-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Tuberculosis in elderly in the Czech Republic
Tuberkulóza u seniorů v České republice
Úvod: Incidence tuberkulózy (TBC) se v České republice (ČR) snižuje od 60. let minulého století. (1965 : 76,9; 1975 : 60,4; 1985 : 45,2; 1995 : 17,7; 2005 : 9,9; 2015 : 4,9 na 100 000 obyvatel). V roce 2017 tvořili muži přes 70 % případů. Nejčastěji jsou postiženi lidé nad 70 let. U starších pacientů se manifestuje více extrapulmonárních a atypických příznaků nemoci, proto může být diagnóza TBC obtížná a přehlížená. U multimorbidních seniorů se často vyskytují chronická onemocnění, malignity a autoimunitní onemocnění, jež znamenají vyšší míru imunosuprese, která nasedá na známý proces immunosenescence. Léčba TBC u seniorů je náročná pro častý výskyt nežádoucích účinků na léky a vysoký výskyt rezistence.
Tento článek se zabývá epidemiologií TBC v ČR, imunologickými a klinickými aspekty, diagnostikou, léčbou a prevencí, dále zde prezentujeme dvě klinické kazuistiky hospitalizovaných seniorů v ČR.
Kazuistiky: V první kazuistice prezentujeme 79letou ženu léčenou pro chronickou obstrukční plicní nemoc (CHOPN), která byla opakovaně hospitalizována pro exacerbaci CHOPN. Následně u ní byla diagnostikována TBC. U pacientky se rozvinuly projevy toxicity a interakce léků v důsledku komorbidit a užívané medikace.
V druhé kazuistice prezentujeme 80letého muže – kuřáka, který byl přijat pro kolapsový stav. TBC se u pacienta rozvinula endogenní cestou z primárního komplexu při postupné progresi bronchogenního karcinomu.
Závěr: Diagnostika a léčba TBC u starších pacientů je náročná. S rostoucím věkem se zvyšuje riziko reaktivace TBC a projevuje se zvýšená náchylnost k tomuto infektu. Ve starší populaci najdeme akumulaci rizikových faktorů pro rozvoj TBC, zejména malnutrici, nízký socio-ekonomický status, kouření a alkoholismus. Mezi nejvíce ohrožené osoby mezi starými lidmi patří křehcí institucializovaní senioři, jejichž incidence TBC je 2–3krát vyšší než u lidí žijících doma. Roste počet seniorů, a tak stoupá i incidence TBC v této skupině, pro optimalizaci zdravotních služeb je proto potřebná detailní znalost epidemiologických rysů TBC v této subpopulaci.
Klíčová slova:
tuberkulóza – stáří – Česká republika – epidemiologie – imunita
Authors: E. Fernandová; K. Bielaková; H. Matějovská-Kubešová
Authors place of work: Department of Internal Medicine, Geriatrics and General Practice, University Hospital Brno and Faculty of Medicine, Masaryk University, Brno, Czech Republic
Published in the journal: Epidemiol. Mikrobiol. Imunol. 68, 2019, č. 4, s. 184-190
Category: Souhrnná sdělení, původní práce, kazuistiky
Summary
Introduction: The incidence of tuberculosis (TB) in the Czech Republic (CR) is decreasing since 1960s. (1965 : 76.9; 1975 : 60.4; 1985 : 45.2; 1995 : 17.7; 2005 : 9.9; 2015 : 4.9 per 100, 000 population). In 2017 men accounted for over than 70% of cases. People aged over 75 years are most frequently affected. Elderly patients tend to develop more of extrapulmonary and atypical manifestation of the disease, the diagnosis of TB can be difficult and consequently overlooked. Multimorbid seniors are suffering from chronic illnesses, malignancies and autoimmune diseases, which translate into higher degree of immunosuppression and add to the generally described process of immunosenescence. Furthermore, therapy of TB in the elderly is challenging because of the increased drug resistance and higher incidence of adverse drug reactions.
This article reviews the epidemiology of TB in the CR, immunological aspects, clinical characteristics, diagnosis, management, prevention of TB infection and presents two clinical cases in hospitalized aging adults in the CR.
Case presentation: We present a case of a 79 year old female suffering from chronic obstructive pulmonary disease (COPD), who was repeatedly hospitalized for acute exacerbations of COPD and was consequently diagnosed with TB. Patient developed manifestation of treatment toxicity and drug interactions due to comorbidities and other medications.
Secondly, we present a case of a 70 year old male, a lifelong smoker, who was initially admitted for collapsing. TB developed via the endogenic route from a Ghon’s complex in association with a slowly progressing bronchogenic carcinoma.
Conclusion: Diagnosis and management of TB in the elderly person can be challenging. Age-related factors increase the risk of TB reactivation as well as enhance susceptibility to TB infection. In elderly population we find accumulation of risk factors for developing TB (malnutrition, low socio economic status, smoking and alcoholism). The people most at risk among elderly include fragile institutionalized seniors whose incidence of TB is 2-3 times higher than those living at home.
Because the number of seniors is growing and the incidence of TB in this subpopulation is increasing, detailed knowledge of the epidemiological features of TB in this group is needed to optimize healthcare services.
Keywords:
Czech Republic – tuberculosis – Epidemiology – elderly – Immunity
INTRODUCTION
Tuberculosis (TB) is defined as an infectious disease caused by Mycobacterium tuberculosis complex. It most often affects the lungs (pulmonary TB) but can also affect other sites (extrapulmonary TB). Globally 1 billion people are affected with TB. It is one of the top 10 causes of death worldwide. TB incidence is globally falling at about 2% per year. In order to eliminate TB, the World Health Organization (WHO) established the End TB Strategy [1]. Ending the TB endemic by 2030 is among the health targets of the Sustainable Development Goals. The predisposition to develop TB in those immunocompromised by age, drugs, disease and malnutrition is well known. TB management in elderly is becoming a public health challenge worldwide [2].
