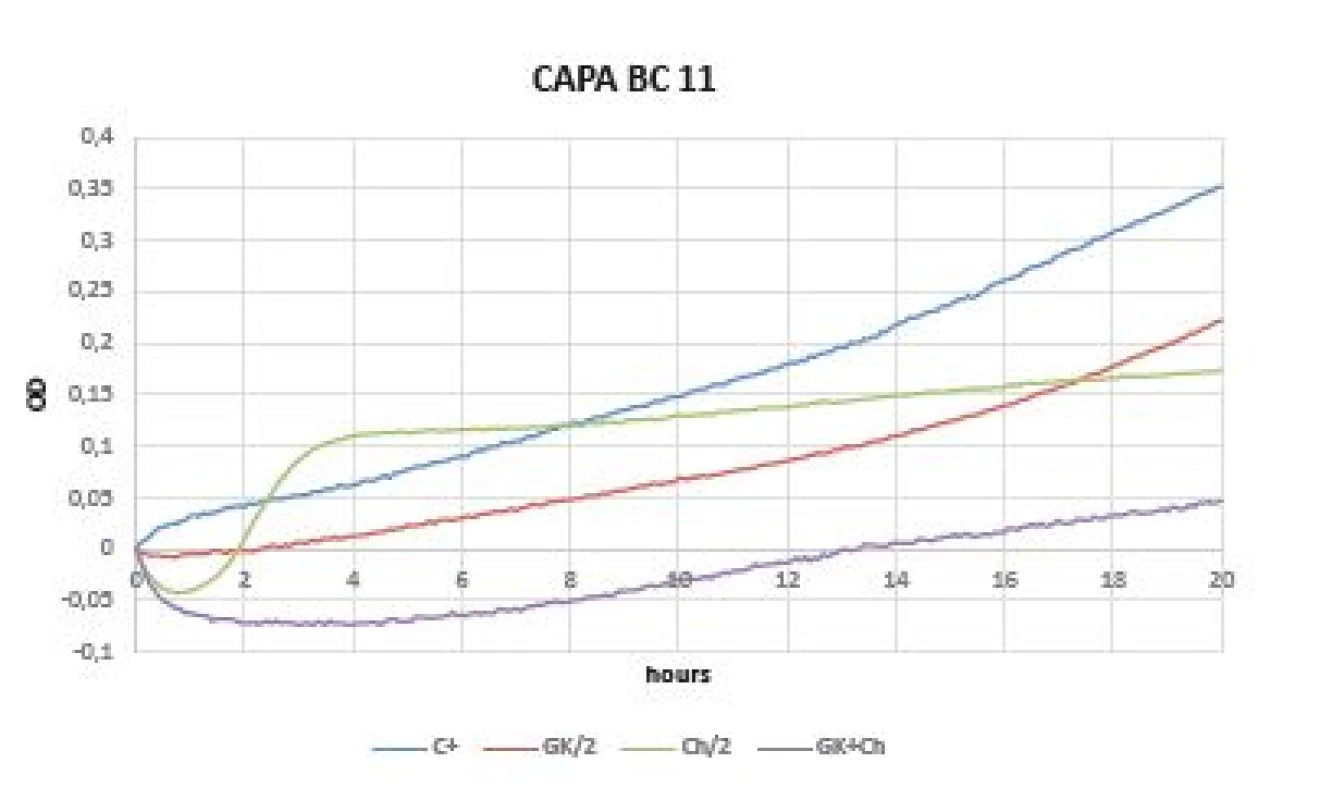
DISCUSSION
Recently, there has been an increased urgent focus on developing novel techniques to deliver drugs more effectively and efficiently. The ideal pharmacokinetic profile can be achieved with the use of polymer based drug delivery devices. A number of polymer-based drug delivery devices and carriers have been proposed for efficient therapy. Among all these drug delivery devices, hydrogels, specifically based on polysaccharides, have attracted considerable attention as excellent candidate for controlled release of therapeutic agents [8].
Hydrogels are three-dimensional polymeric networks quickly swell by imbibing a large amount of water or biological fluids and can de-swell in response to changes in their surroundings. These changes can be induced by changing the surrounding temperature, pH, ionic strength or electro stimulus. Polysaccharide based hydrogels keep moist environment and stimulates faster moist wound healing [9, 10]. Healing under the hydrogel dressing’s wet environment has some advantages, which include suitable environment for skin cell growth (stimulates keratinocytes proliferation), faster healing rate, absorbing wound exudates, protection from bacterial infection, hydrating necrotic tissue, possibility of peeling off without any damage or easier to change the dressing [11].
