-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Comparison of the epidemiological patterns of Lyme borreliosis and tick-borne encephalitis in the Czech Republic in 2007–2016
Srovnání epidemiologických charakteristik výskytu lymské boreliózy a klíšťové encefalitidy v České republice v letech 2007–2016
Východisko:
Lymská borelióza (LB) a klíšťová encefalitida (KE) jsou dvě onemocnění přenášená vektory – klíšťaty. Obě onemocnění jsou endemická a jejich výskyt byl hlášen ve všech krajích České republiky (ČR), včetně městských aglomerací, ale v různém poměru. Vzhledem k podmínkám v příhraničních oblastech, jsou velkému riziku vystaveni také obyvatelé sousedních zemí, jako je Rakousko, Německo, Polsko a Slovensko.
Materiál a metody:
Abychom získali více informací o epidemiologii LB a KE v posledním desetiletí, provedli jsme analýzu dat ze systému národní surveillance z let 2007–2016.
Výsledky:
Incidence LB v ČR činila 37,3/100 000 obyvatel a rok (27,6–46,1/100 000). Incidence KE dosáhla 5,7/100 000 (3,4–8,2/100 000) a vykazovala klesající trend, který ale nebyl statisticky významný (p = 0,155). Rozdíl ve výskytu LB a KE se časem zvyšoval. Celkový poměr infikovaných mužů a žen činil 0,84 u LB a 1,51 u KE. Věkově specifická incidence u obou infekcí vykazovala dva typické vrcholy, první ve věkové skupině 5–9 let v případě LB a 15–19 let v případě KE. Druhý vrchol byl u obou infekcí zaznamenán ve věkové skupině 55–64 let. KE představuje významnou hrozbu zejména u dětí do 15 let věku. U 39 074 případů LB patřily k nejčastějším klinickým projevům erythema migrans 62,1 % a neuroborelióza 25,1 %. U všech 5 969 případů KE se infekce projevila postižením nervového systému, a to ve formě meningoencefalitidy (47,9 %), meningoencefalomyelitidy (21,8 %) či meningitidy (19,8 %).
Závěry:
Data svědčí o vysokém riziku infekce LB a KE v ČR. Incidence obou infekcí během roku vykazuje bimodální rozložení. Výskyt LB je 5–6krát častější, než je tomu v případě KE. V posledních letech byl výskyt LB zhruba stabilní, zatímco výskyt KE vykazoval klesající trend. Naše studie je unikátní v tom, že umožnila porovnání výskytu LB a KE v prostoru a čase.
KLÍČOVÁ SLOVA
Lymská borelióza – klíšťová encefalitida – incidence – životní prostředí a klimatické změny
Epidemiol. Mikrobiol. Imunol., 67, 2018, č. 3, s. 134–140
Authors: B. Kříž 1,2; A. Fialová 1
; H. Šebestová 1; M. Daniel 1; M. Malý 1
Authors place of work: National Institute of Public Health, Prague 1; Third Faculty of Medicine, Charles University, Prague, Bohumír Kříž kept working on the paper until his final day. 2
Published in the journal: Epidemiol. Mikrobiol. Imunol. 67, 2018, č. 3, s. 134-140
Category: Původní práce
Summary
Background:
Lyme borreliosis (LB) and tick-borne encephalitis (TBE) are two vector-borne diseases transmitted by ticks. Both diseases are endemic and have been reported in all regions of the Czech Republic including urban agglomerations, but in varying proportions. Because of the natural conditions in the border areas, the risk of infection is also high for travelers from the neighboring countries such as Austria, Germany, Poland, and Slovakia.
Materials and Methods:
To gain more information on the epidemiology of LB and TBE in the last decade, we analyzed national surveillance data from 2007 to 2016.
Results:
Incidence of LB in the Czech Republic was 37.3/100,000 population and year (27.6 - 46.1/100,000). Incidence of TBE incidence was 5.7/100,000 (3.4–8.2/100,000) and declined although the trend was not significant (p = 0.155). Difference between the incidences of LB and TBE was increasing in time. Overall male-to-female ratio was 0.84 and 1.51 for LB and TBE, respectively. The age-specific incidences of both infections have a typical two-peak shape, with the first peak in the age group 5–9 years for LB and 15–19 years for TBE. The second peak for both LB and TBE is in the age group 55–64 years. TBE poses a considerable risk to children < 15 years. Among 39,074 cases of LB, the most common clinical manifestations were erythema migrants 62.1% and Lyme neuroborreliosis 25.1%. All 5969 TBE cases manifested itself by affecting nervous system, namely meningo-encephalitis 47.9%, meningoencephalomyelitis 21.8% and meningitis 19.8%.
Conclusions:
The data evidence the high chance risk of infection with LB and TBE in the Czech Republic. The incidence of both infections shows a bimodal distribution during the year. LB cases are five to six times as frequent as TBE cases. Over the last years, the incidence of LB has remained roughly stable while TBE has shown a downward trend. The present study is unique in allowing the comparison of the incidence rates of LB and TBE over time and space.
KEYWORDS:
Lyme borreliosis – tick-borne encephalitis – incidence – climate change environment
INTRODUCTION
The main common feature of tick-borne encephalitis (TBE) and Lyme borreliosis (LB), which is important for the comparison of their epidemiological patterns, is the fact that both are zoonoses transmissible to humans by a common vector – the tick (tick-borne diseases). Furthermore, both diseases are characterised by the phenomenon of natural focal point. The causative agents circulate in nature among animal hosts, and humans bitten by an infected tick are considered as their accidental dead end hosts. A tick bite and at least a short blood meal is a prerequisite for both diseases to develop (with the exception of some cases of food-borne infection by tick-borne encephalitis virus (TBEV) [16]. The vector (the tick Ixodes ricinus in the European area where TBE and LB co-occur) is the main factor which maintains the causative agents in the local biocenosis playing a determining role in the contact of the tick with suitable animal hosts. At the same time, the tick mediates the influence of some abiotic factors (particularly meteorological but also orographic and other factors determining the nature of the landscape) on the infection cycle in nature. Such factors underlie the high similarity (or even full congruence) of the landscape parts identified as posing high risk of infection [2, 9]. The risk to humans from both tick-borne infections while entering such areas varies with I. ricinus host-questimg activity which is subject to seasonal changes, as is also reflected, to a large extent, in the seasonal changes of the incidence of tick-borne infections [3]. The impact of human behaviour should also be considered in this context as important reasons for the presence of humans in high-risk areas are recreational outdoor activities. Under local conditions of the Czech Republic (CZ), TBE and LB can be classified as recreational infections.
The above-mentioned common features may be of relevance not only in the comparison of the epidemiological patterns of TBE and LB but also in identification and implementation of the preventive measures to protect humans from contact with infected ticks: warning of increased tick activity, identification of high-risk landscape parts, recommendations on what to do before and during entering such areas and after returning home, etc. Raising awareness about the risks related to ticks is an important part of the prevention of both infections.
The main differences between LB and TBE are due to their causative agents. TBE is caused by the arbovirus of the Flaviviridae family while the causative agents of LB are Gram-negative bacteria of the Borrelia burgdorferi sensu lato complex (Spirochaetaceae). These differences determine the ways of their replication in the body of the vector as well as their relation to the warm-blooded hosts including humans [4].
The first cases of LB in the Czech Republic were reported in the late 1980s of the last century. The cause of the disease is a Gram-negative spirochete from the Borrelia burgdorferi sensu lato complex. In the Czech Republic, cases caused by B. burgdorferi sensu stricto, B. bissettii, B. afzelii, B. garinii, and B. valaisiana have been reported [29]. The vector of the disease in the CZ is almost exclusively the tick Ixodes ricinus. LB is a multiorgan disease which manifests itself by various clinical symptoms. The most common manifestation of LB is erythema migrans (EM) – a characteristic bull’s-eye rash which appears within 3–32 days after the tick bite (within seven to 10 days on average). If an erythematous skin reaction occurs at the site of the tick bite within hours, it is most likely not EM but a hypersensitivity reaction which should not be mistaken for EM. If the rash appears after the above-mentioned incubation period, migrates outward, and reaches 5 cm or more in diameter, it is EM. The central clearing and migrating rash, which are typical for EM, may not occur in all patients with LB. If not treated properly with antibiotics, LB may progress to further stages, manifestation called borrelial lymphocytoma, acrodermatitis chronica atrophicans, inflammation of large joints, neurological symptoms Lyme neuroborreliosis (LNB), heart damage, and eye complications. In untreated or improperly treated patients, these symptoms may persist for weeks or even months and may gradually involve other organs [31, 32].
The tick (I. ricinus) is the main vector of the European variant of tick-borne encephalitis virus from the Flaviviridae family and B. burgdorferi sl in Europe. I. ricinus ticks are present in ecosystems where the environment is suitable for their development and survival. Microclimatic conditions are another crucial factor influencing their occurrence. Other factors, as the size of the wild boar population may also have contributed to the current high levels of TBE incidence [18]. Relatively rarely, humans can acquire TBEV infection through the alimentary route by ingestion of contaminated milk or dairy products. In the CZ, laboratory confirmed cases of TBE have been notified since 1971. The vaccine against this infection is generally available; however, the vaccination coverage is low. In the CZ, there is no TBE vaccination registry, and the estimated TBE vaccine uptake is ca 23%. As data on the number of vaccine doses administered is also lacking, the vaccine protective effect cannot be assessed either.
In the case of natural focal infections, the most relevant epidemiological data is the place of infection acquisition. It provides important information not only on the area where the infection was acquired but also on the risk level and its variation depending on the natural and social circumstances known to play a role in it [17]. Previous studies have shown that the long-term residents of the areas where there are natural foci of TBE are at the highest risk of infection. The patient‘s place of residence is thus relevant not only in terms of infection acquisition area, but also as a source of information on herd immunity resulting from exposure either to the pathogen (both TBE and LB) or to the vaccine (TBE) [19].
MATERIAL AND METHODS
In the CZ, cases of infectious diseases are notifiable by law. The case definitions of LB and TBE are part of the surveillance of these diseases set out in Ministerial Regulation No. 473/2008 [27]. Microbiological diagnosis of LB and TBE is performed primarily by the National Reference Laboratory for Lyme Borreliosis of the National Institute of Public Health, Prague and National Reference Laboratory for Arboviruses of the Public Health Institute, Ostrava. Both laboratories have been involved in national quality control of microbiological laboratories and international external quality control scheme. All general practitioners (GP), paediatricians, and hospital physicians are under the obligation to notify any diagnosed case of the two infections. Relevant epidemiological data including the probable place and date of infection acquisition, onset of the disease, date of hospital admission (if any), clinical form of infection, and date of death (if relevant) are provided by epidemiologists from the regional public health authorities. Some missing detail information was collected directly from the clinicians.
Statistical analysis
Epidemiological data on the numbers of cases of LB and TBE in the CZ classified according to the date of disease onset were obtained from the database of the Czech reporting system of notifiable diseases. Some detail information regarding the clinical forms was collected additionally from the clinicians. The data on the population were taken from sources of the Czech Statistical Office. The data presented are expressed as absolute and relative frequencies and as the incidence rates per 100,000 populations. The comparison of the time course between groups was based on Poisson and linear regression models. To compare percentages of categorical data, the Pearson χ2 test was used. The Kolmogorov-Smirnov test was used to compare the probability distribution of the infections studied. Locally weighted scatterplot smoothing (lowess) was used to generate the curves describing the time trends or distribution of the data. The degree of association between the incidences of the two diseases was characterized by the Spearman’s correlation coefficient. All statistical tests were evaluated as two-sided at a significance level of 0.05. Statistical analysis was performed by the statistical software R, version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) and Stata, release 9.2 (Stata Corp LP, College Station, TX, U.S.A.).
RESULTS
In the Czech Republic, 72,821 cases of LB and 12,082 cases of TBE were notified from 1997 to 2016. In the first half of this period, the incidences of both LB and TBE had an upward trend, with a marked peak for TBE in 2006 (10.0/100,000 population). The incidence of LB was about five to six times as high during the whole study period than TBE. In the second decade, the incidence of LB showed wide year-on-year fluctuations; however, from the long-term perspective outlined by splines in it remained nearly on the same level. The incidence of TBE showed a statistically non-significant decline (p = 0.155) with an average annual decrease of 0.24/100,000; thus the difference between the incidence of LB and that of TBE was increasing during the study period (Figure 1).