EPIDEMIOLOGICAL ASPECTS OF TB IN THE CZECH REPUBLIC
The incidence of TB in the CR has been decreasing consistently since the 1960s. (1965 : 76.9; 1975 : 60.4; 1985 : 45.2; 1995 : 17.7; 2005 : 9.9; 2015 : 4.9 per 100, 000 population) – Figure 1. This decline is in line with the decrease generally observed in the whole European Union (EU) and European Economic Area (EEA) [3]. In 2005, for the first time ever, the number of new reported cases of TB in the CR fell below 1000 cases i.e. below 10 cases/100,000 population [4], which is below the EU/EEA notification rate (11.7 per 100,000 population in 2015). According to the Register of Tuberculosis (RTB), in 2017 in the CR, there were reported 505 TB cases of all types and localizations, i.e. 4.8 cases per 100 000 population. The CR with other 22 countries already fulfills the ‘End TB 2035’ target set by the WHO of TB rates below 10 per 100,000 [5], while other countries report TB rates still above 50 per 100,000 (Lithuania, Romania) 9193. TB mortality was 0.24/100 000 inhabitants in 2017. TB occurs more often in men than in women: men accounted for over 70% of all cases. In women, TB mostly affects the age group 75+, while in men, apart from this category, we may observe a higher incidence in the middle-aged group (≥ 45 years) [7]. Generally, people aged over 75 years are most frequently affected. From the overall number of 505 reported TB cases, pulmonary TB was found in 439 (87%) cases of which 95% involved the lungs. TB of any other localization was reported in 66 (13%) patients. Extrapulmonary localization mainly included involvement of the peripheral lymph nodes, bone and joint TB, and TB of the urinary tract. In the CR, the long-term incidence of TB is highest in Prague, the capital of the CR, and Central Bohemia and lowest in South Bohemia and South Moravia [7].
Fig. 1. The incidence of TB in the CR since 1960s per 100,000 population Source: Institute of Health Information and Statistics of the Czech Republic 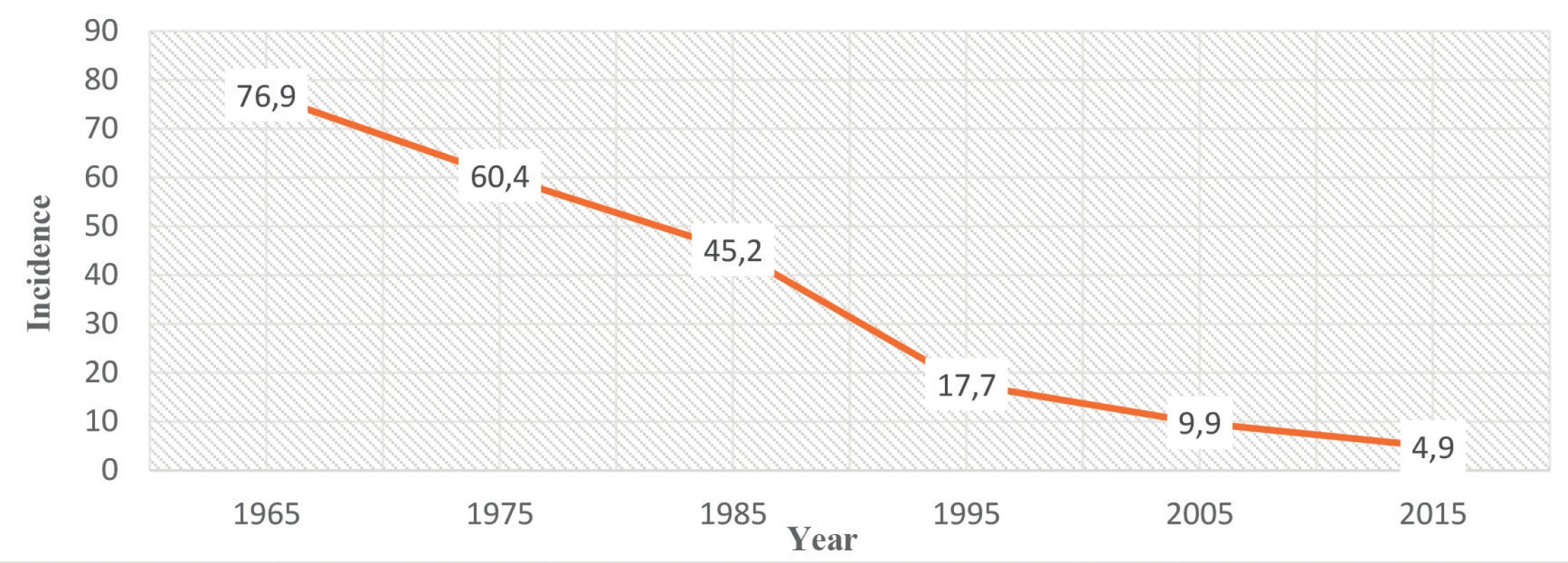
DEMOGRAPHICS AND EXPECTED CHANGES IN ELDERLY
Increasing life-expectancy is leading to a rise in the proportion of inhabitants aged over 75 years, in whom risk factors for the reactivation of latent tuberculosis infection (LTBI) or de novo disease gradually accumulate. LTBI is defined as a state of persistent immune response to M. tuberculosis antigen stimulation without evidence of clinically manifest active TB [8]. People with LTBI are infected with TB bacteria, but do not have signs of active TB, however they are at risk of developing active TB in the future.
