-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
EROSIVE EFFECT OF ACIDIC BEVERAGES ON THE SURFACE OF FILLING MATERIALS
Authors: P. Holík; Y. Morozova; M. Rosa; I. Voborná
Authors place of work: Klinika zubního lékařství, Lékařská fakulta Univerzity Palackého a Fakultní nemocnice, Olomouc
Published in the journal: Česká stomatologie / Praktické zubní lékařství, ročník 122, 2022, 1, s. 17-27
Category: Původní práce
doi: https://doi.org/10.51479/cspzl.2022.003Original article, in vitro study
Summary
Introduction, aim: Erosive-abrasive defects of hard dental tissues are, in addition to tooth decay, one of the most common dental pathologies. Dental filling materials designed to repair these defects must be able to withstand the effects of wear – that is, erosion and abrasion – comparable or better than the enamel they replace. The study is focused on determining the surface changes of filling materials of various categories after exposure to acidic pH. The aim is to verify that the filling materials resist acids better than tooth enamel.
Methods: The immersion of the samples took place in Coca-Cola (pH 2.4) for 5 minutes and for the sake of the erosive process also for 14 days. Image analysis using a confocal laser scanning microscope was used to examine the samples. Changes in surface hardness were also evaluated by nanoindentation and compared with enamel.
Results: The tested filling materials, contrary to the enamel, withstood short-term exposure without significant changes in hardness and appearance. However, micro and macroscopic changes were observed during long-term exposure. These changes were most evident in the group of glass ionomer filling materials.
Conclusion: The observations clearly showed a significantly higher resistance of the filling materials to the acidic environment than that of the enamel.
Keywords:
Erosion – Optical analysis – non-carious defects – filling – nanoindentation
INTRODUCTION AND AIM
Erosion is the loss of hard dental tissues caused by acids of nonbacterial origin. The definition dates from 1949 and the authors are Zipkin and McClure [1, 2]. The acids can be of either endogenous or exogenous origin.
Two or more mechanisms could act simultaneously in the etiology of tooth wear. Multifactorial effects involve three basic mechanisms, such as friction, chemical effects, and stress due to compression, flexion, and tension. Different types of non-carious dental lesions are the result of their isolated or combined action [1].
Erosive lesions can be divided according to their origin into four groups [1]
- Dietary - frequent and excessive consumption of acidic foods,
- Regurgitation - the cause is gastric acids,
- Occupational or industrial erosion - by the action of acids occurring at the workplace or present in the environment,
- Idiopathic - unclear origin, increased content of citric acid in saliva.
The effect of acids on hard dental tissues has been described and investigated many times in laboratory and clinical conditions [3, 4]. The inorganic component of dentin and enamel consists of carbonate hydroxyapatite. The acids occur in the oral cavity in dissociated form in the form of the hydrogen cation H+ and the anion of the acid residue. The hydrogen cation reacts with the phosphate and carbonate anions of hard dental tissues [5]. In addition, anions of organic acids (e.g. citric acid) can form complex compounds with calcium hydroxyapatite. These anions are known as chelating agents [6]. The result of these processes is the release of minerals from the crystal lattice of hard dental tissues, which leads to a reduction in the hardness of the tooth surface [7]. Acids from beverages fall into the category of acids of dietary origin. They decrease the pH of the oral cavity for up to 15 minutes [8]. One of the best known soft drinks in the world is Coca-Cola. Due to its constant composition worldwide, this beverage is also most often used to test hard dental tissues and their resistance to erosion.
However, these acids act not only on the hard dental tissues, but also on all artificial materials found in the mouth. This paper is focused on filling materials.
A situation can be assumed, where an erosive defect of the tooth causes an exposition of dentine and, thus, causes an irritation of the dental pulp. At the same time, this damage changes the shape and then, depending on the extent, the function of the tooth – i.e. the articulation and, last but not least, the aesthetics. This defect needs to be filled with one of the filling materials to create a barrier between the exposed dentin and the environment of the oral cavity. However, this material will also be exposed to acidic pH unless the patient's dietary habits change [9].
