-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Vaginal progesterone pessaries for pregnant women with a previous preterm birth to prevent neonatal respiratory distress syndrome (the PROGRESS Study): A multicentre, randomised, placebo-controlled trial
In a randomized controlled trial, Caroline Crowther and colleagues examine the effect of vaginal progesterone pessaries for pregnant women to prevent neonatal respiratory distress syndrome.
Published in the journal: . PLoS Med 14(9): e32767. doi:10.1371/journal.pmed.1002390
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002390Summary
In a randomized controlled trial, Caroline Crowther and colleagues examine the effect of vaginal progesterone pessaries for pregnant women to prevent neonatal respiratory distress syndrome.
Introduction
The prevention of preterm birth remains a global challenge [1]. Women who have had a previous preterm birth have over twice the risk of giving birth preterm in a subsequent pregnancy [2,3,4]. Babies born preterm are at increased risk of respiratory distress syndrome as a result of immature lung development, and this is a major cause of their early neonatal mortality and morbidity [5] as well as long-term morbidity [6,7].
Progesterone has an important role in uterine quiescence [8,9] and is essential for the maintenance of pregnancy through multiple and complex mechanisms [10,11,12].
An initial systematic review of studies from the 1960s showed that use of progesterone may prevent preterm birth [13]. Over the last decade, there has been renewed interest in the use of vaginal progesterone in pregnancy to prevent recurrence of preterm birth, with several published trials included in the Cochrane systematic review [14]. Some trials suggest that use of vaginal progesterone reduces the risk of preterm birth [15], whilst others do not [16]. This has led to considerable debate and differences in clinical practice recommendations [17,18,19,20].
At the time of planning our trial, there were 2 published clinical trials with relatively small sample sizes that included women with a previous history of preterm birth, and these studies had shown a reduction in preterm birth with the use of both natural vaginal progesterone [15] and intramuscular injection of 17 OH progesterone, a synthetic progestogen [21]. However, intramuscular 17 alpha-hydroxyprogesterone caproate is not available for use in some countries, including Australia and New Zealand. Whilst a reduction in preterm birth may seem beneficial, prolongation of gestation may not lead to health benefits, so it also is important to know the effects on neonatal morbidities, such as respiratory distress syndrome and its sequelae, and on maternal health outcomes.
The primary aim of the PROGRESS randomised, placebo-controlled trial was therefore to assess whether the use of vaginal progesterone pessaries in pregnant women with a history of previous spontaneous preterm birth reduced the risk and severity of respiratory distress syndrome, thus improving the infant’s health. The secondary aims were to examine the effects on other respiratory outcomes; other neonatal morbidities; and maternal health outcomes, especially those related to preterm birth; and to assess any side effects of treatment.
Methods
Design and participants
We conducted a multicentre, placebo-blinded, randomised controlled trial at 39 Australian, New Zealand, and Canadian maternity hospitals. This study is reported as per CONSORT guidelines (S1 Text). The study was approved by the Children’s Youth and Women’s Health Services Human Research Ethics Committee at the Women’s and Children’s Hospital, Adelaide, Australia (approval record number HREC 2006015), and by the ethics committee at each of the 39 collaborating centres (32 in Australia, 5 in New Zealand, and 2 in Canada).
Women were eligible if they had a live singleton or twin pregnancy between 18 and <24 weeks’ gestation and a history of prior preterm birth (either vaginal birth or caesarean birth) at greater than 20 weeks’ gestation and less than 37 weeks’ gestation in their preceding pregnancy where the onset of labour occurred spontaneously or in association with cervical incompetence or following preterm prelabour rupture of membranes. If the women had received progesterone therapy prior to 16 weeks’ gestation, they remained eligible. The protocol for this study has been published [22] (S2 Text).
Women were ineligible if their preceding preterm birth at less than 37 weeks’ gestation was associated with placental abruption or placenta praevia, if it was a multiple pregnancy, or if there had been an iatrogenic decision for early birth, for example, related to fetal distress or preeclampsia.
Women were ineligible if their current pregnancy, at consideration for trial entry, was associated with active vaginal bleeding requiring hospital admission at 18 weeks’ gestation or more, preterm prelabour rupture of membranes, active labour (defined as the presence of uterine activity and cervical dilatation greater than 3 cm), known lethal fetal anomaly or fetal demise, progesterone treatment after 16 weeks’ gestation, or any contraindication to continuation of the pregnancy, such as chorioamnionitis requiring delivery, or contraindication to progesterone therapy (known active liver disease, active or hormone-related thrombophlebitis or thromboembolic disorder, or breast or genital malignancy). The PROGRESS Study protocol did not include the need for cervical length measurement at trial entry or during the pregnancy. The clinician responsible for care of the participant decided whether cervical length screening was undertaken.
The study was approved by the Children’s Youth and Women’s Health Services Human Research Ethics Committee at the Women’s and Children’s Hospital, Adelaide, Australia, and by the ethics committee at each of the 39 collaborating centres (33 in Australia, 4 in New Zealand and 2 in Canada).
Eligible women were provided with written information about the study in the antenatal clinic, counselled by 1 member of the research team, and asked if they would participate. Recruitment started in February 2006 and was completed in September 2012.