Most elderly are at a risk to be infected with M. tuberculosis and to develop a Ghon’s complex – i.e. a small localized caseous necrosis, most often located in the upper or middle lung lobe with subsequent lymphangitis and attainment of the first regional lymph node. If the given individual was in a good physical condition and had no further risk factors, than the process terminated in cicatrisation and a calcification visible on the chest X-ray. The individual with a Ghon’s complex on the chest X-ray could be considered in most cases as having life-long immunity. However, under certain conditions such as exhaustion, malnutrition, immunosuppression, cancer treatment, diabetes etc., such Ghon’s complexes may become the source of post-primary active TB [9, 10].
In general, advanced age is considered to be one of the risk factors for developing TB, together with malnutrition, low socio-economic status, diabetes, malignancies, silicosis, smoking and alcoholism. The most at risk group among the elderly includes fragile, institutionalized senior citizens in whom the incidence of TB is 2–3 times higher than in those living at home [10–12].
IMMUNOLOGICAL ASPECTS IN ELDERLY
The current elderly population differs from the same age category of previous decades due to a relatively more diverse personal medical history of successfully overcome diseases, e.g. malignancies and chronic illnesses including autoimmune diseases. All this translates into a higher degree of immunosuppression, which adds to the generally described process of immune-senescence. Immuno-senescence is defined as the changes in the immune system associated with age. It mainly involves a decrease in macrophage activation and IL 2, IL 12 and IFN γ synthesis, and affects their role as the most important activators of T-cell mediated immune response and natural killers [10, 13, 14].
The presence of diabetes, with a current incidence among the elderly population estimated at 20–25%, further intensifies the mechanisms cited above. Moreover, unless compensation of diabetes does not reach levels below 8% of glycated haemoglobin, there is a negative effect on the proliferation and antigen response of CD4 lymphocytes, which increases susceptibility to infections including TB up to three-fold in the elderly [15, 16].
Further, the deterioration in the ability of the elderly to maintain his/her nutritional status leads to more rapid atrophy of the thymus, to a decrease in the number of circulating T lymphocytes and to a decrease in the antigen response to vaccination [17].
CLINICAL ASPECTS OF TB IN THE ELDERLY
In older age, only 75% of patients suffering from TB manifest with pulmonary involvement. Compared to younger age categories, elderly patients tend to develop extra-pulmonary disease-involvement of lymph nodes, the skeleton, urogenital tract as well as tuberculous meningitis or miliary TB [18, 19]. Also, the manifestations of TB in older age may be atypical – instead of cough, haemoptysis and night sweats, patients may only develop long-term fatigue, deterioration of cognitive functions, anorexia with weight loss and unexplained subfebrile temperatures. If such a condition persists for weeks and months, the differential diagnosis must include TB [20, 21].
DIAGNOSIS IN ELDERLY
TB is diagnosed from a combination of context, symptoms, clinical signs and investigations. Anyone with a cough that lasts for two weeks or more or with unexplained chronic fever and/or weight loss should be evaluated for TB [22]. One of the pitfalls of TB is that we frequently neglect the disease in differential diagnosis. There are many diagnosis difficulties due to presence of chronic diseases in elderly, cognitive and mental disorders, communication problems as hearing loss and presence of atypical radiological findings due to older age pathologies[23]. The golden standard in diagnose active TB are beside the clinical symptoms and radiological examination direct bacteriological examination – sputum smear microscopy, culture and molecular genetic methods. Microscopy of sputum smear is simple, rapid and inexpensive. In 2010, WHO confirmed the diagnostic accuracy of examining two consecutive smears on the same day to diagnose TB. Molecular methods are in advantage used in detecting multi-drug resistant TB(MDR-TB) [24]. Indirect methods in diagnosing active TB are complimentary.
In contrast the golden standard in diagnosing LTBI are indirect methods – Mantoux tuberculin skin test (TST) or interferon-gamma release assay (IGRA). The TST and IGRAs indirectly measure TB infection by detecting memory T-cell response signifying the presence of host sensitization to M. tuberculosis antigens. Direct methods in diagnosing LTBI are still not available. IGRA is an alternative to TST with at least equivalent sensitivity and higher specificity [2, 25]. If compared patients are more compliant with IGRA testing than TST due to technical management [26].
TREATMENT AND DRUG INTERACTIONS IN ELDERLY
The treatment protocol of TB in the elderly does not necessarily differ from the one in adults [2]. What may differ is the duration of therapy, from standardize 6 months it could be prolonged up to 9 months [27]. The current standard WHO approved regimen is rifampicin, isoniazid, pyrazinamide, and ethambutol (HRZE) for two months (intensive phase) followed by isoniazid and rifampicin [with (HRE) or without (HR) ethambutol in areas of high resistance] for four months (continuation phase) – Table 1 [28, 29].
