-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBroad-Scale Recombination Patterns Underlying Proper Disjunction in Humans
Although recombination is essential to the successful completion of human meiosis, it remains unclear how tightly the process is regulated and over what scale. To assess the nature and stringency of constraints on human recombination, we examined crossover patterns in transmissions to viable, non-trisomic offspring, using dense genotyping data collected in a large set of pedigrees. Our analysis supports a requirement for one chiasma per chromosome rather than per arm to ensure proper disjunction, with additional chiasmata occurring in proportion to physical length. The requirement is not absolute, however, as chromosome 21 seems to be frequently transmitted properly in the absence of a chiasma in females, a finding that raises the possibility of a back-up mechanism aiding in its correct segregation. We also found a set of double crossovers in surprisingly close proximity, as expected from a second pathway that is not subject to crossover interference. These findings point to multiple mechanisms that shape the distribution of crossovers, influencing proper disjunction in humans.
Published in the journal: . PLoS Genet 5(9): e32767. doi:10.1371/journal.pgen.1000658
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000658Summary
Although recombination is essential to the successful completion of human meiosis, it remains unclear how tightly the process is regulated and over what scale. To assess the nature and stringency of constraints on human recombination, we examined crossover patterns in transmissions to viable, non-trisomic offspring, using dense genotyping data collected in a large set of pedigrees. Our analysis supports a requirement for one chiasma per chromosome rather than per arm to ensure proper disjunction, with additional chiasmata occurring in proportion to physical length. The requirement is not absolute, however, as chromosome 21 seems to be frequently transmitted properly in the absence of a chiasma in females, a finding that raises the possibility of a back-up mechanism aiding in its correct segregation. We also found a set of double crossovers in surprisingly close proximity, as expected from a second pathway that is not subject to crossover interference. These findings point to multiple mechanisms that shape the distribution of crossovers, influencing proper disjunction in humans.
Introduction
Like most sexually reproducing organisms, humans undergo meiotic recombination. This process plays an important role in evolutionary dynamics, generating new combination of alleles on which natural selection can act ([1] and references therein), and in DNA repair. In humans, recombination is also fundamental to the successful completion of meiosis, helping to align homologous chromosomes and to ensure their proper disjunction [2]. Too little recombination or an abnormal placement of crossovers along the genome often results in aneuploidy, an outcome that leads to fetal loss or to severe developmental disabilities [3]. Thus, errors in the recombination process are clearly highly deleterious.
Nonetheless, there is substantial variation in recombination rates and patterns among humans. Individuals differ in their mean number of crossovers across the genome [4]–[6], in part due to genetic variation [7]. They also differ in the intensity of individual recombination hotspots, and in their use of hotspots genome-wide, variability that is again at least partly heritable [8],[9]. Moreover, this variation has detectable fitness consequences: mothers with a higher mean recombination rate have (slightly) more viable offspring [5],[9]. Together, these findings suggest that human recombination is naturally viewed as a quantitative phenotype subject to selection.
This perspective raises a number of questions about the selective pressures on recombination due to its role in meiosis, notably about their nature and stringency and the extent to which the system is buffered against variation. Answers to these questions have important implications for the relationship between recombination rate variation and the susceptibility to non-disjunction. Such answers are also essential to the study of the evolution of recombination rates and of genome dynamics [10],[11].
Chiasmata, the physical connections between chromatids later processed into crossovers, help to bind the homologs together, thereby aiding in their proper disjunction. In the absence of a chiasma, segregation is thought to be haphazard and to frequently result in aneuploidy, a role for recombination that likely imposes strong selective pressures for at least one chiasma per chromosome, even when the mean number is close to 1 [12].
In most species, however, the total number of chiasmata (in males and females) far exceeds the number of chromosomes (cf. [10]). Foci counts of MLH1, a mismatch repair protein that serves as markers for most (but perhaps not all) human crossovers, indicate that there is a chiasma on each chromosome arm examined [13]. Moreover, across mammals, the number of chromosome arms (i.e., two for metacentric chromosomes) appears to be a better predictor of the mean number of crossovers than is the number of chromosomes [10],[14]. These cytogenetic and evolutionary lines of evidence have led to the suggestion that, with rare exceptions, one chiasma per chromosome arm is required for proper disjunction ([15] and references therein; see also [10],[13],[14],[16],[17]).
The role of recombination in human meiosis shapes the placement of crossovers as well as their number. When more than one crossing-over event occurs on the same chromosome, the events are not spaced randomly, but instead tend to occur farther apart than expected by chance. The more even spacing of events on chromosomes due to this “crossover interference” may serve to further reduce the risk of non-disjunction [18]. While several models of interference have been proposed, the phenomenon remains poorly understood. In particular, evidence from model organisms, including mice, suggests that a small subset of events result from a second pathway, not subject to crossover interference [16]. A central prediction of the two-pathway hypothesis is the presence of rare, double recombinants in close proximity (see [19] for details).
Here, we used human pedigree data to assess whether the number of chiasmata is tightly regulated at the level of chromosomes or chromosome arms and to examine how often proper disjunction occurs in the absence of a chiasma. We also took advantage of the high spatial resolution of the data to investigate the spacing of crossover events.
Results
Our point of departure was the number of crossovers observed in transmissions to viable, non-trisomic offspring (Supplementary Table 1 in Text S1). We constructed this distribution from 576 meioses in a large European-American pedigree (see Methods). The families were genotyped at approximately 400,000 single nucleotide polymorphisms (SNPs), providing excellent coverage of the genome (see Supplementary Table 2 in Text S1) and allowing us to localize crossover locations with high resolution [9]. For chromosome 21, we also considered the number of crossovers in an additional 152 female transmissions; these were inferred in European-American families that had been typed for 133 SNPs [20]. The distributions do not differ significantly between the two data sets (p = 0.86 by a Fisher's Exact Test).
