-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Blood Vessels and Lymphatics in Calcific Aortic Stenosis –In Support of its Inflammatory Pathogenesis
Krevní a lymfatické cévy v kalcifikované aortální stenóze. Příspěvek k teorii o její zánětlivé patogenezi
V rozvinutých zemích je dnes kalcifikovaná aortální stenóza (KAS) nejčastější získanou chlopenní vadou. Je považována za formu aterosklerózy a, stejně jako tato, zánětlivého původu. Většina případů KAS je stařeckého (sklerotického, “degenerativního” typu), vznikající na normální třícípé chlopni, nebo vzniklá na podkladě vrozeně malformované – dvojcípé aortální chlopně.
Vyšetřili jsme 28 případů resekovaných chlopní KAS (18 stařeckého typu, 7 dvojcípých chlopní a 3 neurčitelného typu) – makroskopicky, histologicky a imunohistochemicky (CD 31 na krevní cévy a D2-40 na lymfatika). V kalcifikovaných cípech byly prokázány krevní cévy ve všech 28 případech, lymfatika pak ve 14 z nich. Častost a charakter vaskularizace byly obdobné u obou typů KAS. Je diskutován původ těchto cév.
Nález přítomnosti krevních a lymfatických cév, spolu s přítomností lymfocytárních infiltrátů podporuje teorii zánětlivého původu KAS.Klíčová slova:
kalcifikovaná aortální stenóza – aortální chlopeň – vaskularizace – lymfatické cévy
Authors: I. Šteiner 1; L. Krbal 1; J. Dominik 2
Authors place of work: The Fingerland Department of Pathology and 2Department of Cardiosurgery, Charles University Faculty of Medicine and Faculty Hospital Hradec Králové, Czech Republic 1
Published in the journal: Čes.-slov. Patol., 46, 2010, No. 2, p. 33-36
Category: Původní práce
Summary
In developed countries, calcific aortic stenosis (CAS) has become the most common acquired valvular disease. It is considered a form of atherosclerosis and, like the latter, of inflammatory origin. Majority of cases of CAS are classified etiologically as either senile (“degenerative”) – developing on previously normal aortic valve with three cusps, or based on congenitally malformed – bicuspid aortic valve. Twenty-eight cases of CAS (18 of the senile type, 7 of the bicuspid valve type, and 3 of indeterminable type) were examined by means of histology and immunohistochemistry (CD31 for blood vessels; D2-40 for lymphatics). In the calcified cusps, blood vessels were present in all 28 cases, and lymphatics in 14 of them. Vascularization was associated with lymphocytic infiltrates in 24 cases. There was no difference in the pattern between the two types of CAS. The origin of the cusp vessels is discussed. Our finding in the calcified cusps of both blood and lymphatic vessels together with lymphocytic infiltrates supports the inflammatory theory of the CAS pathogenesis.
Key words:
calcific aortic stenosis – aortic valve – vascularisation – lymphatic vesselsIn developed countries, calcific aortic stenosis (CAS) has become the most common acquired valvular disease and reason for aortic valve replacement. The prevalence of the disease increases with age, reaching 2–4 % in adults over the age of 65 years (3).
Majority of cases of CAS are classified etiologically as either senile (“degenerative”) – of a previously normal aortic valve with three cusps, or based on congenitally malformed – bicuspid aortic valve (Fig. 1).