Tab. 1. Recommended doses of first-line anti-tuberculosis drugs in adults 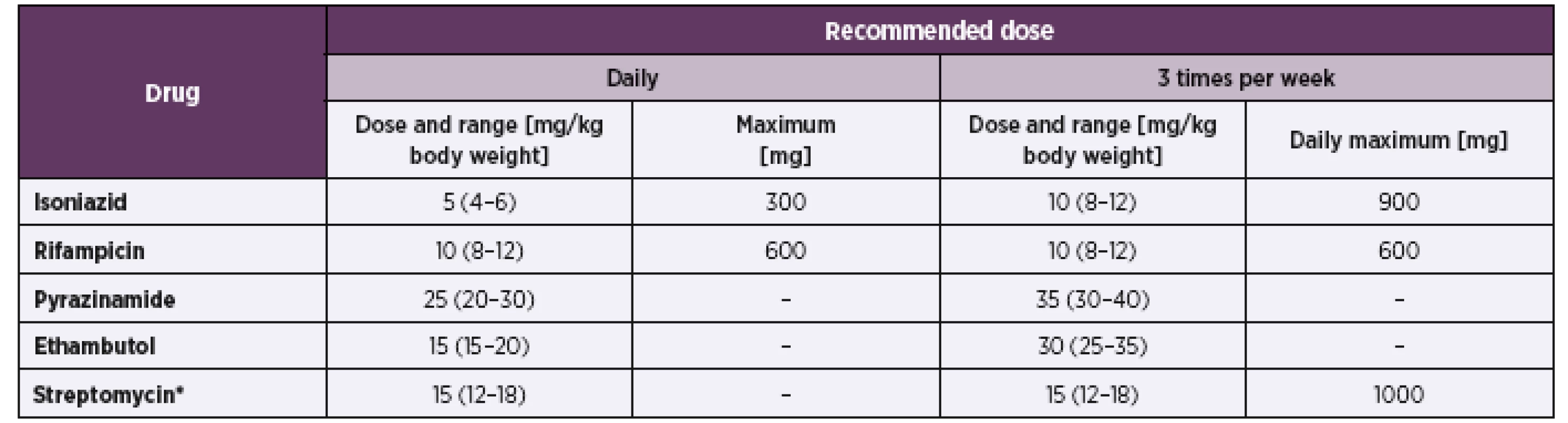
*Patients aged over 60 years may not be able to tolerate more than 500–750 mg daily, so some guidelines recommend reduction of the dose to 10 mg/kg per day in patients in this age group. Patients weighing less than 50 kg may not tolerate doses above 500–750 mg daily (WHO Model Formulary 2008, www.who.int/selection_medicines/list/en/). Source – WHO Guidelines for national programs- fourth edition 2010 When drawing up an optimal therapeutic scheme, the situation is usually complicated by the multiple morbidities of the elderly and the consequent poly-pharmacology of these patients. [18]. Practically, all basic anti-tuberculosis drugs induce drug interactions with medication frequently prescribed to elderly patients and thus affect drug metabolism via cytochromes. Their toxicity may also be enhanced by micronutrient deficiencies – e.g. B6 deficiency during the administration of isoniazid [30]. Pyrazinamide decreases the effect of allopurinol and in - creases the probability of hypoglycemia during treatment with oral antidiabetic agents [31]. Patients over the age of 60 years need not tolerate a streptomycin dose of over 500–750 mg daily and certain guidelines recommend a dose reduction in this age category to 10 mg/kg daily.
The most important interactions of anti-tuberculosis agents are caused by rifampicin. Rifampicin is a strong inductor of cytochrome P450. Maintenance of an adequate clinical effect of drugs metabolized by the same cytochrome may require dosage increases. If rifampicin is discontinued, the effect of induced metabolism recedes within 2 weeks and the dosage of other medication must be reduced.
Rifampicin significantly reduces the concentration and efficacy of the following drug classes:
- antimicrobial drugs – azole antimycotics, clarithromycin, erythromycin, doxycycline, as well as levothyroxine
- anticoagulants – warfarin and newer oral anticoagulants
- immunosuppressive agents – cyclosporine, corticosteroids
- anticonvulsants – phenytoin
- antihypertensive drugs – verapamil, nifedipine, diltiazem, propranolol, metoprolol, enalapril, losartan, quinidine, mexiletine
- antiarrhythmic drugs – digoxin, propafenone
- metabolic medication – sulfonylurea and statins
- psychiatric medication and hypnotics [18, 32].
PREVENTION OF TB IN ELDERLY
The CR was quite progressive in the early detection of TB and its prevention. It was one of the first European countries to introduce in the 1950s nation – wide mandatory abreography, careful and precise check-ups of dairy cows for TB of the udder and vaccination of new-borns using the BCG vaccine with mandatory testing the memory T-cell response of cellular imunity provided by the tuberculin test during school attendance and upon its completion. Further re-vaccination was performed depending on the tuberculin test results.
Given the decrease in the incidence of reported TB cases to below 5/100000 inhabitants, the CR has been ranked since 2001 among those countries with a low incidence of TB and the mandatory vaccination of new-borns as well as revaccination during school attendance have been abolished 32]. However, vaccination of new-born from high risk environments remains mandatory – i.e. where one of the parents had or has active TB, has stayed in a country with a higher incidence of TB or if the child has been in contact with an individual suffering from active TB [33].
Voluntary vaccination may also be used. However, this is not covered by public health insurance.
Non-specific prevention of TB in the elderly undoubtedly includes good nutritional status, with sufficient caloric intake and a diet ensuring a adequate amount of high-quality protein and micronutrients. Another integral component of prevention involves sustained mobility of the elderly, including stays in the countryside, appropriate physical exertion all with the aim of preserving overall fitness, muscle strength, skills and ventilatory functions.