The crossovers observed in gametes cannot be equated to the number of chiasmata in tetrads since, for every chiasma, only two of the four chromatids are recombinants. (The term chiasma is sometimes reserved for the physical manifestation of a crossover detected in cytogenetic studies; here, we employ it to denote a crossover in the tetrad, and use the term crossover to refer specifically to genetic exchanges visible in transmitted gametes.) We used the observed distribution of the number of crossovers in gametes to infer the distribution of chiasmata in the tetrads (also called bivalents) [21]. We were particularly interested in assessing how often there was no chiasma in the tetrad, i.e., how often nullichiasmatic chromosomes segregated properly. Following previous studies (e.g., [22],[23]), we assumed that one of the four chromatids is transmitted at random. We further assumed that there is no chromatid interference, i.e., that when there is more than one chiasma in the tetrad, the pair of chromatids involved in each genetic exchange is chosen independently and at random (cf. [24]). Under this model, given a chiasma, there is always a probability of one half of transmitting a recombinant chromatid. Thus, if there are one or more chiasmata per tetrad, at most half the gametes are expected to be non-recombinants. Assuming that no crossovers are missed due to insufficient marker coverage, a significant excess of non-recombinant gametes beyond one-half provides unequivocal evidence for the existence of tetrads that segregate properly without a chiasma. More generally, the shape of the observed distribution of crossover counts is informative about underlying patterns of recombination in tetrads.
To infer the distribution of the number of chiasmata in tetrads, we used two approaches: a likelihood method, variants of which have been applied in a number of studies [20],[22],[23],[25],[26], as well as a Bayesian approach that we developed and which we believe presents a number of advantages (see Methods). Results from the two methods were similar (see Supplementary Figure 1 in Text S1); to facilitate the comparison to earlier studies, we present those obtained from the likelihood approach.
