-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Suprachoroideal haemorrhage in postoperative period of antiglaucoma surgery, case report
Authors: M. Středová; L. Hejsek; J. Nekolová; N. Jirásková
Authors place of work: Oční klinika Fakultní nemocnice Hradec Králové, Sokolská 581, 500 05 Hradec Králové, přednostka: prof. MUDr. Naďa Jirásková, Ph. D., FEBO
Published in the journal: Čes. a slov. Oftal., 75, 2019, No. 2, p. 92-98
Category: Kazuistika
doi: https://doi.org/10.31348/2019/2/6Summary
Suprachoroidal haemorrhage (SCH) is a serious complication of intraocular procedures. Physiologically there is only a minimal amount of fluid in the suprachoroid space, pathologically the fluid volume increases, which causes ablation of the choroid. SCH could be divided into different cathegories, according to the character of the fluid into serous and haemorrhagic; by the time of occurrence in relation to the surgery into peroperative and postoperative. Diagnosis is based on biomicroscopic and ultrasound examinations. The ocular risk factors for SCH are glaucoma, myopia and aphakia; systemic risk factors include vascular fragility, arterial hypertension and blood coagulation disorders. In the pathogenesis hypotonia of the eye, that causes rupture of the ciliary vessels, plays a very important role. SCH can be treated both conservatively and surgically. As to pharmacotherapy we use gabapentin to suppress neuropathic pain and prednisone, topical mydriatics and anti-inflammatory agents. The type of surgical treatment differs according to time of occurrence, if SCH occurs during the operation, the intervention consists mainly in the wound closure and the repositioning of the weakening tissues; in postoperative forms, we choose drainage procedures, possibly vitreoretinal procedures. Our patient, an 80-year-old myop and chronic glaucomatic treated intensively both topically and systematically underwent trabeculectomy on his left eye due to unsatisfactory intraocular pressure (IOP) and significant glaucoma progression. The surgical intervention went without any complications. In the early post-operative period, there was persisting elevation of IOP, therefore sclera lap was discontinued and 5-fluorouracil was applied under the filter blister. Subsequent hypotonia caused a hemorrhagic SCH with intraocular hypertension, which was resolved by draining the blood with sclerotomias and thus releasing intraocular hypertension. The visual acuity of the left eye gradually improved to almost original values. Intraocular pressure, however, is not well compensated despite many following antiglaucoma surgeries. Therefore, even with the patient‘s maximum therapy, glaucoma continues to progress. In our case, we confirm that it is possible to solve even the relatively most complicated cases of SCH. We stress the necessity to consider the presence of risk factors of the occurrence of SCH before indicating intraocular procedures and also recommend thinking carefully about other less invasive surgical techniques. In glaucoma, it is appropriate taking in account the prediction of life compared to the expected rate of progression of vision loss.
Keywords:
filtering surgery – suprachoroidal haemorrhage – trabeculectomy – glaucoma
INTRODUCTION
Suprachoroidal haemorrhage (SCH), referring to bleeding in the suprachoroidal space between the choroid and the sclera, ranks among feared and serious complications of intraocular surgical procedures [3, 4, 8]. Due to its very adverse prognosis regarding vision, timely diagnosis and treatment is absolutely essential [3, 8].
The suprachoroidal space, a region reaching forward toward the scleral spur and backward toward the edges of the optic nerve papilla, is of a slit shape in a healthy eye, and contains approximately 10 μl of fluid. In pathological conditions the volume of the fluid may increase, causing ablation of the choroid. The detached choroid is of a balloon shape, conditioned by discharges from the vorticose veins, i.e. places where the choroid is firmly attached to the sclera [3].
We distinguish between serous choroidal ablation (choroidal effusion) and haemorrhagic choroidal ablation (suprachoroidal haemorrhage, SCH), the extreme form of which is known as expulsive haemorrhage. These two conditions are mutually differentiated by the character of the fluid in the suprachoroidal space. Choroidal ablation is conditioned by exudation of the serous fluid, and the cause of this condition is most commonly hypotonia of the eyeball or inflammatory reaction. By contrast, in the case of SCH there is blood in the suprachoroidal space [3].
We may encounter SCH perioperatively or following cataract surgery, perforating keratoplasty operations, trabeculectomies or also in pars plana vitrectomies [3, 4].
We can differentiate SCH according to size. SCHs of a smaller scope are generally benign, referred to as suprachoroidal haematomas [3], massive SCHs, namely conditions in which opposite parts of the retina come into apposition, are also referred to as “kissing choroidal ablations” [6]. We divide SCHs occurring in connection with intraocular surgery into perioperative and postoperative, otherwise known as late [3, 4]. Extensive perioperative SCHs are also referred to as expulsive due to their rapid onset and large scope [3]. Late SCH as a rule occurs following uncomplicated glaucoma filtration surgery [3].
Diagnosis of SCH consists in examination of the ocular fundus by indirect ophthalmoscopy and also ultrasound examination, with the aid of which we can distinguish between serous choroidal ablation and SCH, and determine its localisation and scope [3].
A typical objective finding in perioperative and late SCH is shallowing to disappearance of the anterior chamber due to a sudden increase of intraocular pressure, blurring of the red reflex, and sometimes also a prolapse of the intraocular tissues. A growing mass of suprachoroidal bleeding can be seen in the vitreous area [3].
The fundamental risk factors of SCH include above all sclerosis and fragility of the choroidal capillaries, conditioned by advanced age of the patient, systemic arterial hypertension, atherosclerosis [4] and also disorder of blood coagulation, use of anticoagulant drugs and diabetes mellitus [3, 8]. By contrast, Tuli et al. negate high blood pressure or age as a risk of the occurrence of SCH [12]. Of ocular pathologies, a predisposition to this complication is represented by glaucoma, axial myopia, aphakia, post-traumatic conditions and intraocular inflammations or pseudoexfoliation syndrome [3, 4]. These conditions may be accompanied by affliction of the vascular wall, which leads to a reduction of the integrity of the posterior ciliary arteries [4]. According to Tuli et al., ocular risk factors of late SCH following trabulectomy include preoperative anticoagulation, aphakia or presence of an anterior chamber intraocular lens [12]. Further risk factors of the occurrence of SCH include sudden drop of intraocular pressure [12], breach of the posterior capsule of the lens with loss of vitreous body, increase of blood pressure and pulse during intraocular surgical procedure and conditions linked with the Valsalva manoeuvre (sneezing, coughing) [3, 4], which increase episcleral venous pressure, with subsequent increase of the pressure gradient across the wall of the ciliary artery [4].
