-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Unintentional miRNA Ablation Is a Risk Factor in Gene Knockout Studies: A Short Report
One of the most powerful techniques for studying the function of a gene is to disrupt the expression of that gene using genetic engineering strategies such as targeted recombination or viral integration of gene trap cassettes. The tremendous utility of these tools was recognized this year with the awarding of the Nobel Prize in Physiology or Medicine to Capecchi, Evans, and Smithies for their pioneering work in targeted recombination mutagenesis in mammals. Another noteworthy discovery made nearly a decade ago was the identification of a novel class of non-coding genes called microRNAs. MicroRNAs are among the largest known classes of regulatory elements with more than 1000 predicted to exist in the mouse genome. Over 50% of known microRNAs are located within introns of coding genes. Given that currently about half of the genes in mouse have been knocked out, we investigated the possibility that intronic microRNAs may have been coincidentally deleted or disrupted in some of these mouse models. We searched published murine knockout studies and gene trap embryonic stem cell line databases for cases where a microRNA was located within or near the manipulated genomic loci, finding almost 200 cases where microRNA expression may have been disrupted along with another gene. Our results draw attention to the need for careful planning in future knockout studies to minimize the unintentional disruption of microRNAs. These data also raise the possibility that many knockout studies may need to be reexamined to determine if loss of a microRNA contributes to the phenotypic consequences attributed to loss of a protein-encoding gene.
Published in the journal: . PLoS Genet 4(2): e34. doi:10.1371/journal.pgen.0040034
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.0040034Summary
One of the most powerful techniques for studying the function of a gene is to disrupt the expression of that gene using genetic engineering strategies such as targeted recombination or viral integration of gene trap cassettes. The tremendous utility of these tools was recognized this year with the awarding of the Nobel Prize in Physiology or Medicine to Capecchi, Evans, and Smithies for their pioneering work in targeted recombination mutagenesis in mammals. Another noteworthy discovery made nearly a decade ago was the identification of a novel class of non-coding genes called microRNAs. MicroRNAs are among the largest known classes of regulatory elements with more than 1000 predicted to exist in the mouse genome. Over 50% of known microRNAs are located within introns of coding genes. Given that currently about half of the genes in mouse have been knocked out, we investigated the possibility that intronic microRNAs may have been coincidentally deleted or disrupted in some of these mouse models. We searched published murine knockout studies and gene trap embryonic stem cell line databases for cases where a microRNA was located within or near the manipulated genomic loci, finding almost 200 cases where microRNA expression may have been disrupted along with another gene. Our results draw attention to the need for careful planning in future knockout studies to minimize the unintentional disruption of microRNAs. These data also raise the possibility that many knockout studies may need to be reexamined to determine if loss of a microRNA contributes to the phenotypic consequences attributed to loss of a protein-encoding gene.
Introduction
In the mouse, stable disruption of a gene is typically accomplished using gene trap mutagenesis or targeted homologous recombination. We wish to communicate the overlooked possibility of unintentionally disrupting microRNA (miRNA) genes along with a targeted gene. Because miRNAs play key roles in many cellular processes, the unintended ablation of these species may have significant consequences that complicate the interpretation of gene knockout studies.
Methods
Given that many miRNAs are located within introns of longer coding transcripts, we reasoned that a gene trap disrupting a host gene could also alter miRNA expression in one of two ways. The trapping cassette could either ablate miRNA expression with a terminal polyadenylation sequence (Figure 1A) or overexpress an miRNA via an internal promoter (Figure 1B). To determine the potential extent of these unintended changes in miRNA expression, we compared the genomic position all mouse gene traps listed in the International Gene Trap Consortium (IGTC) [1] to the loci of 367 annotated mouse miRNA genes as well as candidate miRNA genes computationally identified by Berezikov et al., 28% of which have been validated to date [2,3]. In the cases where an miRNA was located within an intron of a host gene, we identified any gene traps which inserted within the host gene transcript and upstream of the miRNA. Using the same set of annotated and candidate miRNAs, we next identified all protein-coding genes with an miRNA located within the transcribed loci, in either the sense or the antisense orientation. We cross-referenced these genes with all homologous recombination studies listed in the Mouse Genome Informatics (MGI) database (v. 3.54) [4] to assemble a list of studies where the miRNA and coding gene were potentially co-ablated (Table S1). The boundaries of the deleted loci were bioinformatically verified for each study.
