-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Rare diseases in Italy: analysis of the costs and pharmacotherapy
Vzácná onemocnění v Itálii: analýza nákladů a farmakoterapie
Cílem práce bylo zanalyzovat, jakým způsobem je zajištěna léčba vzácných nemocí v italském regionu Kampánie (konkrétně v zařízení Místní lékařské společnosti v 47. zdravotním okrsku Neapole 1), a to zejména s přihlédnutím na současně platné směrnice v této oblasti; dále ekonomicky vyčíslit incidenci těchto patologií v každém čtvrtletí roků 2007 a 2008. Vzácná onemocnění jsou z každého úhlu pohledu závažnou problematikou. Pacienti narážejí na významné obtíže při získávání informací a při hledání nejvýhodnějšího způsobu zajištění léčby v rámci zdravotnického systému. Lékařské předpisy vystavené v letech 2007 a 2008 byly analyzovány po jednotlivých čtvrtletích s cílem zjistit počet pacientů s každou patologií, použitou léčbu, množství předepsaných léčivých přípravků a náklady na léčbu. Získaná data ukazují významný nárůst nákladů mezi léty 2007 a 2008 a mezi jednotlivými čtvrtletími roku 2008. V důsledku absence specifických guidelines v regionu Kampánie vytvořila Místní lékařská společnost Neapole 1 postup pro pacienty se vzácnými nemocemi, který jim umožní zajistit bezplatnou léčbu těmi přípravky, na které by jinak dopláceli.
Klíčová slova:
vzácná onemocnění • náklady • zajištění léčby • Itálie
Authors: Fabio Petrelli; Iolanda Grappasonni; Lenka Kračmarová; Pasquale Cioffi; Seyed Khosrow Tayebati; Lucia Esposito
Authors place of work: Università degli Studi di Camerino, School of Specialization in Hospital Pharmacy, Camerino (MC), Italy ; Krajská nemocnice T. Bati, a. s., Lékárna, Czech Republic ; Chieti Hospital, Italy ; Università degli Studi di Camerino
Published in the journal: Čes. slov. Farm., 2013; 62, 159-162
Category: Původní práce
Summary
Purpose of this research was to analyse the rare diseases drug supply paths in the Italian region of Campania (Health District 47 of the Local Medical Company Naples 1), with a particular focus on current regulations in this field, and quantify the economic incidence of such pathologies in each quarter of 2007 and 2008. Rare, or orphan, diseases are especially serious and onerous from every point of view. Patients meet significant difficulties in obtaining information and in identifying the most appropriate treatment path within the health care system. Pharmaceutical prescriptions were analysed in order to identify the number of patients for each pathology in each quarter of the years 2007 and 2008, the drugs used, the quantity of each drug, and the costs for treatments. Data show a significant increase of costs during each quarter of the year 2008, as well as from 2007 to 2008. In the absence of specific guidelines for the Campania Region, the Local Medical Company of Naples 1 has established a procedure for patients affected by rare diseases that enables them to receive at no cost products that otherwise would not be distributed for free by the health care system.
Keywords:
rare diseases • costs • drug supply • ItalyIntroduction
Rare, or orphan, diseases (RD) are especially serious and onerous because of their low incidence and chronic nature, and also because of heredity-related issues and problems linked to precocious onset. For most of these pathologies there is no cure today, but only treatments that can help to improve the quality and duration of life. In the United States, it is estimated that at least 20 million people are affected by rare diseases1); in Europe, there are about 25 million people. The aetiology of many orphan diseases is unknown, and there is a dearth of information about possible predisposition or risk factors. Patients and their families meet significant difficulties in obtaining information and in identifying the most appropriate treatment path within the health care system. Internationally, legislation about rare diseases and orphan drugs is broad and very complex; currently there is not a unified classification systems1) further, legislation is usually focused mainly on orphan drugs2). In 1983, the United States were the first to introduce such legislation with the Orphan Drug Act (ODA) in order to facilitate research and development of drugs for rare diseases. The European Union intervened in the field of rare diseases later through Regulation 141/2000/CE, which allows pharmaceutical industries to ask the European Medicine Agency (EMEA) to designate some of their products as “orphan”3,4).
In Italy, the aim of the Rare Disease National Register5), created as part of the National Health Institute (Istituto Superiore di Sanità, ISS), is to obtain epidemiological information (number of RD cases and their distribution throughout the country) and to define the dimension of the problem6).
Every Italian region has followed different paths to establish specialized centres that deal with rare diseases on the basis of different criteria. In addition, they have applied non-homogeneous regulations to the supply of drugs at no cost to patients when such drugs are not registered in Italy but available abroad, and to category C drugs used by these patients (“Health Federalism”)7). Therefore, interpretation of current regulations regarding treatment of rare diseases (Ministry Decree 279/2001) remains a serious problem at the regional level.