Randomisation
Women who gave written informed consent were randomly assigned to either ‘progesterone’ or 'placebo’ using a central telephone randomisation service. The randomisation schedule, prepared by an investigator not involved with clinical care, used balanced variable blocks with stratification by plurality of the pregnancy (singleton versus twin versus triplet) and collaborating centre. Participants, staff, and investigators were masked to study group allocation, and treatment packs appeared identical. The baseline information collected included maternal age, parity, ethnicity, body mass index, plurality, gestational age at trial entry, gestational age, and reason for the previous preterm birth.
Intervention and outcomes
Progesterone group and placebo group
Women randomised to the progesterone and placebo groups were allocated a study number that corresponded to a treatment pack containing the allocated study treatment.
Depending on the study treatment allocation, the treatment packs contained either a 14-week supply of progesterone pessaries (equivalent to 100 mg vaginal progesterone as active substance in hard fat) or similar-appearing placebo pessaries (in hard fat) bought for the study from Orion Laboratories, Western Australia. The manufacturer of the pessaries had no other involvement in the study. Women were asked to self-administer a vaginal pessary each evening from 20 weeks’ gestation, or from randomisation if this occurred after 20 weeks’ gestation, until birth or 34 weeks’ gestation, whichever occurred first. The maximum number of days treatment could be used for was 98 days.
Women were reviewed in the antenatal clinic by the practitioner responsible for their care. Women who presented with preterm prelabour rupture of the membranes after trial entry were advised to discontinue using the vaginal pessaries to reduce the risk of introducing infection. In the event of the development of serious depression or a medical condition that may have been aggravated by fluid retention (asthma, epilepsy, migraine, known cardiac dysfunction, or known renal dysfunction), the clinician was to advise the woman to cease using the trial medication if he or she felt it would be in the woman’s best interests to do so.
At 34 weeks’ gestation, women were asked to complete a questionnaire that assessed health-related quality of life [23], anxiety [24], and depression [25] and asked about any side effects they may have experienced and their compliance with the treatment protocol. After birth, information relating to birth, maternal and infant health, and care was collected from the woman's and infant's case notes by trained research assistants.
Study outcomes
Primary outcome
The primary outcome was the incidence of neonatal respiratory distress syndrome (defined as increasing respiratory distress or oxygen requirement or the need for respiratory support from the first 6 hours of life) and severity of neonatal respiratory disease (defined as mild = mean airway pressure [MAP] < 7 cm H2O and/or fractional inspired oxygen [FiO2] < 0.4; moderate = MAP 7–9.9 cm H2O and/or FiO2 0.40–0.79; severe = MAP ≥ 10 cm H2O, and/or FiO2 ≥ 0.80 with need for ventilation).
Secondary outcomes for the child
The secondary outcomes for the child were as follows:
other respiratory measures, which included the need for and duration of oxygen therapy (including highest FiO2 [%] within 12 hours of birth), need for and duration of mechanical ventilation (including maximum peak pressure [cm H2O] within 12 hours of birth), need for surfactant therapy, nitric oxide for respiratory support, air leak syndrome, and chronic lung disease (defined as the need for any respiratory support, supplemental oxygen, or intermittent positive pressure ventilation or continuous positive airways pressure for a chronic pulmonary disorder on the day the baby reached 36 weeks’ postmenstrual age for infants born before 32 weeks’ gestation, or continued oxygen requirement at 28 days of age for infants born after 36 weeks’ gestation) and
a composite adverse outcome for the infant that included 1 or more of the following: preterm birth (defined as birth at less than 37 weeks’ gestation), perinatal mortality (defined as either a stillbirth [intrauterine fetal death after trial entry and prior to birth] or infant death [death of a live-born infant prior to hospital discharge] and excluding lethal congenital anomalies), severe respiratory disease, chronic lung disease, Apgar score < 4 at 5 minutes of age, birth weight less than the third centile for gestational age at birth and infant sex, intraventricular haemorrhage on early cranial ultrasound, periventricular leucomalacia on later cranial ultrasound, inotropic support for the treatment of patent ductus arteriosus, proven necrotising enterocolitis, proven systemic infection within 48 hours of birth treated with antibiotics, and retinopathy of prematurity.
Secondary study outcomes for the mother
The secondary study outcomes for the mother were as follows:
significant health outcomes, particularly related to preterm birth, such as use of tocolytic therapy or antenatal corticosteroid therapy, defined by 1 or more of the following: maternal death, antepartum haemorrhage, pre-eclampsia, preterm prelabour rupture of membranes, prelabour ruptured membranes at or near term (defined as prelabour rupture of membranes after 36 weeks’ gestation), chorioamnionitis requiring antibiotic use during labour, postpartum haemorrhage, or antibiotic use after birth;
length of any antenatal hospital stay or postnatal stay and psychological health (assessed by quality of life [23], anxiety [24], and depression [25]); and
side effects of progesterone supplementation (including headache, nausea, pain and discomfort, breast tenderness, and coughing) and if any of them were sufficient to stop treatment.