Fig. 1. Surgical specimens of CAS: a) senile – “degenerative” type; b) bicuspid aortic valve 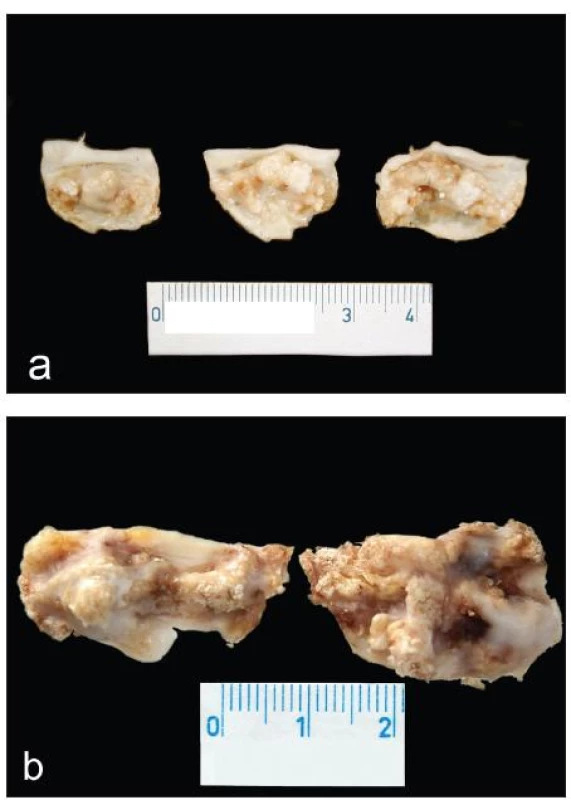
Calcific aortic disease is considered a form of atherosclerosis and, like the latter, of inflammatory origin (6). The aim of our work was to study patterns of blood vessels and lymphatics in CAS.
Material and methods
From December 2008 to March 2009, twenty-eight consecutive calcified aortic valves operatively excised at the Department of Cardiosurgery for pure or predominant nonrheumatic aortic stenosis were submitted for pathological examination.
After gross examination and description, at least one tissue sample was taken vertically from each valve cusp near its center. The formalin-fixed specimens were de-mineralised with the Sakura TDE solution in an automatic decalcifier, processed, embedded in paraffin wax, sectioned and stained with HE and