CASE REPORTS
Case Report No. 1
We present a case of an 80-year-old multimorbid female suffering from chronic obstructive pulmonary disease (COPD), who was repeatedly hospitalized (12 hospitalizations in 5 years) for acute exacerbations of COPD. Beside COPD she had suffered from diabetes, hypertension, chronic heart failure, chronical renal insufficiency, anemia of chronic disease, cerebrovascular disease, hyperuricemia, hypothyroidism, lower back pain, liver steatosis and severe obesity. Her chronic treatment included 10 types of oral medications, insulin and inhalators. At this time, she was admitted for chest pain and dyspnoea.
The chest X-ray showed a bilateral fluidothorax, infiltration of the lungs parenchyma on the left and signs of pulmonary congestion – Figure 2. A pleural puncture was subsequently performed, whereby microscopic examination of the fluid revealed the presence of acid-fast bacilli and the TST was positive. The patient initiated a combination of anti-TB drugs: isoniazid-rifampicin-pyrazinamide-ethambutol and was transfer to sanatorium.
Fig. 2. Chest X-Ray before (Figure 2a) and after (Figure 2b) treatment 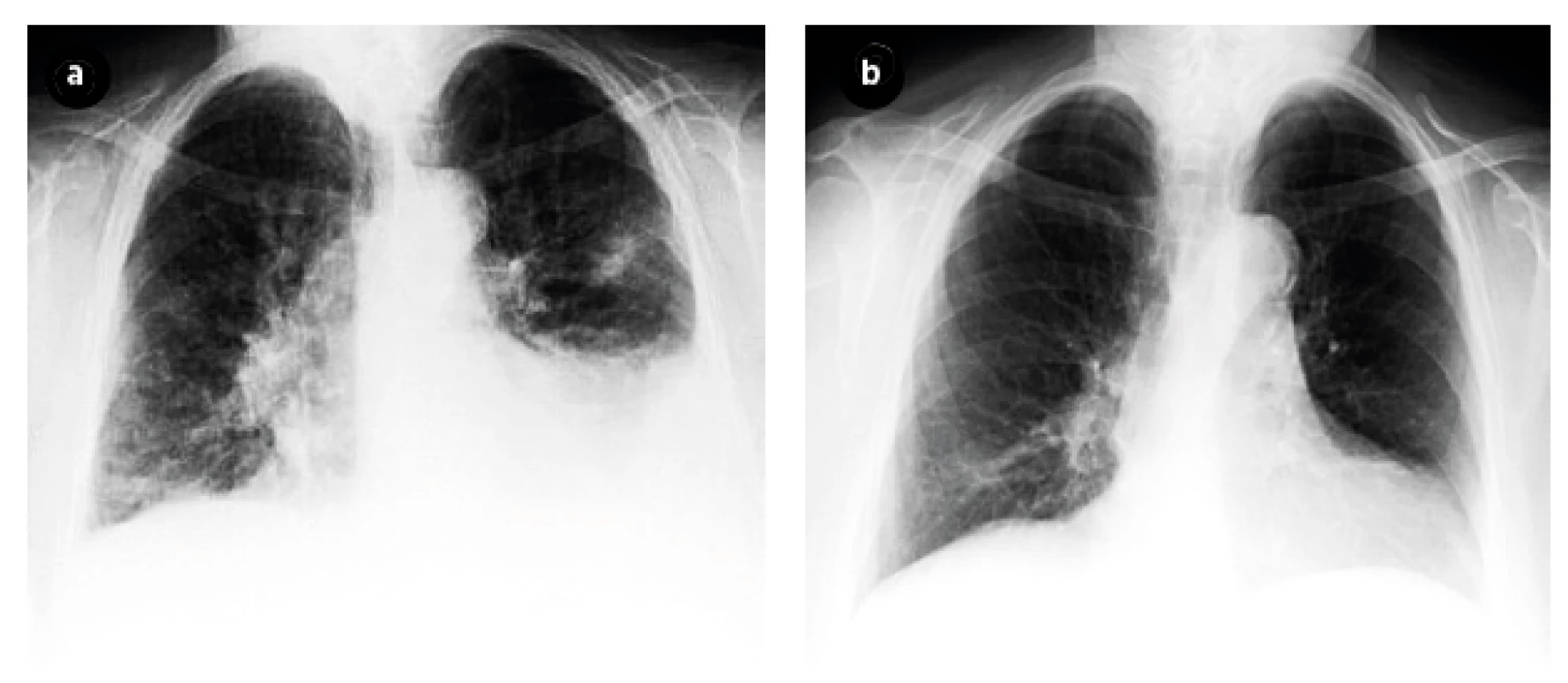
After four weeks patient was readmitted from the sanatorium due to acute renal failure. Treatment with the anti-TB drugs was discontinued and then re-introduced once renal functions have improved. At this time therapy continued only with combination of three drugs, without ethambutol and after another two months the treatment continued with a combination of rifampicin and isoniazid.
The patient was again re-hospitalized after two weeks for recurrent pre-renal failure and then three weeks later for acute heart failure with pulmonary edema. She had to be hospitalized at the sanatorium for the long-term ill, where treatment continued for the next two months with no further complications. Once discharged, the patient stopped taking her anti-tuberculosis medication – after a total of 4 months of treatment.
After two months at home, the patient was again re-admitted with community acquired pneumonia, which was treated with standard antibiotics. Once her condition improved, anti-tuberculosis drugs were re-instituted for another two months.
Summary: We presented an 80-year old uncooperative, multimorbid patient with an accumulation of risk factors for TB and manifestation of treatment toxicity and drug interactions. The treatment scheme had to be adjusted and interrupted twice for several weeks, with a good clinical effect, nonetheless.