Statistical methods
Primary analyses were performed on an intention-to-treat basis, according to the study group allocated at randomisation. As prespecified, unadjusted analyses were performed and then adjusted for the potential confounders of gestational age at randomisation, gestational age of the previous preterm birth, and reason for the previous preterm birth.
Binary outcomes were analysed using log binomial regression, with treatment effects expressed as relative risk (RR) with 95% confidence interval (CI), or Fisher’s exact tests with no adjustment for covariates in the case of rare outcomes. Outcomes measured on a continuous scale were analysed using linear regression, with treatment effects expressed as differences in means. Count outcomes were analysed using Poisson regression or negative binomial regression where overdispersion was present, with treatment effects expressed as ratios of means. Ordinal outcomes were analysed using proportional odds models, with treatment effects expressed as odds ratios of higher severity. For infant outcomes, clustering due to multiple births was taken into account using generalised estimating equations. Statistical significance was assessed at the 2-sided p < 0.05 level, and no adjustment was made for multiple comparisons. No adjustments were made for the 2 primary outcomes, as they were considered strongly related and expected to provide complementary information [26]. All analyses followed a prespecified statistical analysis plan and were performed using SAS software version 9.3 (SAS Institute, Cary, North Carolina, United States).
Sample size
We originally estimated that a sample size of 984 women would be able to show a 40% reduction in neonatal respiratory distress syndrome from 15% to 9% with progesterone supplementation (5% level of significance, 2-tailed alpha, 80% power, 4% loss to follow-up) based upon data from a randomised trial with similar eligibility profile when this trial commenced [21]. In 2009, because of slower than anticipated accrual, we applied for additional funding to complete the study. At this time, the Trial Steering Group asked the following questions of an independent review: (1) ‘Should recruitment stop (because of a significant result or futility)?’ (2) ‘Should we continue recruiting to reach our previous sample size?’ and (3) ‘Does the sample size need refining based on the interim assessment?’ The Trial Steering Group did not see the interim data or the analyses. The independent review undertaken, masked to treatment group, made the following recommendations to the Trial Steering Group: to continue recruitment and to reduce the sample size to 784 women.
Results
Baseline information
Of an estimated 1,919 eligible women able to be approached by the research team between February 2006 and September 2012, a total of 787 (41%) women consented to be enrolled in the study. Reasons for eligible women declining to participate included ‘not interested in research’ (25%), ‘concerned about side effects and risks of use of drugs in pregnancy’ (15%), ‘no reason given’ (13%), ‘did not like the need to use vaginal pessaries’ (9%), ‘too busy’ (8%), ‘did not consider themselves to be at risk of preterm birth’ (6%), ‘partner declined to let them participate’ (5), and ‘other’ (19%).
Of the 787 women recruited, 398 (50.6%) were randomised to the progesterone group, and 389 (49.4%) to the placebo group. There were no losses to follow-up, with clinical outcomes to primary hospital discharge after birth available for all 787 (100%) women and their 799 infants (Fig 1).
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flow diagram of participants in the study. 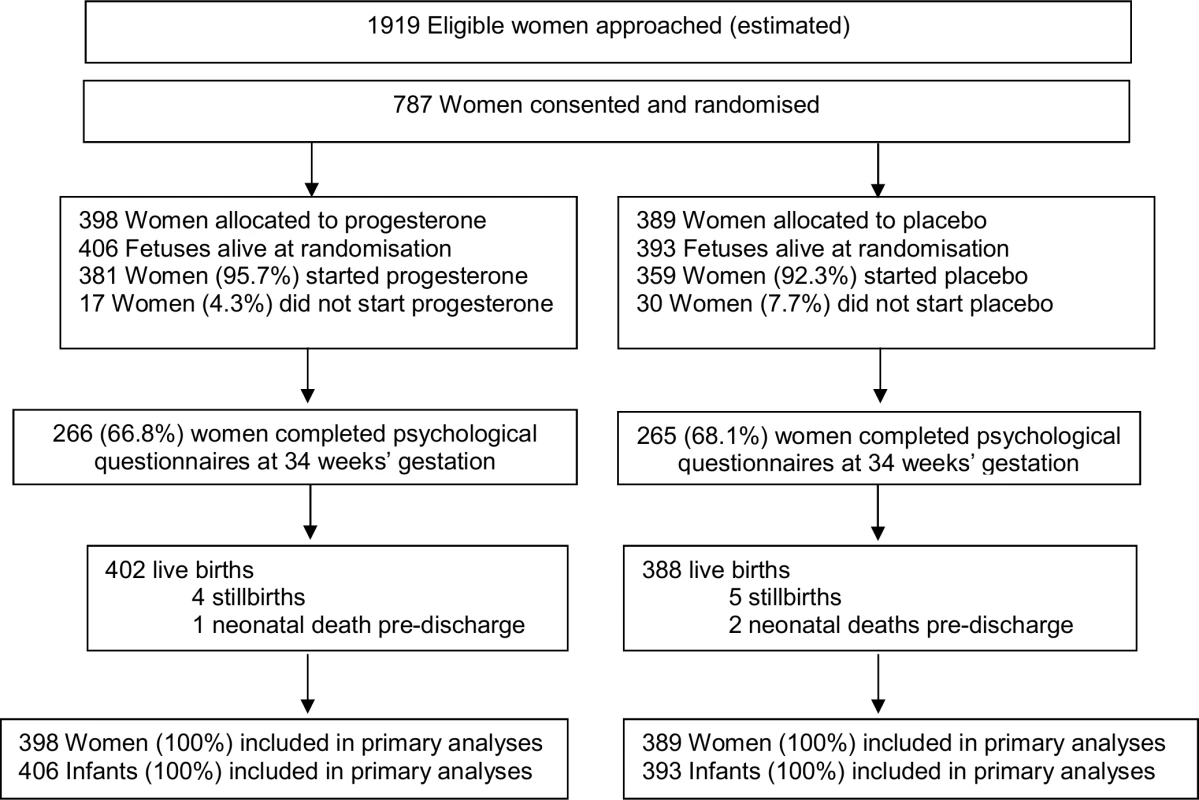
The 2 study groups were similar at the time of study entry for maternal demographics and key variables including gestational age, the reason for the preterm birth in the preceding pregnancy, and the gestational age at which that birth occurred (Table 1). The majority of participants had a singleton pregnancy, with less than 2% having a twin pregnancy (Table 1). Almost all women recruited in both study groups used their allocated study treatment (381 [95.7%] in the progesterone group and 359 [92.3%] in the placebo group), with similar median days of use in both study groups (51.0 days [interquartile range (IQR) 28.0–69.0] in the progesterone group versus 52.0 days [IQR 27.0–76.0] in the placebo group) (Table 1).