Acid resistance is an essential attribute of any filling material. Different resistance of different types of fillings can be pressumed [10]: from amalgam, which is assumed to be completely inert to acids, through composite materials, which depend on the binder and filler used, to glass ionomer materials, which due to their remineralization potential may be prone to rapid demineralization [11, 12].
The aim of this in vitro study was to investigate the changes in surface hardness of various filling materials when exposed to acidic pH. Further aim was to compare the resistance of these materials with the enamel.
METHODS AND MATERIALS
In our in vitro study we used representatives of several categories of filling materials commonly used in the dental office:
- Glass ionomer resin modified dual-cured cement Harvard Ionoresin Fill (Harvard, Germany, tooth-colored filling material, indicated for class I, II and V in deciduous teeth, for permanent teeth for class V, and fillings with low or no occlusal load of class I and II, also for temporary fillings)
- Glass ionomer filling cement setting by acid-base reaction Omnifill C (Omnident, Germany, also filling material in tooth color indicated for the treatment of deciduous teeth, wedge-shape defects, class I and III of a smaller extent)
- Filtek Ultimate nanofilled composite material (3M, ESPE, USA, with a filler content of 55.6 vol. %, aggregated particle size is 0.6 µm‒20 µm, aesthetic filling material with a wide indication)
- Filtek Ultimate Flow nanofilled composite material (3M ESPE, USA, with filler content 46 vol. %, aggregate particle size is from 20 nm to 5 µm, aesthetic filling material indicated in class III and V, in minimally invasive cavities, treatment of small defects in indirect reconstructions, fissure sealing)
- Non-gamma 2 capsulated amalgam, machine mixed GS-80 (SDI, Australia, filling material made of metal-mercury alloy, for its durability and low aesthetics is indicated rather in the posterior teeth)
A disc-shaped sample with a diameter of 5 mm and a thickness of 3 mm was prepared from each material in a silicone matrix, and after setting, the sample was polished with OptiDisc (KerrHawe, Switzerland) Coarse/Medium 40 μm, 20 μm Fine and 10 μm Extra Fine in a micromotor. We compared the erosion resistance of the tested dental materials with the tooth enamel using the data from in vitro study of Morozova et al. (2011), who exposed the enamel samples treted the same way, i.e., immersed in Coca-Cola for 5 minutes [13].
The samples were immersed in Coca-Cola (pH 2.4, Coca-Cola HBC, Czech Republic, composition: water, sugar, carbonic acid, dye E150d, phosphoric acid, aroma, caffeine) [1, 14] for 5 minutes and to illustrate the erosive process also for 14 days. A control sample of each material was kept in water for the same time period.
The samples were then taken out and their hardness was measured by nanoindentation. In our experiments, we used an indentation force of 10 mN applied to a calibrated Berkovich indentor, with loading and subsequent unloading at a rate of 0.5 mN/s. The sample was exposed to the maximum indentation load for 5 seconds. Six measurements were made on each sample. The individual impressions were placed in a linear matrix, the distance between the impressions was 30 μm. The impression hardness (nanoindentation hardness) was determined from the experimental indentation curves using the method proposed by Oliver and Pharr [15]. Only standard shape curves were analyzed. The characteristic mean microhardness values of the respective sample were always calculated from at least four independent measurements and are presented in GPa [13]. Wilcoxon signed rank test with a significance level of α = 0.05 was chosen for statistical analysis of the results.
Optical analysis was also performed with a digital light microscope and a LEXT OLS 3100 confocal laser scanning microscope (CLSM).
RESULTS
Optical analysis of materials
Glass ionomer filling material – Harvard Ionoresin Fill
The control sample of the material appears smooth and shiny on macroscopic observation (Fig. 1), on the tested sample after exposure to Coca-Cola brown lines are visible even without magnification (Fig. 2). The erosive process, despite taking place over the entire surface and causing a slight increase in roughness, took place much more intensely on the predilection lines. An etched surface roughness can be noted, into which the acidic beverage has flowed and a dye has settled in it. An inhomogeneous surface can also be observed in the image of the sample exposed only to water - no scratches are visible in the surface, but rather uneven solidification of the material caused the formation of places with different levels of filler saturation (Fig. 3). After two weeks in Coca-Cola, the surface shows numerous defects extending deeply below the filling surface (Fig. 4). Furthermore, a layer of highly eroded soft material covering the surface was noted.