Hydrogels based on GK are not reported much in literature and GK’s potential as a drug delivery system has not been explored very much [4]. Nevertheless after successful toxicological, teratological and mutagenic tests, GK has been declared as Generally Recognised As Safe (GRAS) by the Food and Drug Administration (FDA). The materials based on GK may also include drugs, such as aceclofenac, nimodipine, famotidine, tetracycline hydrochloride, indomethacin e.g. This fact points to the further use of GK as a drug delivery system [12].
Infectious complications currently represent a dominant share in burn patients’ mortality and morbidity. The most common compartment for the development of infection in this highly specific group of patients is burned regions (skin and soft tissue infections, SSTIs) [13]. A variety of potentially pathogenic microorganisms (PPMs) are used in the etiology of infectious complications. Their representation is highly variable and largely depends on the nature of a skin defect, as well as the location where (in which workplace and in which part of the world) the microbiological surveillance was performed [14, 15]. Still, the most successful PPMs in the case of SSTIs development in burn patients are Staphylococcus aureus and Pseudomonas aeruginosa [16, 17]. There was an increase in the resistance of individual PPMs to various antimicrobial agents especially in the last decade [18, 19]. The only way to ensure good efficiency in fighting PPMs in burned regions in the future is the development and implication of new materials in wound-management with a promising antimicrobial effect. Controlling the development of infectious complications is a key factor in progression within the wound healing phase.
When developing new materials, we are increasingly focusing on fully natural or biosynthetic materials. Material engineering helps us define custom-made products. The most important parameter studied in these materials is its natural antimicrobial capacity. A completely different mechanism of effect on individual PPMs than the one currently in use is optimal.
Materials that do not contain a component with an antimicrobial effect are practically unused in wound-management today. Silver (colloidal, nanocrystalline form) is widespread, and so are other products known from natural sources such as honey [20, 21]. Antiseptic representatives (octenidine dihydrochloride) or antibiotics [22] are also widespread in materials. Antimicrobial activity in other natural materials is also studied intensively. Typical examples are catechins (epigallocatechin gallate, EGCg, the main constituent of tea catechins) [23]. A study by Yoda et al. points to the fact that the antibacterial activity of EGCg is determined to a certain extent by the structure of the bacterial cell wall and the different affinities of EGCg to the cell wall not just to staphylococci but also to gram-negative rods [24]. (EGCg is the main polyphenol isolated from green tea.) Friedman et al. tested EGCg against strains of Bacillus cereus [25]. They concluded that the efficiency of the individual representatives of EGCg (-)-gallocatechin-3-gallate, (-)-epigallocatechin-3-gallate, (-)-catechin-3-gallate, etc. showed antimicrobial activities at nanomolar levels. High efficiency of EGCg against multi-resistant bacteria strains (Pseudomonas aeruginosa) was also gradually demonstrated [26]. The investigation results clearly predict the use of EGCg in wound-management.
An increased effort has been made regarding testing the antimicrobial capacity of different natural polymers particularly over the past two decades. Most of these polymers contain substances that belong to the group of plants derived natural antimicrobials [27].
The main substances with antimicrobial activity that are isolated from plants are phenolic compounds (flavonoids, non-flavonoids), saponins, thiosulfinates, glucosinolates, aldehydes, alcohols, terpenes, polyphenols, etc. [28]. Although these substances have potential antimicrobial activity against bacteria, yeasts and fungi, it is generally believed that plant-derived natural antimicrobials exhibit better inhibitory activity against gram-positive than gram-negative bacteria. Antimicrobial activity is very often associated with the high antioxidant activity of these products [29].
A study by Torquato et al. was published in 2004, including the evaluation of antimicrobial activity of Cashew Tree Gum [30]. It is a product of Anacardium occidentale, a widespread plant in Brazil [31]. Polysaccharide chains of cashew tree gum are from the group of arabinogalactans with different side chains (glucuronic and galacturonic acid residues). An investigation into the antimicrobial activity of cashew tree gum was carried out primarily because it was found that the production of this gum is stimulated by bacteria and fungi [32]. This work followed in the promising results of Marques et al., who pointed to the fact that cashew tree gum had an inhibitory effect on the growth of various bacteria, even fungi [33]. Unfortunately, in the study by Torquato et al., antimicrobial activity was only found in Sacharomyces cerevisiae, no other activity was observed in other PPMs (Staphylococcus aureus, Escherichia coli and Kluyveromyces merxianus, Bacillus cereus, Salmonella typhimurium, Listeria monocytogenes, etc.) and, for example, an increase in biomass was found in Staphylococcus aureus strains with increased cashew gum concentration [30]. One possible explanation for the completely different results is a different approach to the material purification.