Case Report No. 2
We present a case of a 70-year-old male, a lifelong smoker, who was initially admitted for collapsing. Chest X-ray detected non-homogenous condensation of the right lower and middle lobe as well as an oval opacity at the border of the middle and upper lung lobe, clearly demarcated and radiolucent (Figure 3a,b). Patient admitted that he suffered from cough for about 6 months and weakness, anorexia and dyspnea on exertion for about one month. TST was negative but sputum culture detected acid-fast bacilli. Anti-tuberculosis treatment was initiated using the classical combination of streptomycin, isoniazid, pyrazinamide and rifampicin. The patient was discharged after six months of therapy, but one week later he had to be re-admitted because of collapsing due to weakness from inability to eat and drink. Later in the evening patient was found death. The autopsy demonstrated metastatic squamous cell carcinoma of the right lung, grade I. The immediate cause of death was bronchopneumonia.
Fig. 3. Chest X-ray before (Figure 3a) and after (Figure 3b) treatment 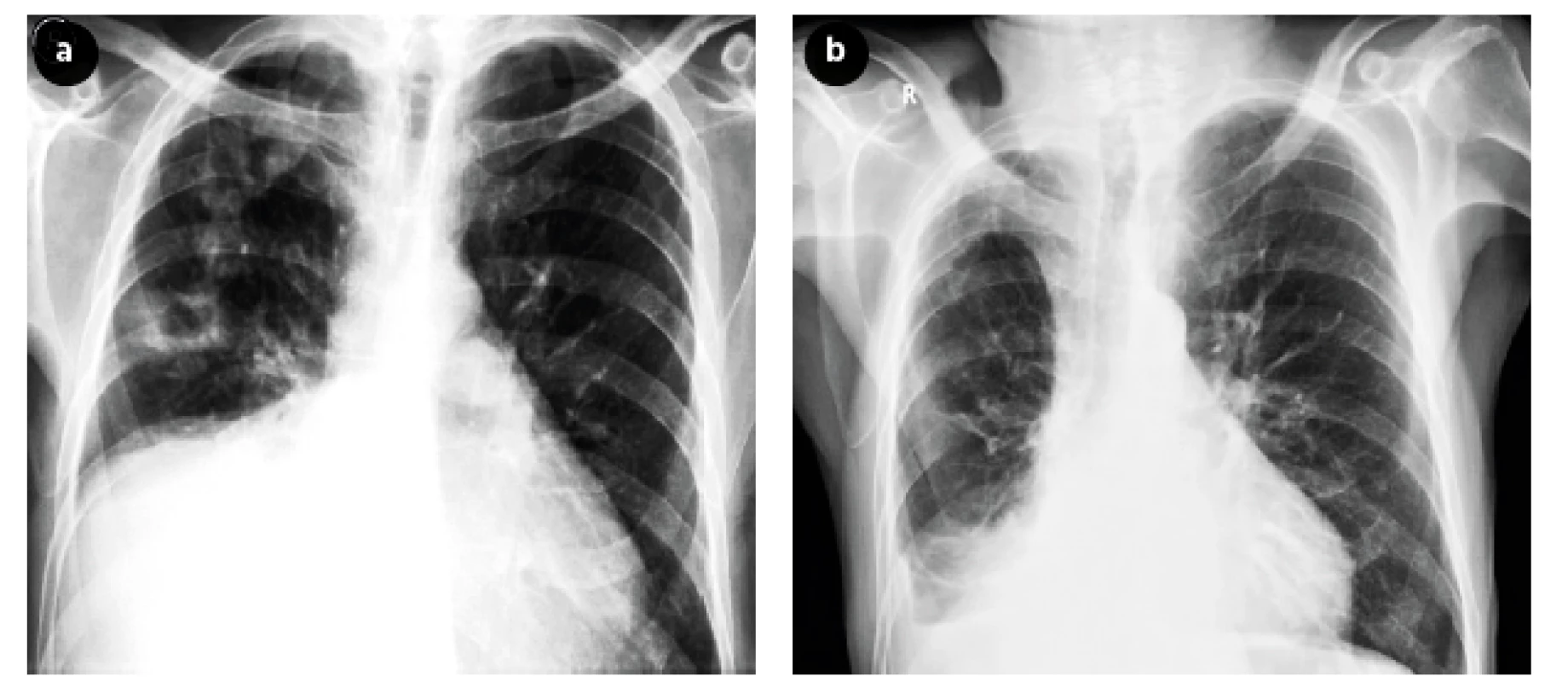
Summary: TB in this patient – a life-long smoker – probably developed via the endogenic route from a Ghon’s complex in association with a slowly progressing bronchogenic carcinoma.
DISCUSSION
M. tuberculosis infects one fourth of global population and 4000 people die of TB everyday [34].
As life expectancy increases, we should anticipate a higher incidence of elderly TB patients, who have an accumulation of risk factors for developing TB disease. This trend is global as several studies show [2]. Moreover, elderly patients often present with a less clear – cut symptomatology of TB and a concurrence with symptoms of other concomitant diseases.
The deterioration of the overall functional status of these patients including the emergence of cognitive dysfunctions may interfere with the implementation of planned diagnostic and therapeutic measures. Diagnosing TB in elderly is challenging. Older adults have reduced ability to produce a high quality sputum specimen and the TST that is used in sputum smear -negative TB tends to produce false negative results.[35]. As in our case report some studies also show that diagnosing TB at autopsy is not an uncommon case [36, 37]. The majority of TB disease in elderly arises from reactivation of previously contained infection [38].
Identifying persons with LTBI is important to the goal of TB control and elimination because treatment of LTBI can prevent infected persons from developing TB disease and stop the further spread of TB.