elastica-Van Gieson stain. Immunohistochemistry was performed using antibodies (Dako Cytomation) to the CD31 (blood vessels), and the D2-40 (lymphatic vessels) antigen.
Results
The type of CAS and the patients’ demographic data are summarized in Table 1.
Tab. 1. Calcific aortic stenosis (n=28) 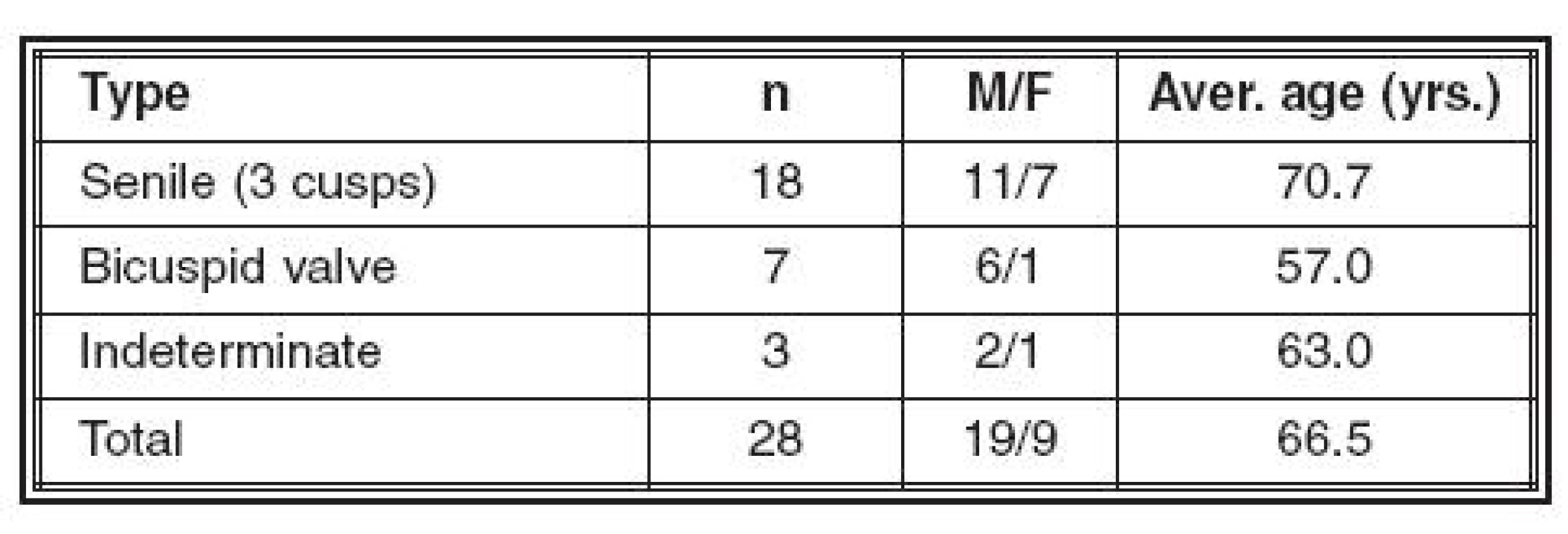
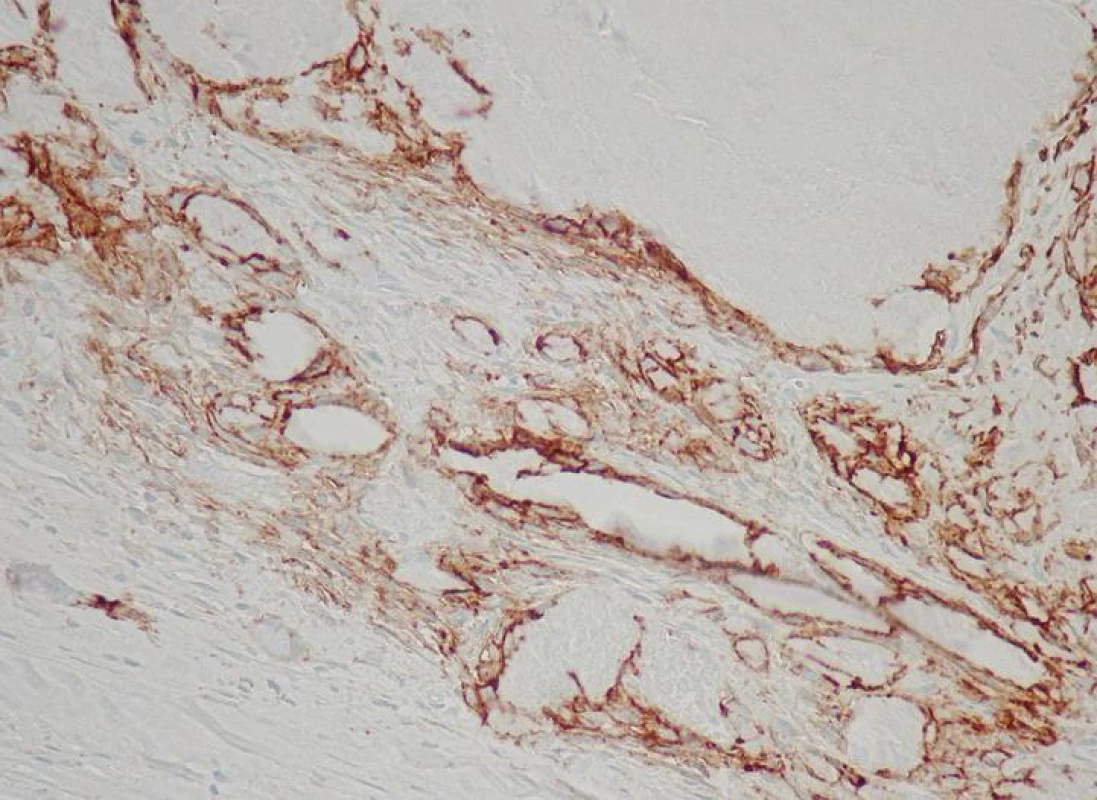
CD31+ blood vessels were present in the cusps in all 28 cases (100 %). They were mostly thin-walled, delicate, irregularly shaped, some of them of sinusoidal character. The vessels were arranged in groups typically localized in the vicinity of calcific foci (Fig. 2) and also beneath the surface area. They were usually empty; in only 10 cases (36 %) some of them did contain blood elements. In 14 cases (50 %) there were, in addition, also thick-walled blood vessels, localized mostly in the basal portion of the cusps.