The measured hardness values show that the hardness did not change significantly after five minutes of exposure to an acidic environment. However, long-term exposure damaged the surface such extensively, that it was impossible to measure the hardness (Tab. 1).
Tab. 1. Values of average hardness along with statistical evaluation – Harvard Ionoresin Fill 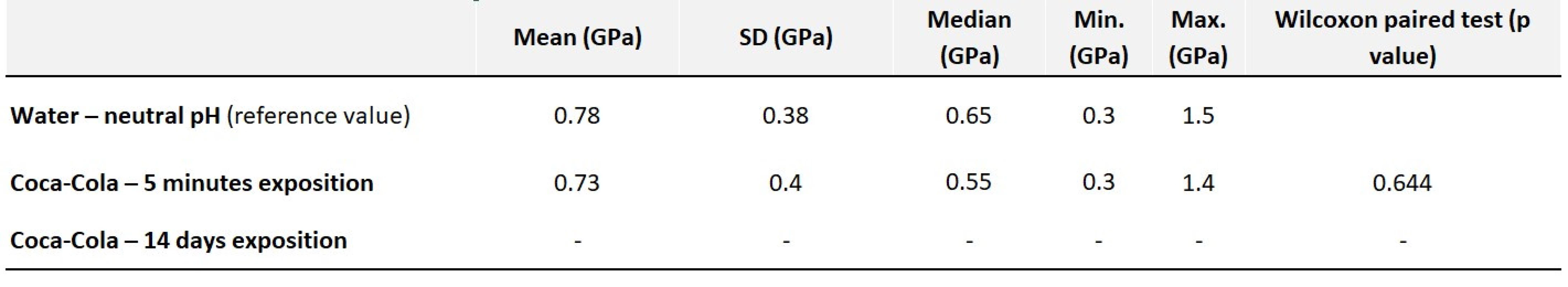
Fig. 1 Harvard Ionoresin Fill control in water 
Fig. 2 Harvard Ionoresin Fill at acidic pH after 14 days 
Fig. 3 Harvard Ionoresin Fill control in water, shown by CLSM 
Fig. 4 Harvard Ionoresin Fill at acidic pH after 14 days, shown by CLSM 
Glass ionomer filling material – Omnifill C
The glass ionomer cement Omnifill C contains small surface defects (Fig. 5), which result in the loss of shine and greater roughness. However, we do not observe a different distribution of filler, the sample seems more homogeneous. There are deep erosive defects on the surface of the eroded sample. However, compared to the previous sample, macroscopic infractions are not visible. The surface is very prone to mechanical wear, therefore even simple abrasion could lead to further significant loss of hard surface (Fig. 6).
Also at the microscopic level, we observe a higher homogeneity and a low incidence of surface irregularities, which, however, could be explained due to the focus of the image (Fig. 7). After 14 days of exposure in Coca-Cola, deep, sharply demarcated defects developed in which the dye from the beverage settled and the entire surface shows minimal hardness (Fig. 8).
Hardness values did not change significantly after five minutes of exposure, as in the previous case. After long-term exposure, the sample eroded so much that the hardness could not be measured by nanoindentation (Tab. 2).
Tab. 2. Values of average hardness along with statistical evaluation – Omnifill C 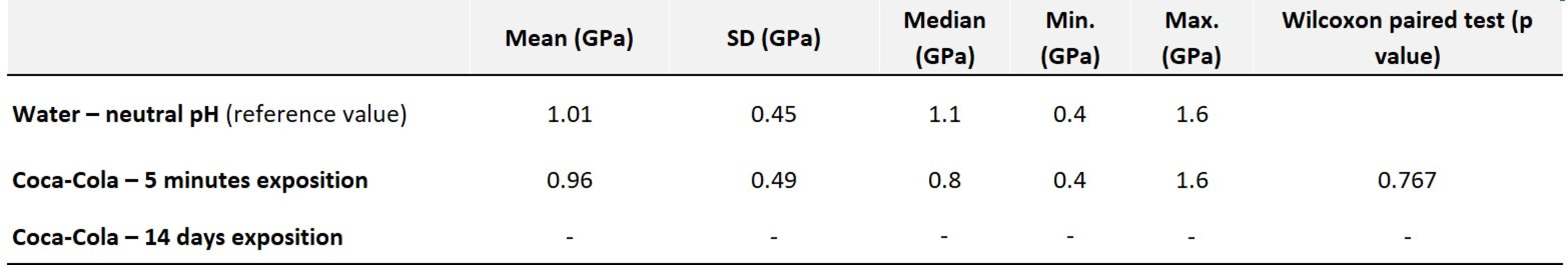
Fig. 5 Omnifill C control sample in water 
Fig. 6 Omnifill C exposed to acidic pH for 14 days 
Fig. 7 Control sample of Omnifill C in water, shown by CLSM 
Fig. 8 Omnifill C exposed to acidic pH for 14 days, shown by CLSM 
Composite filling material – Filtek Ultimate
No difference between accidic and neutral pH samples was observed macroscopically, both retaining shining, smooth surface and hardness (Fig. 9, 10).
