-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mosquito-Disseminated Insecticide for Citywide Vector Control and Its Potential to Block Arbovirus Epidemics: Entomological Observations and Modeling Results from Amazonian Brazil
Fernando Abad-Franch and colleagues report entomological observations before and after citywide mosquito control in Amazonian Brazil, and model the estimated impact on transmission of mosquito-borne viruses.
Published in the journal: . PLoS Med 14(1): e32767. doi:10.1371/journal.pmed.1002213
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002213Summary
Fernando Abad-Franch and colleagues report entomological observations before and after citywide mosquito control in Amazonian Brazil, and model the estimated impact on transmission of mosquito-borne viruses.
Introduction
Fast global spread of mosquito-borne viruses is among the most pressing contemporary public health challenges [1,2]. The dengue, West Nile, and Japanese encephalitis viruses are well-known mosquito-transmitted pathogens, but we are currently witnessing the emergence of novel threats including chikungunya and Zika [1–6]. Both African in origin, these two viruses are causing large epidemics in the Americas and more restricted outbreaks in Europe, Southeast Asia, and the Pacific [5–7]. Ongoing Zika epidemics are particularly worrying because infection with this virus can cause Guillain-Barré syndrome and congenital central nervous system malformations including microcephaly [6–13].
Aedes aegypti and Ae. albopictus are considered the main urban vectors of dengue, Zika, and chikungunya, while Culex spp. mosquitoes transmit West Nile and Japanese encephalitis viruses [1–7]. The presence of urban mosquito vectors also increases the emergence or re-emergence potential of other viruses including yellow fever and Mayaro [1,2]. Effective vaccines exist for yellow fever and Japanese encephalitis, and recent advances in dengue [14] and Zika [15] vaccine development are relatively encouraging. However, major challenges remain (e.g., [16,17]), and for most mosquito-borne viral infections vector control is still the cornerstone of disease prevention [3,18,19]. In theory, effective control of disease spread requires lowering the ratio of competent vectors to susceptible human hosts below a critical threshold value, which, in turn, brings an infection’s basic reproductive number, R0, below unity [20–22]. R0 is a fundamental quantity in infectious disease epidemiology [22]. It measures the number of new (secondary) cases that arise from a primary (index) case entering a susceptible host population; with R0 < 1.0, each case produces, on average, less than one new infection, and the disease fades out from the host population. R0, then, provides also a measure of the control effort needed to effectively stop transmission [20–22].
Despite the large (and mounting) burden imposed by Aedes - and Culex-transmitted viruses [1,2,4–13,23,24], current mosquito control tactics have often failed to reliably reduce vector:human ratios to values that would keep R0 below the 1.0 threshold [18,19,25]. Mosquito control tactics usually combine insecticide spraying to kill adult mosquitoes with the identification and elimination of mosquito breeding sites (i.e., aquatic larval habitats) to limit juvenile mosquito numbers [3,18,19,25]. Unfortunately, insecticide spraying has only transient effects on the adult mosquito population, and the proportion of breeding sites that are detected and treated or eliminated (“breeding-site coverage”) is often so low as to render control campaigns largely ineffective (see [18,25]).
An attractive way to increase breeding-site coverage is to use adult mosquitoes to disseminate tiny particles of juvenile-killing insecticides (larvicides or pupicides) to breeding sites [26,27]. One such insecticide is pyriproxyfen (PPF), an insect juvenile hormone analogue that kills mosquito juveniles at minute doses and can safely be used even in drinking water [3,27,28]. Mosquito-disseminated PPF has been shown to yield high breeding-site coverage and large reductions of adult mosquito emergence across a tropical neighborhood [29]. One crucial open question is whether mosquito-disseminated PPF can effectively reduce mosquito populations at the spatial scale relevant for vector control and disease prevention—the scale of cities and towns. To address this question, we conducted a 2-y trial in a Brazilian Amazon city. First, we asked whether and to what extent some key demographic parameters of local mosquito populations (with a focus on Ae. aegypti and Ae. albopictus) would change following citywide deployment of mosquito-disseminated PPF. We then used simple deterministic models to explore the possible impact of observed changes in female Aedes emergence on the basic reproductive number, R0, for dengue and similar pathogens, including Zika, under epidemiological-entomological scenarios ranging from somewhat optimistic to essentially catastrophic.
Methods
This project was led by the Fundação Oswaldo Cruz (Brazilian Ministry of Health) in a joint initiative with local state and municipal health departments. Formal approval was not required for urban mosquito collection.
Setting and Mosquito Surveillance
We conducted a 2-y trial in Manacapuru, a 60,000-inhabitant city (~13,500 dwellings in ~650 ha) in the Brazilian Amazon (data from the Brazilian Institute of Geography and Statistics; http://www.ibge.gov.br/) (Fig 1). We selected 100 dwellings roughly evenly distributed across the city (Fig 1) for mosquito surveillance including two surveys per month from February 2014 to January 2016 (except that no surveys were conducted in November 2015, and just one survey in February 2015 and January 2016; see S1 Data). Residents in these 100 dwellings gave written informed consent to participate in the study.
Fig. 1. Study site: the city of Manacapuru, state of Amazonas, Brazil. 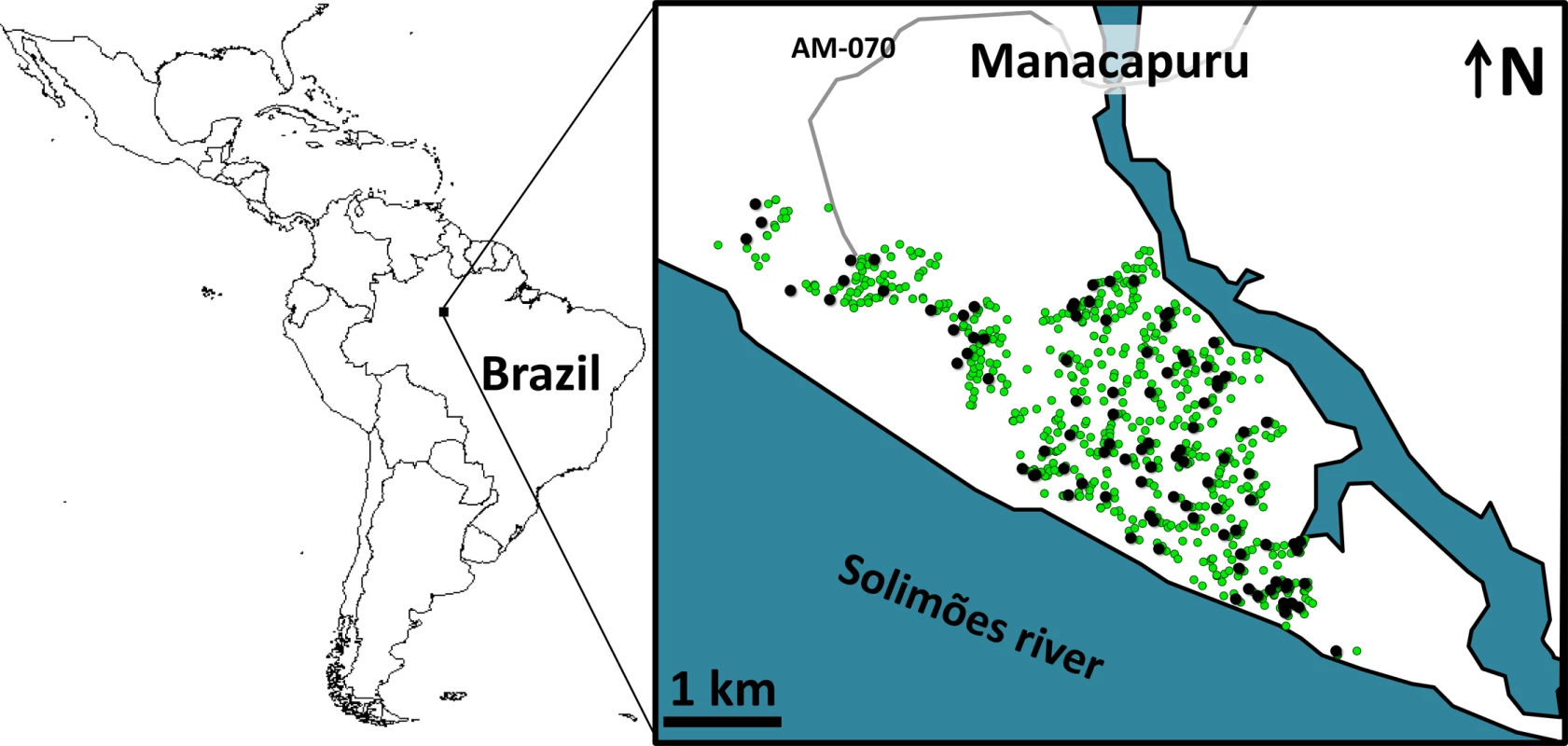
The black circles indicate the location of the 100 dwellings monitored for mosquito vectors; the green circles indicate the location of the 1,000 pyriproxyfen dissemination stations. Circles are overlaid on a schematic of Manacapuru; locations are approximate. The map of Latin America was drawn using the maps library in R 3.1.2 (https://www.r-project.org/). Each month, we set four sentinel breeding sites (SBSs; two per survey) in each surveillance dwelling. SBSs were 580-ml dark-brown plastic cups containing 400 ml of tap water; they were retrieved after 5–6 d of operation, and their contents kept in the laboratory as previously described [29]. We then recorded (a) the number of juvenile mosquitoes developing or dying in each SBS and (b) the number of adult mosquitoes emerging from each SBS. These data allowed us to assess monthly, for four mosquito taxa (Aedes aegypti, Ae. albopictus, Culex spp., and Limatus spp.), the following metrics: (a) house infestation, measured as the percent of surveillance dwellings with at least one juvenile mosquito; (b) juvenile mosquito catch, or the number of larvae in each SBS; (c) juvenile mosquito mortality, or the proportion of mosquito juveniles that died before reaching the adult stage in each SBS; and (d) adult mosquito emergence, or the number of adults emerging from each SBS. Here, we focus on our results on juvenile mosquito catch and adult mosquito emergence—and particularly on the epidemiologically most relevant quantity, female mosquito emergence. Full raw data are provided in S1 Data.