Applying the approach to crossover data for each chromosomal arm separately, we found overwhelming evidence for frequent, proper disjunction in the absence of a chiasma (Figure 1). In both male and female transmissions, estimates of nullichiasmatic arms are as high as 20–40% for the smaller metacentric chromosomes, but are significantly above 0 even for some of the larger chromosome arms. Given the dense marker coverage of each arm (see Supplementary Table 2 in Text S1), we are missing at most a small fraction of crossover events, rendering our estimates robust. Thus, our findings establish that, in humans, proper segregation does not require the tight regulation of the number of chiasmata per chromosome arm.
Fig. 1. For each chromosomal arm, the estimated fraction of bivalents that segregated properly without a chiasma, in male (blue) and female (red) transmissions. 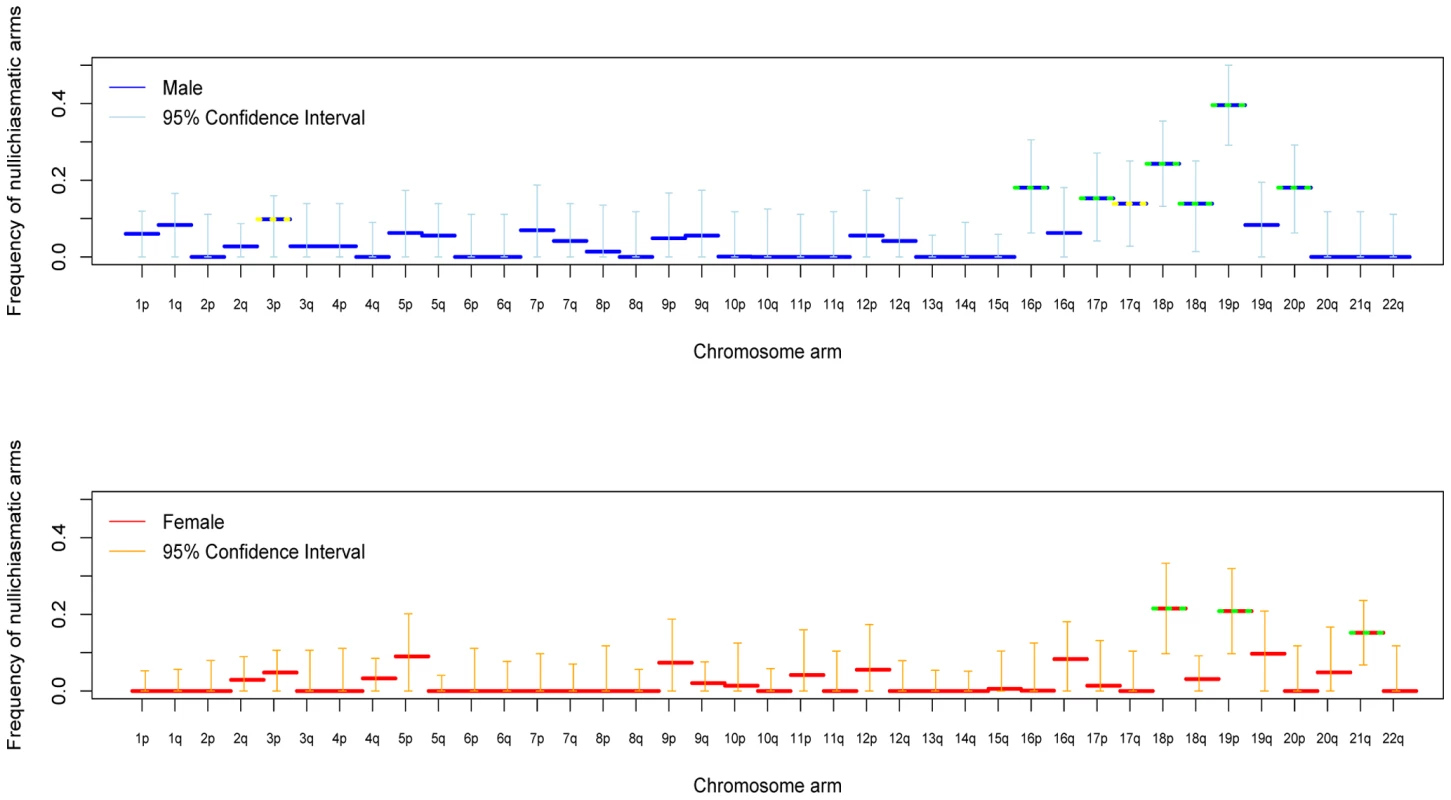
Shown are maximum likelihood estimates and 95% confidence intervals (see Methods). Estimates significantly above 0 at the 5% level are indicated by a yellow dotted line and at the 1% level by a green dotted line. The data for chromosome 21 combine two sets of pedigrees (see Methods). We also assessed the stringency of the rule at the level of the entire chromosome. With these data, we can only test whether nullichiasmatic bivalents can segregate properly for the smaller chromosomes, because for the larger chromosomes, they rarely occur (as the recombination rate is simply too high). Indeed, simulations suggest that, even if nullichiasmatic chromosomes always segregated properly, we would have high power to detect their transmission only for the eight smallest chromosomes in males and for chromosomes 21 and 22 in females (see Supplementary Methods in Text S1). As expected, the larger chromosomes show no evidence of nullichiasmatic transmissions to viable offspring (Figure 2). But among the smaller chromosomes, there are apparent exceptions. In males, we found evidence for proper segregation in the absence of a chiasma for two chromosomes: in over 0.1% (the lower 5%-tile) of cases for chromosome 12 (p = 0.0176), and in over 4.2% of cases for chromosome 18 (p = 0.0120). In females, in turn, we inferred that at least 7.3% (p = 0.0002) of chromosome 21 transmissions occur properly in the absence of a chiasma (Figure 3).
Fig. 2. For each chromosome, the estimated fraction of bivalents that segregated properly without a chiasma, in male (blue) and female (red). 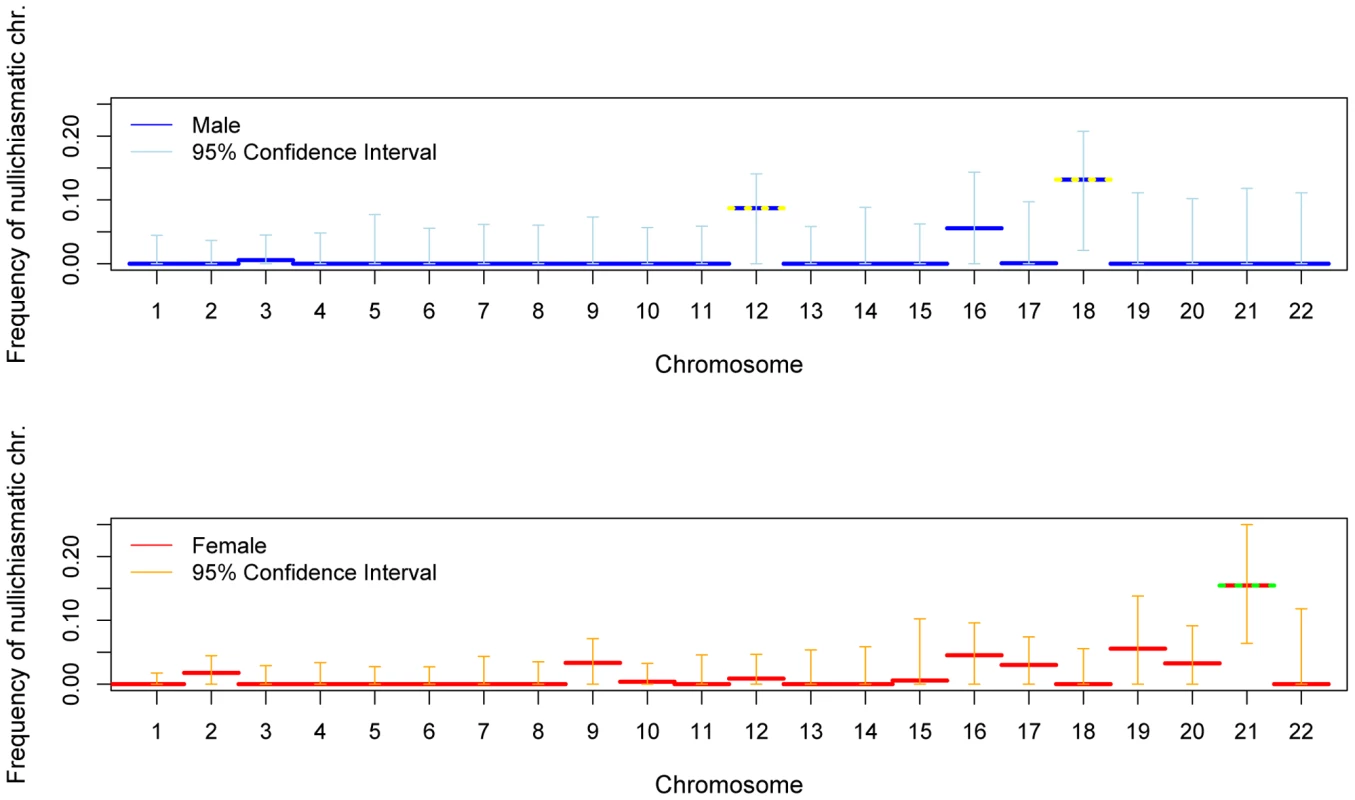
See the legend of Figure 1 for additional details. Fig. 3. The estimated distribution of chiasmata per bivalent, in female transmissions of chromosome 21. 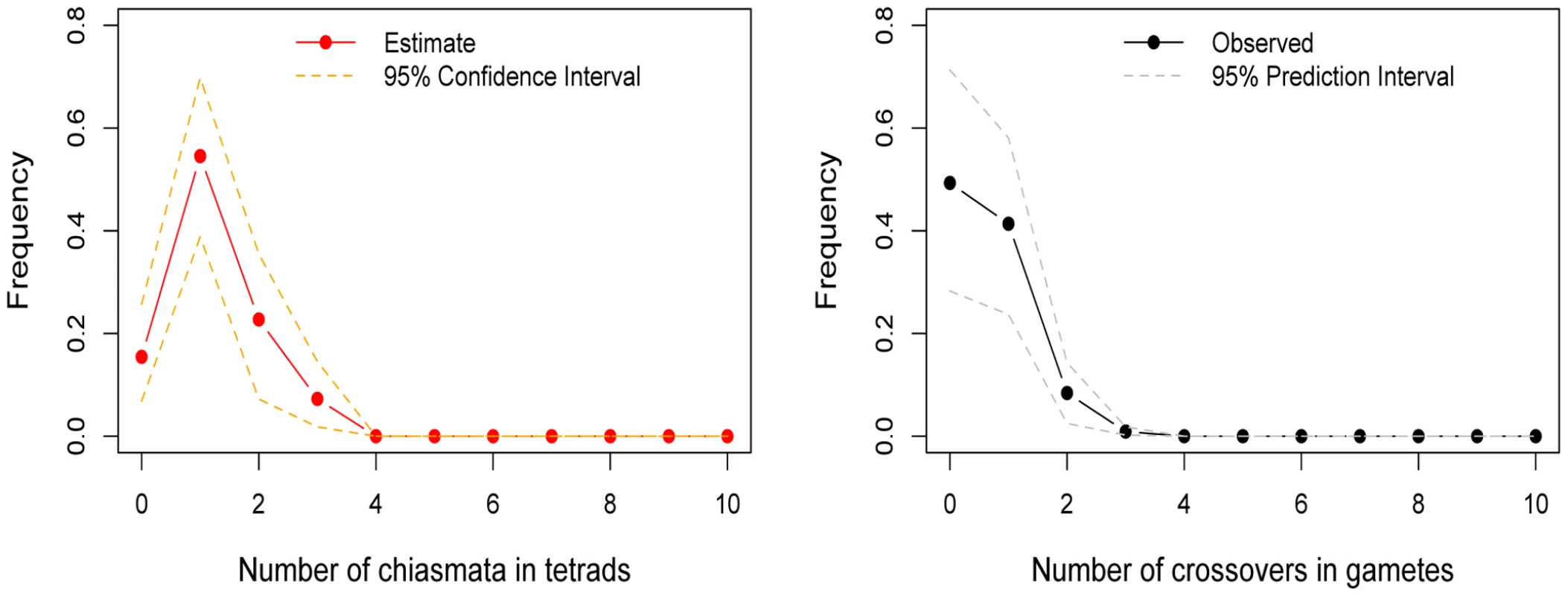