Tab. 1. Comparability of randomised study groups at trial entry and use of study treatment. 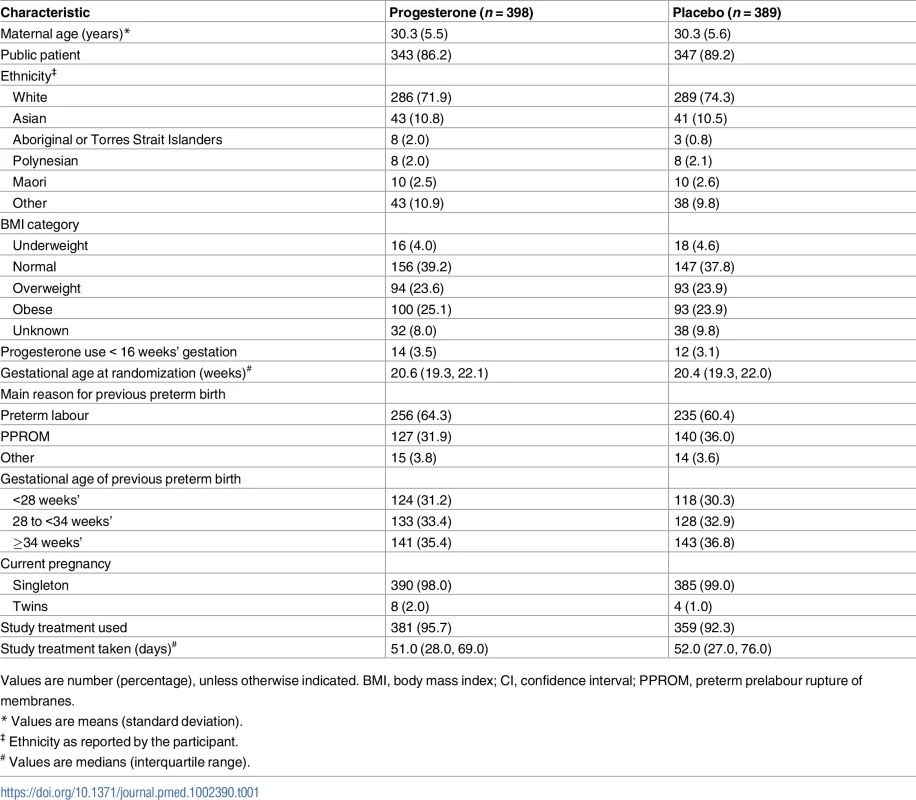
Values are number (percentage), unless otherwise indicated. BMI, body mass index; CI, confidence interval; PPROM, preterm prelabour rupture of membranes. Primary infant outcomes
Risk of respiratory distress syndrome and severity of respiratory disease
The risk of respiratory distress syndrome was similar in both study groups, 10.5% (42/402) in the progesterone group and 10.6% (41/388) in the placebo group (adjusted RR 0.98, 95% CI 0.64–1.49, p = 0.912), as was the severity of any neonatal respiratory disease (adjusted treatment effect 1.02 [95% CI 0.69–1.53, p = 0.905]) (Table 2). Unadjusted analyses showed similar findings to the analyses adjusted for gestational age at randomisation, gestation of previous preterm birth, and reason for previous preterm birth (Table 2).