Fig. 2. CD31+ thin-walled blood vessels in the vicinity of a calcified nodule 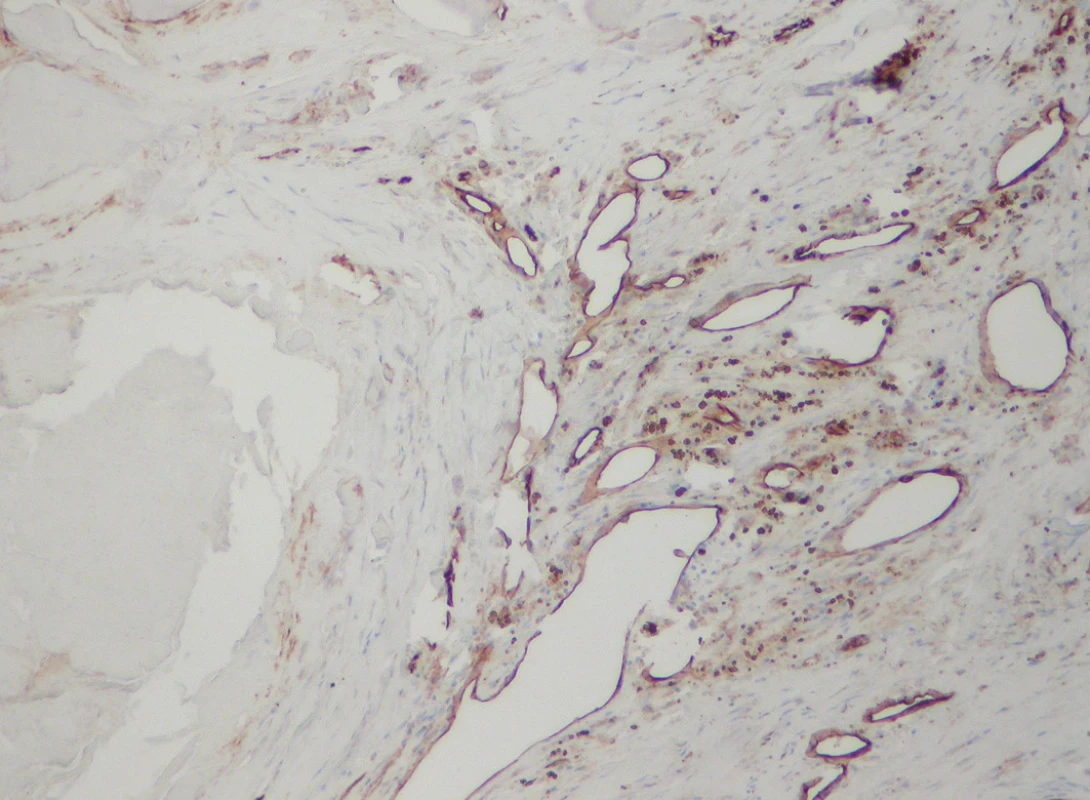
Lymphatic vessels were demonstrated by the D2-40 antibody in 14 cases (50 %), scarcely interspersed among the blood spaces (Figs. 3, 4). Their morphology and distribution was similar to the blood vessels, i.e., they were found related to calcific nodules.