The presumed pathogenesis of SCH is either hypotonia of the eyeball leading to rupture of the walls of the short and long posterior ciliary arteries [4], or hypotonia first of all causing choroidal effusion with enlargement of the suprachoroid space and contraction of the posterior ciliary arteries with their subsequent rupture [3].
Studies dealing with the incidence of SCH in glaucoma filtration surgery state a pronounced difference in the frequency of incidence of perioperative and late SCH. The incidence of perioperative expulsive haemorrhages is approximately 0.15%, while by contrast the incidence of late SCH is as much as 10x higher [3, 4]. The higher incidence of late SCH in glaucoma filtration surgery is conditioned by long-term persistent postoperative hypotonia and inflammatory intraocular processes which may occur in this type of surgical procedure [3, 4]. Tuli et al. state that the incidence of late SCH in various types of intraocular procedures is within the range of 1.6 to 6.2 %, specifically trabeculectomy with application of fluorouracil has an incidence of 6 % [12].
Therapy of SCH is both conservative and surgical. In conservative, adjuvant pharmacological therapy we use gabapentin and prednisone. Gabapentin serves to suppress neuropathic pain. It is used in a total daily dose of 900 mg, initially the dosage may be higher – up to 2700 mg per day. Prednisone is administered in a total daily dose of 40 mg for a period of two to three weeks. In local conservative therapy we use mydriatics – atropine and anti-inflammatory preparations [2]. Reynolds et al. state that SCH of a smaller scale without haemophthalmos, retinal detachment or incarceration of the vitreous can be treated conservatively [9].
Surgical intervention in the case of perioperative form consists in immediate closure of the wound and repositioning of the prolapsed intraocular tissues – if this is possible [3, 10], or even compression of the eyeball with the finger [3]. Closure of the surgical wounds causes an increase of intraocular pressure and therefore also a tamponade of the haemorrhage [3, 4]. The performance of sclerotomy in the acute phase of SCH is controversial with regard to the rapid coagulation of blood and the risk of impossibility of its evacuation. Furthermore, the tamponade effect of increased intraocular pressure may be unstable as a result, and thereby cause repeat haemorrhage [5, 10]. The study by Lakhanpal concludes that the performance of sclerotomy in the acute phase is inappropriate for the eye [5], and is recommended rather in the late phase, at an interval of 7 to 14 days [6, 7, 14].
Any applicable subsequent surgical therapy is highly individual, we take into account all the circumstances (persistent prolapse of intraocular tissues, vitreous body incarcerated in wound, value of intraocular pressure, if applicable residues of lens matter in the eye or signs of retinal detachment on ultrasound) [3, 4]. If a surgical solution is indicated, we choose a drainage procedure, if applicable accompanied by a vitreoretinal procedure [4]. Repositioning of the prolapsed tissues, anterior vitrectomy and lavage of the anterior chamber of the eye should be performed at the moment when there is not a large risk of further suprachoroidal haemorrhage [3, 10], and when the suprachoroidal blood is as thin as possible [2]. In addition to adjustment of the intraocular ratios, drainage of the blood also helps in the treatment of high intraocular pressure [1, 11, 13]. It is possible to drain the blood by sclerotomies in standard localisations or in places of the greatest manifestations of ablation, or with the use of ports for pars plana vitrectomy which can be inserted also transconjunctivally [10]. Guttman states that in addition to the classic scleral approach, surgical (passive) drainage may be performed also by the minimally invasive transconjunctival method with the use of active suction, which enables very good quality drainage management through direct visualisation by microscope. The transconjunctival method is minimally traumatic for the conjunctiva, and for this reason its use in SCH connected with glaucoma filtration surgery is highly advantageous [2, 9].
CASE REPORT
An 80-year-old man with high myopia and chronic glaucoma was hospitalised at the Department of Ophthalmology at the University Hospital in Hradec Králové for evaluation of the intraocular pressure curve and consideration of the following procedure due to long-term unsatisfactory values of intraocular tension, with fluctuations above 30 torr. The patient was being treated for primary open-angle glaucoma bilaterally.
From the ocular anamnesis we select the following: condition following cataract surgery bilaterally at the age of 63 years, subsequently performed repeated suctions of secondary cataract, at the age of 79 selective laser trabeculoplasty was performed bilaterally due to subcompensation of intraocular tensions. The patient was being treated generally for heart arrhythmia and dislipidemia, and used the pharmaceuticals Rytmonorm (propafenone-hydrochloride), Torvacard Neo (calcium salt of atorvastatin) and the food supplements Ascorutin (ascorbic acid), Ocuvite and GS Condro.
We admitted the patient with vision with own correction of 5/5 in the right eye and 5/7.5 in the left eye. Initial intraocular tension in the right eye was 17 torr, in the left eye 15 torr. At the time the patient had been using orally the pharmaceutical Diluran tablet (acetazolamide) long-term 2x per day, with substitution by potassium chloride tablet, concurrent local glaucoma therapy was bilaterally Simbrinza gtt. (bromidine tartrate, brinzolamide) 2x per day and Lumigan gtt. (bimatoprost) 1x per day, i.e. a triple combination of anti-glaucomatous agents – carboanhydrase inhibitors, sympathomimetic drugs, prostaglandin analogue. The patient did not tolerate general therapy with Diluran without complaints, stating worsening of heart arrhythmia and dyspepsia.
On the basis of decompensation of intraocular pressure following the discontinuation of the Diluran tablet and supplementary examinations (of perimeter – fig. 1 and optical coherence tomography of optic nerve papilla), trabeculectomy (TE) was indicated in the left eye under local anaesthesia. The procedure took place without perioperative complications. In the early postoperative period the values of intraocular pressure were above the upper limit of the norm, after massage of the eyeball the values were always normalised. For this reason, on the 3rd postoperative day, the suture of the scleral flap was cut (by injection needle transconjunctivally), with concurrent application of 5-fluorouracil beneath the filtration blister in the left eye. After the procedure temporary hypotonia resulted, a contact lens was applied and afterwards intraocular tension was normalised. On the following, i.e. 4th postoperative day, the patient was discharged for home care.