Tab. 2. Primary and secondary neonatal outcomes by study group. 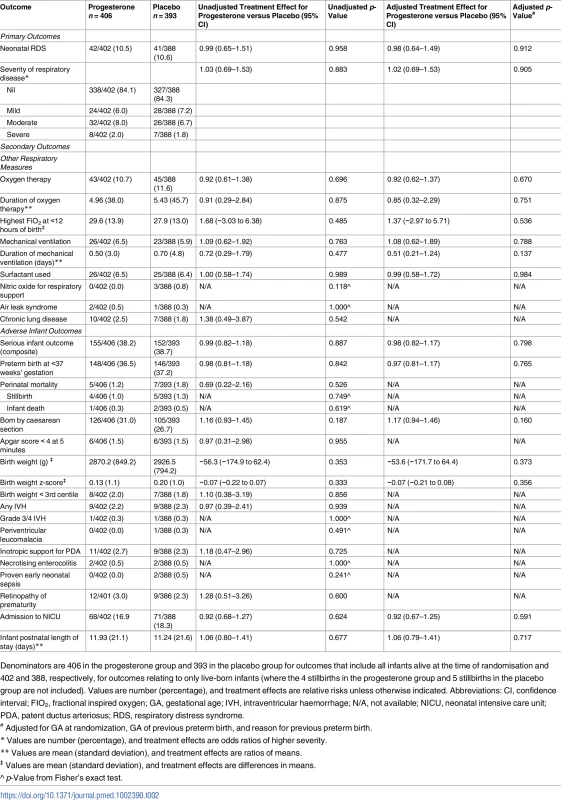
Denominators are 406 in the progesterone group and 393 in the placebo group for outcomes that include all infants alive at the time of randomisation and 402 and 388, respectively, for outcomes relating to only live-born infants (where the 4 stillbirths in the progesterone group and 5 stillbirths in the placebo group are not included). Values are number (percentage), and treatment effects are relative risks unless otherwise indicated. Abbreviations: CI, confidence interval; FIO2, fractional inspired oxygen; GA, gestational age; IVH, intraventricular haemorrhage; N/A, not available; NICU, neonatal intensive care unit; PDA, patent ductus arteriosus; RDS, respiratory distress syndrome. Secondary outcomes for the infant
Other respiratory measures
In keeping with these findings, there were no differences between the study groups for any of the secondary respiratory outcomes that included need for and duration of oxygen therapy, maximum appropriate FIO2 values within 12 hours of birth, use and duration of mechanical ventilation, use of surfactant, use of nitric oxide, air leak syndrome, and chronic lung disease (Table 2).
Adverse infant outcomes
Overall, the risk of any serious adverse outcome for the infant was similar between the study groups (155/406 [38.2%] in the progesterone group and 152/393 [38.7%] in the placebo group, adjusted RR 0.98, 95% CI 0.82–1.17, p = 0.798) (Table 2). There were 12 (1.5%) infant deaths before hospital discharge: 4 stillbirths and 1 death of a live-born infant in the progesterone group and 5 stillbirths and 2 deaths of live-born infants in the placebo group—not a significant difference (Table 2). The proportion of infants born before 37 weeks’ gestation was similar in both study groups (148/406 [36.5%] in the progesterone group and 146/393 [37.2%] in the placebo group, adjusted RR 0.97, 95% CI 0.81–1.17, p = 0.765). A similar proportion of infants were born by caesarean section in both study groups. No differences were evident between the study groups for any of the other individual adverse infant outcomes that included low Apgar score, small for gestational age at birth, intraventricular haemorrhage, periventricular leucomalacia, patent ductus arteriosus requiring treatment, necrotising enterocolitis, proven early neonatal sepsis, retinopathy of prematurity, and need for admission to the neonatal intensive care unit and duration of the infant’s postnatal stay (Table 2).
Secondary outcomes for the women
Significant health outcomes
There were no differences between study groups in the proportion of women experiencing 1 or more significant health outcomes overall (180/398 [45.2%] in the progesterone group and 174/389 [44.7%] in the placebo group, adjusted RR 1.00, 95% CI 0.86–1.17, p = 0.994) or in the individual health outcomes, particularly those related to preterm birth, including use of tocolytic therapy and antenatal corticosteroids prior to the birth, antepartum haemorrhage, preeclampsia, risk of rupture of the membranes preterm or at or near term, chorioamnionitis requiring antibiotics, and postpartum haemorrhage (Table 3). There were no maternal deaths. Antibiotic use after birth was similar between the study groups, as was the need for antenatal admission and the length of any antenatal or postnatal hospital stay (Table 3).
Tab. 3. Secondary maternal outcomes by study group, including those related to preterm birth, psychological health, side effects of study treatment, and compliance. 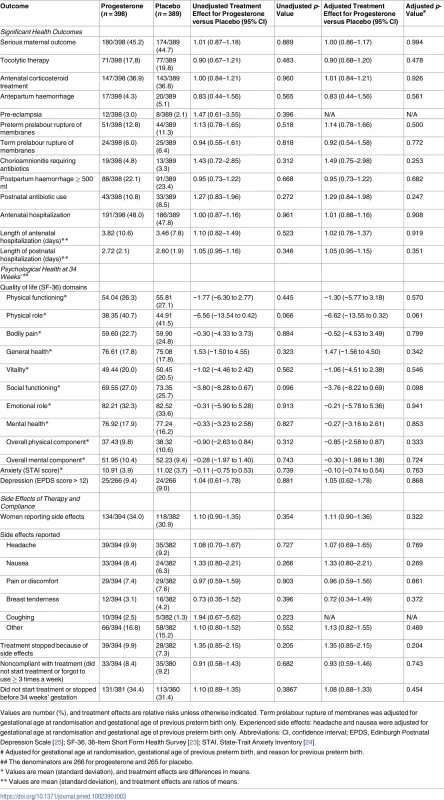
Values are number (%), and treatment effects are relative risks unless otherwise indicated. Term prelabour rupture of membranes was adjusted for gestational age at randomisation and gestational age of previous preterm birth only. Experienced side effects: headache and nausea were adjusted for gestational age at randomisation and gestational age of previous preterm birth only. Abbreviations: CI, confidence interval; EPDS, Edinburgh Postnatal Depression Scale [25]; SF-36, 36-Item Short Form Health Survey [23]; STAI, State-Trait Anxiety Inventory [24]. Psychological health
All measures on the 36-Item Short Form Health Survey (SF-36), including the overall physical and mental components, were similar in both study groups. No differences were seen in the proportion of women with a score on the Edinburgh Postnatal Depression Scale (EPDS) that was suggestive of depression (9.4% in the progesterone group and 9.0% in the placebo group), and the level of anxiety was similar in the 2 study groups (Table 3).
Side effects of study treatment and compliance
The proportion of women reporting any side effects of the treatment at 34 weeks’ gestation was similar between the study groups (134/394 [34.0%] in the progesterone group versus 118/382 [30.9%] in the placebo group), as was the proportion of women who stopped therapy because of side effects (39/394 [9.9%] in the progesterone group versus 28/382 [7.3%] in the placebo group) (Table 3). A similar proportion of women in both study groups either did not start the medication or forgot to use it 3 or more times a week, our measure of compliance (33/394 [8.4%] in the progesterone group and 35/380 [9.2%] in the placebo group) (Table 3). A similar proportion of women in both study groups used the study treatment up to 34 weeks’ gestation and remained undelivered (250/381 [65.6%] in the progesterone group versus 247/360 [68.6%] in the placebo group).
Discussion
Main findings
The PROGRESS Trial showed that in women with a history of previous spontaneous preterm birth, the use of 100-mg vaginal progesterone pessaries daily from 20 weeks’ gestation until 34 weeks’ gestation had no effect on the risk of the baby developing respiratory distress syndrome or on reducing the severity of any neonatal respiratory disease compared with placebo pessaries. In keeping with these findings, no benefits were seen relating to other respiratory outcomes or other neonatal morbidities.
For women, the risk of having a preterm birth was not reduced with the use of progesterone, and the need for interventions related to preterm birth such as tocolysis and antenatal corticosteroids; the need for antenatal hospital admission; and, if admitted, the length of hospital stay were also not reduced. Over 36% of the women in both study groups in the PROGRESS Trial were given antenatal corticosteroids, appropriate for the 36% rate of preterm birth seen in our high-risk population. Although progesterone can suppress proinflammatory cytokines [27], there was no evidence that progesterone exerted an anti-inflammatory effect on infective outcomes for the mother or the baby such as chorioamnionitis requiring the use of antibiotics, need for antibiotic use after birth, or the infant having proven early sepsis. Maternal psychological health status was similar in both study groups, including vulnerability to depression. This is reassuring given the concern that progesterone could aggravate depression.
Generalisability and comparison with other studies
We found no effect of vaginal progesterone on the risk of preterm birth for women with a previous preterm birth, similar to the findings from the O’Brien Trial [16] and the recently published OPPTIMUM Trial [28] but in contrast to other published reports [15, 29, 30, 31,32].
Strengths of the PROGRESS Trial
The clear entry criteria for the PROGRESS Trial were specifically set to easily identify women at high risk of a recurrence of preterm birth based on their previous history and to assess the effects of vaginal progesterone on this population. Inclusion criteria for our study were based on a previous history of preterm birth—a strong predictor for subsequent preterm birth—and not dependent on assessment of cervical length. Women identified and recruited with a history of preterm birth in their preceding pregnancy were at high risk of recurrence, with 36% giving birth before 37 weeks’ gestation, although there was no difference in gestational age at birth or in the proportion born preterm between the study groups. The trial was masked for participants and investigators with a placebo, and the primary outcome of respiratory distress syndrome was reported for all babies.
Potential limitations of current trial
It is possible that the dose of 100 mg progesterone used may have been too low. However, the Da Fonseca Trial [15] used the same 100-mg dose of vaginal progesterone and included women at high risk for preterm birth, defined by at least 1 previous spontaneous preterm birth, prophylactic cervical cerclage, or uterine malformation, but reported a lower rate of preterm birth compared with placebo (13.8% versus 28.5%), as have other trials [29,31]. Of note, a larger daily dose of 200 mg as used in the OPPTIMUM Trial was not found to reduce the risk of preterm birth or improve neonatal or child health at 2 years of age [28].
Our pretrial sample size estimate, based on the reported effect of treatment with progesterone compared with placebo on neonatal respiratory distress syndrome [21], would provide 80% to detect a difference at the 5% significance level. Whilst the reduction in sample size recommended at the masked interim review of data may have reduced power to detect differences, the final trial results do provide reliable study estimates with CIs. To show differences between treatment groups based on these study estimates at the 5% significance level and with 80% power would require a sample size of over 2,966,780 women.
Of eligible women invited to participate in the PROGRESS Trial, only 41% chose to do so, not too dissimilar to the 52% consent rate in the OPPTIMUM Trial [28]. Whether greater involvement of consumers in research proposals and promotion of trials open for recruitment within the community can increase participation in preterm birth research in priority areas, already identified by consumers of care and healthcare practitioners, needs to be established [1,33].
In any intervention study, compliance is crucial to ascertain true effect. Few other studies to date have reported on measures of compliance. In our study, most women started the allocated study treatment, and the median days of use was around 51 days. Nevertheless, a proportion of women in both study groups, almost 9%, either did not start the medication or forgot to use it 3 or more times a week, which was our measure of compliance. Within the OPPTIMUM Trial, compliance—defined slightly differently as 80% or more use of study treatment—was 69% [28]. This is similar to the proportion of women in the PROGRESS Trial who were still taking their study treatment and remained undelivered up to 34 weeks’ gestation (65.2% for women in the progesterone group and 68.6% in the placebo group).
Almost a third of the women reported side effects of treatment with the vaginal pessaries, the most frequent reasons given being headache, nausea, and pain or discomfort, although there were no differences in the proportion of women reporting side effects or the side effects reported by study group. For over 8% of women, these side effects were sufficient for them to stop their study treatment. Cessation of therapy because of side effects has not been well reported in earlier studies.
Clinical relevance
There are ongoing differences in clinical practice recommendations as to whether to recommend use of progesterone or not [17,18,19,20]. The critical issues are whether there are particular subgroups of women who may benefit from use of vaginal progesterone by virtue of their previous obstetric history (such as a history of preterm birth or factors in their current pregnancy, such as shortening of the cervix) and what is the optimal dose and treatment regimen to use (including the gestational age to start treatment, the length of time to use treatment, and the optimal mode of administration: vaginal or intramuscular preparation). There have been calls for an individual participant data meta-analysis (IPD-MA) of the trials already conducted [28, 34] that we strongly endorse. An IPD-MA can assess different participant - and treatment-level characteristics, which is not possible using an aggregate meta-analysis, and thus provide cumulated evidence on these critical issues identified that can be used by women and their families, clinicians, and policy makers as well as identify future research priorities.
Conclusions
Recommendations for clinical practice
Our results do not support the use of vaginal progesterone pessaries in women with a history of a previous spontaneous preterm birth to reduce the risk of respiratory distress syndrome or other neonatal or maternal morbidity. IPD-MA of the relevant trials may identify specific women for whom vaginal progesterone may be of benefit. The search for alternative strategies for the prevention of preterm birth and its sequelae must continue.
Supporting Information
Zdroje
1. March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report of Preterm Birth. Eds Howsom CP, Kinney MV, Lawn JE. WHO, Geneva, 2012.
2. Colicchia LC, Simhan HN. Optimizing subsequent pregnancy outcomes for women with a prior preterm birth. American Journal of Perinatology. 2016;33(3):267–75. doi: 10.1055/s-0035-1571143 26808194
3. Laughon SK, Albert PS, Leishear K, Mendola P. The NICHD Consecutive Pregnancies Study: recurrent preterm delivery by subtype. American Journal of Obstetrics and Gynecology. 2014;210(2):131.e1–8.
4. Yang J, Baer RJ, Berghella V, Chambers C, Chung P, Coker T, et al. Recurrence of preterm birth and early term birth. Obstetrics and Gynecology. 2016.
5. Gibson AM, Doyle LW. Respiratory outcomes for the tiniest or most immature infants. Seminars in Fetal & Neonatal Medicine. 2014;19(2):105–11.
6. Chow SSW, Le Marsney R, Hossain S, Haslam R, K L. Report of the Australian and New Zealand Neonatal Network 2013. Sydney: ANZNN, 2015.
7. Saigal S, Doyle L. An overview of mortality and sequelae of pretrem birth from infancy to adulthood. Lancet 2008;371 : 261–9. doi: 10.1016/S0140-6736(08)60136-1 18207020
8. Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392(6675):509–12. doi: 10.1038/33176 9548257
9. Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(19):7529–34. doi: 10.1073/pnas.1200650109 22529366
10. Nadeem L, Shynlova O, Matysiak-Zablocki E, Mesiano S, Dong X, Lye S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nature communications. 2016;7 : 11565. doi: 10.1038/ncomms11565 27220952
11. Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocrine Reviews. 1995;16(5):608–48. doi: 10.1210/edrv-16-5-608 8529574
12. Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Molecular Human Reproduction. 2001;7(9):875–9. 11517295
13. Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynaecol 1990; 97 : 149–54. 2138496
14. Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No.: CD004947. doi: 10.1002/14651858.CD004947.pub3 23903965
15. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. American Journal of Obstetrics and Gynecology. 2003;188(2):419–24. 12592250
16. O'Brien JM, Adair CD, Lewis DF, Hall DR, Defranco EA, Fusey S, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007;30(5):687–96.
17. American College of Obstetricians and Gynecologists. Prediction and prevention of preterm birth. ACOG 2012.
18. Royal Australian and New Zealand College of Obstetricians and Gynaecologists. Progesterone: Use in the second and third trimester of pregnancy for the prevention of preterm birth. RANZCOG Melbourne: Women's Health Committee, 2013.
19. Royal College of Obstetricians and Gynaecologists. Communication on the Use of Progesterone to Prevent Preterm Delivery. London: RCOG 2010.
20. Society for Maternal-Fetal Medicine Publications Committee, with the assistance of Vincenzo Berghella. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. SMFM Clinical Guideline. American Journal of Obstetrics & Gynecology 2012;206(5):376–386. doi: 10.1016/j.ajog.2012.03.010 22542113
21. Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. The New England Journal of Medicine. 2003;348(24):2379–85. doi: 10.1056/NEJMoa035140 12802023
22. Dodd JM, Crowther CA, McPhee AJ, Flenady V, Robinson JS. Progesterone after previous preterm birth for prevention of neonatal respiratory distress syndrome (PROGRESS): a randomised controlled trial. BMC pregnancy and childbirth. 2009;9 : 6. doi: 10.1186/1471-2393-9-6 19239712
23. Ware JE, Sherbourne CD. The MOS 36 item short form health survey (SF36) conceptual framework and item selection. Medical Care 1992;30 : 473–483. 1593914
24. Marteau TM, Bekker H. The development of a six item form of the State Scale of the Spielberger State Trait Anxiety Inventory (STAI). Br J Clin Psychology 1992;31 : 301.
25. Cox JL. Detection of postnatal depression—development of the 10 item Edinburgh Postnatal Depression Scale (EDPS). Brit J Psych 1987;154 : 782–786.
26. Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet 2005; 365 : 1348–1353. doi: 10.1016/S0140-6736(05)61034-3 15823387
27. Lei B, Mace B, Dawson HN, Warner DS, Laskowitz DT, James ML. Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated bv-2 microglia. PLoS ONE. 2014;9: e103969. doi: 10.1371/journal.pone.0103969 25080336
28. Norman JE, Marlow N, Messow CM, Shennan A, Bennett PR, Thornton S, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet (London, England). 2016;387(10033):2106–16.
29. Akbari S, Birjandi M, Mohtasham N. Evaluation of the effect of progesterone on prevention of preterm delivery and its complications. Scientific Journal of Kurdistan University of Medical Sciences. 2009;14(3):11–9.
30. Majhi P, Bagga R, Kalra J, Sharma M. Intravaginal use of natural micronised progesterone to prevent pre-term birth: A randomised trial in India. Journal of Obstetrics and Gynaecology 2009;29 : 6, 493–498, doi: 10.1080/01443610902980878 19697195
31. Cetingoz E, Cam C, Sakalh M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet 2011;283 : 423–429. doi: 10.1007/s00404-009-1351-2 20091317
32. Azargoon A, Ghorbani R, Aslebahar R. Vaginal progesterone on the prevention of preterm birth and neonatal complications in high risk women: A randomized placebo-controlled double-blin study. Int J Reprod BioMed 2016;14(5):309–316.
33. Duley L, Uhm S, and Oliver S. Top 15 UK research priorities for preterm birth, on behalf of the Preterm Birth Priority Setting Partnership Steering Group. Lancet. 2014; 383 : 2041–2042.
34. Addressing Disparities Program—PCORI www.pcori.org/…/PCORI-Addressing-Disparities-Advisory-Panel-Fall-2016. Pg 106. Pilot IPD-MA project on progesterone and preterm birth with MOD and NIHR.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis
- Preterm birth prevention—Time to PROGRESS beyond progesterone
- Women’s and men’s reports of past-year prevalence of intimate partner violence and rape and women’s risk factors for intimate partner violence: A multicountry cross-sectional study in Asia and the Pacific
- Global services and support for children with developmental delays and disabilities: Bridging research and policy gaps
- Risk, treatment duration, and recurrence risk of postpartum affective disorder in women with no prior psychiatric history: A population-based cohort study
- Chronic disease concordance within Indian households: A cross-sectional study
- Sustained effectiveness and cost-effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
- Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso
- Sustained effectiveness and cost-effectiveness of the Healthy Activity Programme, a brief psychological treatment for depression delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
- Vaginal progesterone pessaries for pregnant women with a previous preterm birth to prevent neonatal respiratory distress syndrome (the PROGRESS Study): A multicentre, randomised, placebo-controlled trial
- Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: A randomized phase 1 study
- Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: Results of the Compass pilot randomised trial
- Keeping it real: A journal editor in clinic
- Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis
- HbA1c for type 2 diabetes diagnosis in Africans and African Americans: Personalized medicine NOW!
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: A randomised 2 × 2 × 3 factorial trial in Burkina Faso
- Oral tetrahydrouridine and decitabine for non-cytotoxic epigenetic gene regulation in sickle cell disease: A randomized phase 1 study
- Keeping it real: A journal editor in clinic
- Sustained effectiveness and cost-effectiveness of Counselling for Alcohol Problems, a brief psychological treatment for harmful drinking in men, delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