Another representative of the natural hydrogels group that was tested for antimicrobial activity is guar gum [34]. Guar gum is a galactomannan, obtained from Cyamopsis tetragonolobus. This material is widely used today, and just like GK, it belongs to GRAS [35]. A study by Tauseef et al. on the pharmaceutical and pharmacological profile of guar gum was published in 2011 [36]. It also discusses the potential of this material’s antimicrobial activity. Methanolic fraction of saponin with extract of guar meal has antibacterial potential against strains of Salmonella typhimurium and Escherichial coli [37].
In his work Al Alawi et al. compares the antimicrobial and cytotoxic activity of Gum acacia (The Gum Arabic) [38]. It is obtained by extracting two species of acacia from the Sub-Saharan Africa, Acaia Senegal and Acaia seyal. Comparison material was obtained from Sudan and Oman Gum acaia. The most important substances from this gum (Hexane, Chloroform, Ethyl acetate, Butanol) were gradually extracted, and were subjected to antimicrobial and cytotoxic activity. Significant cytotoxic activity was not found in any extracts from both acacia species. The largest antimicrobial activity was observed in all chloroform concentrations (2, 1, 0.5, 0.25 mg/ml) extracted from Sudanese Gum acacia against Klebsiella pneumoniae. In contrast, minimal antimicrobial activity was demonstrated in n-butanol extract against Staphylococcus aureus. The antimicrobial activity of other substances is significantly affected by the concentration.
Chitosan is a non-toxic, biocompatible and biodegradable natural polymer with low immunogenicity, antimicrobial action and antioxidant capacity [39]. Another undisputed advantage is its support of phase wound healing. This leads to the use of chitosan as a material for wound dressing applications [40].
The most common source of chitosan for medicinal and non-medicinal applications is an exoskeleton of marine organisms (e.g. crustacean shells, crawfish and squids) [41]. Chitosan produced with insects or fungi is now also commercially available. Like GK, chitosan is also approved by the FDA as a GRAS [42].
The first mention of the antimicrobial action of chitosan and chitosan oligomers was in the 1980s [43, 44]. However, the mechanism of chitosan action against bacteria has not yet been fully described (chitosan has a different activity against gram-positive and gram-negative bacteria). One of the most commonly accepted explanations is that chitosan contains positively charged amino groups that interact with negatively charged microbial cell membranes, leading to fenestrations with subsequent leakage of intracellular bacterial components extracellularly. The antifungal effect of chitosan has been described in various yeasts (Candida albicans, non-albicans candida). In their study, Jeon et al. point to the fact that the antibacterial activity of chitosan and its oligomers is not only highly dependent on the pathogen but also on the molecular mass [45]. Similar results were achieved by No et al. [46]. In both studies, a clear conclusion was reached that the chitosan antimicrobial activity alone is higher than that of chitosan oligomers. However, in general, chitosan exhibits higher antimicrobial activity against gram-positive cocci than gram-negative rods. Chitosan efficiency against bacteria can be further enhanced at a lower pH.
The chitosan actual molecular mass also contributes to its antimibacterial activity. No et al. tested both gram-positive bacteria (Listeria monocytogenes, Bacillus cereus, Bacillus megaterium, Staphylococcus aureus, Lactobacillus spp.) and gram-negative bacteria (Escherichia coli, Pseudomonas fluorescens, Salmonella typhimurium, Vibrio parahaemolyticus). The tested molecular mass of chitosan was from 28 kDa to 1671 kDa. Most of the strains tested had a correlation between the molecular mass of chitosan and its antibacterial efficacy. A study was published in 2010, describing physical and mechanical properties together with the antimicrobial potential of the composite film containing chitosan along with guar gum [47]. Guar gum is a galactomannan (a water-soluble polysaccharide) obtained from the Indian cluster bean, Cyamopsis tetragonoloba [48]. Antimicrobial activity was tested against strains of Staphylococcus aureus and Escherichia coli. It is an interesting fact based on the study that the antimicrobial activity was observed, in particular up to the concentrations of 15-25% (v/v). Another increase in concentration reduced the antimicrobial activity of the test film.