Fig. 4. D2-40+ lymphatics intermingled with negative blood vessels 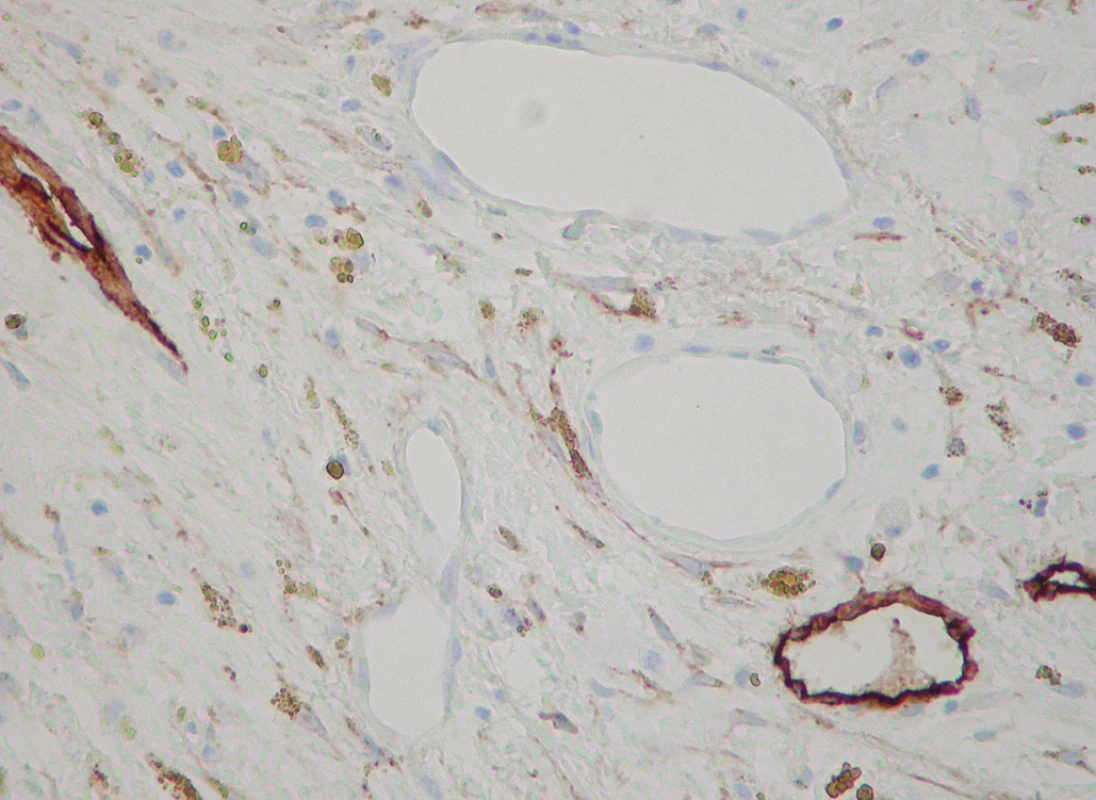
Aggregated areas of inflammatory infiltrates of mostly lymphocytes and macrophages were observed in 24 cases (86 %). They were typically associated with the areas of vascularisation (Fig. 5).
Fig. 5. Chronic inflammatory infiltrate (H&E stain) 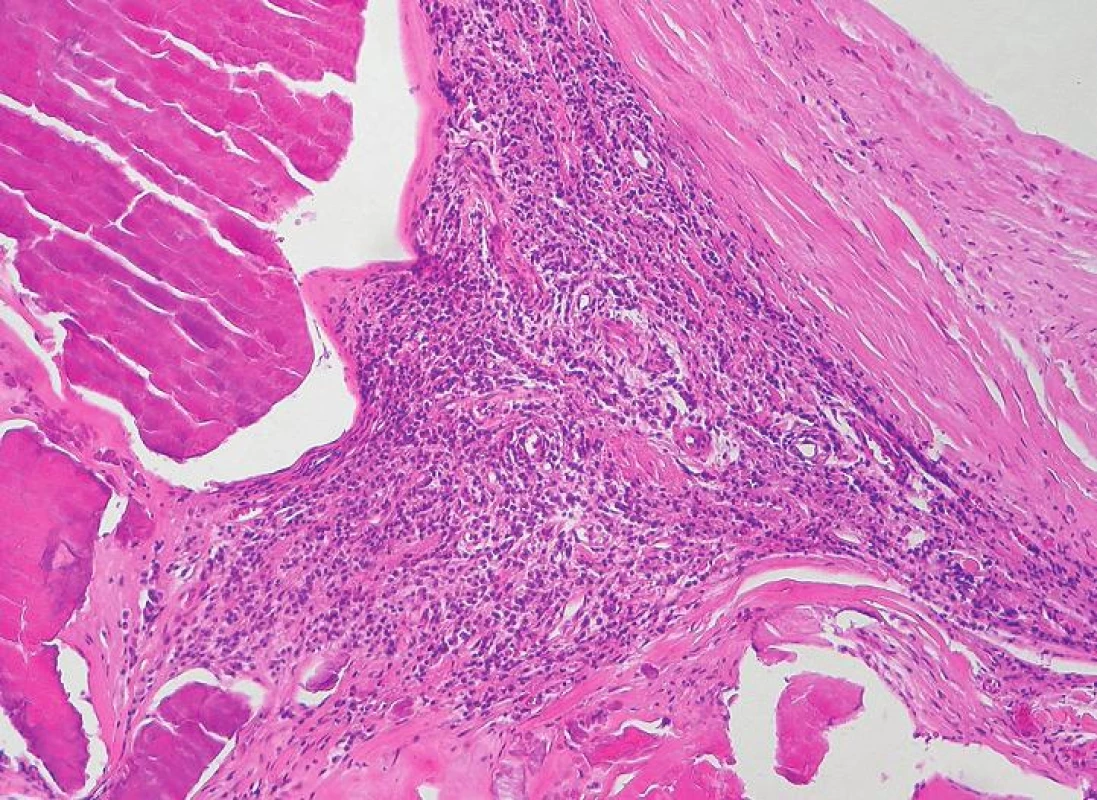
Five valves (18 %) contained foci of metaplastic bone tissue (11).
