-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Low serum deoxyribonuclease I activity is associated with antiTNF-alpha induced skin adverse events in patients with inflammatory bowel disease
Nízká aktivita sérové deoxyribonukleázy-I je u nemocných s idiopatickými střevními záněty spojena se vznikem kožních nežádoucích účinků při antiTNF-alfa léčbě
Východiska:
U významného počtu pacientů s idiopatickými střevními záněty (IBD) se v průběhu léčby infliximabem nebo adalimumabem objevují kožní komplikace. Předpokládá se, že obě léčiva vyvolávají v aktivovaných zánětlivých buňkách apoptózu. Deoxyribonukleáza (DNáza) I je hydrolytický enzym, který je schopen štěpit dvouvláknovou DNA během apoptózy, a její nedostatek doprovází řadu autoimunitních poruch.Cíl:
Posouzení aktivity DNázy I u pacientů s idiopatickými střevními záněty a stanovení jejího vlivu společně s dalšími faktory na vznik imunopatologických kožních komplikací biologické léčby.Metody:
U pacientů s IBD, kteří byli v období 2007–2009 v našem centru léčeni infliximabem nebo adalimumabem, byl zpětně zkoumán výskyt kožních neinfekčních komplikací během této léčby a byla posuzována aktivita DNázy I. Posuzován byl také náhodný vzorek pacientů s IBD, kteří biologickou léčbu absolvovali bez kožních komplikací. Pro určení aktivity DNázy metodou ELISA bylo použito sérum.Výsledky:
Během trvání studie podstoupilo biologickou léčbu 313 pacientů s IBD. U 43 z nich se vyskytly neinfekční kožní komplikace. Zařazeno bylo také 95 pacientů s IBD, kteří biologickou léčbu absolvovali bez kožních komplikací. Univariátní analýza určila jako rizikové faktory pro kožní komplikace ženské pohlaví, léčbu adalimumabem, absenci konkomitantního podávání kortikosteroidů a nízkou aktivitu DNázy I. V rámci multivariátní analýzy byly s nepříznivými kožními reakcemi spojeny pouze nízká aktivita DNázy I (OR 0,45; 95% CI 0,29–0,71; p < 0,001) a léčba adalimumabem (OR 30,8; 95% CI 6,87–138,16; p < 0,001).Závěr:
Nízká aktivita DNázy I může být rizikovým faktorem pro vznik imunitně zprostředkovaných kožních komplikací během biologické léčby pacientů s IBD.Klíčová slova:
idiopatické střevní záněty – infliximab – adalimumab – deoxyribonukleáza (DNáza) I – kožní komplikace
Authors: K. Malickova 1; D. Ďuricová 2
; M. Bortlíkihash2 2,3 1,2
Authors place of work: Institute of Clinical Biochemistry and Laboratory Diagnostics, First Faculty of Medicine of Charles University and General University Hospital, Prague, Czech Republic 1; Clinical and Research Center for Inflammatory Bowel Disease, ISCARE a. s. and Charles University, Prague, Czech Republic 2; Internal Department, First Faculty of Medicine, Charles University and Central Military Hospital, Prague, Czech Republic 3; Department of Probability and Mathematical Statistics, Faculty of Mathematics and Physics of Charles University, Prague, Czech Republic 4
Published in the journal: Gastroent Hepatol 2012; 66(1): 23-31
Category: IBD: původní práce
Summary
Background:
A significant proportion of inflammatory bowel disease (IBD) patients develop skin complications during infliximab or adalimumab therapy. Both drugs are thought to act via induction of apoptosis in activated inflammatory cells. Deoxyribonuclease (DNase) I is endonuclease hydrolyzing double-stranded DNA during apoptosis and its deficiency has been implicated in several autoimmune disorders.Aim:
To assess activity of DNase I in patients with IBD and to identify its impact together with other factors on development of skin immunopathological complications of biological therapy.Methods:
IBD patients treated with infliximab or adalimumab at our centre from 2007–2009 were studied retrospectively for occurrence of skin non-infectious complications during this treatment and assessed for DNase I activity. A random sample of IBD patients treated with biological therapy without skin complications was also assessed. Serum was used to determine DNase I activity by enzyme--linked immunosorbent assay.Results:
During the study period 313 patients with IBD were treated with biological therapy. Of them, 43 patients experienced non-infectious skin complications. 95 IBD patients on biological therapy without skin complications were also included. Univariate analysis identified female gender, adalimumab therapy, no concomitant corticosteroids and low DNase I activity as risk factors for skin complications. In multivariate analysis only low activity of DNase I (OR 0.45; 95% CI 0.29–0.71; p < 0.001) and adalimumab therapy (OR 30.8; 95% CI 6.87–138.16; p < 0.001) were associated with skin adverse events.Conclusions:
Low DNase I activity may be a risk factor for development of immune-mediated skin complications during biological therapy in patients with IBD.Key words:
inflammatory bowel disease – infliximab – adalimumab – deoxyribonuclease (DNase) I – skin complicationsTreatment of inflammatory bowel disease (IBD) such as Crohn‘s disease (CD) and ulcerative colitis (UC) has been revolutionized by the introduction of TNF-alpha (TNF-α) targeted biological agents including infliximab, adalimumab and certolizumab pegol [1].