CONCLUSION
Many publications confirm the antimicrobial properties of various natural polysaccharides or chitosan. No work has been published yet to address the specific antimicrobial problem of GK. Since it is a material whereby its wide potential use is now primarily focused on wound-management of acute and chronic wounds, the data we found is very encouraging for future investigations as well are clinical applications.
The fact that this chitosan-enriched material exhibits synergistic properties in antibacterial capacity is also an important finding, especially when it comes to gram-positive bacteria strains. An effect on the inhibition growth curve of test pathogens was not detected in gram-negative bacteria; therefore, this area would require further intervention in terms of increased GK and chitosan concentrations.
Acknowledgments
Project was supported by funds from Faculty of Medicine MU to junior researcher Bretislav Lipovy 2017 and it was carried out under the specific research project STI-J-17-4776 and project CEITEC 2020 (LQ1601) with financial support from the Ministry of Education, Youth and Sports of the Czech Republic under the National Sustainability Programme II and by the Ministry of Health of the Czech Republic, grant no. 16-29916A.“
Do redakce došlo dne 13. 8. 2018.
Adresa pro korespondenci:
Mgr. Lukáš Vacek
Mikrobiologický ústav LF MU
Fakultní nemocnice u sv. Anny v Brně
Pekařská 53
656 91 Brno
e-mail: 258662@mail.muni.cz
Zdroje
1. Wasiak J, Cleland H, Campbell F, et al. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev. 2013 Mar 28;(3):CD002106. doi: 10.1002/14651858.CD002106.
2. Singh B, Pal L. Sterculia crosslinked PVA and PVA-poly(AAm) hydrogel wound dressings for slow drug delivery: mechanical, mucoadhesive, biocompatible and permeability properties. J Mech Behav Biomed Mater, 2012 May;9 : 9–21. doi: 10.1016/j.jmbbm.2012.01.021.
3. Suleyman G, Alangaden GJ. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect Dis Clin North Am, 2016 Dec;30(4):1023–1052. doi: 10.1016/j.idc.2016.07.008.
4. Singh B, Pal L. Development of sterculia gum based wound dressings for use in drug delivery. European Polymer Journal, 2008;44(10):3222–3230. Dostupné na www: http://dx.doi.org/10.1016/j.eurpolymj.2008.07.013.
5. Mostafa K, Morsy M. Modification of carbohydrate polymers via grafting of methacrylonitrile onto pregelled starch using potassium monopersulfate/Fe2+ redox pair. Polymer International, 2004; 53(7):885–889. doi:10.1002/pi.1449.
6. Costa-Júnior ES, Barbosa-Stancioli EF, Mansur AAP, et al. Preparation and characterization of Chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical application. Carbohydrate Polymers, 2009; 76(3):472–481. Dostupné na www: http://dx.doi.org/10.1016/j.carbpol.2008.11.015.
7. Postulkova H, Chamradova I, Pavlinak D, et al. Study of effects and conditions on the solubility of natural polysaccharide gum karaya. Food Hydrocolloids, 2017;67 : 148–156. Dostupné na www: https://doi.org/10.1016/j.foodhyd.2017.01.011.
8. Singh B, Vashishtha M. Development of novel hydrogels by modification of sterculia gum through radiation cross-linking polymerization for use in drug delivery. Nuclear Inst. and Methods in Physics Research, B [online], 2008; 266(9):2009–2020. doi:10.1016/j.nimb.2008.03.086.
9. Elliot JE, MacDonald M, Nie J, et al. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer, 2004; 45(5):1503–1510. doi:10.1016/j.polymer.2003.12.040.
10. Kim SJ, Lee KJ, Kim SI. Electrostimulus responsive behavior of poly(acrylic acid)/polyacrylonitrile semi-interpenetrating polymer network hydrogels. Journal of Applied Polymer Science, 2004; 92(3):1473–1477. doi:10.1002/app.13718.
11. Jayakumar R, Prabaharan M, Reis RL, et al. Graft copolymerized Chitosan – present status and applications. Carbohydrate Polymers, 2005;62(2):142–158.
12. Boateng JS, Matthews KH, Stevens HN, et al. Wound healing dressings and drug delivery systems: a review. J Pharm Sci, 2008 Aug;97(8):2892–2923.
13. Lipový B, Brychta P, Řihová H, et al. Prevalence of infectious complications in burn patients requiring intensive care: data from a pan-European study. Epidemiol Mikrobiol Imunol, 2016 Mar;65(1):25–32.
14. Azzopardi EA, Azzopardi E, Camilleri L, et al. Gram negative wound infection in hospitalised adult burn patients-systematic review and metanalysis. PLoS One, 2014 Apr 21;9(4):e95042. doi: 10.1371/journal.pone.0095042.
15. Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev, 2006 Apr;19(2):403–434.
16. Coetzee E, Rode H, Kahn D. Pseudomonas aeruginosa burn wound infection in a dedicated paediatric burns unit. S Afr J Surg, 2013 May 3;51(2):50–53. doi: 10.7196/sajs.1134.
17. Posluszny JA Jr, Conrad P, Halerz M, et al. Surgical burn wound infections and their clinical implications. J Burn Care Res, 2011;32(2):324–333. doi:10.1097/BCR.0b013e31820aaffe.
18. Koller J, Boca R, Langsádl L. Changing pattern of infection in the Bratislava Burn Center. Acta Chir Plast, 1999;41(4):112–116.
19. Keen EF 3rd, Robinson BJ, Hospenthal DR, et al. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns, 2010 Sep;36(6):819–825. doi:10.1016/j.burns.2009.10.013.
20. Nímia HH, Carvalho VF, Isaac C, et al. Comparative study of Silver Sulfadiazine with other materials for healing and infection preven-tion in burns: A systematic review and meta-analysis. Burns, 2018 Jun 11. pii: S0305-4179(18)30399-1. doi: 10.1016/j.burns.2018.05.014.
21. Stewart JA, McGrane OL, Wedmore IS. Wound care in the wilderness: is there evidence for honey? Wilderness Environ Med, 2014 Mar;25(1):103–110. doi: 10.1016/j.wem.2013.08.006.
22. Kramer A, Dissemond J, Kim S, et al. Consensus on Wound Antisepsis: Update 2018. Skin Pharmacol Physiol, 2018;31(1):28–58. doi: 10.1159/000481545.
23. Gharib A, Faezizadeh Z, Godarzee M. Therapeutic efficacy of epigallocatechin gallate-loaded nanoliposomes against burn wound infection by methicillin-resistant Staphylococcus aureus. Skin Pharmacol Physiol, 2013;26(2):68–75. doi: 10.1159/000345761.
24. Yoda Y, Hu ZQ, Zhao WH, et al. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J Infect Chemother, 2004 Feb;10(1):55–58.
25. Friedman M, Henika PR, Levin CE, et al. Antimicrobial activities of tea catechins and theaflavins and tea extracts against Bacillus ce-reus. J Food Prot, 2006 Feb;69(2):354–361.
26. Kanagaratnam R, Sheikh R, Alharbi F, et al. An efflux pump (MexAB-OprM) of Pseudomonas aeruginosa is associated with antibacterial activity of Epigallocatechin-3-gallate (EGCG). Phytomedicine, 2017 Dec 1;36 : 194–200. doi: 10.1016/j.phymed.2017.10.010.
27. Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food control, 2014; 46 : 412–429. http://dx.doi.org/10.1016/j.foodcont.2014.05.047.
28. Pisoschi AM, Pop A, Georgescu C, et al. An overview of natural antimicrobials role in food. Eur J Med Chem, 2018 Jan 1;143 : 922–935. doi: 10.1016/j.ejmech.2017.11.095.
29. Pisoschi AM, Pop A, Cimpeanu C, et al. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid Med Cell Longev, 2016;2016 : 9130976. doi: 10.1155/2016/9130976.
30. Torquato DS, Ferreira ML, Sá GC, et al. Evaluation of antimicrobial activity of cashew tree gum. World Journal of Microbiology and Biotechnology, 2004;20(5):505–507.
31. Anderson DMW, Bell PC, Millar RA. Composition of gum exudates from Anacardium occidentale. Phytochemistry, 1974;13 : 2189–2193.
32. Intini M. Phytopathological aspects of cashew (Anacardium occidentale L.) in Tanzania. International Journal of Tropical Plant Disease, 1987;5 : 115–119.
33. Marques MR, Albuquerque LMB, Xavier-Filho J. Antimicrobial and insecticidal activities of cashew tree gum exudate. Annals of Applied Biology, 1992;121 : 371–377.
34. Thombare N, Jha U, Mishra S, Siddiqui MZ. Guar gum as a promising starting material for diverse applications: A review. Int J Biol Macromol, 2016 Jul;88 : 361–372. doi: 10.1016/j.ijbiomac.2016.04.001.