Fig. 1. Perimetric examination of left eye, 31 March 2017 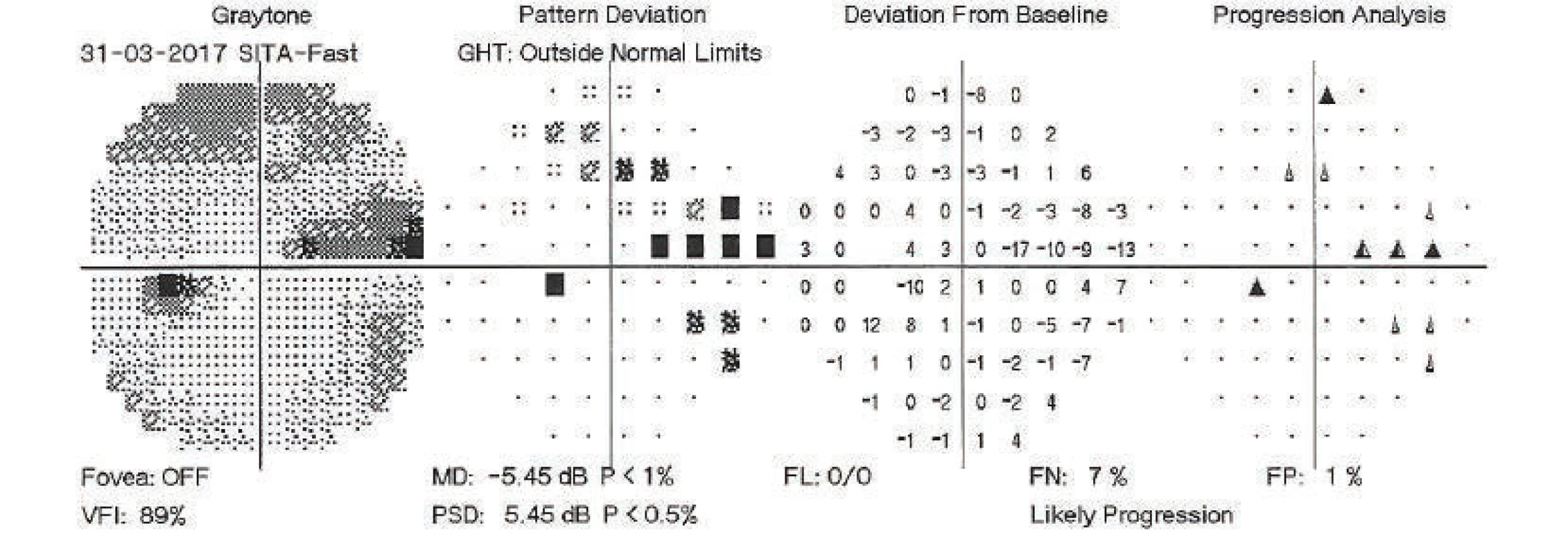
On the fifth postoperative day the patient reported to the emergency section of the Department of Ophthalmology at the University Hospital Hradec Králové due to sudden loss of sight in the left eye, which occurred after leaning forward. Upon admittance to the inpatient section, visual acuity in the left eye was light projection from one side, the eyeball was hypotonic to the touch and the objective finding in the left eye was dominated by a shallow anterior chamber and pronounced choroidal ablation (of the type of “kissing” ablations), reaching as far as the intraocular lens (fig. 2). Conservative therapy by Prednisone was administered in an initial dose of 80 mg. According to the sonographic finding of the left eyeball (fig. 3) we presumed suprachoroidal haemorrhage on the ocular fundus.
Fig. 2. Objective finding in the left eye on 7th day after trabeculectomy (TE) of LE 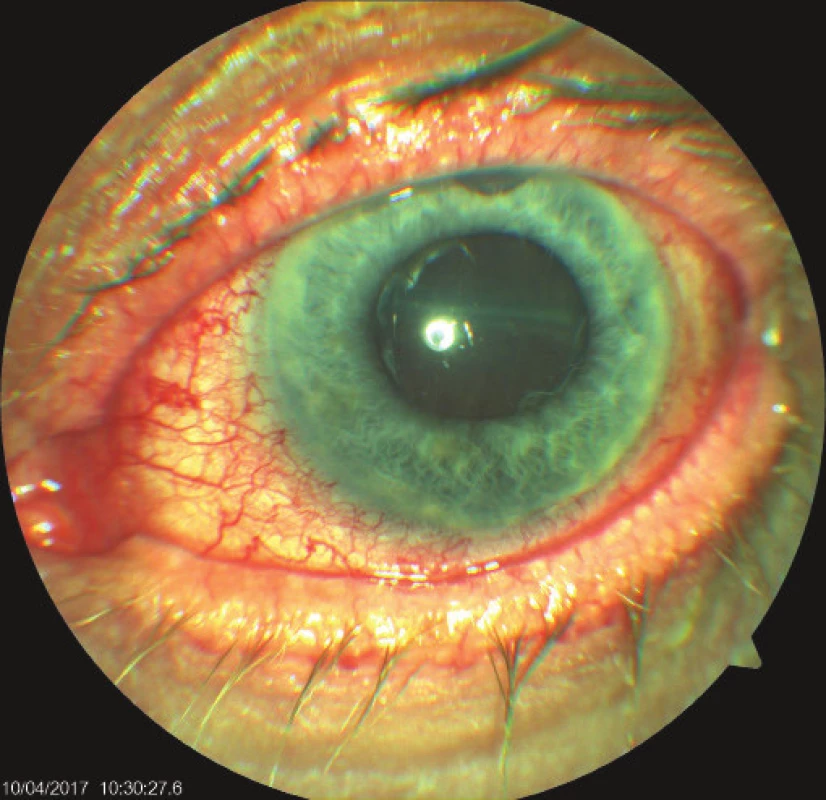
Fig. 3. Ultrasound examination (US) of left eyeball on 7th day after TE of LE 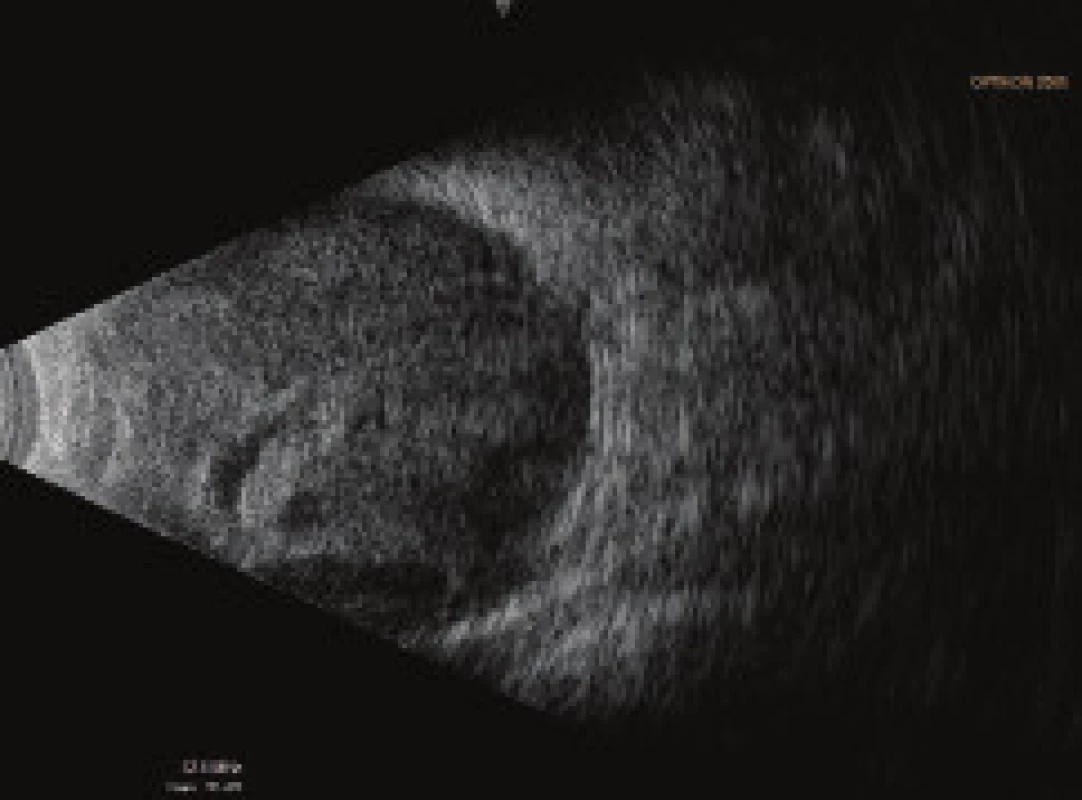
In the following two days intraocular hypertension, which we determined by touch (it was not possible to measure by any instrument), progressed. With regard to the extent of the ablation, in which the opposite sides of the retina were touching, with a risk of their permanent apposition, surgical revision of the left eye was indicated.
We present an abbreviated commentary on the surgical procedure here: first of all a 23G port was inserted 4 mm from the limbus in the temporal quadrant, and active suction of dark blood was conducted with the release of intraocular hypertension. Through the original puncture of the anterior chamber we injected adrenalin, and we formed the disappeared anterior chamber with viscose material. We continued with the revision of the conjunctiva and the scleral flap, which was sutured using a looser suture of 10-0 Ethilon. The colliquated blood was no longer outflowing (even upon active suction), and we therefore widened the scleral incision (following incision of the conjunctiva in the place of insertion of the port). The blood again spontaneously flowed out upon toning of the eyeball by infusion – inserted via the pars plicata into the already released retrolental space. By no. 6 we introduced auxiliary endoillumination (Chandelier) and monitored the development on the ocular fundus upon regression of SCH beneath a surgical microscope. Retino-retinal adhesion was gradually loosened by itself. At the end of the operation, vitreous fibres were evident, compressed from the vitreous area into the coloboma of the iris by no. 12, therefore we cut them with posterior vitrectome (inserted beneath the scleral flap into trabeculectomy). Using this manoeuvre the artificial intraocular lens (IOL) was centred, which had otherwise repeatedly changed its position during the procedure (in all directions from the optical axis). The procedure was completed with a suture of all the surgical entrances, a check of the functionality of trabeculectomy and parabulbar application of gentamicin with diprophos. The findings from the course of operation are presented in fig. 4 to 6.
Fig. 4. Initial phase of surgical procedure, evacuation of haemorrhage by sclerotomy, release of intraocular hypertension, anterior chamber created from viscose material, eyeball later toned with infusion inserted into anterior chamber. In the retrolental space, there is evident high choroidal ablation in proximity behind the IOL. Image from perioperative video – from surgeon’s view 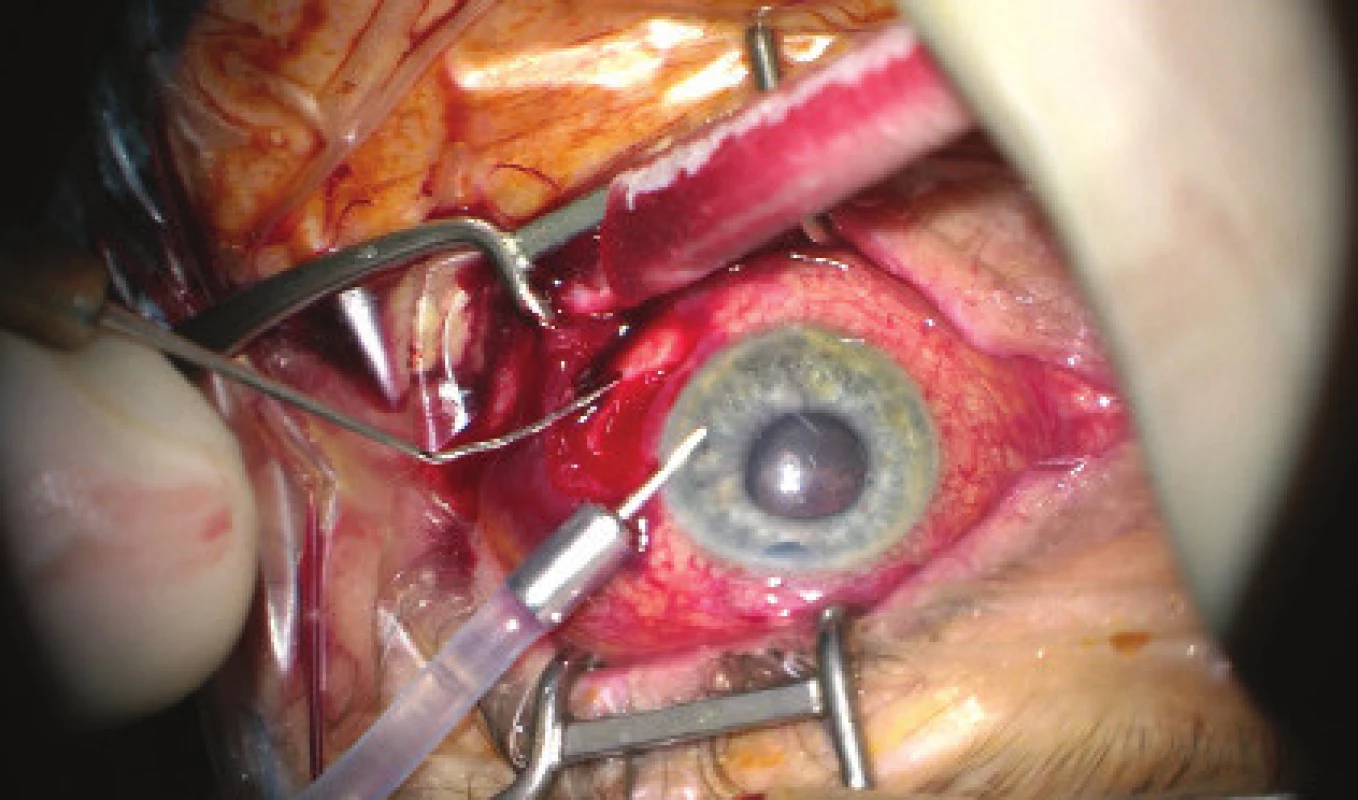
Fig. 5. View of ocular fundus during the course of progressive reduction of height of ablations, apposition of opposite parts of retina gradually “unpeeled”, reflex from ocular fundus begins to be visible. Light is reflected from vitreous fibres which are squeezed into the retrolental space. Image from perioperative video – from surgeon’s view 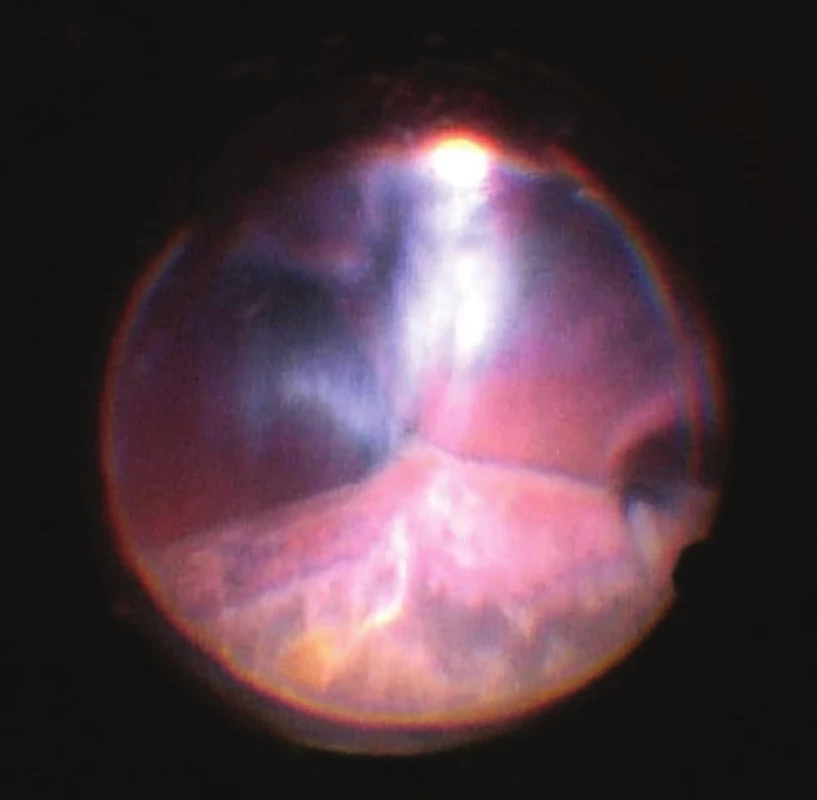
Fig. 6. Progressive evacuation of blood from second sclerotomy (forceps inserted into wound), after disappearance of ablation behind IOL 23G infusion inserted in region of pars plicata (orange port) and 25G light (Chandelier, blue port) for monitoring of the ocular fundus with display system of microscope (Zeiss Resight). Auxiliary traction suture inserted into the region of limbus in no. 8, evident decentration of IOL. Image from perioperative video – from surgeon’s view 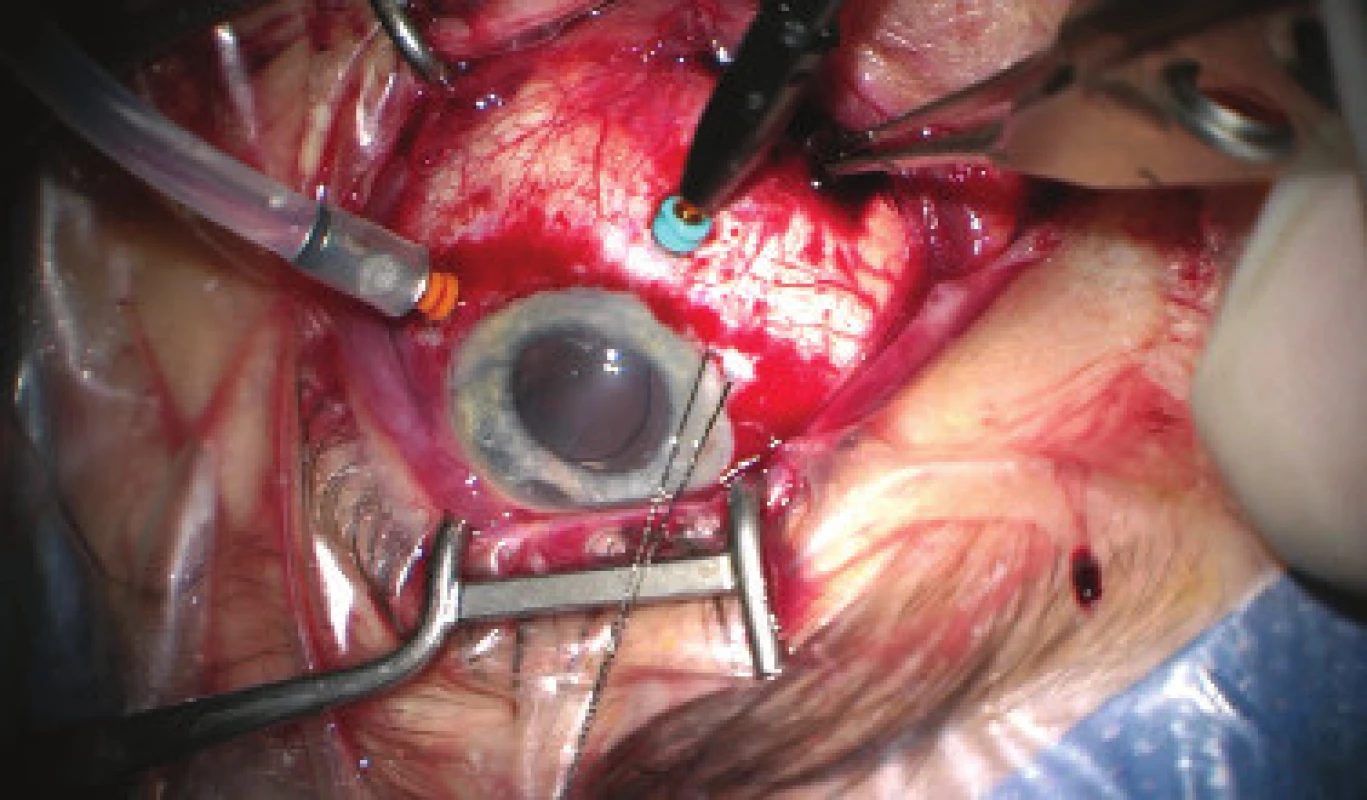
On the first day after the procedure there was a pronounced improvement of vision to 4/50 naturally. Fundus photography of the left eye and the ultrasound image from the same day are presented in fig. 7 and 8. On the second day after the revision there was a further improvement of visual acuity to 6/30 stenopeically. However, in the subsequent period the elevations of intraocular pressure were treated again as they were present even in maximal conservative therapy. On the 27th day following the surgical revision, laser suturolysis was performed, one week later “needling” was performed, which again led to temporary hypotonia of the left eye. In the following 14 days, first of all normalisation was reached, followed by a further elevation of tension in the left eye, and as a result on the 49th day following the surgical revision of suprachoroidal haemorrhage, cyclocryocoagulation of the left eye was performed. This procedure did not achieve an adequate effect, and as a result cyclocryocoagulation of the left eyeball was repeated 16 days later. The patient does not wish to undergo an intraocular surgical solution (for example a further glaucoma operation with drainage implant), which we are not indicating. We are continuing to observe the patient regularly. For a further year vision in the left eye continued at a constant value of 6/30, at the last follow-up examination vision had worsened to 6/60, intraocular pressure in the right eye is compensated, in the left eye subcompensated upon local glaucoma therapy. The perimeter from the last follow-up examination 16 months after trabeculectomy is presented in fig. 9.
Fig. 7. US of left eyeball on 1st day after surgical revision 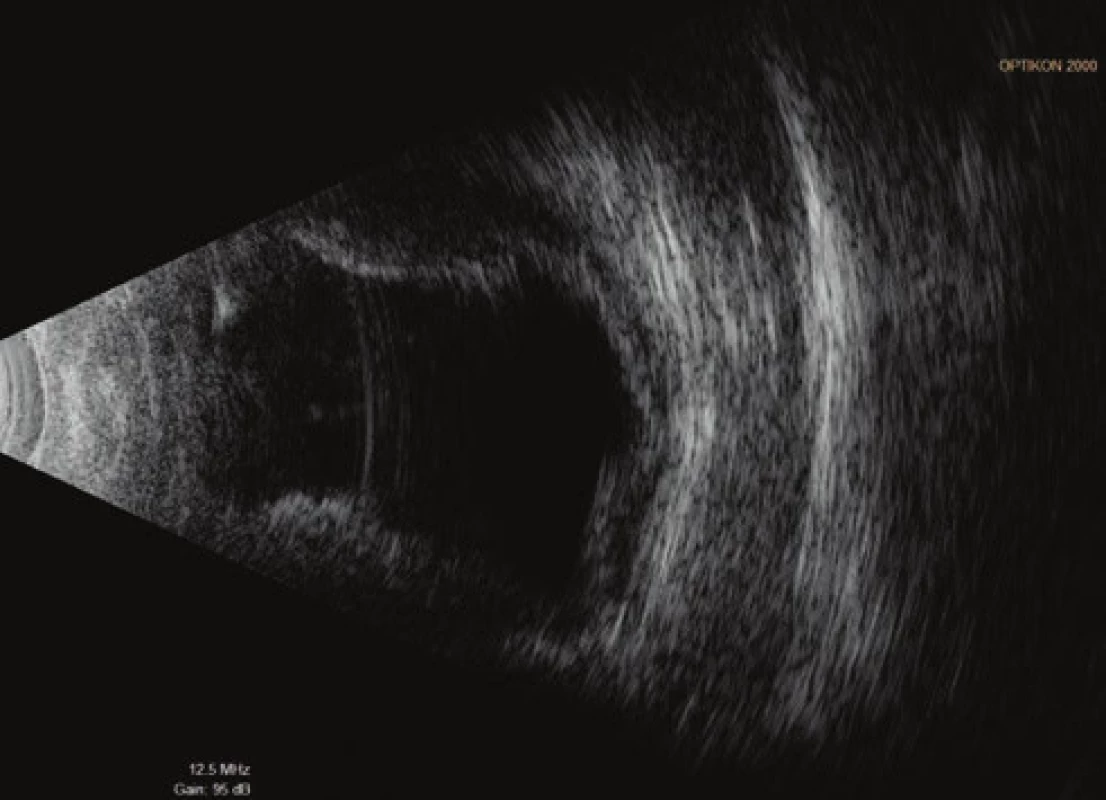
Fig. 8. Photograph of fundus of left eye on 1st day after surgical revision of LE 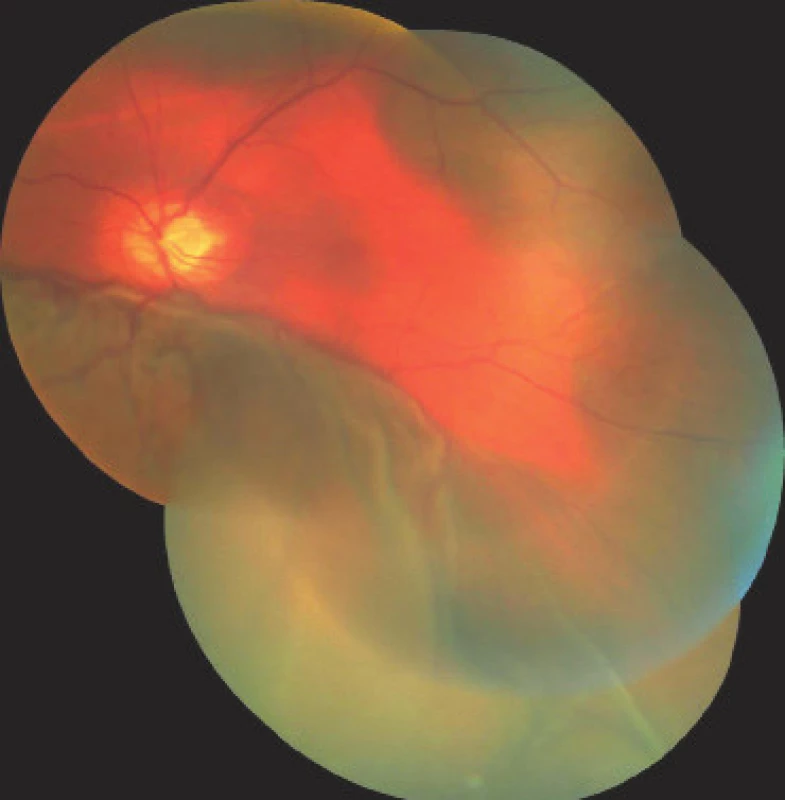
Fig. 9. Perimetric examination of left eye, 8 August 2018 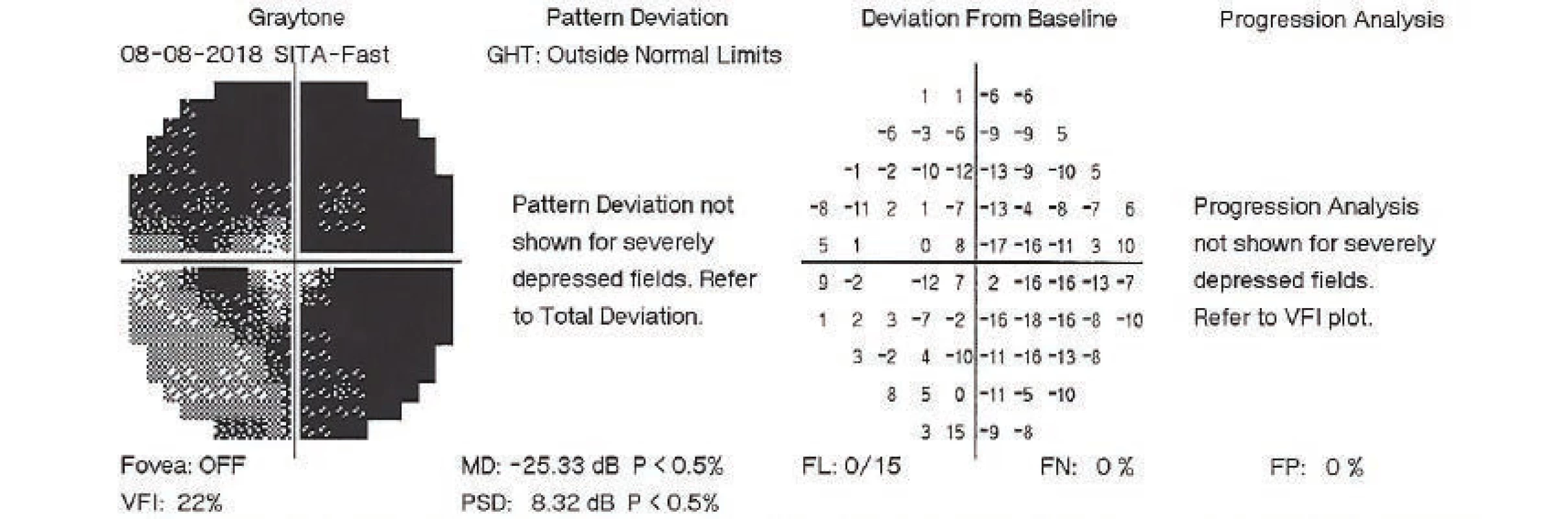
DISCUSSION
In our patient late SCH occurred on the fifth day after trabeculectomy and the second day (upon minimal physical activity) after the severance of the scleral flap and application of fluorouracil beneath the filtration blister.
This patient had fundamental risk factors such as advanced age, glaucoma, axial myopia, condition following cataract surgery with repeated suctions of secondary cataract, and especially postoperative hypotonia.
Of the subjective complaints which patients have upon SCH, the most common is sudden outbreak of pain in the afflicted eye with hemicrania, nausea or vomiting [3]. A peculiar feature of our case was that our patient’s only subjective complaint was rapid and painless loss of sight.
The actual onset of SCH during and after intraocular operations need not be caused by an error or inexperience of the surgeon (above all if the operation takes place as standard), but is generally caused by sudden fluctuations of intraocular pressure in patients with several risk factors [3].
Cases of haemorrhagic choroidal ablation of a smaller scale, not complicated by haemophthalmos, retinal detachment or incarceration of the vitreous body in the surgical wounds can be treated conservatively [9]. Our case report, in accordance with other authors, confirms that it is possible to resolve even the most complicated situations of SCH relatively successfully [6, 7, 14].
We carefully consider indication for glaucoma operations in patients of advanced age, especially so in risk patients with regard to suprachoroidal haemorrhage. In these cases it is also appropriate to take into consideration also less risky surgical procedures to reduce intraocular tension, such as canaloplasty, trabecular microbypass stents, suprachoroidal stents etc. In the case of patients of advanced age with relatively satisfactory visual functions, at the same time we take into account prediction of remaining life in comparison with the expected speed of progression of loss of sight [11].
CONCLUSION
Perioperative and late suprachoroidal haemorrhage is a very serious and feared complication of intraocular surgical procedures. Timely diagnosis and treatment is of key importance for the resulting visual functions of the affected eye. Surgical solution even of the most complicated conditions may have a relatively favourable prognosis.
This article is supported by a project of the Charles University, Prague, Czech Republic (Project PROGRES Q40/07).
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
Received by the Editorial Department on: 27 November 2018
Accepted for printing on: 21 February 2019
MUDr. Markéta Středová
Department of Ophthalmology, University Hospital Hradec Králové, Sokolská 581, 500 05 Hradec Králové
Zdroje
1. Cantor, LB., Katz, LJ., Spaeth, GL. et al.: Complication of surgery in glaucoma: suprachoroidal expulsive hemorrrhage in glaucoma patiens undergoing intraocular surgery. Ophthalmology, 92; 1985 : 1266-1270.
2. Guttham, Ch.: Suprachoroidal hemorrhage management center minimalist techniques. dostupné na WWW <http://www.modernretina.com/modern-medicine-cases/suprachoroidal-hemorrhage-management-centers-minimalist-techniques/page/0/1>
3. Jirásková, N., Rozsíval, P.: Suprachoroideální hemorrhagie – obávaná komplikace nitroočních operací. Trendy soudobé oftalmologie, svazek 7., Galén, 2011, 209-221.
4. Jirasková, N., Rozsíval, P., Pozlerová, J. et al.: Expulsive Hemorrhage after glaucoma filtering surgery. Biomed Pap Med Fac University Palacky Olomouc Czech Repub, 153(3); 2009 : 221-224.
5. Lakpanhal, V.: Experimental and clinical observation on massive suprachoroidal hemorrhage. Trans Am Ophthalmol Soc, 91; 1993 : 545-562.
6. Lakhanpal V., Schocket SS., Elman MJ. et al.: A New Modified Vitreoretinal Surgical Approach in the Management od Massive Suprachoroideal Hemorrhagie. Ophthalmology, 96; 1989 : 793-800
7. Lambrou, FHJr., Meredith, TA., Kaplan, HJ.: Secondary surgical management of expulsive choroidal hemorrhage. Arch Ophthalmol, 105; 1987 : 1195-1198.
8. Rao, A.: Visual restoration after suprachoroidal haemorrhage in glaucoma surgery. BMJ Case Rep; 2014 : 1-4.
9. Reynolds, MG., Haimovici, R., Flynn, HW. et al.: Suprachoroidal Hemorrhage. Ophthalmology, 100; 1993 : 460-465.
10. Sharma, YR., Gaur, A., Azad, RV.: Suprachoroidal haemorrhage. Secondary management. Indian J Ophthalmol, 49; 2001 : 191-192.
11. Stiles, MC.: My Most Difficult Case, A Balancing Act. Glaucoma Today, May/ June 2017; 18-19.
12. Tuli, SS., WuDunn, D., Ciulla, TA. et al.: Delayed Suprachoridal Hemorrhage after Glaucoma Filtration Procedures. Ophthalmology, 108 (10); 2001 : 1808-1811
13. Vail, D.: Posterior sclerotomy as a form of treatment in subchoroidal expulsive hemorrhagie. Am J Ophthalmol, 21; 1938 : 256-260
14. Welch, JC., Spaeth, GL., Benson, WE.: Massive suprachoroidal hemorrhagie: follow-up and outcome of 30 cases. Ophthalmology, 95; 1988 : 1202-1206
Štítky
Oftalmologie
Článek vyšel v časopiseČeská a slovenská oftalmologie
Nejčtenější tento týden
2019 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Familiární středomořská horečka
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Možnosti využití přípravku Desodrop v terapii a prevenci oftalmologických onemocnění
- Selektivní laserová trabekuloplastika nesnižuje nitroční tlak více než argonová laserová trabekuloplastika
-
Všechny články tohoto čísla
- Normal tension vs high tension glaucoma: an – overview
- Correction of myopia and myopic astigmatism by femtosecond laser in situ keratomileusis
- Effect of Botulinum toxin A application in neuro-ophtalmologic indications on Schirmer’s test and tears osmolarity.
- Peripheral exudative hemorrhagic chorioretinopathy
- BILATERAL OPTIC DISC PIT WITH MACULOPATHY – THE CASE REPORT
- Suprachoroideal haemorrhage in postoperative period of antiglaucoma surgery, case report
- Očná klinika Lekárskej fakulty Univerzity Komenského v Bratislave oslavuje 100-TÉ VÝROČIE činnosti – 1. časť
- Česká a slovenská oftalmologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Normal tension vs high tension glaucoma: an – overview
- BILATERAL OPTIC DISC PIT WITH MACULOPATHY – THE CASE REPORT
- Suprachoroideal haemorrhage in postoperative period of antiglaucoma surgery, case report
- Peripheral exudative hemorrhagic chorioretinopathy
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