The survey described in this work sought to:
- analyse the RD drug supply paths in the region of Campania, with a particular focus on current regulations in this field, and quantify the economic incidence of such pathologies in the health district in each quarter of 2007 and 2008, in terms of number of treated patients – i.e. patients who were administered at least one RD drug through the direct supply regime of Health District 47 of the Naples 1 Local Healthcare Agency (ASL) in the reference period;
- identify the costs for such treatments, and compare the pharmaceutical cost trend sustained by the district in each quarter.
Experimental part
Health District 47 of Naples 1 serves about 120 000 individuals. In the period between 2007 and 2008, 22 patients with rare diseases were registered.
In this study, pharmaceutical prescriptions were analysed in order to identify the number of patients for each pathology in each quarter of the years 2007 and 2008, the drugs used, the quantity of each drug, and the costs for treatments.
Results
Examination of the supply of drugs for RD treatments in the district revealed that 15 patients were registered in 2007 (many of whom were treated during the course of the entire year), specifically 12 in the first quarter, 12 in the second, 11 in the third, and 12 in the fourth. The pathologies that affected these patients were mainly haemophilia A, sideroblastic anemia, amyotrophic lateral sclerosis, epidermolysis bullosa, and propionic acidemia (Table 1).
Tab. 1. Number of patients for each pathology (years 2007 and 2008) 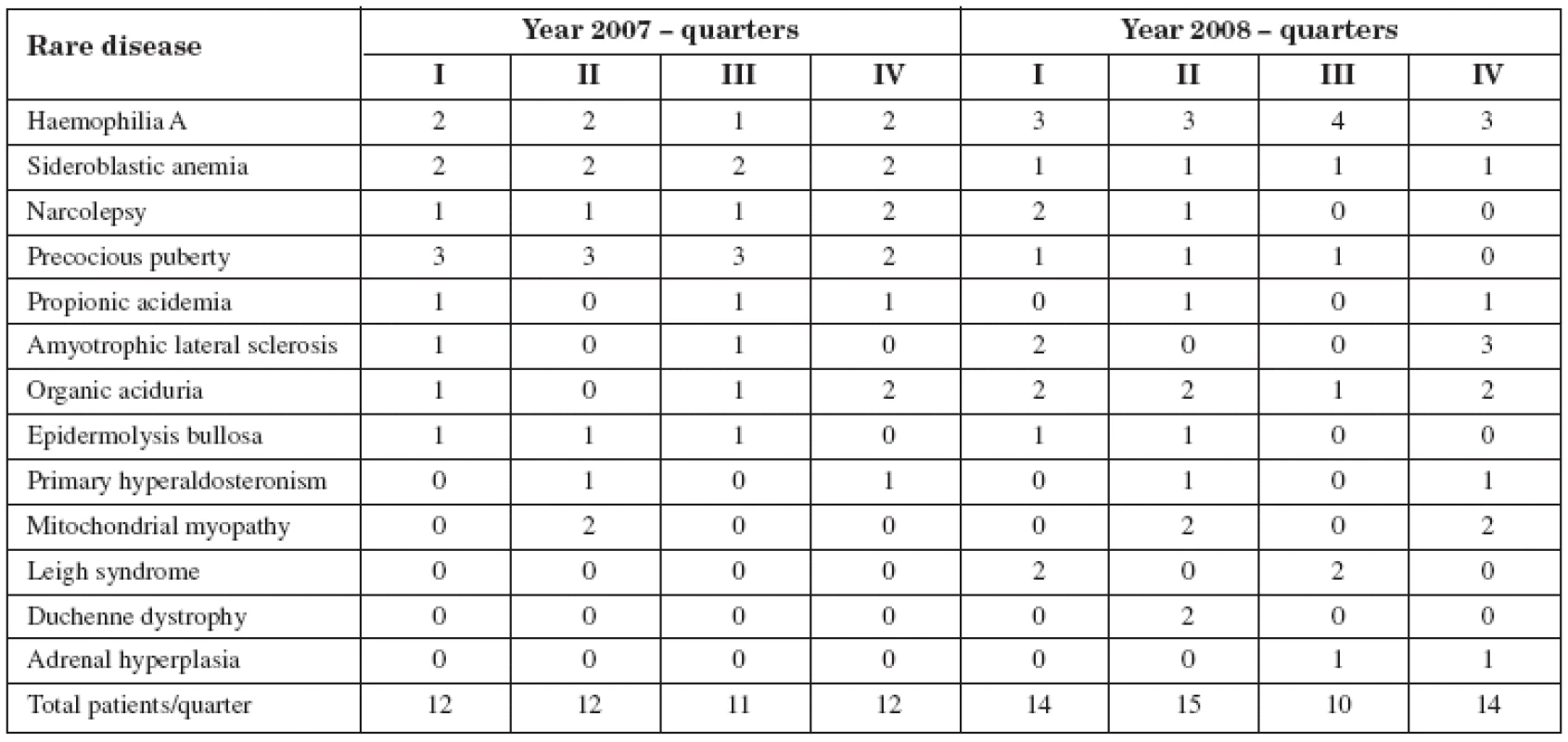
The total RD pharmaceutical expense for 2007 was € 277 181.43 (Fig. 1).