35. Yoon SJ, Chu DC, Raj Juneja L. Chemical and physical properties, safety and application of partially hydrolized guar gum as dietary fiber. J Clin Biochem Nutr, 2008 Jan;42 : 1–7. doi: 10.3164/jcbn.2008001.
36. Tauseef S, Kumar SS. Pharmaceutical and pharmacological profile of guar gum an overview. Int J Pharm Pharm Sci, 2011;3(Suppl 5):38–40.
37. Hassan SM, Byrd JA, Cartwright AL, et al. Hemolytic and antimicrobial activities differ among saponin-rich extracts from guar, quillaja, yucca, and soybean. Appl Biochem Biotechnol, 2010 Oct;162(4):1008–1017. doi: 10.1007/s12010-009-8838-y.
38. Al Alawi SM, Hossain MA, Abusham AA. Antimicrobial and cytotoxic comparative study of different extracts of Omani and Sudanese Gum acacia. Beni-Suef Univ. J. Basic Appl. Sci, 2018;7(1):22–26. Dostupné na www: https://doi.org/10.1016/j.bjbas.2017.10.007.
39. No HK, Park NY, Lee SH, et al. Antibacterial activity of Chitosans and Chitosan oligomers with different molecular weights. Int J Food Microbiol, 2002, 25;74(1-2):65–72.
40. Dragostin OM, Samal SK, Dash M, et al. New antimicrobial Chitosan derivatives for wound dressing applications. Carbohydr Polym, 2016 May 5;141 : 28–40. doi: 10.1016/j.carbpol.2015.12.078.
41. Younes I, Rinaudo M. Chitin and Chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs, 2015 Mar 2;13(3):1133–1174. doi: 10.3390/md13031133.
42. Kean T, Thanou M. Biodegradation, biodistribution and toxicity of Chitosan. Adv Drug Deliv Rev, 2010 Jan 31;62(1):3–11. doi: 10.1016/j.addr.2009.09.004.
43. Kendra DF, Hadwiger LA. Characterization of the smallest Chitosan oligomer that is maximally antifungal to Fusarium solani and elicits pisatin formation in Pisum sativum. Exp. Mycol, 1984;8 : 276–281.
44. Sudarshan NR, Hoover DG, Knorr D. Antibacterial action of Chitosan. Food Biotechnol, 1992;6 : 257–272.
45. Jeon YJ, Park PJ, Kim SK. Antimicrobial effect of chitooligosaccharides produced by bioreactor. Carbohydr. Polym, 2001;44 : 71–76.
46. No HK, Meyers SP. Crawfish Chitosan as a coagulant in recovery of organic compounds from seafood processing streams. J. Agric. Food Chem, 1989;37 : 580–583.
47. Rao MS, Kanatt SR, Chawla SP, et al. Chitosan and guar gum composite films: Preparation, physical, mechanical and antimicrobial properties. Carbohydr Polym, 2010;82(4):1243–1247. doi:10.1016/j.carbpol.2010.06.058.
48. Dea ICM, Morrison A. Chemistry and interactions of seed galactomannans. Adv Carbohyd Chem Bi, 1975;31 : 241–312
Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek Rejstřík
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2018 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- Prion diseases with a focus on Creutzfeldt-Jakob disease, a summary of the incidence of Creutzfeldt-Jakob disease in the Czech Republic over the last 17 years, 2000–2017
- Properties of Staphylococcus aureus strains from food processing staff
- Antimicrobial effect of novel hydrogel matrix based on natural polysaccharide Sterculia urens
- The prevalence of oral HPV infection in healthy populations: A systematic review with a focus on European populations
- Epidemiology of selected Mycobacterium tuberculosis complex members in the Czech Republic in 2000–2016
- Cerebrospinal Fluid Pleocytosis following Meningococcal B vaccination in an Infant
- Microorganisms named after geographical locations and personal names of Czech and Slovak microbiologists
- Koncepce oboru epidemiologie v České republice (2018)
- 28. Pečenkovy epidemiologické dny České Budějovice, 12.–14. září 2018
- Zemřel MUDr. Vladimír Polanecký
- Rejstřík
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Prion diseases with a focus on Creutzfeldt-Jakob disease, a summary of the incidence of Creutzfeldt-Jakob disease in the Czech Republic over the last 17 years, 2000–2017
- The prevalence of oral HPV infection in healthy populations: A systematic review with a focus on European populations
- Properties of Staphylococcus aureus strains from food processing staff
- Epidemiology of selected Mycobacterium tuberculosis complex members in the Czech Republic in 2000–2016
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