There was practically no difference between the senile and the bicuspid type of CAS in the incidence and patterns of vascularisation, of inflammatory infiltration, and of bone formation.
Discussion
In the past, calcific aortic disease was thought to be due to degenerative, time-dependent, wear-and-tear of the leaflets, with passive dystrophic calcium deposition. Now, there is compelling histopathological and clinical data to suggest that CAS is an active disease process akin to atherosclerosis, involving endothelial injury, lipoprotein deposition, foam cells, neovascularisation, chronic inflammation and leaflet calcification, and associated with similar risk factors (6).
Descriptions of the presence of blood vessels in the valves of human hearts vary. The prevailing view maintains that normal aortic valve cusps are nearly avascular (1, 2, 9, 10). Cuspal vascularisation seems to be a feature/consequence of valvular inflammation – endocarditis.
To the best of our knowledge, there is no study discussing origin of the vessels. Partically, there are only three possible pathways to aortic valve vascularisation: [1] ingrowth of vessels from the region of the valve ring, [2] infolding of the valvular superficial endocardium, and [3] in situ origin – neoangiogenesis (Fig. 6).
Fig. 6. a) Thick-walled blood vessels in the base of a cusp (H&E stain); b) Infolding of the endocardium from the valvular surface, possibly giving origin to small vessels (CD31); c) Non-luminized endothelial sprouts and small vessels (CD31) 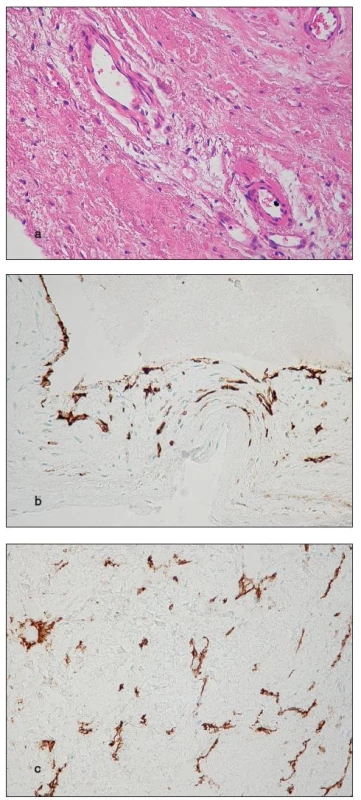
- ad 1) The thick-walled vessels observed in basal parts of cusps in 50 % of our cases (Fig. 6a) are probably of coronary origin, growing from normal vessels present in the region of the valve ring. The finding of blood within the lumina of cuspal vessels in these cases is thus understandable. Among the 10 cases with the content of blood in cuspal vasculature, there were 8 with thick-walled vessels.
- ad 2) The thin-walled vessels were not infrequently found running parallel with the cuspal surface and we have occasionally noticed infolding of the superficial endocardium (Fig. 6b). In such an event, the blood in the vessels may come from the circulation.
- ad 3) There are at least three morphological features in favour of neoangiogenesis: firstly, there was no trace of supplying vessels in the base of the cusps in 50 % of cases; secondly, the vessels correlated with foci of calcium and inflammatory infiltrates; finally, in areas of dense vascularity, the CD31 immunoreaction visualized also non-luminized endothelial sprouts (Fig. 6c). As the source of blood in the neovasculature regards, we speculate that at least part of it communicates with the cuspal surface.
An early event in aortic valve disease appears to be endothelial injury. Lymphocytes and monocytes must enter the valve from the circulation, in response to endothelial dysfunction. Angiogenic growth factors excreted by both endothelial and inflammatory cells are likely to induce angiogenesis in the calcified aortic valve. Angiogenesis further influences progression of CAS (6–8).