Unfocused population – based testing is not cost-effective or useful and leads to unnecessary treatment. TB testing activities should be conducted only among high-risk groups, with the intent to treat if LTBI is detected [39].
The characteristics of basic anti-tuberculosis drugs represent a significant risk of drug interactions with the elderly patients’ chronic medication.
If targeted follow-up and supportive therapy is provided, even the elderly multi-morbid patient is capable of undergoing anti-tuberculosis treatment with a satisfactory result and without any serious complication. A study conducted by Kim et al. revealed high successful treatment rate in elderly regardless of existence of immunocompromising comorbidities. The duration of treatment did not differ between the comorbidity and non-comorbidity groups [40]. Similar results we found in a study conducted by Kwon et al. where 97% of older patients achieved favorable treatment outcomes without adverse drug reaction with exception of GI disorders [41]. But what is a global alarm is Multidrug-resistant TB, according to WHO in 2018 there were 558 000 new cases with resistance to rifampicin – the most effective first –line drug.
Preventive measures against TB in the elderly population include apart from smoking cessation, a high-quality diet encompassing, a sufficient amount of proteins and micronutrients and observance of a regimen that includes regular physical exercise in the countryside.
CONCLUSIONS
The CR with TB incidence 4.9 per 100.000 populations fulfills the WHO target ‘End TB 2035’ (TB rates below 10 per 100,000). However, TB remains a complex health concern due to aging population, persistence of TB in aging populations and the rise of drug-resistant TB. Elderly are the most affected and vulnerable group with challenging TB diagnosis. However, unlike many other diseases, TB is potentially curable if diagnosed and treated early. It is therefore crucial that caregivers pay special attention to diagnosis and treatment of TB in older adults since prompt diagnosis of active pulmonary TB is essential for treating the individual as well as for public health control to stop further spread.
This publication was created as part of the MUNI/A/1382/2018 project.
We declare that we have no conflict of interest.
Do redakce došlo dne 31. 7. 2019.
Adresa pro korespondenci:
MUDr. Emmanuela Fernandová
Klinika interní, geriatrie a praktického lékařství
Fakultní nemocnice Brno
Jihlavská 340/20
625 00 Brno
e-mail: emmanuelafdo@gmail.com
Zdroje
1. WHO. Global strategy and targets for tuberculosis prevention, care and control after 2015[online]. Dostupné na www: https://www.who.int/tb/post2015_strategy/en/ (accessed 14 July 2019).
2. Li J, Chung P-H, Leung CLK, et al. The strategic framework of tuberculosis control and prevention in the elderly: a scoping review towards End TB targets. Infect Dis Poverty, 2017;6 : 70.
3. Hollo V, Beauté J, Ködmön C, et al. Tuberculosis notification rate decreases faster in residents of native origin than in residents of fo-reign origin in the EU/EEA, 2010 to 2015. Euro Surveill; 22. Epub ahead of print 23 March 2017. DOI: 10.2807/1560-7917. ES.2017.22.12.30486.
4. Potrepčiaková S, Skřičková J. Tuberkulóza. Prakticus odborný časopis Společnosti všeobecného lékařství ČLS JEP, 2008;7 : 24–29.
5. Organisation mondiale de la santé. Global Tuberculose Report: 2015. Genève (Suisse): Word Health Organization, 2015.
6. Surveillance report. Tuberculosis surveillance and monitoring in Europe 2017. 162.
7. Institute of Health Information and Statistics of the Czech Republic. Basic Overview of Tuberculosis Epidemiology in the Czech Republic in 2017 [online]. Dostupné na www: http://www.uzis.cz/system/files/tbc2017_an.pdf (2018).
8. Vašáková M. Doporučený postup diagnostiky a léčby latentní tuberkulózní infekce. Česká pneumologická a ftizeologická společnost[online]. Dostupné na www: http://www.pneumologie.cz/stranka/62/sekce-pro-tuberkulozu/.
9. Vlachová M. Tuberculosis in senior age. Geriatrie a gerontologie 2015;4(1): 26–30.
10. Menon S, Rossi R, Nshimyumukiza L, et al. Convergence of a diabetes mellitus, protein energy malnutrition, and TB epidemic: the neglected elderly population. BMC Infect Dis. 2016;16 : 361.
11. Vašáková M. Tuberculosis in the Czech Republic. Current status. Diagnosis, treatment and prevention. Vnitr Lek, 2013;59 : 284–289.
12. Rajagopalan S. Tuberculosis and aging: a global health problem. Clin Infect Dis, 2001;33 : 1034–1039.
13. Wang CH, Yu CT, Lin HC, et al. Hypodense alveolar macrophages in patients with diabetes mellitus and active pulmonary tuberculosis. Tuber Lung Dis, 1999;79 : 235–242.
14. Seo GH, Kim MJ, Seo S, et al. Cancer-specific incidence rates of tuberculosis: A 5-year nationwide population-based study in a country with an intermediate tuberculosis burden. Medicine (Baltimore), 2016;95:e4919.
15. Leung CC, Lam TH, Chan WM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol, 2008;167 : 1486–1494.
16. Leow MKS, Dalan R, Chee CBE, et al. Latent tuberculosis in patients with diabetes mellitus: prevalence, progression and public health implications. Exp Clin Endocrinol Diabetes, 2014;122 : 528–532.
17. Victoria J, Drake VJ. Nutrition and Immunity. Linus Pauling Institute; 2010. Dostupné na www: http://lpi.oregonstate.edu/mic/micronutrients-health/immunity.