Fig. 1. Annual incidences of Lyme borreliosis (n = 72,821) and tick-borne encephalitis (n=12,082) in the Czech Republic in 1997–2016 and their trends characterized by locally weighted scatterplot smoothing (lowess) 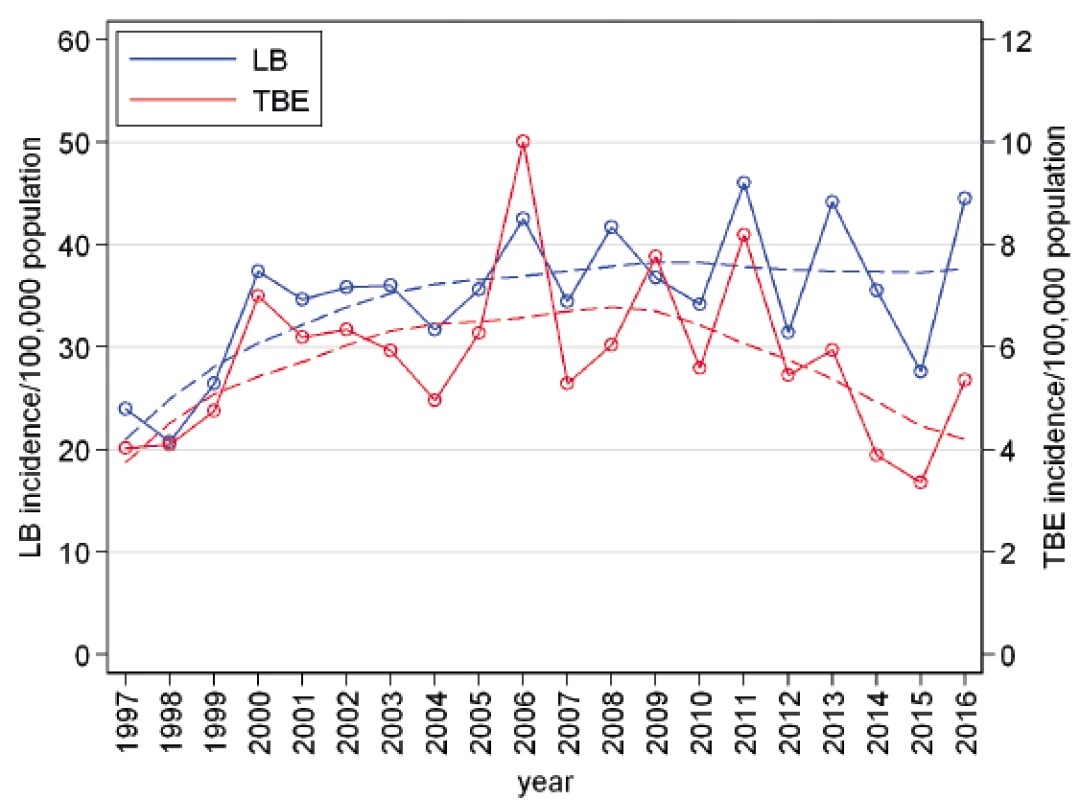
The present study focused on the ten-year period 2007 to 2016, for which more detailed data have been available. In this period, 39,074 cases of LB were reported, with an average incidence of 37.3/100,000 population and year (max 46.1/100,000, min 27.6/100,000). During this time period 3 cases of LB died. The case fatality rate was 0.008%. The respective data for TBE were 5 969 cases reported in 2007–2016, with an average incidence of 5.7/100,000 population and year (max 8.2/100,000, min 3.4/100,000) and a case fatality rate of 0.54% (32 deaths).
The most common clinical manifestation of LB was erythema migrans 62.1%. Neurological manifestations occurred in 25.1% of patients and muscle and joint disorders in 11.3% of patients. The most common manifestations of TBE were meningo-encephalitis and meningoencephalomyelitis observed in 47.9% and 21.8% patients, respectively (Table 1).
Tab. 1. Distribution of clinical manifestations of LB and TBE in the Czech Republic in 2007–2016 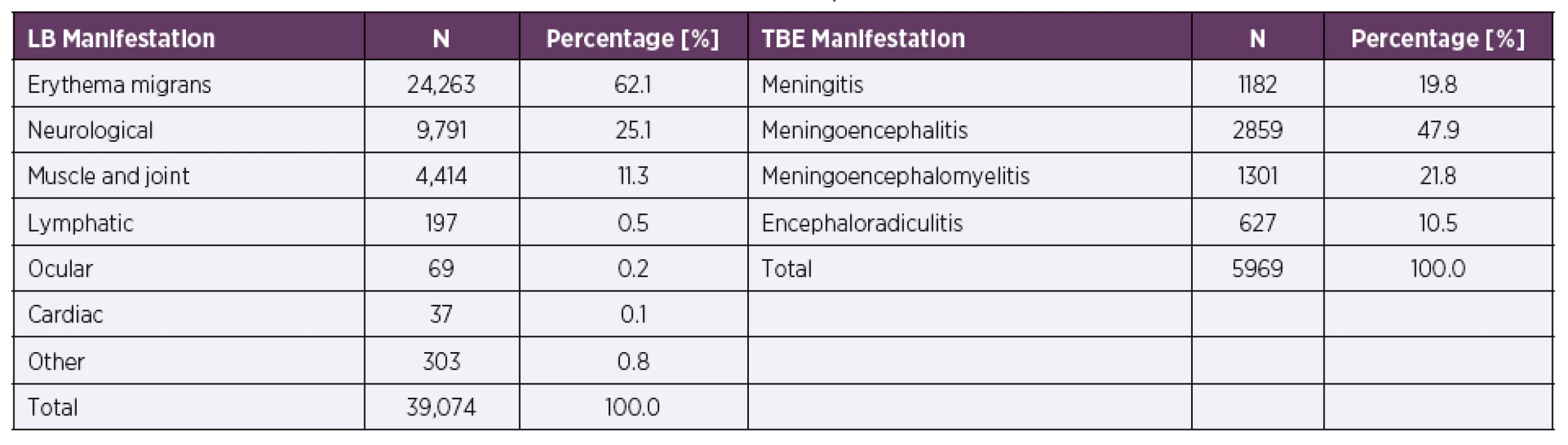
The overall incidence of LB is higher in females than in males (39.7 vs. 34.7/100,000), p < 0.001. The difference is due mainly to the contribution of women aged 45–74 years, with the highest female-to-male ratio being observed in this age group. In contrast, among children aged 5–14 years, more patients are males. The overall incidence of TBE is statistically significantly higher in males than in females (7.0 vs. 4.5/100,000), p < 0.001. This holds true for all age groups except children aged 0 years (Table 2).
Tab. 2. Incidences of LB and TBE per 100,000 population and year, by gender and age group, Czech Republic, 2007–2016 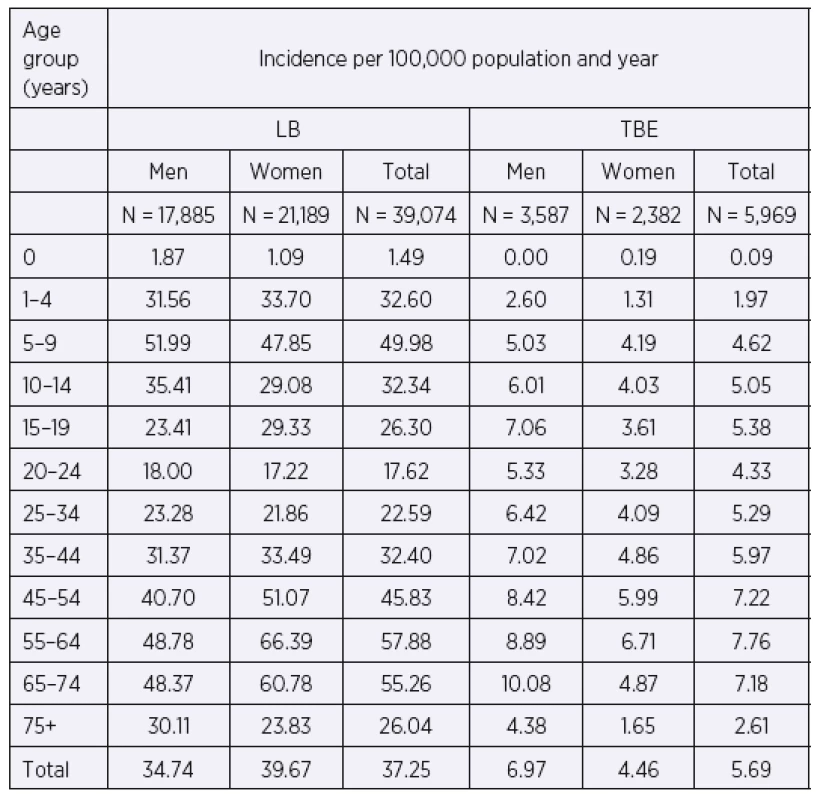
The age group-specific incidence of LB in 2007–2016 shows two peaks, one in the age group 5–9 years, followed by a decline until the age of 20–24 years, and the other peak in the age group 55–64 years. The differences between age groups are statistically significant (p < 0.001). TBE, as compared to LB, shows smaller but statistically demonstrable differences (p < 0.001) between age groups. The trends in the age-specific incidence differ significantly between LB and TBE (p < 0.001). TBE reaches the first peak in the adolescent group 15–19 years, with a subsequent slight decline in the age group 20–24 years, and the other peak in the age group 55–64 years (Figure 2).
Fig. 2. Incidences of Lyme borreliosis (n = 39,074) and tick-borne encephalitis (n = 5 969) per 100,000 population and year, by age group, Czech Republic, 2007–2016 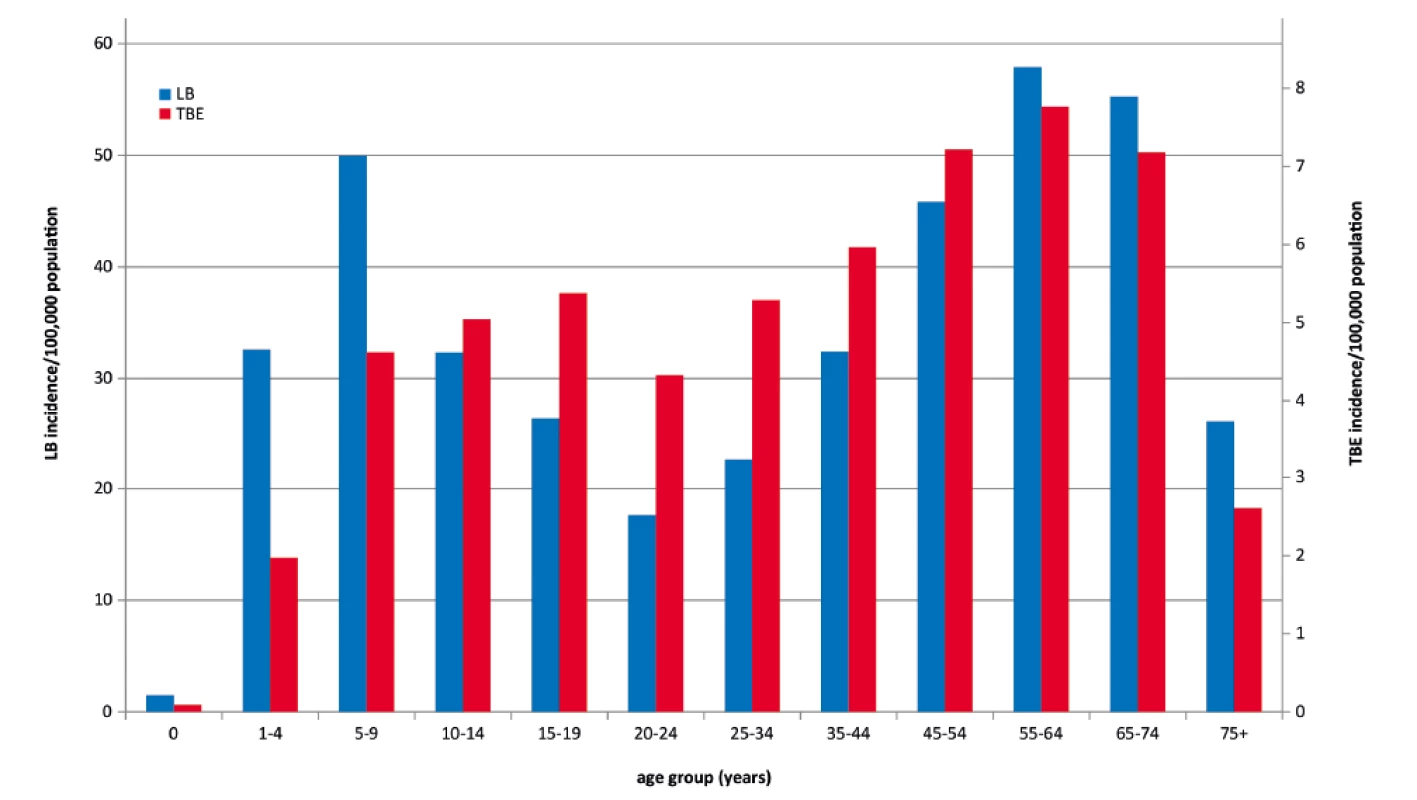
The average weekly incidence of LB (for the entire period 2007–2016) was in a range from 0.15 to 0.31/100,000 in the first 15 weeks of the year. In the following weeks, there was an upward trend, with an increase from 0.38/100,000 in week 16 to the peaks of 1.65 and 1.67/100,000 in weeks 27 and 31, respectively. The incidence with week-on-week fluctuations then declines to 0.18/100,000 in the last week of the year.
The weekly incidence of TBE was greater than 0.01/100,000 from late March (week 13). It gradually increased to reach a peak of 0.37/100,000 in beginning July (week 27). The steady downward trend, after a considerable drop in weeks late August to late September (weeks 34–38) was discontinued by a local rise to 0.14–0.16/100,000 in beginning October (weeks 39 and 40). The incidence then decreased continuously 0.01/100,000 in late November (week 48). From April to November, the curves for LB and TBE have similar shapes, but differ from each other in weeks of sharp rise or rapid drop. Despite their similarity, the statistical test captured differences (p < 0.001), which is true, among others, for the second autumn peak recorded for TBE and not for LB or for the excess incidence of LB in final weeks of the year that continues during the first three months of the following year (Figure 3).
Fig. 3. The weekly incidences of Lyme borreliosis (n = 39,074) and tick-borne encephalitis (n = 5 969) per 100,000 population and year, by week of onset, Czech Republic, 2007–2016 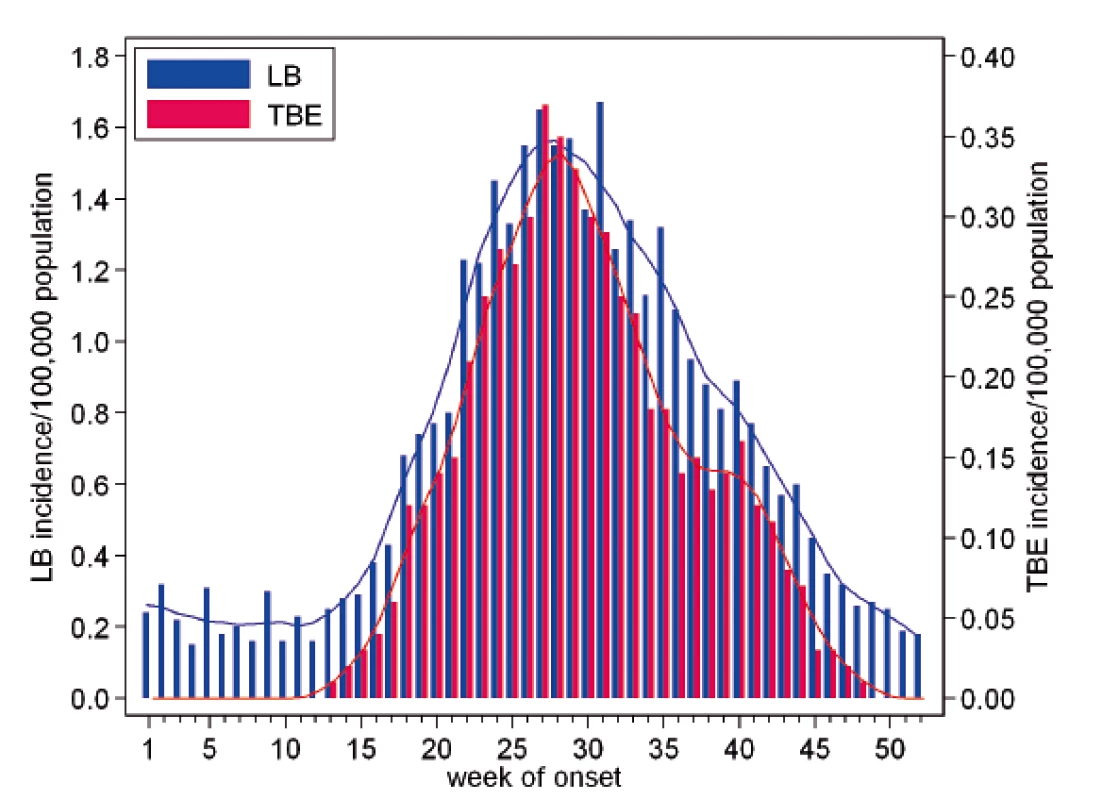
The distribution of LB and TBE cases by region (NUTS 3) shows that both infections occur in all regions of the CZ, but in different proportions. Generally, the correlation between LB and TBE in the regions is low (Spearman’s correlation coefficient of 0.15). The areas with the highest co-incidence of LB and TBE cases are the South Bohemian Region (LB 44.8/100,000, TBE 18.1/100,000) at the borders with Germany and Austria and the Highlands Region (LB 75.5/100,000, TBE 12.7/100,000) in the southern part of the country which attracts tourists due to its closeness to the border with Austria because of the natural conditions favourable for both tics and tourists. Many cases of LB are also reported in other regions bordering Poland, Slovakia and Saxony, a federal state of Germany (Figure 4).
Fig. 4. Incidences of Lyme borreliosis (n = 39,074) and tick-borne encephalitis (n = 5 969) per 100,000 population and year, by NUTS 3 regions of the Czech Republic, 2007–2016 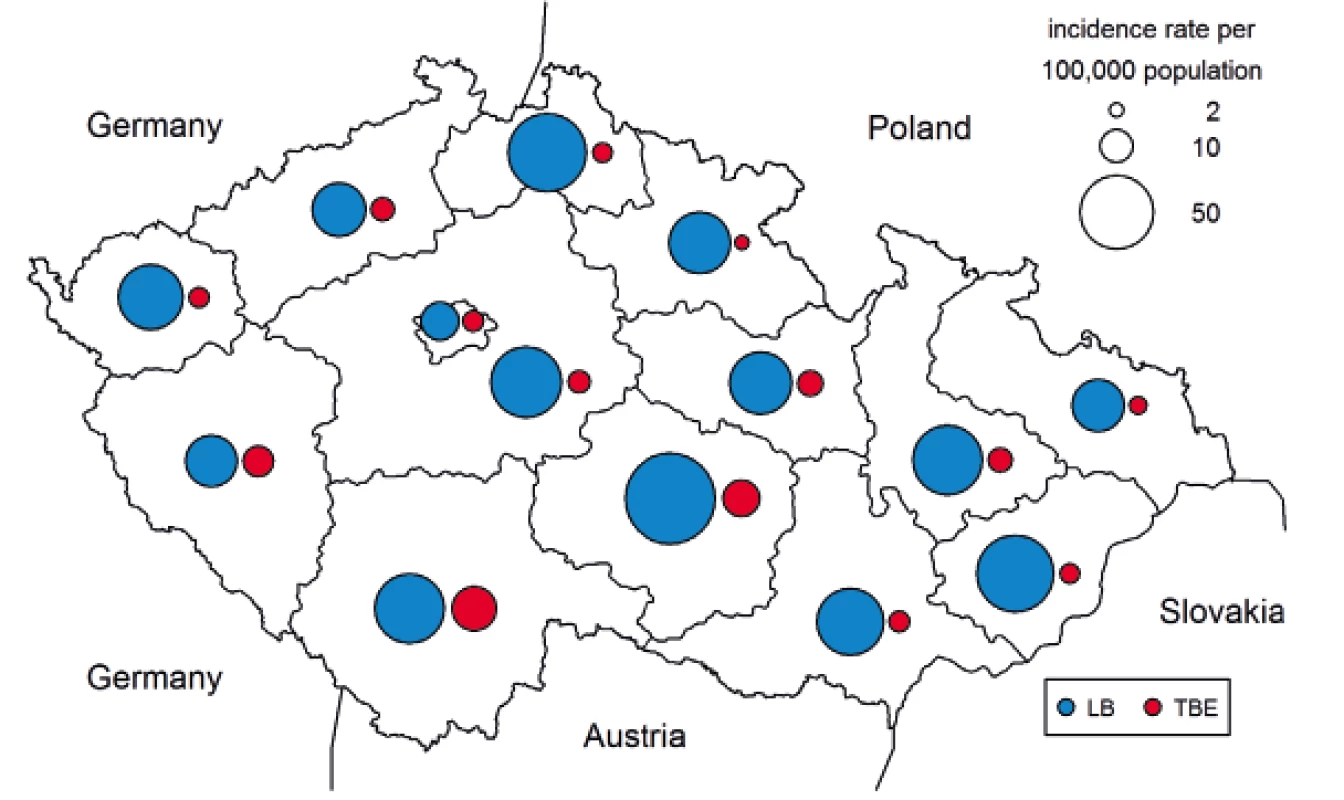
DISCUSSION
The results of the present study show that in the last decade, the incidence of LB in the CZ has a steady trend with year-on-year fluctuations. The overall incidence of LB is higher in females than in males. The age-specific incidence typically has two peaks, one in the age group 5–9 years (50.0/100,000) and the other in the age group 55–64 years (57.9/100,000). In contrast to LB, more patients with TBE are males in the CZ. The age-specific TBE incidence has two peaks, first one in the age group 15–19 years (5.4/100,000) and the second in the age group 55–64 years (7.8/100,000).
In the CZ, the LB antibodies were detected in 14.8% in the sera collected in 2001 in study subjects divided into six age categories between 10 and 59 years. The highest positivity rate 26.2% was detected in the 10–14 year age group [22] which roughly corresponds to increased incidence in children found in the present study.
LB is the most common tick-borne disease worldwide. The incidence is estimated to be 85,000 cases per year in the world and 65,000 cases per year in Europe [10]. It varies between countries in a range from 0.01/100,000 population to 350/100,000. The incidence above 100/100,000 has been reported in parts of Slovenia, Germany, Austria, and southern Sweden [28]. A study in six states of eastern Germany has reported the average incidence rates to be 17.8 and 23.3 cases per 100,000 population in 2002 and 2003, respectively. The age-specific incidence curve showed two peaks, one in children aged 5–9 years and the other at the age of 60–64 years in 2002 and 65–69 years in 2003. The average incidence of LB in northern Slovakia over 22 years was 16.2/100,000, being the highest in the age group 45–54 years (21.2/100,000) [1]. The average annual incidence of LB in France over six years was 42 per 100,000 population, being the highest in the age groups 5–10 years and 50–70 years [34]. The incidence of LB of 37.7/100,000 population found in this study ranks the Czech Republic among the European countries with a higher occurrence of the disease.
Attention should be drawn not only to clinical symptoms but also to time from the tick bite to onset of illness. A plausible explanation for the emergence of LB infections in winter months (December to March) can be the long (and difficult to determine) incubation period and/or late diagnosis in some patients often observed in Lyme neuroborreliosis and arthritic forms of LB. Neither can a possible bite by a questing tick be ruled out at temperatures above 5 °C which may occur even in winter.
Erythema migrans was reported in 87–89% of patients with LB in Germany [24]. In European countries, EM has been recorded in 60–80% of cases of LB [28]. The value of 62% found in the present study is at the lower limit of the range.
LNB is the most common form of the disseminated stage of LB in Europe [26]. LNB has been reported in 10–20% of symptomatic patients in European countries [28]. In an 11-year study conducted in the CZ, 27% of 23 631 hospitalised patients with LB were diagnosed with LNB [21]. Up to 25% of patients with LB developed LNB in another study in the CZ [15]. This figure corresponds with 25% of patients with neurological manifestations found in the present study. In Norway, 1 506 cases of disseminated and chronic LB were reported in 1998–2004. The most common manifestations were LNB (71%), arthritis (22%), and acrodermatitis chronica atrophicans (5%) [25]. The three-year average incidence of LNB in Denmark was 3.2/100,000 population [5]. In a Polish study, 13% of patients with LB presented with LNB accompanied by concentration and memory disorders [35].
Tick-borne encephalitis is a notifiable disease in 16 European countries. Over twenty years (1990–2009), 169,937 cases of TBE were reported in Europe and European part of Russia. In endemic countries, the peak incidence of TBE reached, in the short term in some years, 10/100,000 population in the Czech Republic, 12–14/100,000 population in Slovenia, 27.8/100,000 population in Estonia, 53/100,000 population in Latvia, and 22/100,000 population in Lithuania [33]. In Austria, where, similarly to the CZ, there are natural foci of TBE, the incidence of TBE in the unvaccinated population is about 6/100,000. However, it is as low as 0.9/100,000 in the 85% vaccinated population [11, 12]. The figure of 5.7% found in present study is similar to that for unvaccinated Austrian population which is consistent with the fact that the vaccination coverage is low in the CZ. In Switzerland, 110–120 cases of TBE per year were reported before 2011 which gives the incidence of 1.4/100,000. Natural foci of TBE are found in all regions of the country [8].
The upward trend in the incidence of TBE in the CZ observed from the early seventies of the last century until 2011 [20] was followed by a certain decline in the last years. Over the last forty years, similar increasing trend until the end of the first decade of the current century was also reported in other European countries. Climate change has played a role in this increase during the period [20]. Suitable environmental and climatic conditions for reservoir animals, tick vectors, and circulation of TBEV as well as human leisure activities contribute to the rise of TBE [33]. Local autumn peaks of TBE were observed in previous years (2001–2006) in the CZ, and the phenomenon has recently been studied in detail [4].
Until the late 1990s, the highest age-specific incidence in the CZ was recorded in 15–19-year-olds. Later, the main peak of incidence moved to the age group of 60–64 years [17] which is still true, as the data from this study shows.
In Europe, more than 10,000 patients with TBE are admitted to hospital every year. One in three patients develops a long-term cognitive impairment [7]. In European countries, TBE manifests itself as meningitis in 50% of patients, as meningoencephalitis in 40% of patients, and as meningoencephalomyelitis in 10% of patients [13]. In comparison presented Czech TBE data show lower proportion of patients with meningitis and higher proportion with meningoencephalomyelitis. Pareses, ataxia, and difficulty walking may persist for months to years [13]. One third of TBE patients in Sweden have long-term sequelae, cognitive dysfunction, and lowered quality of life [23].
TBE in children is, to a certain extent, similar to that of adults, but tends to have better prognosis. Nevertheless, children can develop cognitive dysfunctions [30]. In a Swedish study, 55 children with TBE who presented with central nervous system impairment were followed up for two to seven years. Two thirds of them had cognitive problems, tiredness, headache, irritability, and memory disorders [6].
The data analyzed come from a routine reporting to the surveillance system of infectious diseases. Even though reporting of the diseases in question is mandatory in the Czech Republic, it cannot be ruled out that some particularly light cases are not reported to the system. This would correspond to the relatively high incidence of antibodies in the population. However, estimates of the proportion of unreported cases are not available. The Slovak study [1] has documented that nearly half of the patients treated for LB were not registered by public health office.
In the CZ, the incidence of LB is five to six times as high as that of TBE. The incidence rates of the two diseases show similar trends based on weekly data, which, however, differ significantly from each other in certain segments, namely in the spring and autumn periods. The second autumn wave of TBE was positively influenced by atmospheric precipitation in the months August and September while the correlation between precipitation and TBE in the first half of the year was considerably lower [20]. The rise in cases of TBE in the late summer is considered as linked to the peak activity of Ixodes ricinus nymphs [14].
CONCLUSIONS
Both LB and TBE have been reported in all regions of the Czech Republic including urban agglomerations, but in varying proportions. The data evidence the high chance risk of infection with LB and TBE in the Czech Republic. The incidence of both infections shows a bimodal distribution during the year. LB cases are five to six times as frequent as TBE cases. Over the last years, the incidence of LB has remained roughly stable while TBE has shown a downward trend. The present study is unique in allowing the comparison of the incidence rates of LB and TBE over time and space. Because of the natural conditions in the border areas, the risk of infection is also high for travellers from the neighbouring countries such as Austria, Germany, Poland, and Slovakia. The present study is unique in allowing the comparison of the incidence rates of LB and TBE over time and space.
Acknowledgement
Supported by GAČR project no. 18-22125S.
Do redakce došlo dne 23. 4. 2018.
Adresa pro korespondenci:
RNDr. Marek Malý, CSc.
Státní zdravotní ústav
Šrobárova 48
100 42 Praha 10
Zdroje
1. Bochnicková M, Szilágyiová M, Gardlík R. Lyme borreliosis-epidemiological analysis of incidence in the northern region of Slovakia. Epidemiol Mikrobiol Imunol, 2012;61(1–2):3–8.
2. Daniel M, Kolář J, Zeman P, et al. Tick-borne encephalitis and Lyme borreliosis: comparison of habitat risk assessments using satelite data. Centr Eur J Publ Hlth, 1999;7(1):35–39.
3. Daniel M, Malý M, Danielová V, et al. Abiotic predictors and annual seasonal dynamics of Ixodes ricinus, the major disease vector of Central Europe. Parasites &Vectors, 2015;8 : 478. DOI 10.1186/s13071-015-1092-y.
4. Daniel M, Danielová V, Fialová A, et al. Increased Relative Risk of Tick-Borne Encephalitis in Warmer Weather. Front Cell Infect Microbiol,2018;8 : 90.
5. Dessau RB, Espenhain L, Mølbak K, et al. Improving national surveillance of Lyme neuroborreliosis in Denmark through electronic reporting of specific antibody index testing from 2010 to 2012. Euro Surveill, 2015;20(28).
6. Fowler Å, Forsman L, Eriksson M, Wickström R. Tick-borne encephalitis carries a high risk of incomplete recovery in children. J Pediatr, 2013;163(2):555–560.
7. Gaudelus J. Tick borne encephalitis in children. Arch Pediatr, 2009;16(Suppl 2):S108–14 (In French).
8. Gäumann R, Růžek D, Mühlemann K, Strasser M, Beuret CM. Phylogenetic and virulence analysis of tick-borne encephalitis virus field isolates from Switzerland. J Med Virol, 2011;83(5):853–863.
9. Gray JS, Kahl O, Robertson JN, et al. Lyme borreliosis habitat assessment. Zent Bl Bakteriol, 1998;287 : 211–228.
10. Hubálek Z. Epidemiology of lyme borreliosis. Curr Probl Dermatol, 2009;37 : 31–50.
11. Heinz FX, Stiasny K, Holzmann H, et al. Vaccination and tick-borne encephalitis, Central Europe. Emerg Infect Dis, 2013;19(1):69–76.
12. Heinz FX, Stiasny K, Holzmann H, et al. Emergence of tick-borne encephalitis in new endemic areas in Austria: 42 years of surveillance. Euro Surveill, 2015;20(13):9–16.
13. Kaiser R. Tick-borne encephalitis: Clinical findings and prognosis in adults. Wien Med Wochenschr, 2012;162(11–12):239–243.
14. Karbowiak G, Biernat B. The role of particular tick developmental stages in the corculation of tick-borne pathogens affecting humans in Central Europe. 2.Tick-borne encephalitis virus. Ann Parasitol, 2016;62(1):3–9.
15. Krbková L, Bednářová J, Čermáková Z. Improvement of diagnostic approach to Lyme neuroborreliosis in children by using recombinant antigens in detection of intrathecally produced IgM/IgG. Epidemiol Mikrobiol Imunol, 2016;65(2):112–117.
16. Kříž B, Beneš Č, Daniel M. Alimentary transmission of tick-borne encephalitis in the Czech Republic. Epidemiol Mikrobiol Imunol, 2009;58(2):98–103.
17. Kriz B, Maly M, Benes C, Daniel M. Epidemiology of tick-borne encephalitis in the Czech Republic 1970–2008.Vector Borne Zoonotic Dis, 2012;12(11):994–999.
18. Kriz B, Daniel M, Benes C, Maly. The role of game (wild boar and roe deer) in the spread of tick-borne encephalitis in the Czech Republic. Vector Borne Zoonotic Dis, 2014;14(11):801–807.
19. Kriz B, Hubalek Z, Marek M, et al. Results of the screening of tick-borne encephalitis virus antibodies in human sera from eight districts collected two decades apart. Vector Borne Zoonotic Dis, 2015;15(8):489–493.
20. Kříž B, Kott I, Daniel M, et al. Impact of climate changes on the incidence of tick-borne encephalitis in the Czech Republic in 1982–2011. Epidemiol Mikrobiol Imunol, 2015;64(1):24–32.
21. Kriz B, Malý M, Daniel D. Neuroborreliosis in patients hospitalised for Lyme borreliosis in the Czech Republic in 2003–2013. Epidemiol Mikrobiol Imunol, 2017;66(3):116–123.
22. Kříž B, Malý M, Balátová P, et al. A serological study of antibodies to Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in the sera of healthy individuals collected two decades apart. Acta Parasitol, 2018;63(1):33–39.
23. Lindquist L, Vapalahti O. Tick-borne encephalitis. Lancet, 2008;371(9627):1861–1871.
24. Mehnert WH, Krause G. Surveillance of Lyme borreliosis in Germany, 2002 and 2003. Euro Surveill, 2005;10(4):83–85.
25. Nygård K, Brantsaeter AB, Mehl R. Disseminated and chronic Lyme borreliosis in Norway, 1995–2004. Euro Surveill, 2005;10(10):235–238.
26. Picha D, Moravcová L, Smíšková D. Diagnostic relevance of the chenokine CXCL13 and anti-C6 peptide antibodies in patients with neuroboreliosis. Epidemiol Mikrobiol Imunol, 2017;66(2):112–117 (in Czech).
27. Regulation no.473/2008 annexe 23 Surveillance L.borreliosis, annexe 28 Surveilance Tick-borne encephalitis.
28. Rizzoli A, Hauffe H, Carpi G, et al. Lyme borreliosis in Europe. Euro Surveill, 2011;16(27):pii=19906.
29. Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis, 2011;2(3):123–128.
30. Rostasy K. Tick-borne encephalitis in children. Wien Med Wochenschr, 2012;162(11–12):244–247.
31. Stanek G, Fingerle V, Hunfeld KP, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect, 2011;17(1):69–79.
32. Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet, 2012;379(9814):461–473.
33. Süss J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks Tick Borne Dis, 2011;2(1):2–15.
34. Vandenesch A, Turbelin C, Couturier E, et al. Incidence and hospitalisation rates of Lyme borreliosis, France, 2004 to 2012. Euro Surveill, 2014;19(34).
35. Zajkowska J, Czupryna P, Kuśmierczyk J, et al. Clinical forms of neuroborreliosis-the analysis of patients diagnosed in department of infectious diseases and neuroinfection medical academy in Bialystok between 2000–2005. Przegl Epidemiol, 2007;61(1):59–65 (In Polish).
Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2018 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
-
Všechny články tohoto čísla
- Fecal bacteriotherapy in the treatment of Clostridium difficile infection
- Human rotavirus A detection: Comparison of enzymatic immunoassay and rapid chromatographic test with two quantitative RT-PCR assays
- Human Mozdok leptospirosis first diagnosed by serum agglutinin-absorption tests in the Slovak Republic
- Susceptibility of clinical isolates of Bordetella pertussis to chemicals
- Laboratory evaluation of repellency of traditional Czech homemade repellents against Aedes aegypti
- Comparison of the epidemiological patterns of Lyme borreliosis and tick-borne encephalitis in the Czech Republic in 2007–2016
- Successful rituximab treatment of granulomatous/lymphocytic interstitial lung disease in common variable immunodeficiency
- Zemřel doc. MUDr. Bohumír Kříž, CSc.
- Molecular characterization of Streptococcus pneumoniae isolates recovered from cases of pneumococcal vaccine failure in children under five years of age in the Czech Republic in 2012–2014
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Comparison of the epidemiological patterns of Lyme borreliosis and tick-borne encephalitis in the Czech Republic in 2007–2016
- Fecal bacteriotherapy in the treatment of Clostridium difficile infection
- Successful rituximab treatment of granulomatous/lymphocytic interstitial lung disease in common variable immunodeficiency
- Susceptibility of clinical isolates of Bordetella pertussis to chemicals
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