The safety profile of biological therapy has been a major concern since the start of their use. So far, both infliximab and adalimumab seem to be safe as outlined by national and centre cohorts, safety registries and also by recently published meta-analysis [2–5].
However, an increasing number of probably immune-mediated complications during the biological therapy such as various skin exanthema and severe arthralgias have been observed [4,6–8]. Up to 20% of patients on infliximab treatment have been recently reported to experience a various skin non-infectious complications in a large cohort study [4].
The modes of action of antiTNF-α agents are still not well understood. The main mechanism of action of infliximab and adalimumab is thought to be via induction of apoptosis in activated inflammatory cells [9,10]. Both agents have been shown to cause apoptosis in monocytes through a caspase 3-dependent pathway [9,10]. Moreover, a significant increase in apoptotic CD3+ lymphocytes in the colonic lamina propria has been shown in IBD patients treated with these preparations [11]. Degradation of DNA into nucleosomal units during apoptotic cell death is carried out by several enzymes called desoxyribonucleases (DNases) [12–14]. DNase I is a Ca2+/Mg2+/Mn2+ endonuclease which hydrolyses double-stranded DNA. Its deficiency leads to difficulties in removing DNA from nuclear antigens and promotes susceptibility to autoimmune disorders [15]. A deficiency of DNase I was found in patients with systemic lupus erythematodes (SLE) correlating with high titers of antibodies against nucleosomal antigens [16,17].
We have thus hypothesized that decreased activity of DNase I could lead also to drug-induced immunopathological skin complications in patients with IBD treated with infliximab and adalimumab. The primary aim of our study was to assess the activity of DNase I in patients with IBD and to examine the impact of DNase I activity on occurrence of skin non-infectious complications during biological therapy. The secondary aims were to search for other factors with a possible influence on development of skin complications and to assess the impact of biological therapy on DNase I activity.
Patients and methods
Patients
Patients with IBD treated with infliximab or adalimumab at our centre from July 2007–September 2009 were retrospectively studied for occurrence of skin complications during this treatment. The diagnosis of IBD of all patients was made in accordance with international diagnostic criteria [18,19]. Since the first reports of skin adverse events during antiTNF-α therapy in 2007, patients treated at our centre with biological treatment were intentionally and more closely followed-up with respect to skin complications and questioned on skin problems during out-patient visits. The date of onset, localization, character of the lesions, management and outcome of skin complications were intended to be recorded in medical files.
In patients with skin complications during biological therapy which were considered to be of non-infection origin, the activity of DNase I was measured. Patients with infectious skin complications (herpetic, bacterial, mycotic) and those on adalimumab therapy with skin reactions at the application site only were not included. The demographic and clinical data on disease characteristics, concomitant therapy, number of infusions/injections, treatment response, type of skin complications, treatment of skin lesions and outcome of skin adverse events were retrieved from medical files.
Furthermore, a random sample of IBD patients treated with biological therapy without occurrence of skin complications was assessed for DNase I activity. The clinical and demographic characteristics of the examined individuals were drawn from the medical files.
DNase I of all patients was measured from serum samples stored in our blood bank and taken prior to (81%) or after start of biological therapy (19%).
In order to assess the impact of biological therapy on DNase I activity, in 43 patients on infliximab treatment (11 with and 32 without skin complications), in whom blood samples taken prior to and 6 weeks after treatment start were available, the activity of DNase I and C-reactive protein (CRP) were measured repetitively.
20 healthy blood donors (10 females) were used as the age-matched control group.
The treatment strategy of all patients was standardized and consisted of induction therapy (infliximab 5 mg/kg at weeks 0, 2, 6 and adalimumab 160/80 mg or 80/40 mg at weeks 0 and 2) followed by maintenance regime (infliximab every eight weeks and adalimumab every other week) in initial responders. Efficacy of antiTNF-α therapy was retrospectively assessed and considered as non-response in case of sustained or increased activity, partial response in case of improvement without complete relief of symptoms and complete response in case of complete resolution of symptoms.
Some patients obtained several treatment courses of biological therapy during the study period. A second, third, etc. treatment course was defined as a repetitive drug application after bowel surgery or > 3 months after discontinuation of previous one for other reason or introduction of another preparation due to allergy, loss of response to previous drug or other reason. Because part of the patients obtained both infliximab and adalimumab and the occurrence of skin complications was not necessarily observed in all treatment courses given to one patient, the analyses were carried out per treatment course instead of per patient.
The study was approved by the Institutional Ethical Committee. The purpose and procedures of the study were explained to participants and signed informed consents were obtained from each.
Laboratory measurement
Serum was used for DNase I activity measurement. Blood samples were taken from the cubital vein and centrifuged for 10 minutes at ambient temperature and 1,300 g and separated serum aliquots were frozen at -80 °C. Analyses of samples were performed within twelve months.
Serum DNase I activity was determined by a validated standardized enzyme-linked immunosorbent assay (DNase I Activity, Orgentec, Germany). In this assay, specific DNase substrate is bound to microwells. Any present DNase I activity in serum sample reacts with the specific immobilized DNase substrate for 60 minutes at 37 °C. Washing of the microwells removes non-reactive serum components. Horseradish peroxidase (HRP) conjugated anti-DNase substrate immunologically detects the remaining DNase I substrate immobilized on the microplate. Washing of the microwells removes unbound conjugate. An enzyme substrate in the presence of bound conjugate hydrolyzes to form a blue color. The addition of an acid stops the reaction forming a yellow end-product. The intensity of this yellow color was measured photometrically at 450 nm by MRXII (Dynatech, UK) photometer and analyzed by the Revelation (Dynatech, UK) software. The amount of color is inversely proportional to the DNase I activity. Cut-off value of 75% of DNase I activity (which corresponds to the 25% activity reduction) was assessed according to the manufacturer’s recommendations.
Statistical analysis
Standard descriptive statistical analyses were performed, including frequency distributions for categorical data and calculation of median and (interquartile) range for continuous variables.
Univariate and multivariate logistic regression has been performed to calculate marginal and adjusted odds ratios for skin complications among IBD patients with respect to following clinical factors: gender, age, diagnosis, disease duration, type of antiTNF-α preparation, change of type of antiTNF-α preparation, number of applications, concomitant corticosteroid or immunosuppressive therapy, personal history of psoriasis/atopic eczema. No correction for multiple testing was made. The fact that some patients contributed with replicate observations has been accounted for using the method of Generalized Estimating Equations [20]. A p value smaller than 0.05 was considered significant. The area under the receiver operating curve (ROC) with 95% confidence interval and positive and negative predictive values to predict immunopathological skin complications for different values of cutoffs of DNase I activity were calculated.
The analyses have been performed using the R software version 2.11.0, R package geepack and R package ROCR.
Results
313 patients with IBD (172 females) were treated with biological therapy at our centre from July 2007–September 2009. Infliximab was given to 176 patients, adalimumab to 104 patients and 33 patients were treated with both infliximab and adalimumab.
A total of 43 patients (14%), 34 with CD and 9 with UC, were found to have experienced some skin complications, judged as of non-infection origin, during the biological therapy. A random sample of IBD patients who never experienced skin adverse events during biological treatment and in whom the serum sample for DNase I activity measurement was available included 95 patients, 59 with CD and 36 with UC.
Applications of biological therapy
43 patients who ever experienced skin complications obtained a total of 49 courses of biological treatment (23 with infliximab and 26 with adalimumab) with 6 patients having two treatment courses (4 with infliximab followed by adalimumab and 2 with adalimumab followed by infliximab). Of these 49 treatment courses, only 44 were complicated by skin eruptions. Out of 6 patients with two treatment courses, 1 patient had skin complications during both preparations, whereas 5 patients developed skin adverse events only during 1 course of biological therapy (2 patients were on adalimumab and had uncomplicated previous treatment with infliximab; 2 patients were on infliximab without any skin complications after switch to adalimumab and 1 patient was on adalimumab therapy without recurrence of skin lesions after later administration of infliximab).
To 95 patients who never experienced skin complications during antiTNF-α therapy, 117 courses of biological therapy were given (98 with infliximab and 19 with adalimumab).
Hence, the final population for statistical analysis included 44 applications of biological therapy with skin adverse events and 122 applications without skin complications (fig. 1).
Fig. 1. Recruitment of population for statistical analysis – applications of biological therapy. Obr. 1. Nábor subjektů pro statistickou analýzu – nasazení biologické léčby. 
Demographic and clinical characteristics of patients at the start of each biological treatment course are outlined in tab. 1.
Tab. 1. Demographic and clinical characteristics of patients at start of each application of biological therapy. Tab. 1. Demografické a klinické charakteristiky pacientů při zahájení biologické léčby. 
AE, adverse events; *median (interquartile range); **azathioprine/6-mercaptopurine Skin complications
The median number (range) of infusions or injections until occurrence of skin adverse events was 4 (1–11) in infliximab and 8 (1–54) in adalimumab therapy. 26 patients (59%) had dermatology examination and in only 6 of them skin biopsy was performed. The rest of patients had mild skin eruptions and did not require specialist consultation. In the majority of patients skin lesions had psoriasiform character although in only 4 cases psoriasis or suspicious psoriasis was confirmed and the majority of patients did not have exact dermatology diagnosis even despite the dermatology examination (tab. 2). The typical localization of skin lesions were face, scalp, palms and soles, but in general any part of the body was involved including trunk and extremities. Local therapy only was given to 15 patients, 19 patients obtained systemic therapy with or without local therapy and 10 patients did not require any treatment. In 12 patients (27%) the biological treatment had to be stopped due to skin complications. 32 patients (72%) had either complete or partial resolution of skin lesions, whereas in 6 patients (14%) the eruptions were refractory or persisted despite the therapy. Six patients (14%) experienced resolution of skin complications with subsequent recurrences.
Tab. 2. Skin complications in patients with inflammatory bowel disease treated with infliximab and adalimumab. Tab. 2. Kožní komplikace u pacientů s idiopatickými střevními záněty léčených infliximabem a adalimumabem. 
Impact of biological therapy on DNase I activity
No difference in DNase I activity was observed between the blood samples taken prior to and 6 weeks after start of infliximab treatment (both in patients with and those without skin complications), contrary to a statistically significant reduction in CRP levels (tab. 3).
Tab. 3. Impact of infliximab therapy on the activity of desoxyribonuclease I (DNase I) and C-reactive protein (CRP) level in 43 patients with inflammatory bowel disease. Tab. 3. Vliv léčby infliximabem na aktivitu deoxyribonukleázy I (DNázy I) a hladinu C-reaktivního proteinu (CRP) u 43 pacientů s idiopatickými střevními záněty. 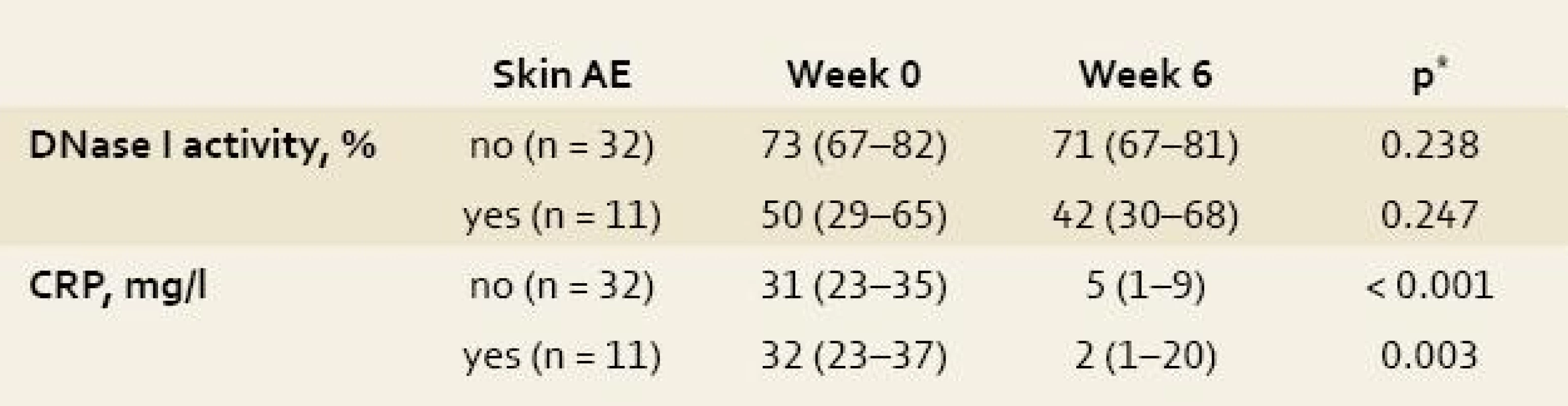
Date are expressed as median (interquartile range); *Wilcoxon test; AE, adverse events. DNase I activity thus seems not to oscillate during the treatment with infliximab and to be independent of a decrease in inflammatory activity as expressed by decline in CRP level.
Activity of DNase I enzyme
Only 31 out of 138 IBD patients included (22%) had baseline DNase I activity above the reference cut-off value of 75%. DNase I activity in IBD patients (median 68%; IQR: 50–75%) was significantly lower compared to healthy blood donors (96%, 87–98 %); p < 0.001. Patients with UC had a higher DNase I activity (72%, 63–79%), compared to CD patients (67%, 49–73%); p = 0.008. No significant gender related difference was observed.
Univariate analysis
Univariate analysis among IBD patients identified low DNase I activity, female gender, no concomitant therapy with corticosteroids and use of adalimumab as risk factors for development of skin adverse events, whereas change of type of biological preparation showed just borderline statistical significance (tab. 4). The median (IQR) activity of DNase I in patients with skin complications was 47.5% (42–54%) compared to 71% (67–79%) in those without skin adverse events (fig. 2). When analyzing infliximab and adalimumab separately patients with skin complications on either infliximab or adalimumab had significantly lower activity of DNase I than those with no skin complications (median 52% vs. 71%; p < 0.001 for infliximab and 46% vs.72%; p < 0.001 for adalimumab).
Fig. 2. Deoxyribonuclease (DNase) I activity in inflammatory bowel disease patients with and without skin complications during biological therapy. Obr. 2. Aktivita deoxyribonukleázy (DNázy) I u pacientů s idiopatickými střevními záněty, u nichž se v průběhu biologické léčby vyskytly nebo nevyskytly kožní komplikace. 
No other clinical factors, such as naivety to biological therapy, concomitant therapy with azathioprine/6-mercaptopurine, age at start of biological therapy, disease duration or number of applications were identified (tab. 4).
Tab. 4. Univariate logistic regression analysis of the impact of clinical factors on the occurrence of skin complications. Tab. 4. Univariátní logistická regresní analýza vlivu klinických faktorů na výskyt kožních komplikací. 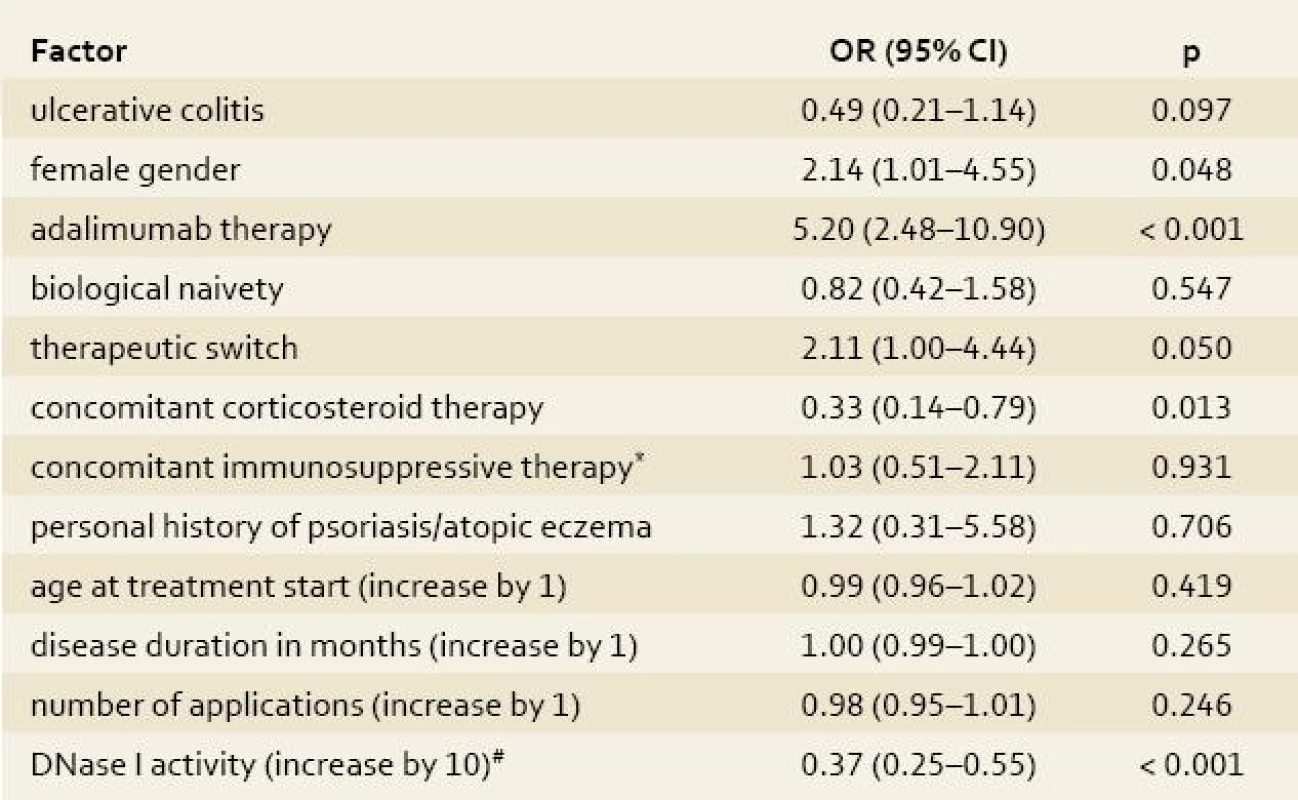
*azathioprine/6-mercaptopurine; #the risk of skin adverse events decreases by each 10% increase of DNase I activity (within the measured range) by OR of 0.37 DNase I, deoxyribonuclease I; OR, odds ratio; CI, confidence interval Multivariate analysis
In multivariate analysis, only low activity of DNase I and adalimumab preparation were significantly associated with occurrence of skin complications (tab. 5).
Tab. 5. Multivariate logistic regression analysis of the impact of clinical factors on the occurrence of skin complications. Tab. 5. Multivariátní logistická regresní analýza vlivu klinických faktorů na výskyt kožních komplikací. 
DNase I, deoxyribonulcease I; OR, odds ratio; CI, confidence interval, *each OR is adjusted with respect to all other factors in the table; **azathioprine/6-mercaptopurine; #the risk of skin adverse events decreases by each 10% increase of DNase I activity (within the measured range) by OR of 0.45. The area under the ROC curve (AUC) for the prediction of immunopathological skin complications among IBD patients using DNase I activity was 0.856 (95% CI, 0.785–0.927), fig. 3. The highest positive and negative predictive values were 44% and 97% respectively for cutoff 65% of DNase I activity under the prevalence of 15%.
Fig. 3. Receiver operating curve (ROC ) for the prediction of immunopathological skin complications among patients with inflammatory bowel disease using serum deoxyribonuclease (DNase) I activity. Obr. 3. ROC křivka pro predikci imunopatologických kožních komplikací u pacientů s idiopatickými střevními záněty s využitím aktivity deoxyribonukleázy (DNázy) I v séru. 
Discussion
The present study assessed the activity of DNase I enzyme in IBD patients treated with biological therapy and investigated its impact, together with other clinical factors, on the occurrence of skin complications during this treatment. Patients with IBD in general were found to have significantly lower activity of DNase I compared to healthy individuals. UC patients had higher DNase I activity than CD patients, whereas no gender related difference was observed. Similarly, no influence of biological therapy on DNase I activity was seen. Multivariate analysis identified low activity of DNase I and adalimumab preparation to be significantly associated with development of skin non-infectious complications during biological therapy.
Skin non-infectious complications during biological therapy represent an emerging problem of the recent period. The majority of reports arise from patients with rheumatic diseases, although there have been increasing evidence also in patients with IBD [4,6,7,21–23]. The presentation and severity of skin lesions differ among the patients, nevertheless a great proportion have psoriasiform character, observed also in our study. In a recent, large cohort study evaluating the safety of infliximab in patients with IBD, up to 20% of patients were reported to experience wide variety of skin lesions during this treatment. 64% of them underwent dermatology examination and of them 61% were diagnosed with psoriasiform dermatitis [4].
The underlying pathophysiological mechanism responsible for this phenomenon remains unknown. Several lines of evidence suggest that, under certain conditions, antiTNF-α treatment promotes the activation of autoreactive T-cells and leads to tissue damage via autoimmune mechanisms [24]. This theory is supported by the common induction of organ non-specific autoantibodies (such as antinuclear antibodies) in a substantial number of patients receiving antiTNF-α agents. Anti-nuclear antibodies develop in up to 50% of infliximab-treated patients and up to 37% of adalimumab-treated patients [25]. In principle, this formation of autoantibodies does not affect the efficacy of antiTNF-α therapy and seems not to predispose to autoimmune diseases [26].
However, a different situation could arise in case of the defective clearance of nucleosomal antigens. Such an abnormal accumulation of self-ligands could allow T-cell exposure for a sufficient time or a self-ligand quantity to induce pathological T-cell differentiation. DNase I is one of the several endonucleases involved in degradation of DNA during apoptosis in humans [14]. Its deficiency in mice models has been linked to development of autoimmunity conditions [14,27]. In humans, deficiency of DNase I has been implicated in the pathophysiology of SLE. Patients with SLE have been consistently found to have low activity of DNase I correlating with high titers of serum nucleosomal antigens and antinucleosomal antibodies [16,17,28]. Low DNase I activity has been found also in another systemic and organ specific autoimmune diseases, such as Sjögren’s disease and thyroid autoimmunity [29,30].
The exact mode of action of infliximab and adalimumab is still unknown. Currently, the most accepted theory of the main mechanism is via induction of apoptosis in activated inflammatory cells [11]. Interestingly, a recently published study found significantly increased levels of plasma nucleosomes, potential autoantigens, in patients with rheumatoid arthritis after infliximab treatment [31]. The present study was thus based on hypothesis that deficiency of DNase I and subsequent alteration of the apoptotic cells clearance might play a role also in development of skin complications during biological therapy.
Our results showed that patients with skin complications during biological therapy had significantly lower activity of DNase I compared to individuals without skin adverse events and thus suggested low activity of DNase I as a possible risk factor of skin complications.
Activity of DNase I was not influenced by anti-inflammatory effect of infliximab and did not correlate with decrease in CRP levels. Similar observation was reported in previously published study of SLE patients where no relationship between DNase I activity and medication use or activity of the disease could be found [16]. These results imply that serum DNase I activity is more or less stable and might propose reduced DNase I activity as an early predictor of probably biological therapy-associated immunopathological skin complications.
On the other hand, it has to be emphasized that similar skin complications as seen in infliximab and adalimumab therapy have been observed also during certolizumab treatment despite the fact that certolizumab has not been proven as a pro-apoptotic agent [32]. This suggests that other mechanisms are also involved in the pathogenesis of skin autoimmune phenomena induced by antiTNF-α therapy.
DNase I activity in all IBD patients, regardless of skin involvement, was significantly lower than in healthy individuals. The majority, 68% of patients had DNase I activity below the cut-off 75% set as a norm. CD patients showed significantly lower DNase I activity than UC patients. Hypothetically, different genetic background of these two nosologic units could contribute to this observation.
The finding of adalimumab preparation as a risk factor for skin adverse events has to be interpreted with caution since the proportion of patients on adalimumab therapy was not equal in a group of patients with and without skin complications which could bias the result. Nevertheless, a recently published article on new-onset psoriasis in rheumatoid arthritis patients treated with antiTNF-α preparations (infliximab, adalimumab and etanercept) found an increased incidence rates of psoriasis in patients treated with adalimumab compared to other two preparations [21].
The limitation of the study is the retrospective character and a relatively small sample size. Furthermore, only 59% of patients had dermatology examination and of them only about one forth had skin biopsy. Hence, the infectious origin of all skin lesions, judged by the clinicians as non-infectious, cannot be definitely excluded.
In conclusion, up to 14% of IBD patients developed skin non-infectious complications during biological therapy. Patients with skin complications had significantly lower activity of DNase I compared to those with uncomplicated treatment course. Activity of DNase I might thus serve as a predictor of skin complications during biological therapy. Further studies, however, have to confirm these results.
Acknowledgements
We would like to thank Mrs. Marcela Jarolimova for her invaluable help with the management of patients´ sera.
This study was supported by Research project No. 0021620807 of the Czech Ministry of Education.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.Doručeno/Submitted: 23. 11. 2011
Přijato/Accepted: 12. 1. 2012
Professor Milan Lukáš, MD, PhD
Clinical and Research Center for Inflammatory Bowel DiseaseISCARE IVF a. s. and Charles University
Jankovcova 1569/2c170 04 Prague 7, Czech Republic
milan.lukas@email.cz
Zdroje
1. Rutgeerts P, Vermeire S, Van AG. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target? Gut 2007; 56(4): 453–455.
2. Caspersen S, Elkjaer M, Riis L et al. Infliximab for inflammatory bowel disease in Denmark 1999-2005: clinical outcome and follow-up evaluation of malignancy and mortality. Clin Gastroenterol Hepatol 2008; 6(11): 1212–1217.
3. Colombel JF, Loftus EV Jr., Tremaine WJ et al. The safety profile of infliximab in patients with Crohn‘s disease: the Mayo clinic experience in 500 patients. Gastroenterology 2004; 126(1): 19–31.
4. Fidder H, Schnitzler F, Ferrante M et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut 2009; 58(4): 501–508.
5. Peyrin-Biroulet L, Deltenre P, Branche J et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn‘s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol 2008; 6(6): 644–653.
6. Rahier JF, Buche S, Peyrin-Biroulet L et al. Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin Gastroenterol Hepatol 2010; 8(12): 1048–1055.
7. Fiorino G, Allez M, Malesci A et al. Review article: antiTNF-alpha induced psoriasis in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2009; 29(9): 921–927.
8. Van Moerkercke W, Ackaert C, Jurgens M et al. Anti-TNFa Induced Severe Arthralgia as a manifestation of Autoimmunity? Gastroenterology 2010; 138(5): S60–S61.
9. Lugering A, Schmidt M, Lugering N et al. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn‘s disease by using a caspase-dependent pathway. Gastroenterology 2001; 121(5): 1145–1157.
10. Shen C, Assche GV, Colpaert S et al. Adalimumab induces apoptosis of human monocytes: a comparative study with infliximab and etanercept. Aliment Pharmacol Ther 2005; 21(3): 251–258.
11. Lugering A, Lebiedz P, Koch S et al. Apoptosis as a therapeutic tool in IBD? Ann N Y Acad Sci 2006; 1072 : 62–77.
12. Enari M, Sakahira H, Yokoyama H et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 1998; 391(6662): 43–50.
13. Napirei M, Ricken A, Eulitz D et al. Expression pattern of the deoxyribonuclease 1 gene: lessons from the Dnase1 knockout mouse. Biochem J 2004; 380 (Pt 3): 929–937.
14. Zhang J, Xu M. Apoptotic DNA fragmentation and tissue homeostasis. Trends Cell Biol 2002; 12(2): 84–89.
15. Ueki M, Takeshita H, Fujihara J et al. Caucasian-specific allele in non-synonymous single nucleotide polymorphisms of the gene encoding deoxyribonuclease I-like 3, potentially relevant to autoimmunity, produces an inactive enzyme. Clin Chim Acta 2009; 407(1–2): 20–24.
16. Martinez-Valle F, Balada E, Ordi-Ros J et al. DNase 1 activity in patients with systemic lupus erythematosus: relationship with epidemiological, clinical, immunological and therapeutical features. Lupus 2009; 18(5): 418–423.
17. Martinez VF, Balada E, Ordi-Ros J et al. DNase 1 and systemic lupus erythematosus. Autoimmun Rev 2008; 7(5): 359–363.
18. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol 1989; 170 : 2–6.
19. Stange EF, Travis SP, Vermeire S et al. European evidence based consensus on the diagnosis and management of Crohn‘s disease: definitions and diagnosis. Gut 2006; 55 (Suppl 1): 1–15.
20. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42(1): 121–130.
21. Harrison MJ, Dixon WG, Watson KD et al. Rates of new-onset psoriasis in patients with rheumatoid arthritis receiving anti--tumour necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2009; 68(2): 209–215.
22. Lee HH, Song IH, Friedrich M et al. Cutaneous side-effects in patients with rheumatic diseases during application of tumour necrosis factor-alpha antagonists. Br J Dermatol 200; 156(3): 486–491.
23. Grinblat B, Scheinberg M. The enigmatic development of psoriasis and psoriasiform lesions during anti-TNF therapy: a review. Semin Arthritis Rheum 2008; 37(4): 251–255.
24. Sfikakis PP, Kollias G. Tumor necrosis factor biology in experimental and clinical arthritis. Curr Opin Rheumatol 2003; 15(4): 380–386.
25. Vermeire S, Noman M, Van AG et al. Autoimmunity associated with anti-tumor necrosis factor alpha treatment in Crohn‘s disease: a prospective cohort study. Gastroenterology 2003; 125(1): 32–39.
26. Hoentjen F, van Bodegraven AA. Safety of anti-tumor necrosis factor therapy in inflammatory bowel disease. World J Gastroenterol 2009; 15(17): 2067–2073.
27. Walport MJ. Lupus. DNase and defective disposal of cellular debris. Nat Genet 2000; 25(2): 135–136.
28. Kim I, Hur NW, Shin HD et al. Associations of DNase IV polymorphisms with autoantibodies in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2008; 47(7): 996–999.
29. Belguith-Maalej S, Hadj-Kacem H, Kaddour N et al. DNase1 exon2 analysis in Tunisian patients with rheumatoid arthritis, systemic lupus erythematosus and Sjogren syndrome and healthy subjects. Rheumatol Int 2009; 30(1): 69–74.
30. Dittmar M, Bischofs C, Matheis N et al. A novel mutation in the DNASE1 gene is related with protein instability and decreased enzyme activity in thyroid autoimmunity. J Autoimmun 2009; 32(1): 7–13.
31. D‘Auria F, Rovere-Querini P, Giazzon M et al. Accumulation of plasma nucleosomes upon treatment with anti-tumour necrosis factor-alpha antibodies. J Intern Med 2004; 255(3): 409–418.
32. Bourne T, Fossati G, Nesbitt A. A PEGylated Fab‘ fragment against tumor necrosis factor for the treatment of Crohn disease: exploring a new mechanism of action. BioDrugs 2008; 22(5): 331–337.
Štítky
Dětská gastroenterologie Gastroenterologie a hepatologie Chirurgie všeobecná
Článek vyšel v časopiseGastroenterologie a hepatologie
Nejčtenější tento týden
2012 Číslo 1- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
-
Všechny články tohoto čísla
-
Kvíz – případ z klinické praxe
Selhání biologické léčby u nemocného s ulcerózní kolitidou - A word to new subscribers to the journal – members of the Czech Hepatological Society ČLS JEP
- Guidelines for the administration of biological therapy in patients with inflammatory bowel diseases: 2nd edition
- Low serum deoxyribonuclease I activity is associated with antiTNF-alpha induced skin adverse events in patients with inflammatory bowel disease
- Serum levels of infliximab and antibodies to infliximab, clinical using
- The role of vitamin D for inflammatory bowel diseases
- Matrix metalloproteinases and their inhibitors in correlation to proliferative and classical tumour markers during surgical therapy of colorectal liver metastases
- Gastrocolic fistula
- Endoscopic submucosal dissection of early gastric cancer
- Bowel preparation for colonoscopy
- Václav Havel – a look back
- What did Václav Havel for us and what can we do for his heritage?
-
How important is the multidisciplinary approach to the colorectal cancer patients?
The 1st National Colorectal Cancer Congress/1. Postgraduate Course of SGO report - Správná odpověď na kvíz
- New standards in Crohn’s disease treatment
- Journal at the beginning of 66th annual volume: the current state and perspectives
- Recollection from Gastrofórum
- Mutaflor – Escherichia coli kmen Nissle 1917, sérotyp O6:K5:H1
- News from the IBD world
-
Kvíz – případ z klinické praxe
- Gastroenterologie a hepatologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bowel preparation for colonoscopy
- Gastrocolic fistula
- Serum levels of infliximab and antibodies to infliximab, clinical using
- The role of vitamin D for inflammatory bowel diseases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