To the left is the inferred distribution of chiasmata per bivalent, obtained from a maximum-likelihood method. The point estimates are shown as filled red circles and the 95% confidence intervals as orange dotted lines (see Methods). To the right is the observed distribution of crossovers among female transmissions to viable, non-trisomic offspring, in two sets of pedigrees (see Methods). As a test of the goodness of fit of the model, we also show (in gray dotted lines) the prediction intervals obtained from 5000 simulations where we binomially sample the number of crossovers per gamete distribution given the estimated distribution of chiasmata per bivalent; as can be seen, the data fit well within the 95% prediction intervals (see Methods). The data indicate that a substantial and significant (p = 0.0002) fraction of female transmissions involved a nullichiasmatic chromosome 21. Given that the estimated fractions of nullichiasmatic bivalents are relatively small, they may be sensitive to a modest number of missing crossover events. To evaluate this possibility, we performed a number of checks. For chromosomes 12 and 18, these analyses suggested that our results are tentative, as a subset of families are missing informative markers for some of the telomeric regions—enough to potentially inflate the apparent number of non-recombinant gametes and lead to an over-estimate of the fraction of nullichiasmatic tetrads (see Supplementary Methods in Text S1). For chromosome 21, however, the two data sets that we analyzed have good marker coverage and are missing at most a small number of events, indicating that our results are reliable (see Supplementary Methods in Text S1).
In interpreting the chromosome 21 results, a second consideration is the number of tests performed. As noted above, for the larger chromosomes, the probability of the data will be very similar under a model allowing for nullichiasmatic chromosomes and one that does not; only for the small chromosomes could the p-value derived from our likelihood ratio test be small. This reasoning suggests that we should be correcting for many fewer than 22 tests—possibly as few as two. But even if we were to conservatively correct for 22 tests, the results for chromosome 21 remain significant (e.g., if we use a Bonferroni correction, p = 0.0044). Thus, in females at least, the requirement for one chiasma per chromosome is not absolute.