18. Tuberculosis of adults – standard of treatment plan. Czech pneumological and ftizeological society [online]. Dostupné na www: http://www.pneumologie.cz/guidelines.
19. Culqui-Lévano DR, Rodriguez-Valín E, Donado-Campos J de M. Analysis of extrapulmonary tuberculosis in Spain: 2007–2012 National Study. Enferm Infecc Microbiol Clin, 2017;35 : 82–87.
20. Kalvach Z, Zadak Z, Jirak R, et al. Geriatrie a Gerontologie. Praha, Grada, 2004 : 541. ISBN 80-247-0548-6.
21. Mamo JP, Brij SO. Treating tuberculosis in the elderly population: a lesson in multi-disciplinary care. Scott Med J, 2013;58:e15–16.
22. Ryu YJ. Diagnosis of Pulmonary Tuberculosis: Recent Advances and Diagnostic Algorithms. Tuberc Respir Dis (Seoul), 2015;78 : 64–71.
23. Sood R. The Problem of Geriatric Tuberculosis. Journal of Indian Academy of Clinical Medicine;5 : 7.
24. Implementing Tuberculosis Diagnostics. Dostupné na www: https://apps.who.int/iris/bitstream/handle/10665/162712/9789241508612_eng.pdf;jsessionid=7E84B47FA396F89C52864E4491E12CEB?sequence=1 (accessed 8 September 2019).
25. Mori T, Leung CC. Tuberculosis in the global aging population. Infect Dis Clin North Am 2010; 24 : 751–768.
26. Michael S. Niederman. Tuberculosis:Diagnosis and Management. Dostupné na www: https://mhb.openmedicalinstitute.org/media/2019/675/data/lectures/18/index.html (accessed 8 September 2019).
27. Thrupp L, Bradley S, Smith P, et al. Tuberculosis prevention and control in long-term-care facilities for older adults. Infect Control Hosp Epidemiol, 2004;25 : 1097–1108.
28. Grace A, Mittal A, Jain S, et al. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database of Systematic Reviews; 2018. Epub ahead of print 12 January 2018. DOI: 10.1002/14651858.CD012918.
29. World Health Organization. Stop TB Initiative (World Health Organization) (eds). Treatment of tuberculosis: guidelines. 4th ed. Geneva: World Health Organization, 2010.
30. NIDRAZID-Státní ústav pro kontrolu léčiv [online]. Dostupné na www: http://www.sukl.cz/modules/medication/detail.php?code=0003303&tab=texts (accessed 15 July 2019).
31. Pyrazinamid. Státní ústav pro kontrolu léčiv [online]. Dostupné na www: http://www.sukl.cz/modules/medication/detail.php?code=0231976&tab=texts (accessed 15 July 2019).
32. Benemicin. Státní ústav pro kontrolu léčiv [online]. Dostupné na www: http://www.sukl.cz/modules/medication/detail.php?code=0093921&tab=texts (accessed 15 July 2019).
33. Metodika k provádění pravidelného očkování proti tuberkulóze (TBC) v ČR. Dostupné na www: http://www.pneumologie.cz/stranka/62/sekce-pro-tuberkulozu/ (accessed 15 July 2019).
34. Snapshot. Dostupné na www: https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (accessed 10 September 2019).
35. Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults-time to take notice. Int J Infect Dis, 2015;32 : 135–137.
36. Yew WW, Yoshiyama T, Leung CC, et al. Epidemiological, clinical and mechanistic perspectives of tuberculosis in older people. Respirology, 2018;23 : 567–575.
37. Wu Y-C, Lo H-Y, Yang S-L, et al. Factors correlated with tuberculosis reported after death. Int J Tuberc Lung Dis, 2014;18 : 1485–1490.
38. Hochberg NS, Horsburgh CR. Prevention of tuberculosis in older adults in the United States: obstacles and opportunities. Clin Infect Dis, 2013;56 : 1240–1247.
39. Targeted Testing for Tuberculosis | LTBI: A Guide for Primary Health Care Providers| Guides & Toolkits | Publications & Products | TB | CDC. Dostupné na www: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm (2018, accessed 15 September 2019).
40. Kim SY, Lee S-M, Yim J-J, et al. Treatment response and adverse reactions in older tuberculosis patients with immunocompromising comorbidities. Yonsei Med J, 2013;54 : 1227–1233.
41. Kwon YS, Chi SY, Oh IJ, et al. Clinical characteristics and treatment outcomes of tuberculosis in the elderly: a case control study. BMC Infect Dis, 2013;13 : 121.
Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2019 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- Západonilská horečka – autochtonní výskyt na jižní Moravě v roce 2018 z pohledu epidemiologa
- Odhadovanie vplyvu nadváhy a obezity na riziko vzniku rakoviny u českej a slovenskej populácie
- Epidemiologie virové hepatitidy E
- Tuberkulóza u seniorů v České republice
- 110. výročí narození prof. Karla Rašky a 40. výročí vymýcení pravých neštovic
- Sedmdesát let od vzniku Společnosti pro epidemiologii a mikrobiologii České lékařské společnosti J. E. Purkyně
- K životnému jubileu profesorky MUDr. Daniely Kotulovej, CSc.
- Rejstřík
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Západonilská horečka – autochtonní výskyt na jižní Moravě v roce 2018 z pohledu epidemiologa
- Epidemiologie virové hepatitidy E
- Tuberkulóza u seniorů v České republice
- Odhadovanie vplyvu nadváhy a obezity na riziko vzniku rakoviny u českej a slovenskej populácie
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