Intervention
In March 2015, after 1 y of monthly monitoring, the intervention started. Citywide PPF dissemination occurred from March through July 2015. Working under our supervision, municipal vector control staff deployed 1,000 PPF dissemination stations (DSs) scattered over the entire urban area (Fig 1); all site owners gave oral informed consent. DSs were 2-l plastic cups containing 600–700 ml of tap water and with the inner wall lined with black, Oxford-type polyester cloth dusted with 5 g of PPF 0.5% (SumiLarv 0.5G; Sumitomo Chemical, Tokyo) ground to fine powder (see also [29]). Municipal vector control staff visited DSs fortnightly for maintenance (re-dusting with PPF and refilling with water). Logistic constraints, however, precluded DS maintenance in some city sectors at some time points (see S1 Data and S1 Fig); we investigated the possible effects of these operational failures using generalized linear mixed models (GLMMs) (see below).
From August through October 2015, PPF dissemination was scheduled to be “focal”—i.e., limited to dwellings with evidence of infestation by Aedes spp. based on the SBS surveillance. Again, logistic constraints did not allow for full coverage, with focal dissemination not taking place in eight of the 37 dwellings found to be infested at least once over this 3-mo period; in addition, our field team noted that PPF used in focal dissemination in October 2015 (26 dwellings) was not ground to sufficiently fine powder (see S1 Data and S1 Fig). Final PPF dissemination occurred in October 2015, with SBS-based monitoring maintained until January 2016. Thus, the trial spanned 12 mo before PPF dissemination, 5 mo of citywide PPF dissemination, 3 mo of focal PPF dissemination, and 3 mo after PPF dissemination stopped. Importantly, conventional Aedes control measures (active breeding-site searches and breeding-site elimination by municipal vector control staff) were in place over the first 12 and last 6 mo of the trial—i.e., over the periods with no citywide PPF dissemination.
Descriptive Analyses
We first described our data using graphs and tables, and calculated summary statistics including percentages with score 95% confidence intervals, means with standard errors, and quantiles.
Statistical Modeling
We used GLMMs to quantify changes in (a) juvenile mosquito catch (number of larvae caught in SBSs) and (b) adult Aedes emergence (number of adults emerging from SBSs) following PPF dissemination. The Akaike information criterion (AIC) and the Bayesian information criterion (BIC) [30,31] unambiguously selected the negative binomial error structure (versus Poisson) as the best fit for our count data (see S1 Table). Our GLMMs accounted for unequal sampling effort due to missing SBS surveillance data by including the (log)number of operational SBSs in each dwelling each month as an offset. Since repeated observations were made over time in each dwelling, we specified dwelling ID as a random factor. Six dwelling-months produced no data (closed dwellings) and were excluded from the analyses, for a total of 2,294 SBS surveillance data points clustered in 100 dwellings. We specified intervention as a factor, indexing four consecutive periods: (1) before the intervention, or baseline; (2) citywide PPF dissemination (with some operational failures as noted above); (3) focal PPF dissemination (also with some operational failures); and (4) after PPF dissemination. We also tested alternative models excluding intervention effects (“null” models) or specifying, for each dwelling and month, (a) whether PPF dissemination (including DS maintenance) had/had not taken place at least once in the previous month (coded 1/0) or (b) the intensity/quality of dissemination, with 0 = no dissemination, 1 = unsupervised dissemination with possible operational failures, and 2 = supervised dissemination. For the second variable, for each month we summed the scores of the two fortnightly dissemination/maintenance events of the previous month, so this variable could take on integer values from 0 (no events) to 4 (two supervised events) (see S1 Data and S1 Fig). Dissemination/maintenance was recorded at the city sector level during citywide PPF dissemination and at the dwelling level during focal PPF dissemination. All these alternative GLMMs had, however, much larger AIC and BIC scores (consistently >50 units; see S1 Table) than the basic four-period models, on which we therefore base inference [30,31]. Our models controlled for the effects of rainfall (monthly total) and temperature (monthly average of maximum daily values); since these covariates were correlated (Pearson’s ρ = −0.704), we fit separate GLMMs adjusting for (standardized) rainfall and temperature. The Brazilian National Institute of Meteorology (INMET), which operates a meteorological station at the study locality, provided daily weather data (see S1 Data). GLMMs were fit using package lme4 1.1–10 in R 3.1.2 [32,33]. See S1 Table for details on the structure and relative performance of the full set of models used in each analysis, and S1 Text for a brief description of our original statistical modeling plan.
Deterministic Modeling
Using our empirical data and a simple Ross-Macdonald–type model [20–22], we explored the potential effects of observed changes in Aedes spp. female emergence on pathogen transmission. We calculated the basic reproductive number, R0, for pathogens resembling Aedes-borne viruses, including dengue (see Table 1), and the ratio (denoted m) of female Aedes mosquitoes to susceptible humans, which was the parameter we aimed to affect with our intervention. R0 is given by
with parameters as defined in Table 1. We estimated monthly m ratios as the number of Aedes females emerging from SBSs each month in each dwelling divided by 4.5, the average number of people per dwelling in our study setting. We hence assumed that 100% of the local human population was susceptible to the pathogen, mirroring the current spread of Zika and chikungunya outside Africa [5–7]. To provide much more conservative estimates of intervention effects on R0, we repeated these analyses using three times as many emerging females as observed—i.e., using 3m instead of m. This represents the (unlikely) possibility that eight further breeding sites with mean productivity similar to that of our SBSs were present, on average, in each dwelling each month.Tab. 1. Parameter values used to investigate the expected variation of the basic reproductive number, <i>R</i><sub>0</sub>, of a mosquito-borne viral infection as a function of the ratio of emerging <i>Aedes</i> females to humans under five hypothetical scenarios. 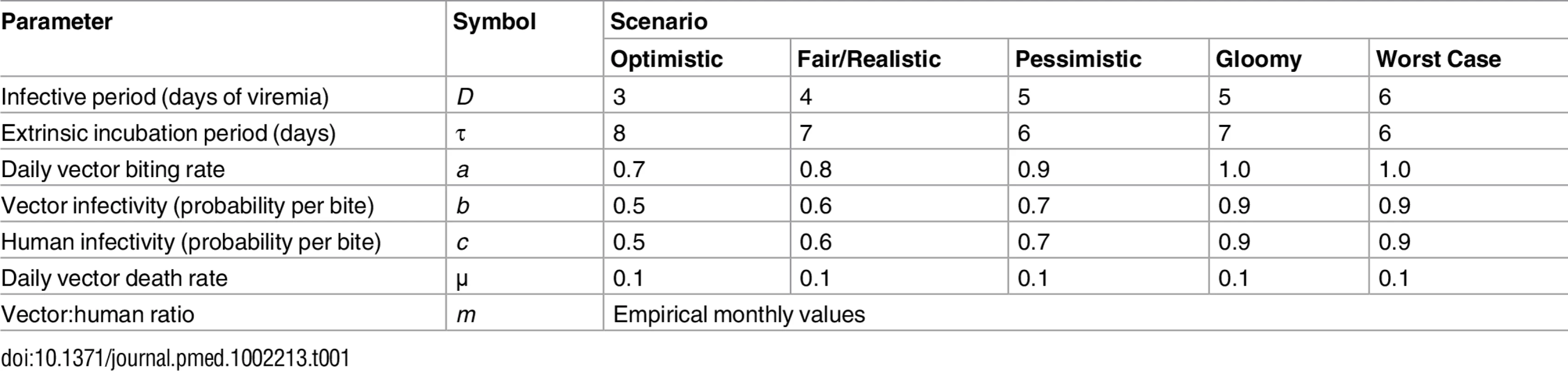
Results
Descriptive Analyses
Ae. albopictus was the dominant mosquito species at the study site; overall, we caught 12,817 Ae. albopictus and 5,346 Ae. aegypti juveniles in our SBSs. House infestation by Aedes spp. fell from monthly values consistently about 70%–90% at baseline (mean 84.5%, median 87%, range 67%–97%) to a mean of 33% during citywide PPF dissemination (median 24%, range 15%–61%) and to a lowest value of 9% in the first month of focal dissemination (mean 16%, median 13%, range 9%–26%); afterwards, infestation gradually recovered to baseline values (Fig 2A). We also collected 58 Culex spp. (Cx. quinquefasciatus and a few Cx. nigripalpus) and 1,213 Limatus spp. (mainly L. durhami) larvae during the trial. House infestation by Culex spp. was consistently low before dissemination (median 1%, range 0%–6%); afterwards, just four dwellings were positive in just one month (April 2015). Dwelling infestation by Limatus spp. was recorded only before PPF dissemination (median 17.6%, range 0%–83%).
Fig. 2. Changes in mosquito population metrics following deployment of mosquito-disseminated pyriproxyfen: descriptive graphs. 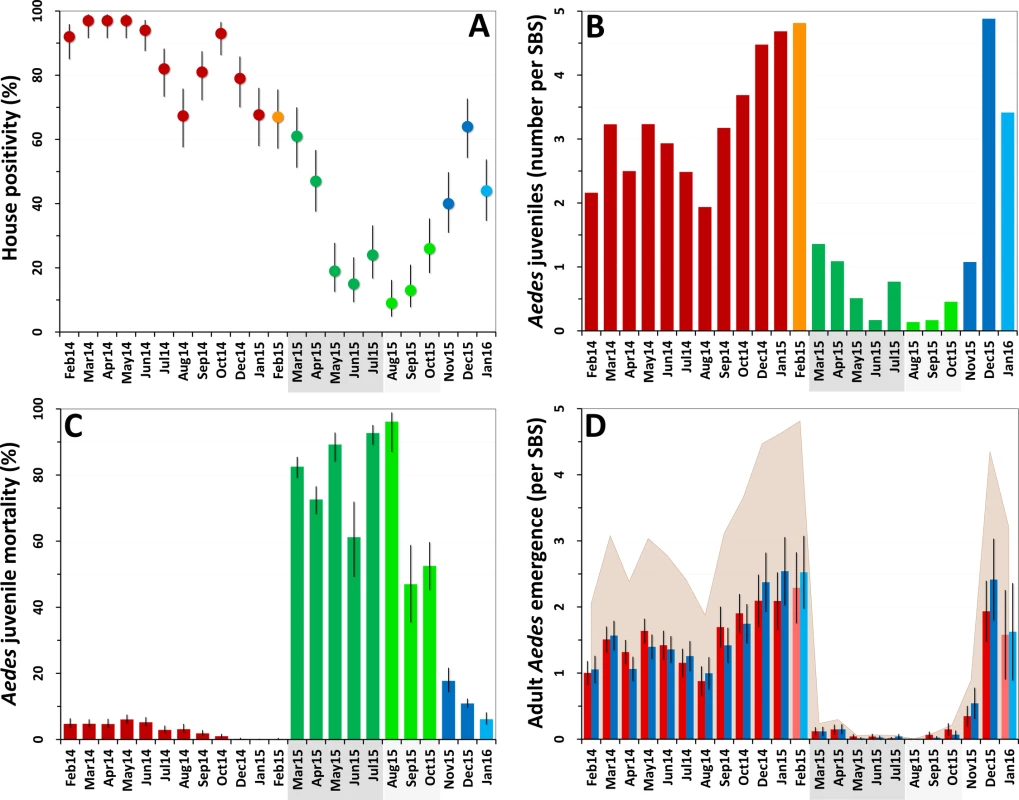
(A) Monthly dwelling infestation by Aedes spp. (percent of dwellings in which at least one Ae. albopictus or Ae. aegypti juvenile was present in sentinel breeding sites [SBSs]); error bars are score 95% confidence intervals. (B) Mean monthly numbers of Aedes juveniles per SBS. (C) Monthly Aedes juvenile mortality (overall percent of juveniles that died before reaching adulthood); error bars are score 95% confidence intervals. (D) Mean monthly adult Aedes emergence (number of juvenile Aedes that developed into adults in each SBS); error bars are two standard errors. In all panels, the periods of citywide (dark grey) and focal (light grey) pyriproxyfen (PPF) dissemination are highlighted on the x-axes. Color coding in (A–C): red, pre-intervention (baseline) period, with orange indicating that just one survey was conducted in February 2015; dark green, citywide PPF dissemination; light green, focal PPF dissemination; blue, post-intervention period, with light blue indicating that just one survey was conducted in January 2016. Color coding in (D): red, females; blue, males; shaded area, total adult emergence; lighter red/blue, single-survey months (February 2015 and January 2016). Juvenile Aedes catch fell from a median value of 3.20 individuals per SBS per month before the intervention (range 1.94–4.82, mean 3.28) to less than one juvenile per SBS per month during citywide (median 0.77, range 0.17–1.36, mean 0.78) and focal (median 0.17, range 0.14–0.46, mean 0.26) PPF dissemination. Aedes catch rose back to a mean of more than three larvae per SBS over the last 3 mo of the trial (Fig 2B). At the dwelling level, these figures translate into typical mean catches of about 7–17 juvenile Aedes per month before PPF dissemination, falling to a minimum of 0.52 (52 Aedes juveniles in 100 dwellings) in the first month of focal dissemination. Mean monthly catch per dwelling was 1.01 for Limatus spp. and 0.04 for Culex spp. before dissemination; except for seven Culex larvae caught in the second month of focal dissemination, neither genus appeared in samples taken during or after the intervention.
Before PPF dissemination, most Aedes juveniles survived to adulthood in our SBSs. Mean baseline monthly mortality was 1.9% (median 2.4%, range 0.0%–3.8%) for Ae. albopictus and 6.6% (median 5.5%, range 0.0%–17.8%) for Ae. aegypti. Monthly Aedes spp. mortality soared to 79.7% on average (range 61.2%–92.7%) during citywide PPF dissemination and reached a peak value of 96.2% (95% CI 87.0%–98.9%) in the first month of focal dissemination (Fig 2C). We could not investigate possible changes in juvenile Limatus mortality (mean at baseline 3.95%) because no larvae were caught after dissemination started. All Culex spp. juveniles caught before, but just three of seven caught during, PPF dissemination survived to adulthood.
The combined effects of much lower juvenile mosquito catches (Fig 2B) and much higher juvenile mortality (Fig 2C) yielded a striking citywide decrease of adult mosquito emergence during PPF dissemination (Fig 2D). Mean monthly Aedes adult emergence from SBSs was 1,077 (median 1,034, range 653–1,635) at baseline, for a mean of 3.2 adults per SBS per month (median 3.1, range 1.9–4.8) and 10.8 adults per dwelling per month (median 10.3, range 6.7–16.5). During citywide PPF dissemination, monthly emergence fell about 40-fold to just 56 adults on average (median 26, range 21–117), or 0.14 adults per SBS (median 0.07, range 0.06–0.30) and 0.56 adults per dwelling (median 0.26, range 0.21–1.17). Comparing extreme values (1,635 adults in January 2015 versus 21 adults in May 2015), adult Aedes emergence fell about 80-fold during citywide PPF dissemination. Further decreases were recorded during focal dissemination, down to a minimum of just two adult Aedes in total (a male and a female Ae. albopictus) emerging from SBSs, each in a different dwelling, in August 2015—an 800-fold reduction relative to January 2015. As with other metrics, adult Aedes emergence rose back to baseline values after PPF dissemination stopped (Fig 2D).
Since mosquito females but not males transmit human pathogens, we separately assessed Aedes female emergence from our SBSs. Table 2 summarizes monthly female emergence, and Figs 3 and 4 show, respectively, the numbers of Ae. albopictus and Ae. aegypti females emerging from SBSs in each dwelling and month. Monthly Aedes female emergence fell from an average of 536.6 (median 530, range 306–750) before to 28.8 (median 16, range 6–58) during citywide PPF dissemination; median values were therefore 33-fold lower, and extreme values 125-fold lower, during than before citywide dissemination. Again, this reduction became even larger over the focal dissemination period, with just one Aedes female emerging from the SBSs in August 2015—a >500-fold decrease compared to January 2015 (Table 2; Figs 2D, 3 and 4).
Fig. 3. Monthly female Aedes albopictus emergence in each of the 100 surveillance dwellings. 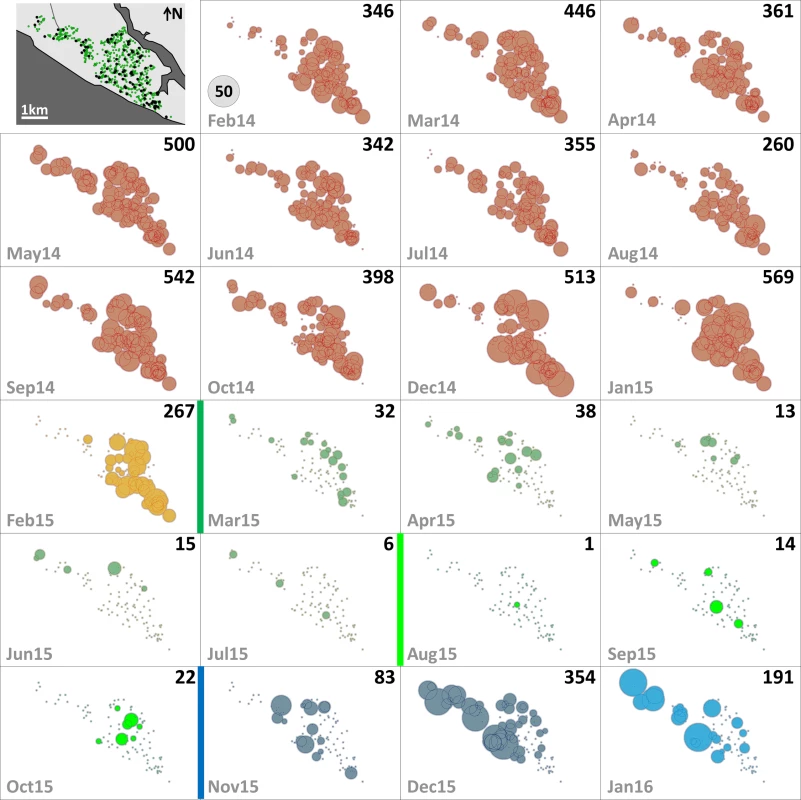
The distribution of dwellings (black dots) and pyriproxyfen dissemination stations (green dots) is shown in the first panel, where dots are overlaid on a schematic of Manacapuru. In the remaining panels, bubble size is proportional to the number of emerging Ae. albopictus females; the scale is shown as a grey bubble in the second panel. For each month, the total number of emerging Ae. albopictus females is shown in the upper right corner of the panel. Color coding: brown, pre-intervention (baseline) period, with yellow indicating a single-survey month; dark green, citywide PPF dissemination; light green, focal PPF dissemination; blue, post-intervention period, with light blue indicating a single-survey month. Temporal boundaries between periods are highlighted by colored vertical bars. Fig. 4. Monthly female Aedes aegypti emergence in each of the 100 surveillance dwellings. 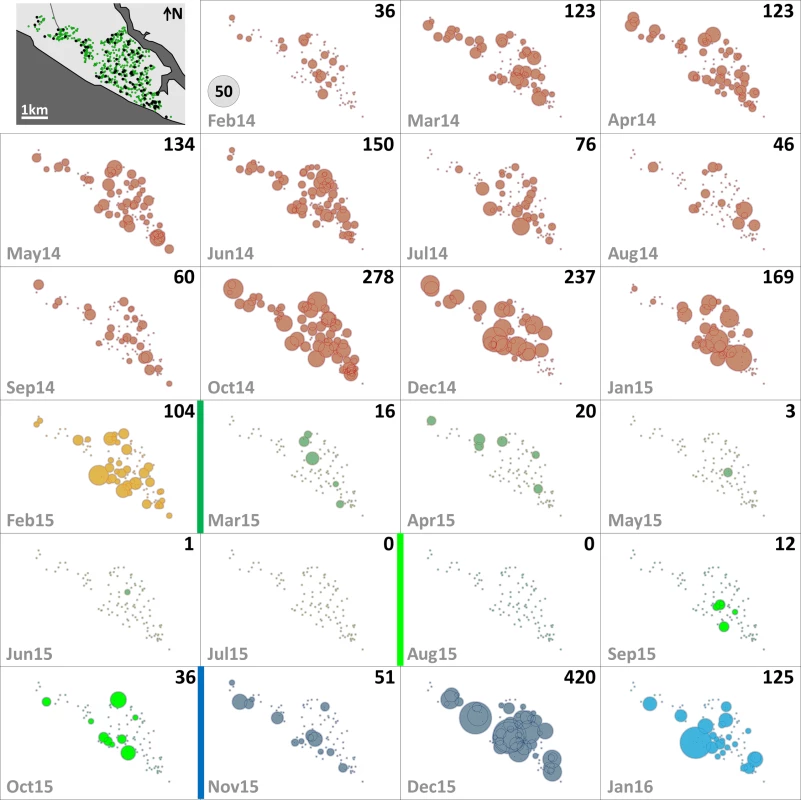
The distribution of dwellings (black dots) and pyriproxyfen dissemination stations (green dots) is shown in the first panel, where dots are overlaid on a schematic of Manacapuru. In the remaining panels, bubble size is proportional to the number of emerging Ae. aegypti females; the scale is shown as a grey bubble in the second panel. For each month, the total number of emerging Ae. aegypti females is shown in the upper right corner of the panel. Color coding: brown, pre-intervention (baseline) period, with yellow indicating a single-survey month; dark green, citywide PPF dissemination; light green, focal PPF dissemination; blue, post-intervention period, with light blue indicating a single-survey month. Temporal boundaries between periods are highlighted by colored vertical bars. Tab. 2. Monthly female Aedes spp. emergence from sentinel breeding sites set in 100 surveillance dwellings, Manacapuru, Amazonas, Brazil, February 2014–January 2016. 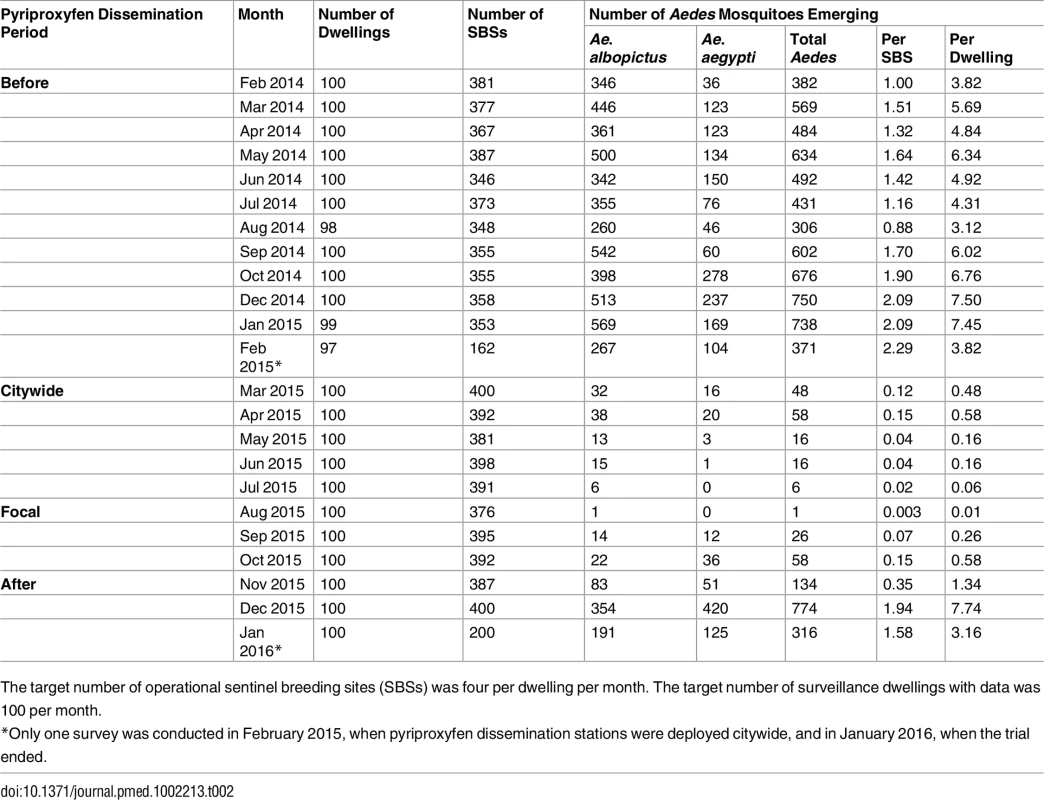
The target number of operational sentinel breeding sites (SBSs) was four per dwelling per month. The target number of surveillance dwellings with data was 100 per month. Statistical Modeling
GLMMs estimated strong negative effects of PPF dissemination on juvenile mosquito catch (Table 3; Fig 5A). Compared with baseline values, mean juvenile catch (all species) was estimated to fall by 80.2% (95% CI 76.3%–83.5%) and 92.1% (95% CI 89.9%–94.0%) during, respectively, citywide and focal PPF dissemination. The largest effect estimate was for Ae. albopictus catch, with a 94.1% reduction (95% CI 92.0%–95.6%) during focal dissemination and with negative effects still evident after dissemination stopped (36.2% reduction, 95% CI 18.9%–53.7%) (Fig 5B). Ae. aegypti mean catch was estimated to fall by 72.7% (95% CI 63.9%–79.4%) and 83.1% (95% CI 74.8%–88.7%) during citywide and focal dissemination, respectively (Fig 5B); the estimated increase in Ae. aegypti catch after PPF dissemination (Fig 5B) is driven by four outlier dwellings with a mean monthly catch of 30.2 Ae. aegypti juveniles per SBS (see S2 Fig).
Fig. 5. Estimated impact of mosquito-disseminated pyriproxyfen on Aedes populations: results of generalized linear mixed models. 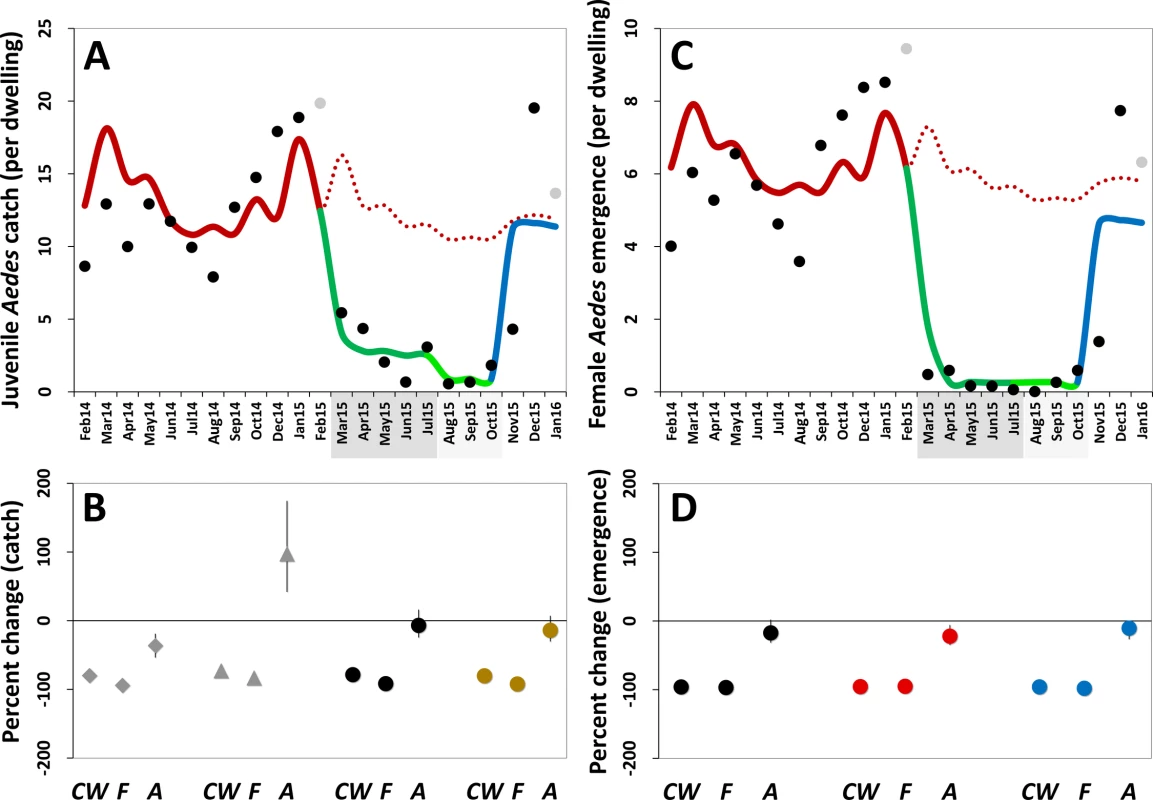
(A) Mean monthly Aedes juvenile catch per dwelling: dots, observed values (in grey, values adjusted for single-survey months); solid line, model predictions (red, pre-intervention period; dark green, citywide pyriproxyfen [PPF] dissemination; light green, focal PPF dissemination; blue, post-intervention period); dotted line, model-predicted trajectory in the absence of intervention as a function of monthly rainfall and adjusted for the number of operational sentinel breeding sites and dwelling-level clustering. (B) Model-predicted percent change (with 95% confidence intervals) in juvenile mosquito catch relative to the pre-intervention period (CW, citywide PPF dissemination; F, focal dissemination; A, after PPF dissemination): diamonds, Ae. albopictus; triangles, Ae. aegypti; black circles, Aedes spp.; gold circles, all mosquito species. (C) Mean monthly Aedes adult emergence per dwelling, with coding as in (A). (D) Model-predicted percent change (with 95% confidence intervals) in adult Aedes emergence relative to the pre-intervention period, with periods coded as in (B); black circles, all Aedes adults; red circles, Aedes females; blue circles, Aedes males. In (A) and (C), the periods of citywide (dark grey) and focal (light grey) PPF dissemination are highlighted on the x-axes. Tab. 3. Estimated effects of mosquito-disseminated pyriproxyfen on juvenile mosquito catch: results from generalized linear mixed models. 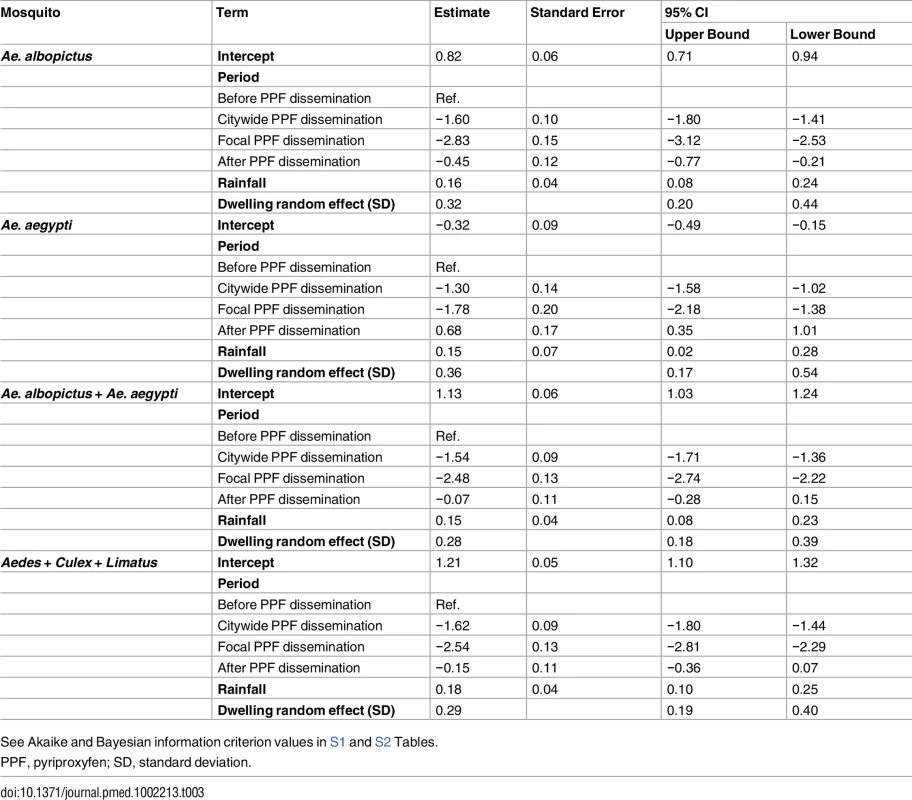
See Akaike and Bayesian information criterion values in S1 and S2 Tables. Mean reductions in adult Aedes emergence relative to baseline, as estimated by a GLMM (Table 4), were 96.0% (95% CI 95.2%–96.8%) during citywide and 96.8% (95% CI 95.8%–97.6%) during focal PPF dissemination, with emergence rising back to baseline values after dissemination ended (17.3% mean reduction relative to baseline but with a 95% CI ranging from a 31.6% decrease to a 2.0% increase). For Aedes females, GLMM estimates suggest emergence reductions of 95.6% (95% CI 94.6%–96.5%) during citywide and 95.1% (95% CI 93.4%–96.3%) during focal dissemination, with emergence still 22.1% (95% CI 5.8%–34.9%) lower in the post-intervention period than at baseline (Table 4; Fig 5C and 5D). Results were similar for Aedes males, with a maximum estimated reduction in emergence of 98.0% (95% CI 97.1%–98.7%) in the focal dissemination period (Table 4; Fig 5D).
Tab. 4. Estimated effects of mosquito-disseminated pyriproxyfen on adult Aedes emergence: results from generalized linear mixed models. 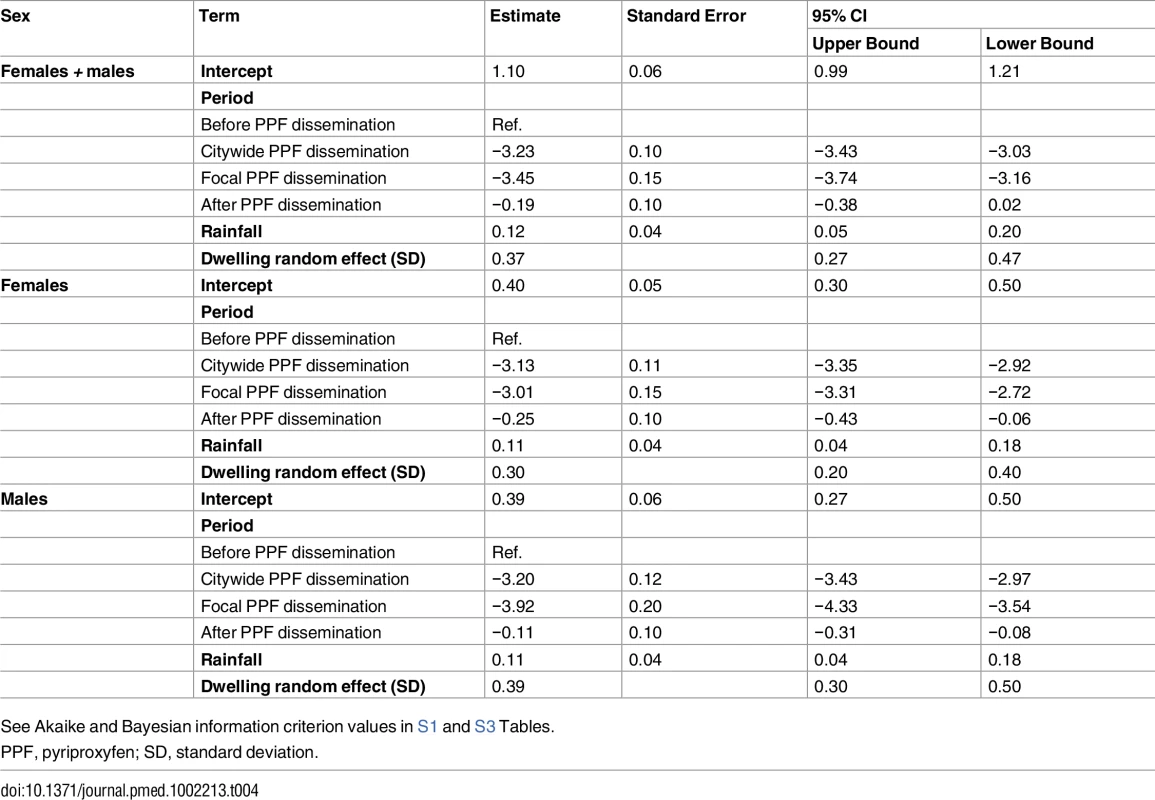
See Akaike and Bayesian information criterion values in S1 and S3 Tables. All the above intervention effect estimates were fully consistent with those derived from models in which we used temperature instead of rainfall to provide adjustment for weather conditions; as expected, our GLMMs overall suggest moderate positive effects of rainfall and weaker negative effects of maximum temperature on mosquito population metrics (see S2 and S3 Tables).
Deterministic Modeling
Monthly values of m, an estimate of the mean number of Aedes females emerging per person, fell from 1.2 (median 1.2; range 0.7–1.7) before PPF dissemination to 0.06 during both citywide (median 0.04, range 0.01–0.13) and focal (median 0.06, range 0.002–0.13) PPF dissemination. Fig 6 shows R0 values as a function of monthly m ratios in five scenarios ranging from optimistic to worst case (see Table 1). Recall that R0 measures the number of new infections arising from a primary case [20–22], so an infection can persist in a host population only if R0 > 1.0; recall also that our models assume a naïve human population with no immunity against the pathogen. Fig 6 shows that, across scenarios, the reduction of monthly m ratio seen during PPF dissemination is predicted to consistently bring R0 to <1.0, whereas baseline m values (first 12 mo) predict R0 values typically between 2.0 (optimistic scenario; range 1.15–2.75) and 5.5 (fair/realistic scenario; 3.18–7.63). Even in the worst-case scenario, with baseline R0 = 32 (range 19–45), the intervention would bring R0 to <1.0 for 4 and 9 mo assuming, respectively, daily vector death rates of μ = 0.1 and μ = 0.3 (see [34–37]) (Fig 6). A reanalysis using three times as many emerging Aedes females as observed predicts R0 < 1.0 for 1 mo (worst-case scenario, with baseline R0 from 56 to 133) to 8 mo (optimistic scenario, with baseline R0 ranging from 3 to 8) (see S3 Fig).
Fig. 6. Monthly estimates of the basic reproductive number (R0) of mosquito-borne viruses similar to dengue, Zika, or chikungunya. 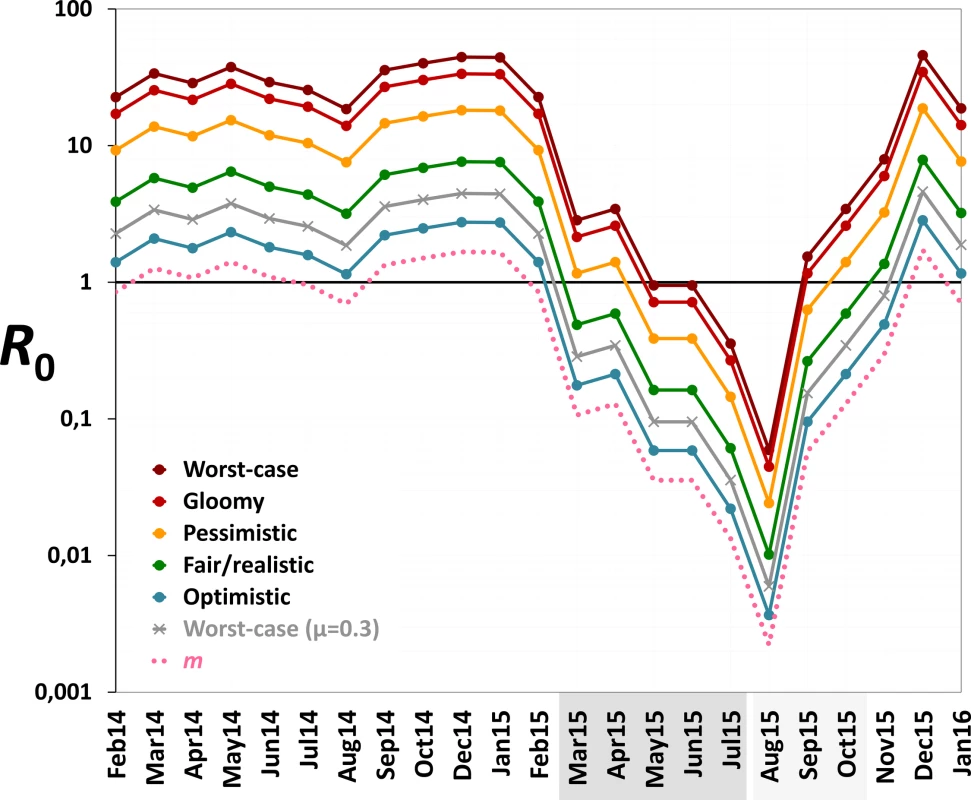
We considered scenarios ranging from optimistic to very adverse (see parameter values for each scenario in Table 1); the grey line corresponds to the worst-case scenario but with a higher value of the mean daily female mosquito death rate (μ = 0.3 instead of 0.1) to approximate data from wild Ae. aegypti populations (see [34–37]). The pink dotted line shows empirical monthly values of the number of Aedes females per person (parameter m) in our study setting and period. The periods of citywide (dark grey) and focal (light grey) PPF dissemination are highlighted on the x-axis. Discussion
In this study we have shown that a sharp citywide decrease in mosquito vector populations followed the application of a low-technology tactic based on mosquito-disseminated PPF in a tropical town. Population declines were observed for Aedes and Culex spp., two foremost vectors of human disease, and for Limatus spp. The 95%–96% reduction in Aedes female emergence we report has the potential of blocking arbovirus transmission under scenarios ranging from somewhat optimistic to overtly adverse. The control of urban Culex spp. could have similar effects on the spread of important pathogens ranging from West Nile virus to lymphatic filariae; Culex spp. might in addition transmit Zika virus [38], although this is yet to be confirmed. Suppression of urban Limatus populations has less clear public health implications, but several bunyaviruses capable of infecting mammals have been isolated from mosquitoes of this day-biting genus in Amazonia [39].
Our findings suggest that mosquito-disseminated PPF could be particularly relevant for the control of epidemic outbreaks such as those seen when Zika, dengue, or chikungunya virus sweeps through immunologically naïve populations. Given partial herd immunity, the more stable endemic-epidemic transmission of, for example, dengue in many countries [40] would be even easier to interrupt. We note, in addition, that some of the parameter values used in our calculations (Table 1) probably exceed typical real values; in fact, our baseline R0 estimates are higher than those reported for dengue epidemics in Brazil [34]. Daily death rates of Aedes females, for example, have been estimated as μ ≈ 0.2–0.4 in Brazil and Puerto Rico [35–37]. If, moreover, PPF reduces the lifespan of female Aedes as it does with Anopheles gambiae [41], this would further increase μ. Using μ = 0.3 instead of 0.1, our models suggest that R0 would be brought to <1.0 for 9 mo under the worst-case scenario—and for 6 mo even assuming three times as many emerging females as observed (Figs 6 and S3). Further, Aedes infectivity (parameter b in Table 1) is probably lower, on average, than we assumed in our calculations (see, e.g., [42,43] for Zika virus). Thus, in general, our models likely underestimate the potential intervention effects on R0. This suggests that mosquito-disseminated PPF might block arbovirus transmission citywide even under very adverse circumstances—an entirely susceptible population, long-lasting viremias, frequent mosquito biting, short extrinsic incubation periods, and high probabilities of virus transmission from vector to human and vice versa (Table 1; Figs 6 and S3).
The findings we report come, however, with several important caveats. The most obvious is that ours was a before–after, single-site trial lacking independent replicates, which limits the strength of the evidence we present. To partly mitigate this limitation, we estimated intervention effects using detailed 12-mo baseline data, and accounted for dwelling-level repeated measures, weather conditions, and unequal sampling effort using GLMMs. The size, sign, and consistency of effect estimates—which fully align with expectations based on mosquito biology and previous reports of PPF effects [26,27,29,44]—reinforce our confidence in the outcome of our analyses. In addition, that our findings are consistent with results from a neighborhood in a different city [29] suggests that PPF dissemination effects might be replicable elsewhere. Control results might however depend on the local availability of alternative breeding sites, which we did not measure. The impact of the intervention could thus be reduced in places where competing larval habitats are widespread enough to distract egg-laying females away from DSs. A second key limitation is that our SBS data are difficult to interpret in terms of true adult mosquito density or abundance [45]. While this should not greatly affect our estimates of relative change in juvenile catch and adult emergence, it calls for a cautious reading of our R0 results. Even if our complementary analyses using three times as many emerging females as observed are reassuring, we regard our R0 estimates as explicit, plausible hypotheses to be tested in future trials—ideally, cluster-randomized trials with replicate intervention and control sites and including a blind prospective assessment of arboviral infection incidence [46]. Finally, we note that we did not have the means to measure PPF in our SBSs, and therefore lack direct evidence of PPF dissemination. However, we did not record any extreme weather event or vector control intervention that could account for our observations. Further, alternative GLMMs investigating dissemination intensity/quality (coded as a 1-mo-lagged 0–4 variable; see Methods) suggested “dose-dependent” effects—with, for instance, a 57.1% (95% CI 54.3%–59.8%) reduction in monthly adult Aedes emergence for each unit increase in dissemination intensity/quality (see S4 Fig). In sum, we are confident that the striking changes in mosquito demographics we report were real and were a direct consequence of our intervention. The caveats discussed above call, however, for a cautious interpretation of our results, particularly regarding virus transmission—which we did not measure empirically.
In our trial, local vector control staff deployed and maintained PPF DSs, with the research team providing initial training and nearly continuous supervision—in which we monitored, but did not interfere with, PPF dissemination or DS maintenance. This led to some operational problems, including failure to maintain or deploy some DSs as scheduled (mainly due to lack of fuel for reaching the more distant northwestern city sector) and suboptimal PPF grinding (see S1 Data and S1 Fig). Model comparisons showed, however, that four-intervention-period GLMMs performed much better (as measured by much lower AIC and BIC scores [30,31]) than alternative GLMMs with more detailed descriptions of PPF dissemination dynamics (S1 Table). This suggests that operational problems had little impact on overall intervention effects, and hence that the strategy may work under the constraints of real-life vector control efforts. In practical terms, the most relevant obstacle was that the PPF we used is formulated as coarse sand-like granules that had to be manually ground to talc-like powder; this was time-consuming and yielded dust particles of variable, unknown size. In preparation for larger-scale trials, we are using mechanical micronizers to get PPF dust of standardized particle size.
One additional asset of our approach is that it may easily be combined with other interventions, traditional or novel, in integrated mosquito control strategies. For example, control agents and community members could focus on treating or destroying large, conspicuous, and accessible breeding sites, while mosquitoes disseminate PPF to the small, cryptic, and inaccessible larval habitats often used by Aedes spp. The community could engage in DS maintenance with support from local health agents; this would empower communities and may enhance acceptability while reducing costs. During outbreaks, indoor insecticide spraying could synergize PPF dissemination to quickly block transmission; in sites with effective early warning systems, spatially targeted interventions could be deployed as soon as the first cases of infection (in humans, vectors, or sentinel hosts) are detected. In general, flexible PPF dissemination strategies can be designed to suit particular needs in time and space. For example, focally deploying DSs at high densities could protect people in transmission-prone places such as hospitals, schools, stadiums, markets, churches, cemeteries, hotels, or prisons. Specific dissemination schemes in airports, bus/train stations, or ports (even on ships) might help limit man-mediated Aedes spread. Mosquito-disseminated PPF also holds promise for sites without mosquito-borne disease transmission but where mosquito bites cause skin lesions, allergies, distress, or economic losses (e.g., by affecting tourism). We also note that the 95%–98% reduction in adult Aedes emergence we recorded (Fig 5) could allow PPF dissemination to contribute to strategies based on the release of sterile, transgenic, or Wolbachia-transinfected mosquitoes, which require a high enough ratio of modified to wild mosquitoes [47–50]. The scale of mosquito releases (and associated costs including those of mass rearing) could be considerably reduced after a pulse of mosquito-disseminated PPF crashes local wild populations. Finally, we stress that combining PPF with products or tactics with different modes of action [46] would help reduce the odds of selecting resistant mosquitoes—a concern we also plan to address by testing larvicides other than PPF in experimental dissemination trials.
Here we have shown, in summary, that mosquito-disseminated PPF has the potential to become a major tool for urban mosquito control and, consequently, for the prevention of mosquito-borne diseases. These findings might be equally relevant for rapidly spreading emerging arboviral infections, including Zika and chikungunya, and for better-established endemic pathogens, including dengue, West Nile, and Japanese encephalitis viruses. Cluster-randomized, multi-site controlled trials are now necessary to provide stronger evidence for (or against) these hypotheses [46]. We plan to conduct one such trial in the context of the Brazilian dengue control program, which recently recommended considering our approach for inclusion in national guidelines [51]. Based on the present findings, we anticipate that randomized controlled trials will show that mosquito-disseminated PPF can develop into a new, crucial means for improving global public health.
Supporting Information
Zdroje
1. Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85 : 328–45. doi: 10.1016/j.antiviral.2009.10.008 19857523
2. Institute of Medicine. Vector-borne diseases: understanding the environmental, human health, and ecological connections. Washington (DC): National Academies Press; 2008.
3. World Health Organization, Special Programme for Research and Training in Tropical Diseases. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization; 2009.
4. Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310 : 308–15. doi: 10.1001/jama.2013.8042 23860989
5. Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372 : 1231–9. doi: 10.1056/NEJMra1406035 25806915
6. Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med. 2016;374 : 601–4. doi: 10.1056/NEJMp1600297 26761185
7. Faria NR, Azevedo R do S, Kraemer MU, Souza R, Cunha MS, Hill SC, et al. Zika virus in the Americas: early epidemiological and genetic findings. Science. 2016;352 : 345–9. doi: 10.1126/science.aaf5036 27013429
8. Mlakar J, Korva M, Tul N, Popović M. Zika virus associated with microcephaly. N Engl J Med. 2016;374 : 951–8. doi: 10.1056/NEJMoa1600651 26862926
9. Rubin EJ, Greene MF, Baden LR. Zika virus and microcephaly. N Engl J Med. 2016;374 : 984–5. doi: 10.1056/NEJMe1601862 26862812
10. Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–2015: a retrospective study. Lancet. 2016;387 : 2125–32. doi: 10.1016/S0140-6736(16)00651-6 26993883
11. Besnard M, Eyrolle-Guignot D, Guillemette-Artur P, Lastère S, Bost-Bezeaud F, Marcelis L, et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill. 2016;21 : 30181.
12. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374 : 1981–7. doi: 10.1056/NEJMsr1604338 27074377
13. Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387 : 1531–9. doi: 10.1016/S0140-6736(16)00562-6 26948433
14. Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015; 372 : 113–23. doi: 10.1056/NEJMoa1411037 25365753
15. Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353 : 1129–32. doi: 10.1126/science.aah6157 27492477
16. Thomas SJ. Preventing dengue—is the possibility now a reality? N Engl J Med. 2015;372 : 172–3. doi: 10.1056/NEJMe1413146 25365706
17. Lipsitch M, Cowling BJ. Zika vaccine trials. Science. 2016;353 : 1094–5. doi: 10.1126/science.aai8126 27609872
18. Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008;5:e68. doi: 10.1371/journal.pmed.0050068 18351798
19. Wilder-Smith A, Renhorn KE, Tissera H, Abu Bakar S, Alphey L, Kittayapong P, et al. DengueTools: innovative tools and strategies for the surveillance and control of dengue. Glob Health Action. 2012;5 : 17273.
20. Smith DL, Battle KE, Hay SI, Barker CM, Scott TW, McKenzie FE. Ross, Macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS Pathog. 2012;8:e1002588. doi: 10.1371/journal.ppat.1002588 22496640
21. Heffernan JM, Smith RJ, Wahl LM. Perspectives on the basic reproductive ratio. J R Soc Interface. 2005;2 : 281–93. doi: 10.1098/rsif.2005.0042 16849186
22. Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford: Oxford University Press; 1991.
23. Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg. 2011;84 : 200–7. doi: 10.4269/ajtmh.2011.10-0503 21292885
24. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496 : 504–7. doi: 10.1038/nature12060 23563266
25. Bowman LR, Donegan S, McCall PJ. Is dengue vector control deficient in effectiveness or evidence?: systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10:e0004551. doi: 10.1371/journal.pntd.0004551 26986468
26. Itoh T. Utilization of blood-fed females of Aedes aegypti as a vehicle for the transfer of the insect growth regulator, pyriproxyfen, to larval habitats. Trop Med. 1994;36 : 243–8.
27. Devine GJ, Perea EZ, Killeen GF, Stancil JD, Clark SJ, Morrison AC. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc Natl Acad Sci U S A. 2009;106 : 11530–4. doi: 10.1073/pnas.0901369106 19561295
28. World Health Organization. Pyriproxyfen in drinking-water: use for vector control in drinking-water sources and containers. Background document for development of WHO guidelines for drinking-water quality. Geneva: World Health Organization; 2008.
29. Abad-Franch F, Zamora-Perea E, Ferraz G, Padilla-Torres SD, Luz SLB. Mosquito-disseminated pyriproxyfen yields high breeding-site coverage and boosts juvenile mosquito mortality at the neighborhood scale. PLoS Negl Trop Dis. 2015;9:e0003702. doi: 10.1371/journal.pntd.0003702 25849040
30. Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer; 2002.
31. Vaida F, Blanchard S. Conditional Akaike information for mixed-effects models. Biometrika. 2005;92 : 351–70.
32. Bates D, Maechler M, Bolker B, Walker S. lme4: mixed-effects models in R using S4 classes and methods with RcppEigen. 2016 [cited 2016 Dec 6]. Available from: https://github.com/lme4/lme4/.
33. R Development Core Team. R: a language and environment for statistical computing. Version 3.1.2. Vienna: R Foundation for Statistical Computing; 2014 [cited 2016 Dec 6]. Available from: https://www.R-project.org.
34. Favier C, Degallier N, Rosa-Freitas MG, Boulanger JP, Costa Lima JR, Luitgards-Moura JF, et al. Early determination of the reproductive number for vector-borne diseases: the case of dengue in Brazil. Trop Med Int Health. 2006;11 : 332–40. doi: 10.1111/j.1365-3156.2006.01560.x 16553913
35. David MR, Lourenço-de-Oliveira R, Maciel de Freitas R. Container productivity, daily survival rates and dispersal of Aedes aegypti mosquitoes in a high income dengue epidemic neighbourhood of Rio de Janeiro: presumed influence of differential urban structure on mosquito biology. Mem Inst Oswaldo Cruz. 2009;104 : 927–32. 19876569
36. Harrington LC, Buonaccorsi JP, Edman JD, Costero A, Kittayapong P, Clark GG, et al. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidae) from Puerto Rico and Thailand. J Med Entomol. 2001;38 : 537–47. 11476334
37. Dutra HLC, dos Santos LMB, Caragata EP, Silva JBL, Villela DAM, Maciel-de-Freitas R, et al. From lab to field: the influence of urban landscapes on the invasive potential of Wolbachia in Brazilian Aedes aegypti mosquitoes. PLoS Negl Trop Dis. 2015;9:e0003689. doi: 10.1371/journal.pntd.0003689 25905888
38. Franca RFO, Neves MHL, Ayres CFJ, Melo-Neto OP, Brandão Filho SP. First International Workshop on Zika Virus held by Oswaldo Cruz Foundation FIOCRUZ in northeast Brazil March 2016—a meeting report. PLoS Negl Trop Dis. 2016;10:e0004760. doi: 10.1371/journal.pntd.0004760 27258065
39. Hervé JP, Dégallier N, Travassos da Rosa APA, Pinheiro FP, Sá Filho GC. Arboviroses—aspectos ecológicos. In: Instituto Evandro Chagas—50 anos de contribuição às ciências biológicas e à medicina tropical. Vol 1. Belém: Fundação Serviços de Saúde Pública, Ministério da Saúde do Brasil; 1986. pp. 409–37.
40. Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol. 2008;62 : 71–92. doi: 10.1146/annurev.micro.62.081307.163005 18429680
41. Ohashi K, Nakada K, Ishiwatari T, Miyaguchi J, Shono Y, Lucas JR, et al. Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae). J Med Entomol. 2012;49 : 1052–8. 23025186
42. Di Luca M, Severini F, Toma L, Boccolini D, Romi R, Remoli ME, et al. Experimental studies of susceptibility of Italian Aedes albopictus to Zika virus. Euro Surveill. 2016;21 : 30223.
43. Chouin-Carneiro T, Vega-Rua A, Vazeille M, Yebakima A, Girod R, Goindin D, et al. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl Trop Dis; 2016;10:e0004543. doi: 10.1371/journal.pntd.0004543 26938868
44. Sihuincha M, Zamora-Perea E, Orellana-Rios W, Stancil JD, López-Sifuentes V, Vidal-Oré C, et al. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J Med Entomol. 2005;42 : 620–30. 16119551
45. Focks DA. A review of entomological sampling methods and indicators for dengue vectors. Geneva: World Health Organization; 2003.
46. Vontas J, Moore S, Kleinschmidt I, Ranson H, Lindsay S, Lengeler C, et al. Framework for rapid assessment and adoption of new vector control tools. Trends Parasitol. 2014;30 : 191–204. doi: 10.1016/j.pt.2014.02.005 24657042
47. Alphey L. Genetic control of mosquitoes. Annu Rev Entomol. 2014;59 : 205–24. doi: 10.1146/annurev-ento-011613-162002 24160434
48. Carvalho DO, McKemey AR, Garziera L, Lacroix R, Donnelly CA, Alphey L, et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl Trop Dis. 2015;9:e0003864. doi: 10.1371/journal.pntd.0003864 26135160
49. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476 : 454–7. doi: 10.1038/nature10356 21866160
50. Jeffery JAL, Yen NT, Nam VS, Nghia LT, Hoffmann AA, Kay BH, et al. Characterizing the Aedes aegypti population in a Vietnamese village in preparation for a Wolbachia-based mosquito control strategy to eliminate dengue. PLoS Negl Trop Dis. 2009;3:e0000552.
51. Secretaria de Vigilância em Saúde, Ministério da Saúde do Brasil. Relatório da reunião internacional para implementação de alternativas para o controle do Aedes aegypti no Brasil. Bol Epidemiol. 2016;47 : 1–9.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 1- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Hojení análních fisur urychlí čípky a gel
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
-
Všechny články tohoto čísla
- Evaluating Hospital-Based Surveillance for Outbreak Detection in Bangladesh: Analysis of Healthcare Utilization Data
- Effect of a Primary Care Walking Intervention with and without Nurse Support on Physical Activity Levels in 45- to 75-Year-Olds: The edometer nd onsultation valuation (PACE-UP) Cluster Randomised Clinical Trial
- Patient Safety Incidents Involving Sick Children in Primary Care in England and Wales: A Mixed Methods Analysis
- Biomarker-Defined Subsets of Common Diseases: Policy and Economic Implications of Orphan Drug Act Coverage
- Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis
- Priority-Setting for Novel Drug Regimens to Treat Tuberculosis: An Epidemiologic Model
- Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review
- Association of Body Mass Index with DNA Methylation and Gene Expression in Blood Cells and Relations to Cardiometabolic Disease: A Mendelian Randomization Approach
- Socioeconomic Inequalities in Body Mass Index across Adulthood: Coordinated Analyses of Individual Participant Data from Three British Birth Cohort Studies Initiated in 1946, 1958 and 1970
- Mosquito-Disseminated Insecticide for Citywide Vector Control and Its Potential to Block Arbovirus Epidemics: Entomological Observations and Modeling Results from Amazonian Brazil
- Using Genetic Variation to Explore the Causal Effect of Maternal Pregnancy Adiposity on Future Offspring Adiposity: A Mendelian Randomisation Study
- The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight
- Customised and Noncustomised Birth Weight Centiles and Prediction of Stillbirth and Infant Mortality and Morbidity: A Cohort Study of 979,912 Term Singleton Pregnancies in Scotland
- Master Regulators of Oncogenic Response in Pancreatic Cancer: An Integrative Network Biology Analysis
- Sick Children Crying for Help: Fostering Adverse Event Reports
- What Is the Purpose of the Orphan Drug Act?
- Novel Vector Control Approaches: The Future for Prevention of Zika Virus Transmission?
- Artificially Sweetened Beverages and the Response to the Global Obesity Crisis
- Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp)
- Bolstering Community Cooperation in Ebola Resurgence Protocols: Combining Field Blood Draw and Point-of-Care Diagnosis
- Defining Abnormal Fetal Growth and Perinatal Risk: Population or Customized Standards?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight
- What Is the Purpose of the Orphan Drug Act?
- Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis
- Using Genetic Variation to Explore the Causal Effect of Maternal Pregnancy Adiposity on Future Offspring Adiposity: A Mendelian Randomisation Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