Using CLSM it can be confirmed that the homogeneity and compactness of the composite filling surface remain excellent even after 14 days of Coca-Cola exposure and the predilection sites of higher erosive damage are only small (Fig. 11, 12). It should be noted that due to the composition of the material, this can be expected and that the erosion will be only slight. After 14 days of exposure to Coca-Cola, several small particles or rather clusters of these particles were released, but otherwise the surface is unchanged even after this long-term exposure.
The hardness of the samples stored in water and in Coca-Cola after short-term and long-term exposure is practically the same (Tab. 3). Thus, no significant change in hardness was demonstrated.
Tab. 3. Values of average hardness along with statistical evaluation – Filtek Ultimate 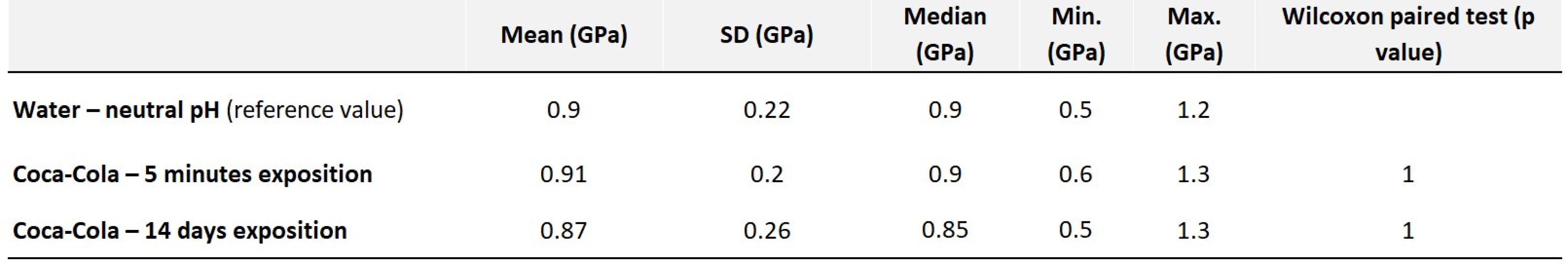
Fig. 9 Filtek Ultimate control sample in water 
Fig. 10 Filtek Ultimate exposed to acidic pH for 14 days 
Fig. 11 Filtek Ultimate in water sample, shown by CLSM 
Fig. 12 Filtek Ultimate exposed to acidic pH for 14 days, shown by CLSM 
Composite material in flow consistency – Filtek Ultimate Flow
The material is macroscopically very smooth and shiny (Fig. 13). However, exposure to Coca-Cola caused a loss of shine on some parts of the sample surface (Fig. 14).
Surface erosion is already visible in the microscope after 14 days, but does not interfere with the deeper structures of the material. Compared to the control sample (Fig. 15), some larger clusters of filler particles are also missing (Fig. 16). We observe the so-called delamination of layers [16]. This mechanism of erosion, in which the individual layers of the filler peel off, reveals deeper layers which do not have reduced hardness. Conversely, in hybrid materials where the filler distribution is not homogeneous, an area with a higher concentration of filler particles and, thus, harder in the indentation measurement can be measured below the desquamated layer. These results can also be found in the study of Hong-Yi Fan (2014) [17]. The measured hardness after 5 minutes of exposure to Coca-Cola was a slightly increased camparing with that measured in the study mentioned above. However, it could be explained due to the different focus of the sample during the measurements. We do not consider this change to be significant from a statistical point of view either. After 14 days of exposure the hardness did not change significantly again (Tab. 4).
Tab. 4. Values of average hardness along with statistical evaluation – Filtek Ultimate Flow 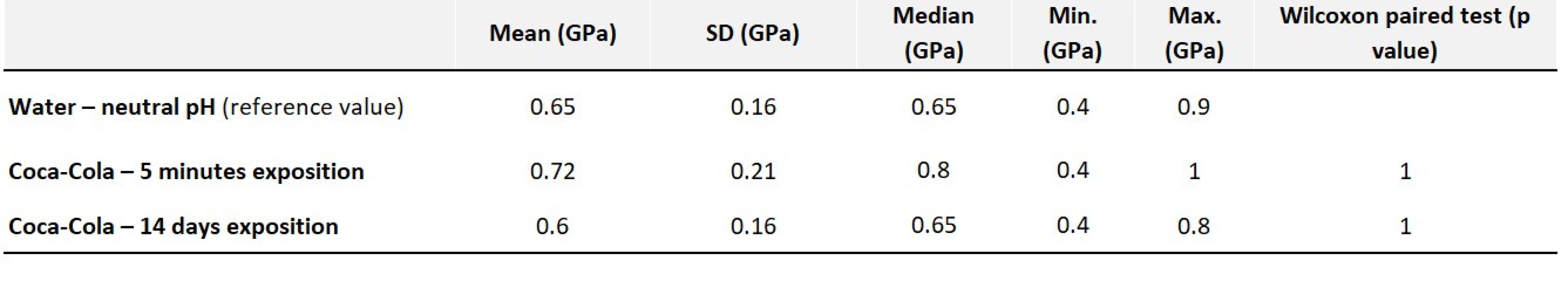
Fig. 13 Filtek Ultimate Flow control sample in water 
Fig. 14 Filtek Ultimate Flow exposed to acidic pH for 14 days 
Fig. 15 Filtek Ultimate Flow control sample in water, shown by CLSM 
Fig. 16 Filtek Ultimate Flow exposed t o acidic pH for 14 days, shown by CLSM 
Amalgam
We used amalgam fillings samples for the completeness of our measurement. The results confuremed our assumption, that the non-gamma-2 amalgam was not affected in any way by an acidic environment. No macroscopic changes can be observed in the images between the control sample and the sample exposed to acidic pH (Fig. 17, 18).
The changes between the samples detected by CLSM are only minimal and can be attributed to the different local character of the examined sample area. No defect that could potentially compromise the filling, or at least be macroscopically visible, was noted. CLSM images are taken at a higher magnification for better detection of small defects (Fig. 19, 20).
There were no changes in hardness values, even after long-term exposure in an erosive environment (Tab. 5). The dental malgam appears to be the most resistant to erosion.
Tab. 5. Values of average hardness along with statistical evaluation – amalgam 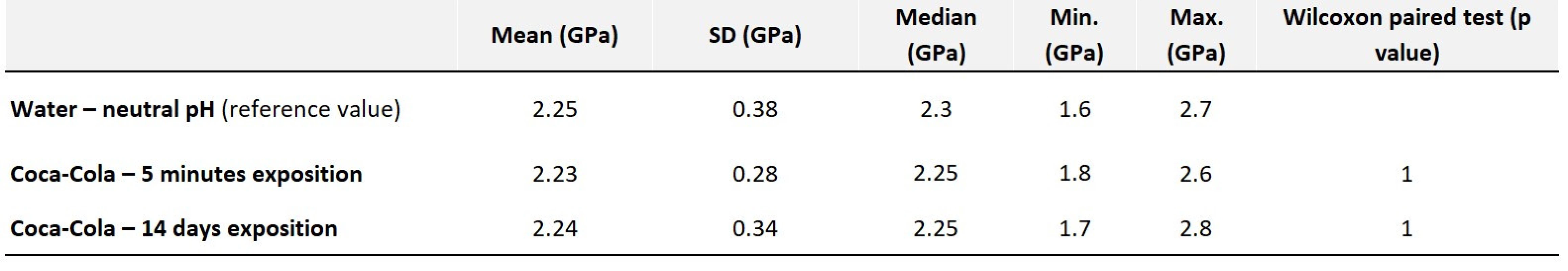
Fig. 17 Amalgam control sample in water 
Fig. 18 Amalgam exposed to acidic pH for 14 days 
Fig. 19 Amalgam control sample in water, shown by CLSM 
Fig. 20 Amalgam exposed to acidic pH for 14 days, shown by CLSM 
A summary of the results of hardness testing by nanoindentation is given in the Table 6.
Tab. 6. Hardness measurement by nanoindentation – summary 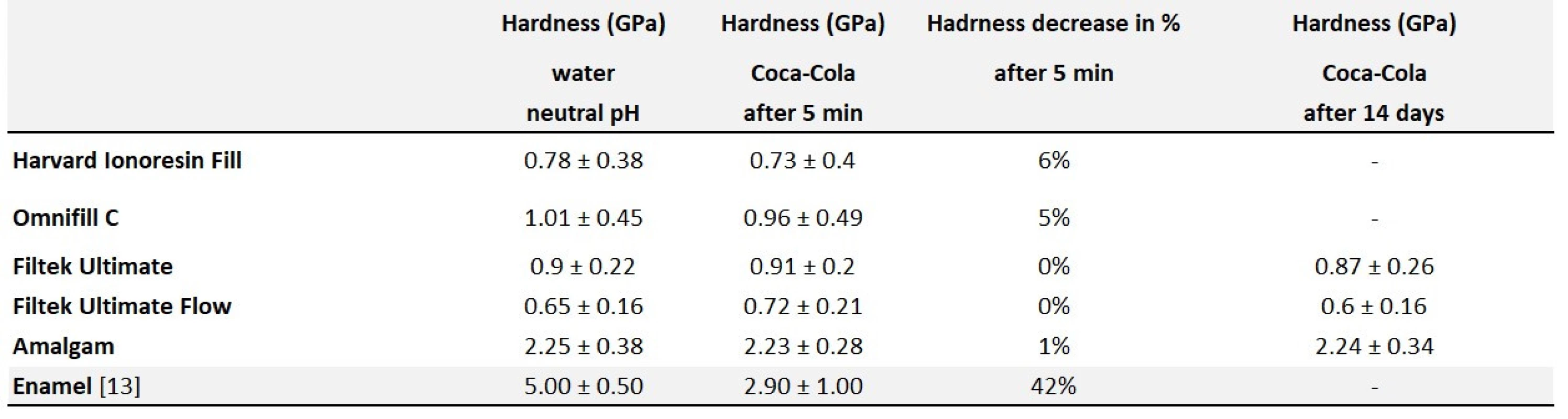
DISCUSSION
According to our observations, filling materials are only minimally affected by acidic environments compared to hard dental tissues. Due to their calcium content, glass ionomer cements are prone to surface erosion even after relatively short exposure, but compared to hard dental tissues, surface erosion probably will not be the cause of fatal filling failure [18]. Rather, it will be a precursor to other related damage, such as mechanical wear, scratches, and especially predilection fracture sites. Clinical observations suggest that glass ionomer filling fail under mechanical stress mostly by fractures [19].
Amalgam has proven to be the best acid-resistant material. No signs of surface erosion were observed. Thus, compared to the enamel, it can be assumed, and confirmed by clinical observations, that amalgam resists acids better than the tooth surface or other filling material [20]. However, due to the demanding cavity preparation and necessary retention matters for amalgam, as well as poor aesthetics, it is not and will not be an option for reconstruction of erosive defects [21].
Composite fillings samples withstood acidic environments better than the enamel. There were only microscopic surface changes and desquamation of the surface filler particles. Macroscopic changes were not apparent. However, their composition has a certain effect on the erosion of the surface of composite. Organic acids dissolve Bis-GMA polymers more easily and filler particles are released. This is probably due to the fact that UDMA/TEGMA achieve a higher degree of conversion than Bis-GMA. Depending on the type of filler and monomer a different wear rate has been clinically observed [22]. However, in resistance to acids, the composite material performed well, as evidenced by other studies [17, 18, 23]. Furthermore, due to the adhesion, this type of material seems to be an ideal choice for dental erosions reconstruction (small erosive defects even without preparation) [9]. It can also be used preventively as a protective layer against acids attacks [24].
However, the identification and elimination of the cause remains the primary and most important principle in the treatment of erosions [25].
The largest limitation of this study was the limited range of materials. The following research should focus on a detailed examination and comparison of more different materials in each group. As mentioned above, different types of monomers react to acidic media with different sensitivity. Likewise, glass ionomer materials are now produced in a whole range of modifications that could affect the result of the acidic test.
CONCLUSION
The hardness of none of the tested filling materials exposed to Coca-Cola for five minutes was reduced. All the tested filling materials showed less change in hardness after five minutes of Coca-Cola exposure comparing with the dental enamel.
In the case of long-term exposure (14 days), the samples of both glass ionomer filling materials (Harvard Ionoresin Fill, Omnifill C) were eroded to such extent, that their surface hardness was no longer measurable by nanoindentation. In contrast, the samples of amalgam and composite materials (Filtek Ultimate, Filtek Ultimate Flow) did not show a significant change in hardness compared to the sample in water.
Zdroje
- Morozova J. Erozivní defekty tvrdých zubních tkání: Část 1. Čes stomatol Prakt zubní lék. 2011; 59(1): 4–13.
- Lussi A. Eroze zubů: vyšetření, diagnóza, rizikové faktory. Prophylaxis dialogue. Zvláštní vydání o erozi. 2009/2010 : 13–16.
- Saads Carvalho T, Lussi A. Acidic beverages and foods associated with dental erosion and erosive tooth wear. In: Zohoori FV, Duckworth RM. The impact of nutrition and diet on oral health. Basel: Karger; 2020, 91–98.
- Joshi M, Joshi N, Kathariya R, Angadi P, Raikar S. Techniques to evaluate dental erosion: A systematic review of literature. J Clin Diagn Res. 2016; 10(10): ZE01–ZE07. doi:10.7860/JCDR/2016/17996.8634
- Lussi A, Ganss C. Erosive tooth wear. Basel: Karger; 2014, 163–179.
- Lussi A, Addy M, Angmar-Mansson B. Dental erosion from diagnosis to therapy. Basel: Karger; 2006, 67–69, 130–135.
- Rajeev G, Lewis AJ. A time based objective evaluation of the erosive effects of various beverages on enamel and cementum of deciduous and permanent teeth. J Clin Exp Dent. 2020; 12(1): e1–e8. doi:10.4317/jced.55910
- Hans R, Thomas S, Garla B, Dagli RJ, Hans MK. Effect of various sugary beverages on salivary pH, flow rate, and oral clearance rate amongst adults. Scientifica (Cairo). 2016; 2016 : 5027283. doi: 10.1155/2016/5027283
- Loomans B, Opdam N, Attin T, Bartlett D,Edelhoff D, Frankenberger R, Benic G, Ramseyer S, Wetselaar P, Sterenborg B, Hickel R, Pallesen U, Mehta S, Banerji S,Lussi A, Wilson N. Severe tooth wear: European consensus statement on management guidelines. J Adhes Dent. 2017; 19(2): 111–119. doi: 10.3290/j.jad.a38102
- Ahmed ME. Surface hardness assessment of tooth substrates and different esthetic restorative materials after Immersion in different acidic media. Int J Dent Oral Heal. 2018; 4(11): 178–183.
- Aliping-McKenzie M, Linden RW, Nicholson JW. The effect of Coca-Cola and fruit juices on the surface hardness of glass-ionomers and 'compomers'. J Oral Rehabil. 2004; 34(11): 1046–1052.
- Ozdemir-Ozenen D, Sungurtekin-Ekci E, Ozenen G. Effect of common daily acidic beverages on the surface roughness of glass ionomer-based dental restorative biomaterials. Glass Phys Chem. 2019; 45(6): 496–502.
- Morozova J, Zapletalová Z, Čtvrtlík R, Ranc V. Effect of selected acidic foodstuffs and beverages on enamel mechanical properties of human extracted teeth and their role in dental erosion origin. Čes stomatol Prakt zubní lék. 2012; 112(3): 77–87.
- Attin T, Weiss K, Becker K, Buchalla W, Wiegand A. Impact of modified acidic soft drinks on enamel erosion. Oral Dis. 2005; 11(1): 7–12.
- Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992; 7(6): 1564–1583.
- Onat A. Mechanical and dry sliding wear properties of silicon carbide particulate reinforced aluminium-copper alloy matrix composites produced by direct squeeze casting method. J Alloys Compd. 2010; 489(1): 119–124.
- Hong-Yi F, Xue-Qi G, Yang L, Zhuo-Li Z, Hai-Yang Y. The nanomechanical and tribological properties of restorative dental composites after exposure in different types of media. J Nanomater. 2014; 2014(2): 759038. doi:10.1155/2014/759038
- Wongkhantee S, Patanapiradej V, Maneenut C, Tantbirojn D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J Dent. 2006; 34(3): 214–220.
- Heck K, Frasheri I, Diegritz C, Manhart J, Hickel R, Fotiadou C. Six-year results of a randomized controlled clinical trial of two glass ionomer cements in class II cavities. J Dent. 2020; 97 : 103333. doi: 10.1016/j.jdent.2020.103333
- Honório HM, Rios D, Francisconi LF, Magalhaes AC, Machado MAAM, Buzalaf MAR. Effect of prolonged erosive pH cycling on different restorative materials. J Oral Rehab. 2008; 35(12): 947–953.
- Patki B. Direct permanent restoratives--amalgam vs composite. J Evol Med Dent Sci. 2013; 2(46): 8912–8919.
- Söderholm KJ, Lambrechts P, Sarrett D, Abe Y, Yang MC, Labella R, Yildiz E, Willems G. Clinical wear performance of eight experimental dental composites over three years determined by two measuring methods. Eur J Oral Sci. 2001; 109(4): 273–281.
- Attin T, Wegehaupt FJ. Impact of erosive conditions on tooth-colored restorative materials. Dent Mater. 2014; 30(1): 43–49.
- Zhao X, Pan J, Malmstrom HS, Yan-Fang R. Protective effects of resin sealant and flowable composite coatings against erosive and abrasive wear of dental hard tissues. J Dent. 2016; 49 : 68–74. doi: 10.1016/j.jdent.2016.01.013
- Šedý J. Abrazivně-erozivní poškození tvrdých zubních tkání. In: Šedý J. Kompendium stomatologie II. 1. vydání. Praha: Triton; 2017, 200–208.
Štítky
Chirurgie maxilofaciální Ortodoncie Stomatologie
Článek vyšel v časopiseČeská stomatologie / Praktické zubní lékařství
Nejčtenější tento týden
2022 Číslo 1- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Orální lichen planus v kostce: Jak v praxi na toto multifaktoriální onemocnění s různorodými symptomy?
- Význam ústní sprchy pro čištění mezizubních prostor
- MIH – komplexní problém s nutností komplexního přístupu
- Benzydamin v léčbě zánětů v dutině ústní
-
Všechny články tohoto čísla
- Editorial
- ZIRCONIA CERAMICS: PROPERTIES AND CLASSIFICATION
- EROSIVE EFFECT OF ACIDIC BEVERAGES ON THE SURFACE OF FILLING MATERIALS
- Změna na postu šéfredaktora časopisu Česká stomatologie a praktické zubní lékařství
- IN MEMORIAM DOC. MUDr. IVO DŘÍZHAL, CSc.
- HISTOLOGICAL EXAMINATION OF THE LINGUAL FRENULUM IN PATIENTS WITH ANKYLOGLOSSIA
- Česká stomatologie / Praktické zubní lékařství
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- ZIRCONIA CERAMICS: PROPERTIES AND CLASSIFICATION
- HISTOLOGICAL EXAMINATION OF THE LINGUAL FRENULUM IN PATIENTS WITH ANKYLOGLOSSIA
- EROSIVE EFFECT OF ACIDIC BEVERAGES ON THE SURFACE OF FILLING MATERIALS
- IN MEMORIAM DOC. MUDr. IVO DŘÍZHAL, CSc.
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