To learn more about patterns of recombination underlying proper disjunction in humans, we used the high resolution of crossover locations in our data in order to better characterize crossover interference. Overall estimates of the strength of crossover interference are similar to those previously reported based on a smaller data set [27], although potential differences emerge between sexes and among chromosomes (see Supplementary Figure 2 in Text S1). Also consistent with earlier studies [27],[28], we find that the centromere is not a barrier to interference (see Supplementary Figure 3 in Text S1). This suggests that when a chiasma occurs close to the centromere of one arm, it will increase the odds of the other arm being nullichiasmatic.
Because of the high density of our markers, we were able to identify a set of double crossovers in close proximity (<5 cM), distributed across families and genomic locations (see Supplementary Table 4 in Text S1). This observation is highly unexpected under the standard statistical model of interference, the gamma renewal model (Figure 4). The excess of tight double crossovers is still apparent even when we focus on a set of stringently vetted double crossovers, which likely under-estimates the true number (Supplementary Figure 4 in Text S1). Thus, in humans, a non-negligible number of double crossovers occur surprisingly close together.
Fig. 4. Double crossovers in close proximity. 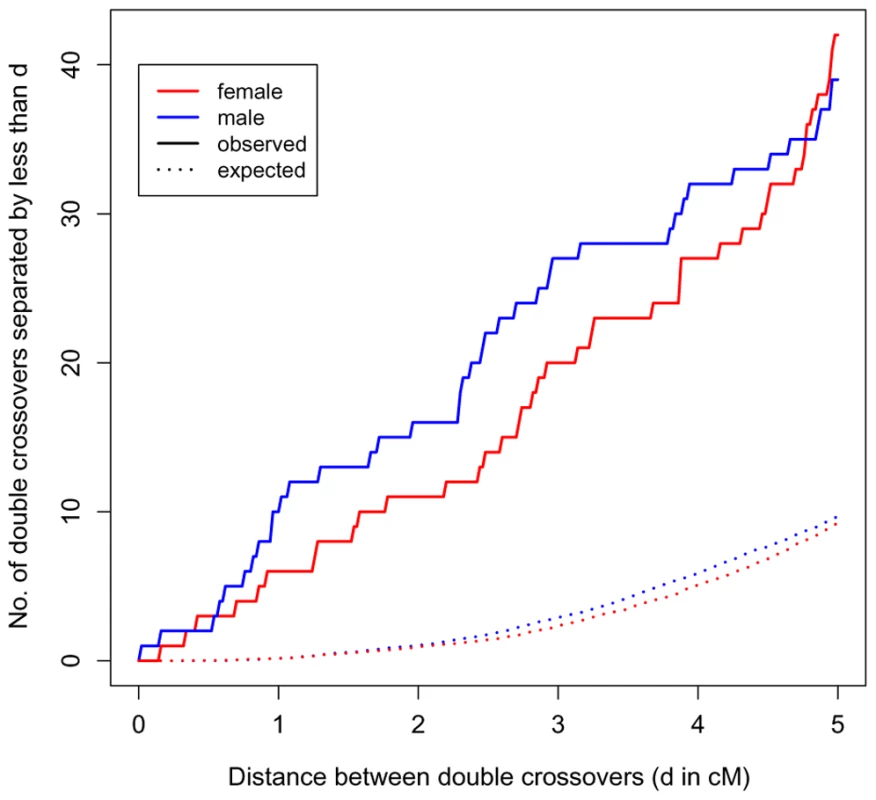
The number of double crossovers within 5 cM or less versus the number expected under a gamma renewal model, as estimated from the data (distances are based on sex-specific genetic maps; see Supplementary Methods in Text S1). In red are events in female transmissions; in blue are events in male transmissions. A version of this figure with stringently vetted double crossovers is presented in Supplementary Figure 4 in Text S1. Discussion
Our statistical analysis indicates that human crossovers are not regulated on the scale of an arm but on that of an entire chromosome (as found also in another mammal, shrews, using a cytogenetic approach [29]). In fact, the genetic length of a chromosome is extremely well predicted by a model that incorporates only two features: the need for one event and the length of the chromosome [19],[30] (see also Figure 5). The tight fit of the model further suggests that there are few chromosome-specific factors affecting the total recombination rate per chromosome and that beyond one event, additional crossovers occur in rough proportion to physical length.
Fig. 5. The total recombination rate per chromosome, in males and females, as predicted by an obligate chiasma per bivalent (i.e, an intercept at 0.5 crossovers per gamete) and the physical length of the chromosome. 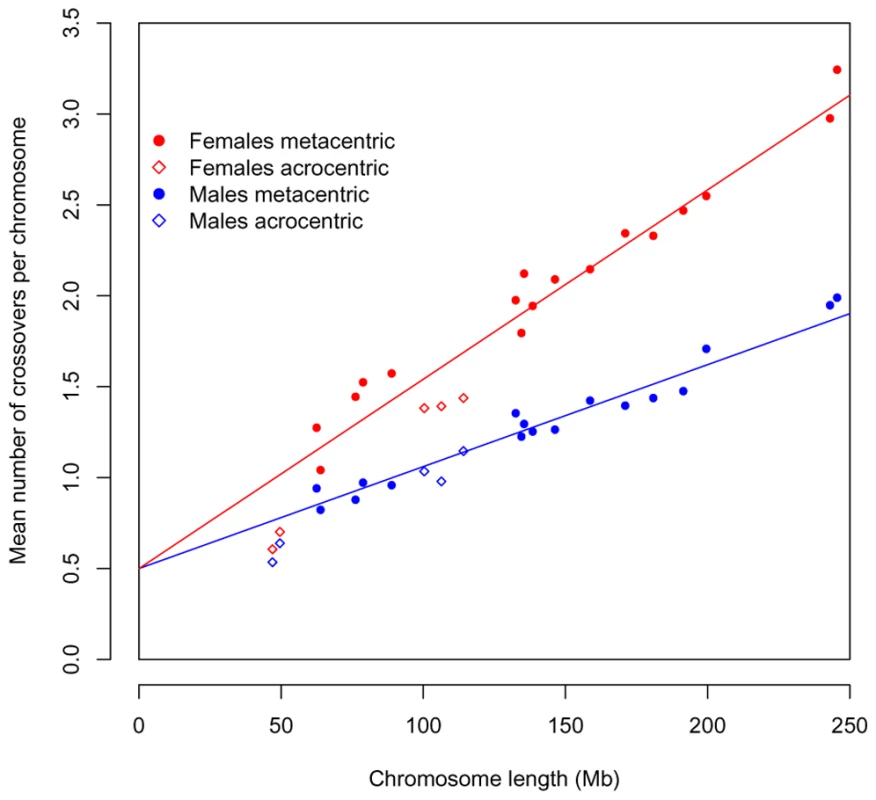
In red are the observed values for females and in blue the ones for males; circles denote metacentric chromosomes and diamonds acrocentric ones. The regression line is shown for each sex separately. In both cases, there is an excellent fit of this simple model to the data (R2 = 0.94 for females; R2 = 0.95 for males). As known for over half a century, the placement of these additional events is subject to positive crossover interference. Interestingly, however, this may not be true of all crossovers (see [19] for discussion). Indeed, we find an excess of rare, double crossovers in close proximity, which are better fit by the model of Housworth and Stahl (2003) [19], where there are two types of crossovers, only one of which is subject to interference, than by the standard interference model (see Supplementary Methods in Text S1). Thus, while other explanations remain, our results are consistent with the existence of a second crossover pathway in humans.
We also see exceptions to the rule of one chiasma per chromosome, most clearly for female (but not male; see Figure 2) transmissions of chromosome 21. Our analysis relies on assumptions of no chromatid interference and no meiotic drive of chromatids [31]. Violations of these assumptions could increase the variance in the transmission of non-recombinant chromatids, potentially serving as an alternative explanation for our findings. We extended our model to mimic this effect (see Supplementary Methods in Text S1) and found that a large variance in transmission probability could account for the observed distribution of crossovers on chromosome 21 even if there were an obligate chiasma to ensure proper disjunction (Supplementary Figure 5 in Text S1). However, studies in yeast, where this hypothesis can be tested directly, found no evidence of chromatid interference [24],[32]. In turn, if meiotic drive explains our findings, we might expect to see over-transmission of certain genotypes in females. Yet there is no evidence for transmission distortion of markers on chromosome 21 (see Supplementary Methods in Text S1). Additional explanations for our finding are strong, female-specific selection against recombinant gametes or the preferential transmission of non-recombinant chromatids. While both remain formal possibilities, of interest in their own right, there is no evidence for such selection in humans, nor is there (to our knowledge) a clear mechanism that would lead to the over transmission of non-recombinant chromatids.
Given that the model assumptions are well supported by studies in model organisms, and the frequent observation of trisomy 21 in humans [3], the most plausible interpretation of our findings is that nullichiasmatic chromosomes 21 occasionally experience proper disjunction. This conclusion is in qualitative agreement with the results of a subset of cytogenetic studies, which find that shorter bivalents sometimes lack an MLH1 focus (e.g., [33]–[35]); for example, Tease et al. [35] found that ∼3.5% of ooyctes from one female lacked a MLH1 focus on the chromosome 21 bivalent. However, the results of such studies vary markedly, likely due to the small number of cells and individuals that can be considered (e.g., [28], [34], [36]–[38]). Moreover, if there is a second crossover pathway in humans, MLH1 foci may not mark all crossovers [16]. Perhaps most importantly, in studies of pachytene cells, the fate of the daughter cells remains unknown [33]; in contrast, we are ascertaining viable offspring, where proper disjunction has clearly occurred.
Intriguingly, the pedigree data suggest that the proper segregation of nullichiasmatic chromosome 21 is fairly frequent. In the absence of a back-up mechanism to aid in the proper disjunction of nullichiasmatic chromosomes, chromatids are expected to segregate randomly to the poles, resulting in chromosomally unbalanced daughter cells in half the outcomes and in balanced cells in the other half. Thus, the rate of proper segregation of nullichiasmatic chromosomes should equal the rate of aneuploidy (in fact, it should be quite a bit lower, since aneuploidy has other sources). Yet fewer than 1% of all conceptions are thought to be aneuploid for chromosome 21—an estimate markedly lower than the fraction of nullichiasmatic transmissions that seem to segregate properly. This large discrepancy raises the possibility of a back-up mechanism in humans, similar to those that exist in Drosophila and yeast [33].
The absence of a chiasma on maternal chromosome 21 is known to be a risk factor for trisomy 21—the main cause of Down's syndrome [20],[22],[39]. If there is a back-up mechanism that aids in the proper disjunction of nullichiasmatic chromosomes, variation in its effectiveness (either across females or with age) could contribute to the risk of forming aneuploid gametes [31].
Methods
The observed distribution of the number of crossovers
We previously estimated the location of crossover events in a large Hutterite (a founder population of European origin) pedigree that had been genotyped using Affymetrix 500 K genotyping arrays [9]. To this end, we required K = 5 or more consecutive informative markers to call a crossover event [9]. At the scale of a megabase or more, our sex-specific genetic maps were highly concordant with the those of Kong et al. [40], which are based on more meioses but fewer markers [9].
For these analyses, we focused on 52 overlapping, nuclear families of four or more genotyped offspring, as simulations suggested that our algorithm is highly reliable for families of more than three children (for K = 5) [9]. Based on the total 576 meioses, we constructed the distribution of the number of crossovers in (male or female) gametes, for each chromosome. For female transmissions of chromosome 21, we supplemented our data set with crossover numbers reported in Oliver et al. (2008) [20]. All transmissions were to viable, non-trisomic offspring.
To assign crossovers to chromosome arms, we relied on the centromere gap location in build May 2004 of the human genome (as provided by http://genome.ucsc.edu/). Events were assigned to the p arm if the start of the interval within which they were localized was left of the centromere; remaining events were assigned to the q arm. When the intervals spanned the centromere boundary, we conservatively added the events to both arms; few events fell in this category (see Supplementary Table 1 in Text S1).
Estimating the number of chiasmata in bivalents
Our starting point for inference was the model of Ott (1996) [25], in which the binomial distribution with parameter 0.5 describes the number of crossovers, Y, in a gamete given X chiasmata in the bivalent:(1)
This model assumes no chromatid interference in the distribution of chiasmata across chromatid pairs; in the Supplementary Methods in Text S1, we describe a simple extension that mimics some of the effects of chromatid interference. The object of inference was the probability distribution of the number of chiasmata in bivalents, described by the vector p, where(2)is the probability of x chiasmata in a bivalent. For computational convenience, we assumed that x lies in a finite range 0…xmax; we chose xmax = 20. To obtain maximum likelihood estimates of p for each chromosome and chromosome arm, we employed the EM algorithm of [25]. Confidence intervals were estimated using the parametric bootstrap procedure described in Yu and Feingold (2001) [23], based on 5000 permutations for chromosomes and 1000 for chromosome arms.
Testing for the proper disjunction of nullichiasmatic bivalents
To test for the presence of nullichiasmatic bivalents among our sample of gametes (that necessarily underwent proper disjunction), we conducted a likelihood ratio test (LRT) of the unconstrained model (in which p0≥0) to the constrained model (p0 = 0). To fit the latter, we utilized the same EM algorithm subject to the additional constraint that p0 = 0. Because the asymptotic distribution for the LRT statistic is known to provide a conservative test [23], we calculated p-values using parametric bootstrap (again with 5000 permutations for chromosomes and 1000 for chromosome arms).
A Bayesian approach to the problem
By taking a complementary, Bayesian approach to inference, we were able to incorporate extensive prior information about recombination patterns in order to improve inferential power, to quantify the deviation of p from a naïve model with no chromosome interference and to avoid possible statistical problems arising from a high-dimensional parameter and modest sample size.
Specifically, using the Kong et al. (2002) [40] estimate of the expected number of crossovers, λ, in a particular chromosome or chromosome arm, we employed a Dirichlet prior on p with parameter vector α, where(3)
The prior expectation of px is αx/J, which equals the Poisson probability of x chiasmata (given a mean of 2λ) and arises from a model of no chromosome interference. J controls the weight of prior information, and allows for deviation from this simple model. We explored values of J = 0.1, 1 and 5. Results were similar across values of J (not shown); for ease of interpretation, we present the results for J = 1. The Bayesian model was fit using Markov chain Monte Carlo (MCMC), which is described fully in the Supplementary Methods in Text S1.
Characterizing the strength and nature of crossover interference
The gamma model, a standard model for crossover interference, was previously found to be a good fit to inter-crossover distances in humans and in a number of other organisms (e.g., [27],[41]). In the gamma model, the locations of the chiasmata on tetrads occur according to a stationary gamma renewal process, where the genetic distances between chiasmata follow a gamma distribution with shape and rate parameters ν and 2ν, respectively. Under the assumption of no chromatid interference, the locations of the crossovers are obtained by thinning the chiasma locations independently, with a probability of 1/2.
Following Broman and Weber (2000) [27], we estimated the parameter ν from our data, for each sex and each chromosome separately (Supplementary Figure 3 in Text S1). The value of ν estimated from the Hutterite data is similar to that obtained by [27], but with tighter confidence intervals due to the larger number of meioses available in the Hutterites. We find some evidence for variation between chromosomes and sexes in the strength of interference (i.e., variation in ν). However, given the lack of fit of the gamma model (Figure 4), these findings should be interpreted with caution.
Supporting Information
Zdroje
1. OttoSP
LenormandT
2002 Resolving the paradox of sex and recombination. Nat Rev Genet 3 252 261
2. HuntPA
HassoldTJ
2002 Sex matters in meiosis. Science 296 2181 2183
3. HassoldT
HuntP
2001 To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2 280 291
4. BromanKW
MurrayJC
SheffieldVC
WhiteRL
WeberJL
1998 Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 63 861 869
5. KongA
BarnardJ
GudbjartssonDF
ThorleifssonG
JonsdottirG
2004 Recombination rate and reproductive success in humans. Nat Genet 36 1203 1206
6. LynnA
AshleyT
HassoldT
2004 Variation in human meiotic recombination. Annu Rev Genomics Hum Genet 5 317 349
7. KongA
ThorleifssonG
StefanssonH
MassonG
HelgasonA
2008 Sequence variants in the RNF212 gene associate with genome-wide recombination rate. Science 319 1398 1401
8. JeffreysAJ
NeumannR
2005 Factors influencing recombination frequency and distribution in a human meiotic crossover hotspot. Hum Mol Genet
9. CoopG
WenW
OberC
PritchardJ
PrzeworskiM
2008 High resolution mapping of crossovers reveals extensive variation in fine-scale recombination patterns among humans. Science 319 1395 1398
10. CoopG
PrzeworskiM
2007 An evolutionary view of human recombination. Nat Rev Genet 8 23 34
11. GautBS
WrightSI
RizzonC
DvorakJ
AndersonLK
2007 Recombination: an underappreciated factor in the evolution of plant genomes. Nat Rev Genet 8 77 84
12. MatherK
1938 Crossing-over. Biol Rev 13 252 292
13. HassoldT
JudisL
ChanER
SchwartzS
SeftelA
2004 Cytological studies of meiotic recombination in human males. Cytogenet Genome Res 107 249 255
14. Pardo-Manuel de VillenaF
SapienzaC
2001 Recombination is proportional to the number of chromosome arms in mammals. Mamm Genome 12 318 322
15. DumasD
Britton-DavidianJ
2002 Chromosomal rearrangements and evolution of recombination: comparison of chiasma distribution patterns in standard and robertsonian populations of the house mouse. Genetics 162 1355 1366
16. BaudatF
de MassyB
2007 Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosome Res 15 565 577
17. HassoldT
HansenT
HuntP
VandeVoortC
2009 Cytological studies of recombination in rhesus males. Cytogenet Genome Res 124 132 138
18. van VeenJE
HawleyRS
2003 Meiosis: when even two is a crowd. Curr Biol 13 R831 833
19. HousworthEA
StahlFW
2003 Crossover interference in humans. Am J Hum Genet 73 188 197
20. OliverTR
FeingoldE
YuK
CheungV
TinkerS
2008 New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet 4 e1000033 doi:10.1371/journal.pgen.1000033
21. WeinsteinA
1936 The theory of multiple strand crossing-over. Genetics 21 155 199
22. LambNE
FeingoldE
ShermanSL
1997 Estimating meiotic exchange patterns from recombination data: an application to humans. Genetics 146 1011 1017
23. YuK
FeingoldE
2001 Estimating the frequency distribution of crossovers during meiosis from recombination data. Biometrics 57 427 434
24. ZhaoH
McPeekMS
SpeedTP
1995 Statistical analysis of chromatid interference. Genetics 139 1057 1065
25. OttJ
1996 Genetic mapping and DNA sequencing.
SpeedT
WatermanMS
The IMA Volumes in Mathematics and its Applications New York Springer Verlag 49 63
26. BuggeM
CollinsA
PetersenMB
FisherJ
BrandtC
1998 Non-disjunction of chromosome 18. Hum Mol Genet 7 661 669
27. BromanKW
WeberJL
2000 Characterization of human crossover interference. Am J Hum Genet 66 1911 1926
28. LaurieDA
HultenMA
1985 Further studies on chiasma distribution and interference in the human male. Ann Hum Genet 49 203 214
29. BorodinPM
KaramyshevaTV
BelonogovaNM
TorgashevaAA
RubtsovNB
2008 Recombination map of the common shrew, Sorex araneus (Eulipotyphla, Mammalia). Genetics 178 621 632
30. KabackDB
GuacciV
BarberD
MahonJW
1992 Chromosome size-dependent control of meiotic recombination. Science 256 228 232
31. ZwickME
SalstromJL
LangleyCH
1999 Genetic variation in rates of nondisjunction: association of two naturally occurring polymorphisms in the chromokinesin nod with increased rates of nondisjunction in Drosophila melanogaster. Genetics 152 1605 1614
32. ManceraE
BourgonR
BrozziA
HuberW
SteinmetzLM
2008 High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 454 479 485
33. KoehlerKE
HassoldTJ
1998 Human aneuploidy: lessons from achiasmate segregation in Drosophila melanogaster. Ann Hum Genet 62 467 479
34. SunF
Oliver-BonetM
LiehrT
StarkeH
TurekP
2006 Analysis of non-crossover bivalents in pachytene cells from 10 normal men. Hum Reprod 21 2335 2339
35. TeaseC
HartshorneGM
HultenMA
2002 Patterns of meiotic recombination in human fetal oocytes. Am J Hum Genet 70 1469 1479
36. Codina-PascualM
CampilloM
KrausJ
SpeicherMR
EgozcueJ
2006 Crossover frequency and synaptonemal complex length: their variability and effects on human male meiosis. Mol Hum Reprod 12 123 133
37. SunF
Mikhaail-PhilipsM
Oliver-BonetM
KoE
RademakerA
2008 The relationship between meiotic recombination in human spermatocytes and aneuploidy in sperm. Hum Reprod 23 1691 1697
38. FergusonKA
LeungS
JiangD
MaS
2009 Distribution of MLH1 foci and inter-focal distances in spermatocytes of infertile men. Hum Reprod 24 1313 1321
39. WarrenAC
ChakravartiA
WongC
SlaugenhauptSA
HalloranSL
1987 Evidence for reduced recombination on the nondisjoined chromosomes 21 in Down syndrome. Science 237 652 654
40. KongA
GudbjartssonDF
SainzJ
JonsdottirGM
GudjonssonSA
2002 A high-resolution recombination map of the human genome. Nat Genet 31 241 247
41. McPeekMS
SpeedTP
1995 Modeling interference in genetic recombination. Genetics 139 1031 1044
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2009 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
Nejčtenější v tomto čísle- Identification of a Cardiac Disease Modifier Gene Using Forward Genetics in the Mouse
- Molecular Tooth Decay
- Broad-Scale Recombination Patterns Underlying Proper Disjunction in Humans
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