The cardiac lymphatic system has been investigated by observations using dye-injection techniques, electron microscopy and lymphangiography (4). In recent years, lymphatic endothelial cell specific proteins have been identified immunohistochemically, including D2-40 antibody (5). Lymphatics have not been found in normal aortic valves. The presence of lymphatics in CAS was noticed by Kholova (personal communication, 2008). To the best of our knowledge, however, our paper is the first systematic study on this topic.
The immunohistochemistry showed sparse lymphatics intermingled with predominating blood vessels. The two types of vessels had the same distribution – around calcific focuses. It is thus reasonable to suspect their common origin in situ.
To conclude, the finding in CAS of blood and lymphatic vessels together with lymphocytic infiltrates supports the inflammatory theory on its pathogenesis. The designation “degenerative” for CAS thus seems inappropriate. We suggest alternative terms – “sclerotic”, or “senile” CAS.
Acknowledgment
The study was supported by the Research Project MZO (Grant 00179906)
Address for Correspondence:
Prof. Ivo Šteiner, MD, PhD.
The Fingerland Department of Pathology
Faculty Hospital
500 05 Hradec Králové, Czech Republic
E-mail: steiner@lfhk.cuni.cz
Zdroje
1. Clarke, J. A.: An x-ray microscopic study of the blood supply to the valves of the human heart. Brit. Heart J., 27, 1965, s. 420–423
2. Duran, C. M. G. Gunning, A. J.: The vascularization of the heart valves: a comparative study. Cardiovasc. Res., 3, 1968, s. 290–296
3. Freeman, R. V., Otto, C. M.: Spectrum of calcific aortic valve disease. Pathogenesis, disease progression, and treatment strategies. Circulation, 111, 2005, s. 3316–3326
4. Johnson, R. A., Blake, T. M.: Lymphatics of the heart. Circulation, 33, 1966 s. 137 – 142
5. Kahn, H. J., Bailey, D., Marks, A.: Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and subset of angiosarcomas. Mod. Pathol., 15, 2002, s. 434–440
6. Mazzone, A., Epistolato, M. C., Gianetti, J. et al.: Biologic features (inflammation and neoangiogenesis) and atherosclerotic risk factors in carotid plaques and calcified aortic valve stenosis. Two different sites of the same disease? Am. J. Clin. Pathol., 126, 2006 s. 494–502
7. Mohler, E. R., Gannon, F., Reynolds, C., et al.: Bone formation and inflammation in cardiac valves. Circulation, 103, 2001, s. 1522–1528
8. Mohler, E. R.: Mechanisms of aortic valve calcification, Am. J. Cardiol. 94, 2004, s. 1396–1402
9. Silver, M. D., Gotlieb, A. I., Schoen, F. J.: Cardiovascular Pathology, 3rd edition. Churchill Livingstone, New York, Edinburgh, London, Philadelphia, 2001
10. Soini, Y., Salo, T., Satta, J.: Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum. Pathol., 34, 2003, s. 756–763
11. Steiner, I., Kašparová, P., Kohout, A., et al.: Bone formation in cardiac valves: a histopathological study of 128 cases. Virchows Arch., 450, 2007, s. 653–657
Štítky
Patologie Soudní lékařství Toxikologie
Článek Plán akcí IPVZČlánek Jaká je vaše diagnóza?
Článek vyšel v časopiseČesko-slovenská patologie

2010 Číslo 2-
Všechny články tohoto čísla
- Fibroblasts – Known or Unknown Cells
- Blood Vessels and Lymphatics in Calcific Aortic Stenosis –In Support of its Inflammatory Pathogenesis
- Prof. MUDr. Jozef Babala, CSc. – k nedožitým osemdesiatinám
- Histologic Findings after Sodium Phosphate Bowel Preparation for ColonoscopyDiagnostic Pitfalls of Colonoscopic Biopsies
- Plán akcí IPVZ
- Jaká je vaše diagnóza?
- Guidelines for autopsy investigation of sudden cardiac death
- U. von Streitberg, G. Seitz, Bamberg: Dobrý let
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fibroblasts – Known or Unknown Cells
- Histologic Findings after Sodium Phosphate Bowel Preparation for ColonoscopyDiagnostic Pitfalls of Colonoscopic Biopsies
- Jaká je vaše diagnóza?
- Prof. MUDr. Jozef Babala, CSc. – k nedožitým osemdesiatinám
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání