-
Medical journals
- Career
Development and uniformity evaluation of low-dose medicated chewing gums prepared by compression method
Authors: Yuliia Maslii 1; Olena Ruban 1; Olga Yevtifieieva 2; Volodymyr Hrudko 2; Svitlana Gureyeva 3; Andriy Goy 3; Tetiana Kolisnyk 1
Authors‘ workplace: Department of Industrial Technology of Drugs, National University of Pharmacy, Valentynіvska str. 4, 61168 Kharkiv, Ukraine 1; Department of Pharmaceutical Chemistry, National University of Pharmacy, Kharkiv, Ukraine 2; JSC Farmak, Kyiv, Ukraine 3
Published in: Čes. slov. Farm., 2020; 69, 33-42
Category: Original Articles
Overview
The aim of this work was to develop medicated chewing gums (MCGs) containing 10 mg of lysozyme hydrochloride (LH) and 20 mg of ascorbic acid (AsA) obtained by the compression method with Health in Gum® (HiG®) PWD 01 as a compressible gum base. Because of a low content of active ingredients, it was essential to choose the way of adding them to the tableting mass and evaluate their distribution homogeneity in the dosage units. The blends for compression were prepared by two methods: the first one was simple mixing of all components; the second one included the step of wet granulation of a three-component mixture – LH, sucralose and a taste additive. Flow properties of LH, AsA, HiG®, LH granules and blends for compression were studied. MCGs were evaluated according to Ph.Eur. 9.0 Chapters 2.9.5, 2.9.6 and 2.9.40. AsA and HiG® were characterized as free flowing, while LH had insufficient flow properties. Compared with a simple mixed blend, the granulation step allowed significantly improving flow properties of the final blend for compression. Unlike MCGs compressed from the simple mixed blend, MCGs prepared through the granulation step met Ph.Eur. 9.0 Chapter 2.9.40 requirements. The propriety of MCG preparation method involving the step of wet granulation also has been confirmed by mass and drug content uniformity tests.
Keywords:
ascorbic acid – medicated chewing gums – lysozyme hydrochloride – mass uniformity – drug content uniformity – uniformity of dosage units
Introduction
One of the key factors primarily affecting the choice of medication for many patients is the type of dosage form. As it is known, solid dosage forms for oral administration are the most convenient and preferable. Among modern solid oral dosage forms, medicated chewing gums (MCGs) offer valuable advantages such as easy and pleasant administration, suitable consumption at any time without need of water and swallowing, as well as very fast onset of action due to the oral mucosa absorption and therefore less first-pass metabolism1–3).
Owing to a comparatively long retention time in the oral cavity, MCGs are attractive dosage form for the use in dentistry4–6). Depending on active ingredients which incorporated into MCGs can exert a therapeutic or disease-preventive action on both soft oral tissues and dentin. In particular, MCGs can be a good delivery platform for releasing drugs against such common oral diseases as gingivitis, periodontitis, stomatitis and dental caries. A good candidate for medicated chewing gum formulation is lysozyme – a natural antimicrobial compound normally occurring in human saliva. Lysozyme possesses activity against gram-positive and some gram-negative pathogenic bacteria, fungi and viruses and has anti-inflammatory and immunomodulating effects7–9). It also serves as a protective agent violating the ability of microorganisms to be attached to a tooth surface that is caries prevention10–11). Lysozyme hydrochloride (LH), a derivative of lysozyme, is known to be an active ingredient of several marketed medications (Lysobact®, Bosnalijek, Bosnia and Herzegovina; Lysopain® N, Sanofi-Aventis, Switzerland; Glossithiase®, Jolly-Jatel, France; etc.), in which its dose per intake varies from 5 to 20 mg.
Other oral problems which can be cured by MCGs are hyposalivation and xerostomia, experienced as mouth dryness. Inadequate saliva production reduces the defence mechanisms of the oral cavity, significantly increasing the risk of oral mucosa diseases, oral ulcers and inflammation symptoms. Administration of MCGs directly increases salivation in response to mechanical (chewing) stimulus12). In addition to chewing, salivary flow can be enhanced by using saliva stimulants – organic acids, e.g. ascorbic acid (AsA), which also plays a prominent role in oral health maintenance. AsA deficiency has been proved to cause dryness of oral mucosa, gingival bleeding and petechial hemorrhages in various parts of the mouth, resulting in xerostomia, ulcerative gingivitis and stomatitis development13). However, it should be borne in mind that high concentrations of AsA may demineralize teeth, leading to dental erosions. In the view of this, low doses of AsA should be considered for inclusion in chewing products. For example, the amounts of AsA of 30 and 60 mg incorporated into MCGs were shown to be “too low to affect hard tissues”14).
There are three main methods for the production of MCGs: traditional (melting) method (I), cooling, grinding and tableting method (II), and direct compression method (III). The principal drawback of the first two methods is the difficulty to achieve high accuracy of content uniformity of active pharmaceutical ingredients (APIs) in MCGs. For instance, in the case of the most commonly used traditional method the heating process and further mixing of highly viscous mass lead to the risk of alterations in the shape, size and/or weight of the final gums, which complicates the control of dosing homogeneity. Moreover, the melting technology is associated with raised temperatures, which may cause instability of thermosensitive ingredients, as well as with the use of complex equipment for hot melt processing and cooling. In contrast, the direct compression method allows not only simplifying and speeding up the manufacturing process of MCGs but also excluding the above-mentioned risks and drawbacks, especially by using directly compressible co-processed gum bases like Health in Gum® (HiG®) developed by Cafosa Gum SA (Spain)1–3, 15).
Despite apparent advantages, the direct compression method is often associated with challenges. For instance, segregation of the components can occur, leading to inhomogeneous distribution of ingredients in the mass for compression and then in the dosage units. One of the principal risk factors for segregation is the wide range of particle sizes in the direct compression blends, in which APIs tend to have the finest particles. Besides, other powder properties, which are important for a successful compression process, such as good flowability, also depend a lot on particle size uniformity16).
Improving powder flowability is achieved when using a granulation technique that narrows particle size distribution and in such a way eliminates segregation problems. The most effective way to turn the powders into granules is by wetting powder blend with granulating liquid and further milling and drying the wetted mass, i.e. the-so called “wet granulation”.
Although wet granulation provides apparent improvement of certain parameters, it requires more time and equipment units compared to direct compression and there is a risk of product loss during the different processing steps (granulation, drying, sieving). All of these factors can increase costs of the final product. Wet granulation is also contraindicated for moisture sensitive materials.
Thus, the aim of this work was to develop MCGs containing lysozyme hydrochloride 10 mg and ascorbic acid 20 mg obtained by the compression method using HiG® as the compressible gum base. Because of a low content of APIs, it was essential to choose the way of addition of APIs to the chewing gum mass. In the view of this, two batches of MCGs were prepared – by the direct compression method and with the step of wet granulation – and subjected to the evaluation of API distribution homogeneity in the dosage units.
Experimental part
Materials
Description of APIs and excipients used in the study is given in Table 1. All the chemicals and reagents used were of analytical grade.
1. Description of APIs and excipients used in the study 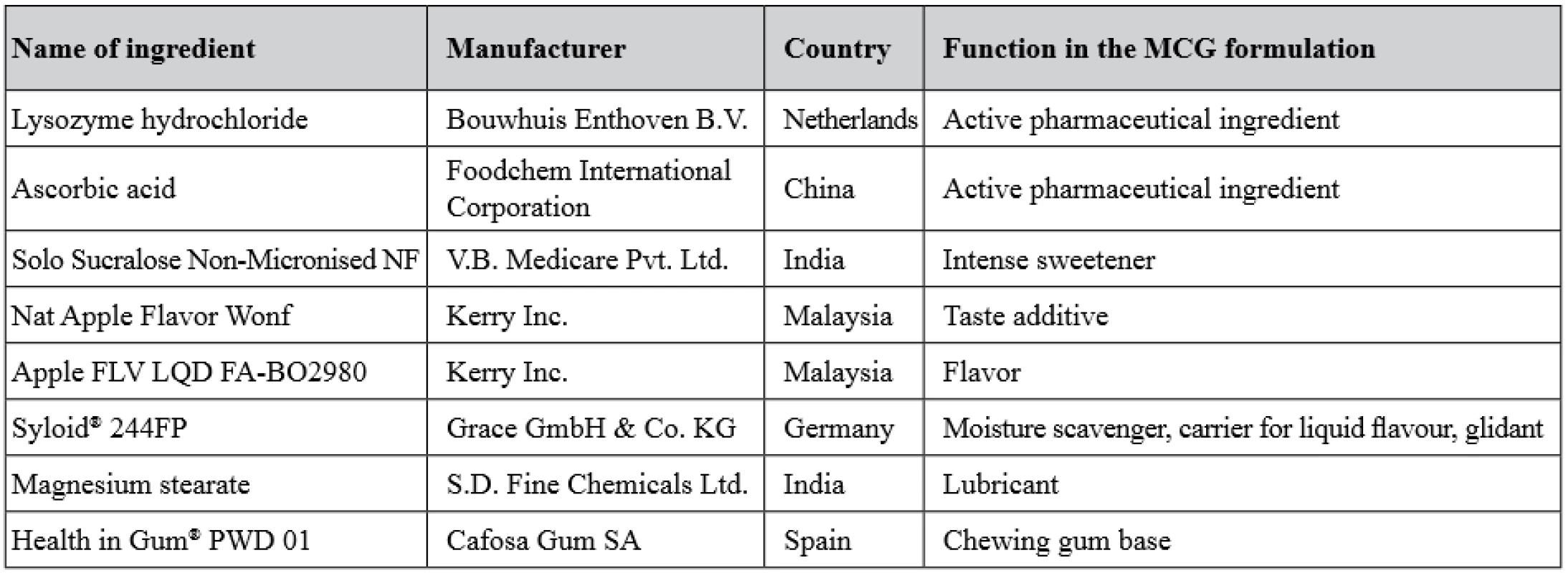
Preparation of medicated chewing gums
Two batches of MCGs were obtained by different methods. To prepare the first MCG batch, all ingredients were sieved through a sieve with a pore diameter of 1.0 mm, then dry-mixed and directly compressed (i.e., direct compression method) – batch DC. The second method included the step of wet granulation of the mixture of LH, a taste additive and sucralose. In this method, the three-component mixture was wetted using 96.0% ethanol, granulated through a sieve with a pore diameter of 2.0 mm, then dried at a room temperature and sifting through a sieve with a pore diameter of 1.0 mm. After that the granules were individually mixed with the rest of ingredients – batch WG. Both batches of MCGs were produced on a laboratory single-punch tablet press (model HTM-01E, Mariupol Plant of Technological Equipment, Ukraine) to obtain gums with a mass of 1000 mg.
The composition of the batches is given in Table 2.
2. Composition of MCG batches obtained by the direct compression (DC) method and with a step of wet granulation (WG) 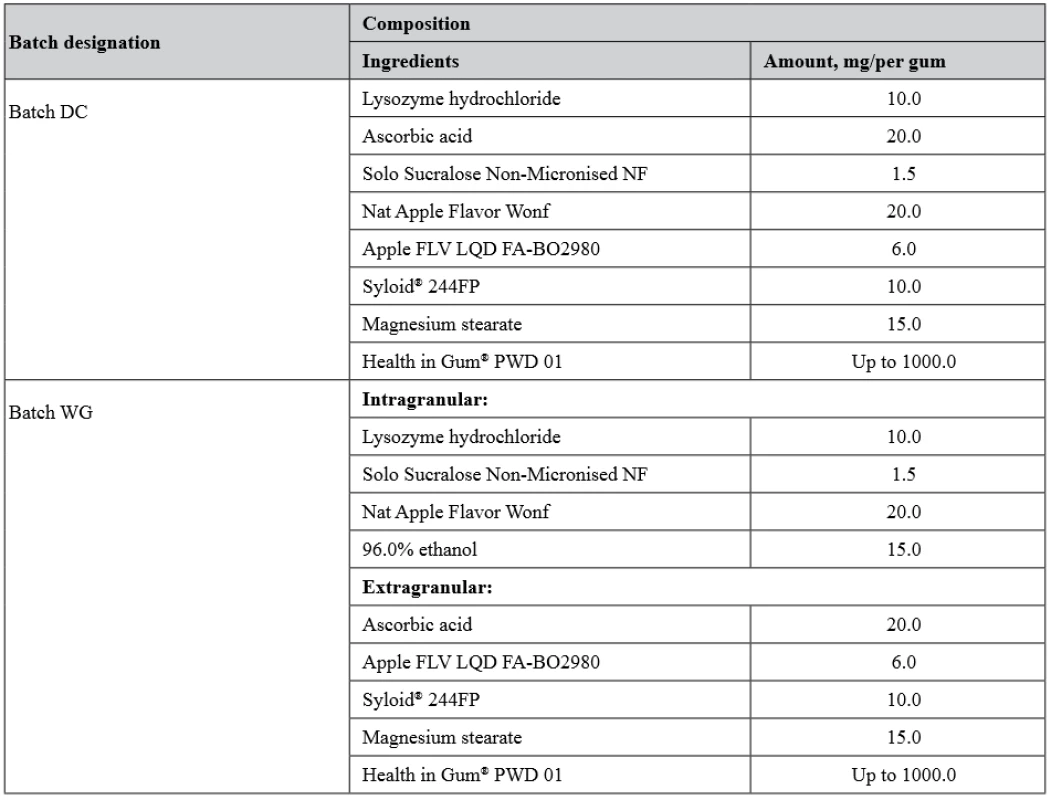
Bulk and flow properties
To choose the proper method for gum mass preparation, bulk and flow properties of APIs, granules, HiG® and final blends for compression (batch DC and batch WG) such as bulk density, tapped density, compressibility index and Hausner ratio, flowability, and angle of repose were determined.
A Pharma Test Tap Density Tester (Model PT-TD1, Germany) was used to determine bulk and tapped densities of the samples as described in the European Pharmacopoeia (Ph.Eur.) 9.0 Chapter 2.9.34 “Bulk density and tapped density of powders”. Compressibility index and Hausner ratio were calculated from bulk and tapped densities according Ph.Eur. 9.0 Chapter 2.9.36 “Powder flow”.
Flowability was determined as the time required for pouring out of 100.0 g of the sample. Flow rate measurements were performed using a fixed conical funnel (diameter of the outflow opening, 15 mm) with a damper of a laboratory device for powder flowability determination (Model VP-12A, Mariupol Plant of Technological Equipment, Ukraine). The diameter of the cone formed by the heaped material was registered. Angle of repose was calculated as the inverse tangent (arctan) of the ratio of the cone maximum height to its radius.
Optical microscopy
A Konus 1000× Academy Biological Monocular Microscope (Konus Italia Group S.P.A., Italy) equipped with a camera ScopeTek was used for particle characterization of the samples. The processing of photos was carried out using Scope Photo software (version 3.0.12.498).
Evaluation of prepared MCGs
The prepared gums were evaluated according to Ph.Eur. 9.0 Chapters 2.9.5 “Uniformity of mass of single-dose preparations”, 2.9.6 “Uniformity of content of single-dose preparations”, and 2.9.40 “Uniformity of dosage units”.
Assay
The LH assay was carried out spectrophotometrically using a Specord 200 spectrophotometer (Analytik Jena AG, Austria) as described in the Japanese Pharmacopoeia (JP XVII) monograph for LH. To perform the assay test, one gum was crushed to powder by using a laboratory crushing machine Knife Mill Pulverisette 11 (Fritsch GmbH, Germany) to obtain the sample solution with an approximate LH concentration of 0.01 mg/ml. Lysozyme JP Reference Standard (RS) (PMRJ, Japan) with 0.98 mg of lysozyme per mg (calculated on the dried basis) was used in this experiment to obtain the standard solution (approximate concentration, 0.01 mg/ml).
The AsA assay was performed by redox-based iodometric titration. One gum (previously crushed to powder) was placed into a flask and for better wetting of the powder 20 ml of 40.0% ethanol was added. The content of the flask was heated to 40–50 °C, shaken and then cooled to a room temperature. 10 ml of a 0.1 M hydrochloric acid solution, 0.5 ml of a 10 g/l potassium iodide solution, and 2 ml of a starch solution as the indicator were added to the flask. The mixture was shaken and titrated with a 0.0167 M potassium iodate solution until a persistent violet-blue color was obtained. 1 ml of 0.0167 M potassium iodate is equivalent to 8.8271 mg of AsA.
Statistical analysis
Statistical analysis was carried out using Microsoft Excel 2010 software.
For the test “Uniformity of dosage units” (Ph.Eur. Chapter 2.9.40), the contents of AsA and LH were determined in each of 10 randomly selected MCGs of the batch. The acceptance value (AV) was calculated depending on the mean of individual contents (X̅,%) as described in Ph.Eur. The batch was considered to meet the requirements of this test if AV was less than or equal to the maximum allowed acceptance value L1 = 15.
For the test “Uniformity of mass of single-dose preparations” (Ph.Eur. Chapter 2.9.5) the average mass, SD, RSD, and RSD maximum level (RSDmax) were calculated. RSDmax was determined as RSDmax = maxΔx/t(95, 19), where maxΔx – maximum percentage deviation, t(95, 19) – Student’s distribution at 95% confidence level and the degree of freedom of 1917). RSD calculated for this batch of MCGs was compared with RSDmax, which is 2.4 with maxΔx = 5.0% and t = 2.093.
For the test “Uniformity of content of single-dose preparations” (Ph.Eur. Chapter 2.9.6), the contents of LH and AsA were determined in each of 10 MCGs. For each individual MCG, the content of AsA in mg per a dosage unit , the percentage content of AsA with reference to the average content , and the percentage content of AsA with reference to the nominal content were calculated using the formulas [1], [2] and [3], respectively:
where VT is the volume of 0.0167 M potassium iodate solution, ml; F is the titrant correction factor; mi is the mass of one MCG unit, mg; C̅ and Cnom are the average content (mg) and the nominal content (mg) of AsA, respectively.
The obtained results were averaged by calculating the average values ,,, , as well as the relative standard deviation values, and the relative confidence intervals .
The evaluation of LH content uniformity in the dosage units was carried out in the same way as for AsA, wherein the amount of LH (mg) per 1 mg of MCG (CLH) and the content of LH in mg per a dosage unit (Cmg/mi) were calculated by the following formula [4]:
where MS – amount of Lysozyme RS taken (calculated on the dried basis), mg; MT – amount of the sample taken (calculated on the dried basis), mg; AS1 and AS2 – the absorbance at 640 nm of the standard solutions with the concentrations of 0.005 mg/ml and 0.01 mg/ml, respectively; AT – the absorbance at 640 nm of the sample solution; mi – the mass of one MCG unit, mg.
Results and discussion
To find out whether it is possible to use direct compression for obtaining MCGs with LH and AsA, the bulk and flow properties of APIs and the gum base as the dominant excipient in the gum mass were studied (Table 3).
3. Bulk and flow properties of APIs and the compressible gum base Health in Gum® 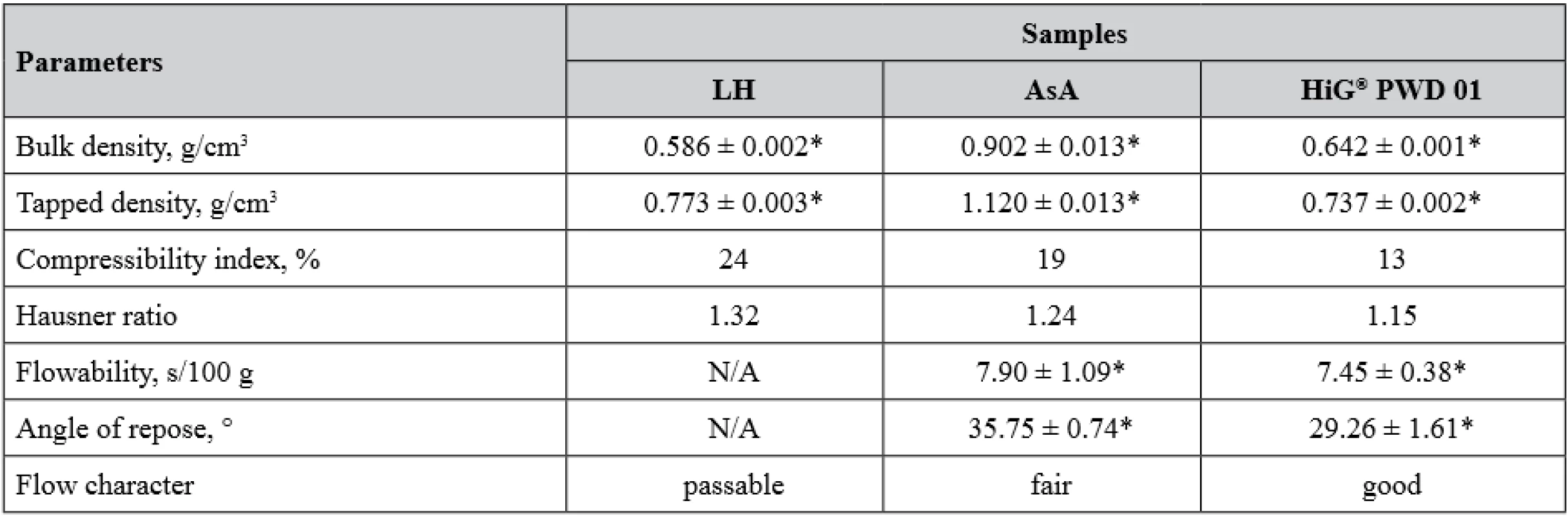
LH – lysozyme hydrochloride, AsA – ascorbic acid, N/A – not applicable, the powder does not flow *n = 5, p < 0.05, values are expressed as mean ± confidence interval From Table 3, it is obvious that LH exhibited insufficient flow properties – it does not flow through the funnel at all and the values of compressibility index and Hausner ratio indicate a passable flow character. Another situation was observed in the cases of AsA and gum base HiG® which were free-flowing with close average values of flow rate. Possible explanations of these findings may include several points. First, poor flow of LH powder can be associated with much finer particle size distribution compared to AsA and HiG®. As it is known, flowability is the capacity of powders to pour out and the driving force of this process is gravity, hence the greater the mass of powder particles, then the better their ability to flow. Obviously, LH powder had the smallest value of bulk density, since its particles are the lightest and their gravity was not enough to fill all cavities between them. When tapping was applied, additional force contributed to further particle downward movement and powder density changed dramatically resulting in a greater Hausner ratio. The second point is the difference in the surface character of the particles. If the powder particles have a rough, unsmooth surface, then they will form many bridges due to the cohesive bonds at the point of contact between one another under loose packing. Under tapping, these cohesive bonds are broken and again higher powder density is obtained. On the other hand, less cohesive powders will not undergo so much change in bulk density, as they do not form the bridging cohesive bonds18).
However, it should be noted that the flow characters of AsA and HiG® slightly differ according to the calculated compressibility indexes and Hausner ratios, as well as angle of repose values, and are regarded as fair and good, respectively. These data are in accordance with the declared applicability of HiG® as a gum base intended for direct compression1, 3, 19).
Taking into account insufficient flow properties of LH, which may cause its inhomogeneous distribution in MCGs16), two different methods for preparation of gum mass were tested. The first method was simple dry mixing of all ingredients as it is usually practiced in direct compression technology (batch DC). The second method included the step of wet granulation of a three-component mixture (LH, sucralose and taste additive). AsA was not considered to undergo wet granulation because of its satisfactory flow properties and also in order to avoid its oxidation upon contact with the granulating liquid20). Therefore, AsA powder was mixed with the granules and gum base, after that the rest of the excipients were added (batch WG). Bulk and flow properties of LH granules and blends for compression of two batches are given in Table 4.
4. Bulk and flow properties of lysozyme hydrochloride granules and two final blends 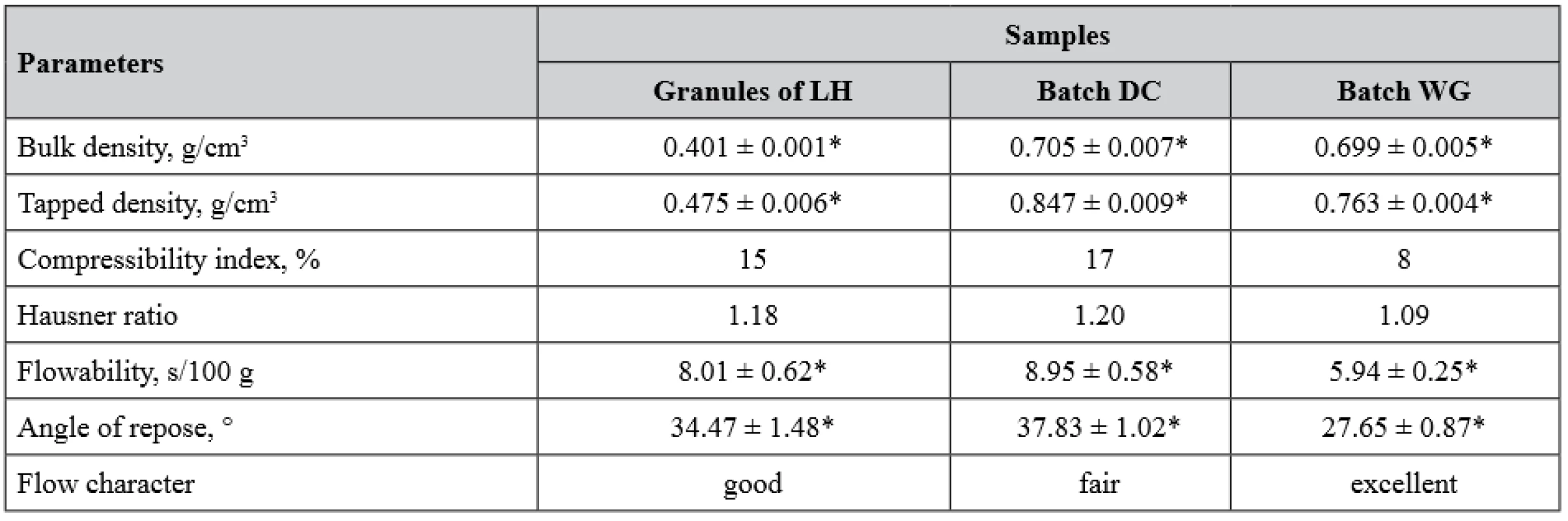
LH – lysozyme hydrochloride
*n = 5, p < 0.05, values are expressed as mean ± confidence intervalAs it is clearly seen from Table 4, the granulation step expectedly improved flow properties of LH and the blend for compression of the batch WG, whose flow character was achieved to be excellent contrary to the simple mixed blend of the batch DC with only fair flow. To determine how granulation step affects particle homogeneity in the blend for compression, we carried out optical microscopic examination for both blends (Fig. 1).
According to Fig. 1, it can be concluded that the step of wet granulation has led to more homogenous particles in the final blend for compression (batch WG), which also resulted in improving flow properties of this blend.