Fig. 1. Configurations of Protein:miRNA Gene Disruptions. 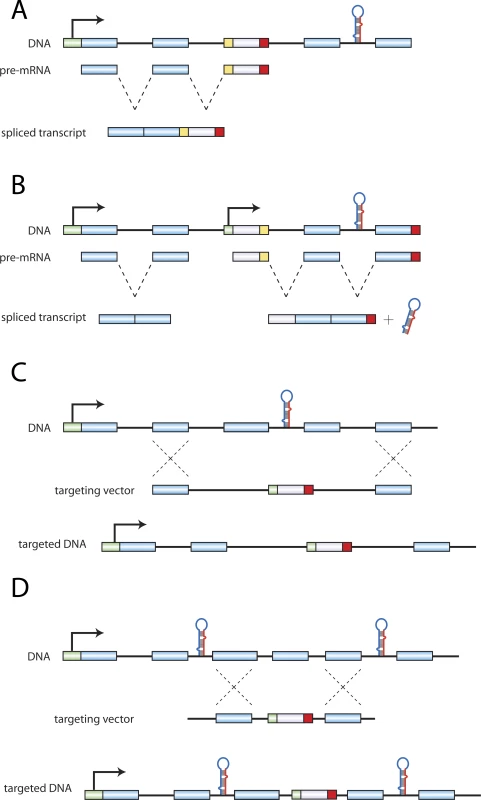
Retroviral gene traps have the potential to (A) ablate miRNA transcription or (B) lead to constitutive overexpression of the miRNA. Results/Discussion
Our analysis of the IGTC database revealed 98 annotated or candidate miRNAs potentially misregulated in 420 gene trap cell lines (Table S2). A study of the slit3 gene [5] is an example of a potential unintended double-knockout scenario produced from a gene trap cell line. To ablate slit3, the authors used a trap located upstream of exon 6, which produced a truncated slit3 mRNA. Mir-218–2 is located within intron 14 of slit3, and the potential loss of mir-218–2 expression may contribute to the phenotype resulting from the loss of functional slit3.
The analysis of the MGI database yielded a small but significant number of studies where miRNAs may have been unintentionally disrupted (Table S1). In addition to 20 studies where an annotated or candidate miRNA was completely ablated by the targeting strategy (Figure 1C), there were also numerous studies describing the deletion of regions immediately upstream (78 cases) or downstream (55 cases) of a miRNA (Figure 1D), or in the promoter of the host gene (4 cases). MiRNAs have been shown to be transcribed in conjunction with a host transcript or from an independent promoter [6]. Therefore, the disruption of host promoters or of regions adjacent to miRNAs may compromise promoter and/or enhancer sites for these miRNAs.
While 71 of the studies in our analysis were published prior to the expansion of the miRNA field in 2002, the fact that 90 were published since may indicate that miRNAs in targeted loci continue to be overlooked. To avoid inadvertent double-knockout scenarios, we wish to alert investigators to consider non-coding elements in the locus to be deleted. Because not all non-coding elements have been annotated, it may be preferable to employ methods that minimize the deletion of endogenous DNA. We also wish to raise the interesting possibility that a number of studies may need to be reevaluated to dissociate the consequences of ablating an miRNA from the consequences of ablating the targeted gene.
Supporting Information
Zdroje
1. Griffiths-JonesSGrocockRJvan DongenSBatemanAEnrightAJ
2006
miRBase: microRNA sequences, targets and gene nomenclature.
Nucleic Acids Res
34
D140
D144
2. BerezikovEGoryevVvan de BeltJWienholdsEPlasterkRH
2005
Phylogenetic shadowing and computational identification of human microRNA genes.
Cell
120
21
24
3. NordASChangPJConklinBRCoxAVHarperCA
2006
The International Gene Trap Consortium Website: a portal to all publicly available gene trap cell lines in mouse.
Nucleic Acids Res
34
D642
D648
4. EppigJTBultCJKadinJARichardsonJEBlakeJAand the members of the Mouse Genome Database Group
2005
The Mouse Genome Database (MGD): from genes to mice—a community resource for mouse biology.
Nucleic Acids Res
33
D471
D475
5. LiuJZhangLWangDShenHJiangM
2003
Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice.
Mech Dev
120
1059
1070
6. RodriguezAGriffiths-JonesSAshurstJLBradleyA
2004
Identification of mammalian microRNA host genes and transcription units.
Genome Res
14
1902
1910
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2008 Číslo 2- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
Nejčtenější v tomto čísle- Unintentional miRNA Ablation Is a Risk Factor in Gene Knockout Studies: A Short Report
- Ready for Her Close-Up: An Interview with Elaine Strass
- The Evolution of Spinnable Cotton Fiber Entailed Prolonged Development and a Novel Metabolism
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