Fig. 1. Pharmaceutical costs for rare diseases (years 2007 and 2008) 
In 2008, 21 patients were registered, as follows: 14 in the first, 15 in the second, 10 in the third and 14 in the fourth quarter. The pathologies that affected these patients were mainly haemophilia A, amyotrophic lateral sclerosis, epidermolysis bullosa, organic acidemia, Kearns-Sayre syndrome and Leigh’s disease (Table 1). The fluctuation of number of patients in individual quarters is linked to a series of reasons. The adhesion to the therapy was facultative and the patient could decide whether follow it or not. It is also possible that the fluctuation could be influenced by the decision to interrupt, change in ASL, therapeutic plan modification, death. These changes were not recorded.
The total RD pharmaceutical expense for 2008 was € 746 123.98 (Fig. 1).
Usually in Italy prices of drugs change every year. The great difference between 2007 and 2008 can not be ascribed only to the prices increase. One can imagine the change could be linked to the therapeutic plan and prescription growth.
In the absence of a specific regional procedure regarding RD drug supply, the Naples 1 ASL adopted the following course:
- a) The citizen, on the basis of the prescription written by a Presidium of the rare disease national network, must present a request for drugs, food and other products that are considered essential and not otherwise supplied by the National Healthcare System to the UOASB (Operative Unit for Basic Medical Assistance) of his or her own district.
- b) An essential requirement for the procedure to commence is that UOASB verifies – by referring to lists available on the ISS website – that the Presidium is a part of the national network. Subsequently, once the documents are acquired, the UOASB must complete a form containing the patient’s personal data as well as the data of the Presidium, the diagnosis and the rare pathology code.
- c) The form is authorised by the UOASB Manager and by the DSB Director (Base Healthcare District).
- d) The District Pharmacy is in charge of purchasing and supplying the authorised products.
Discussion
In the absence of specific guidelines for the Campania Region, the Naples 1 ASL has established a procedure for patients affected by rare diseases that enables them to receive at no cost products that otherwise would not be distributed for free by the health care system. The Naples 1 ASL procedure was developed on the basis of a careful assessment of national and regional legal requirements in relation to rare diseases. In particular, art. 6 of the Ministry Decree (MD) n. 279/2001 establishes that: “the regions, on the basis of their own need and population, must provide for acquisition and distribution of specific drugs to patients, including direct supply of these drugs through public pharmacies”.
The Campania Region has appointed the Regional Pharmaceutical Service to monitor the distribution of drugs to all individuals affected by rare diseases. With regards to the legal aspect, the healthcare agency management has established a dedicated Coordination Committee (CdC) made up of two physicians and two pharmacists to carry out the procedure; they have met several times in order to formulate proposals to submit to the healthcare agency management.
When a patient is affected by a rare disease recognized in MD n. 279/2001, attachment 1 of 279/2001, the patient’s primary care physician refers the patient to a specialist in a public hospital as identified by Regional General Decree N. 1362 of 21. 10. 2005. If the diagnosis is not certain, the patient can request medical examinations at no cost through a specialist of an authorized centre that uses the R00-R99 code. If the patient has a hereditary rare disease, the genetic examinations to be conducted are free for the patient and family members as required to establish the diagnosis.
The Campania Region supplies at no cost to the patient all drugs (bands A and H) and food products (MD 8. 6. 2001) indicated in the therapeutic plan prescribed by the specialist. H drugs for home use are distributed by the local hospital pharmacies. Foreign drugs that are not available for sale in Italy are paid for by the NHS only if supplied within hospital facilities. Band C drugs and/or food supplements are usually paid for by the NHS. Patients affected by rare diseases can request supply of these drugs by providing a health certificate attesting to their need for these drugs and their absolutely irreplaceable effect, together with a document indicating their economic status, to their local ASL, which will evaluate the possibility of providing these drugs for free.
For patients treated outside their own Region or ASL, the free therapeutic treatments provided by the NHS are charged to the Region or ASL of origin through the inter-regional or inter-agency compensation institute.
Conclusion
Number of cases and costs for RD are growing. Patients face not only the burden of the disease and high costs of treatments, but also must deal with problems of bureaucracy and poor management. The data analysis of this study confirms these high costs for the period 2007–2008. The healthcare district total expenditure to supply RD drugs to 15 patients in 2007 and 21 in 2008 was respectively € 277 181.43 and € 746 393.37; there was a significant increase during each quarter of 2008 one by one, as well as from one year to the next.
The regional procedure adopted for disbursement of orphan drugs serves the mission of the local healthcare authority to guarantee healthcare for all individuals.
However, as execution of the healthcare agency procedure is quite recent, reliable data from monitoring the procedure itself, as envisaged by the CdC, are not available yet.
Acknowledgement
Authors want to express their thanks to the Health District 47 of Naples 1 for using necessary data and enabling this research.
Conflicts of interest: none.
Received 31 May 2013
Accepted 2 June 2013
Fabio Petrelli • prof. Iolanda Grappasonni (∗) • Seyed Khosrow Tayebati
Università degli Studi di Camerino
School of Pharmaceutical Sciences and Health Products
Via Madonna delle Carceri 9, 62032 Camerino (MC), Italy
e-mail: iolanda.grappasonni@unicam.it
Lenka Kračmarová
Krajská nemocnice T. Bati, a.s., Lékárna, Czech Republic
Pasquale Cioffi
Chieti Hospital, Italy
Lucia Esposito
Università degli Studi di Camerino, School of Specialization in Hospital Pharmacy, Camerino (MC), Italy
Zdroje
1. BCC Research. Global markets for orphan drugs, report. Wellesley: BCC 2007. http://www.bccresearch.com/report/ /PHM038B.html (13. 2. 2009)
2. Regulation (EC) n. 847/2000 of the Commission of 27 April 2000. Application of the criteria for the designation of orphan medicinal product and definitions of the concepts ‘similar medicinal product’ and ‘clinical superiority’. O.J. L103 28.04.2000 : 5–8.
3. Drummond M. F., Wilson D. A., Kanavos P., Ubel P., Rovira J. Assessing the economic challenges posed by orphan drugs. Int. J. Technol. Assess. Health. Care 2007; 23, 36–42.
4. McCabe C., Claxton K., Tsuchiya A. Orphan drugs and the NHS: should we value rarity? BMJ 2005; 331, 1016–1019.
5. Regolamento di istituzione della Rete Nazionale delle Malattie Rare e di esenzione dalla partecipazione al costo delle relative prestazioni sanitarie per circa 350 MR. DM n. 279 del 18 maggio 2001. GURI n. 160 del 12 luglio 2001 (Suppl Ord n. 180/L).
6. Bianchi F., Taruscio D. Registro Nazionale Malattie Rare. Epidemiologia di 44 malformazioni congenite rare in Italia. Rome: Istituto Superiore di Sanità 2002; Rapporti ISTISAN 02/36.
7. Riforma del Titolo V della Costituzione. Legge Costituzionale 8 ottobre 2001, n. 3. GURI n. 248 del 24 ottobre 2001.
Štítky
Farmacie Farmakologie
Článek Nové knihy
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2013 Číslo 4- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Rare diseases in Italy: analysis of the costs and pharmacotherapy
- Development of financial participation of patients in the costs of pharmacotherapy in the years 2008–2012
- Effect of abiotic elicitation on the sanguinarine production and polyphenol oxidase activity in the suspension culture of Eschscholtzia californica CHAM.
- Antioxidant activity of extracts and HPLC analysis of flavonoids from Capella bursa-pastoris (L.) Medik
- Content uniformity of warfarin-containing mixtures and tablets
- Drugs of a Baroque monastery pharmacy
-
XXXV. pracovní dny Radiofarmaceutické sekce
České společnosti nukleární medicíny ČLS JEP - Prof. RNDr. Ľudovít Krasnec – 100. výročie narodenia
- Prof. Ing. Jozef Lehotay, DrSc. – sedemdesiatnik
- Nové knihy
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drugs of a Baroque monastery pharmacy
- Content uniformity of warfarin-containing mixtures and tablets
- Antioxidant activity of extracts and HPLC analysis of flavonoids from Capella bursa-pastoris (L.) Medik
- Effect of abiotic elicitation on the sanguinarine production and polyphenol oxidase activity in the suspension culture of Eschscholtzia californica CHAM.
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