1. Optical microscopy characterization (3.3 times zoom) of particles: I – simple mixed blend (batch DC), II – blend prepared with the step of wet granulation (batch WG), A – HiG® particles, B – ascorbic acid particles, C – lysozyme hydrochloride and the rest excipient particles, D – granules obtained from lysozyme hydrochloride, sucralose, and taste additive 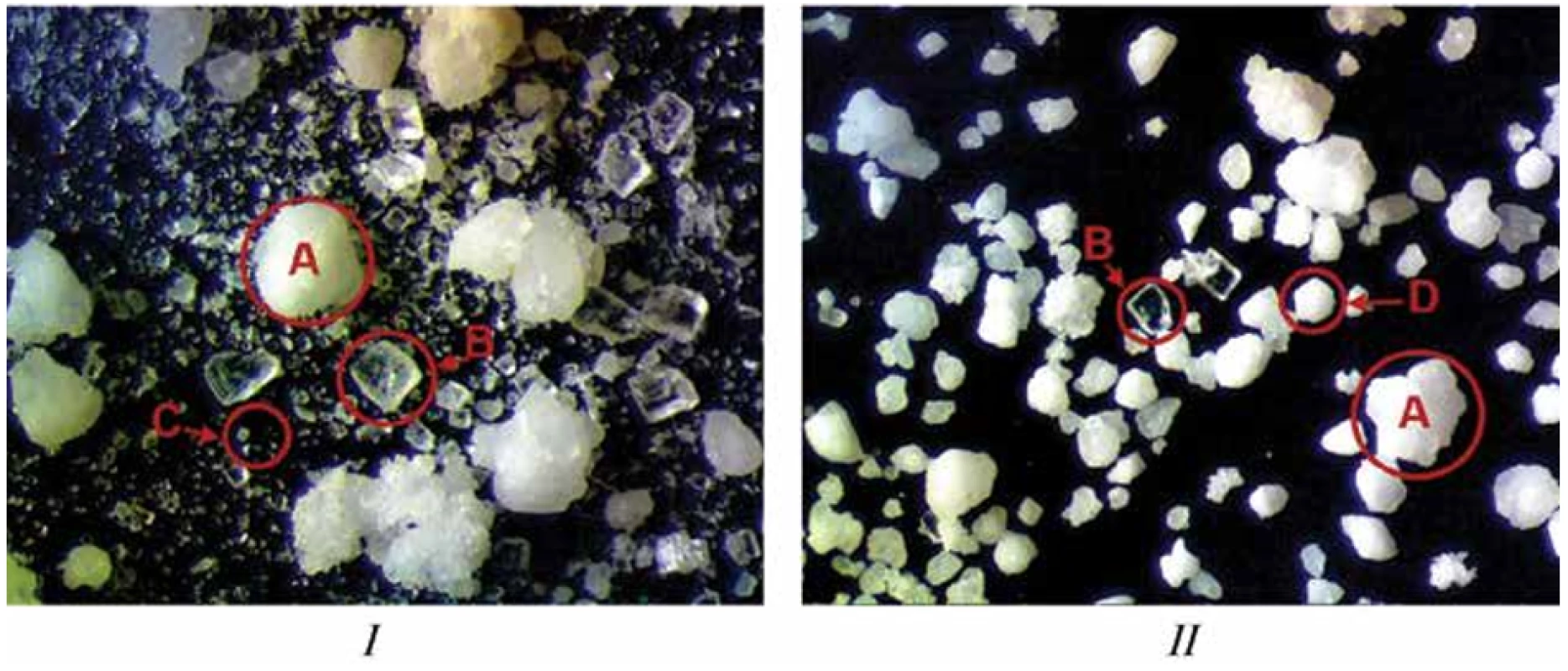
We further studied whether the method for preparation of the blend for compression is crucial for the uniformity of compressed chewing gums.
According to Ph.Eur. 9.0, MCGs should comply with the test for uniformity of dosage units (UDU), which characterizes the degree of distribution homogeneity of APIs among dosage units (chapter 2.9.40). For single-dose solid dosage forms with multiple components, content uniformity test is applicable. This test is based on the assay of the APIs in each of 10 units of the dosage form to determine whether the individual contents are within the limits set21). Table 5 shows the results of content uniformity test of dosage units for MCGs of two different batches.
5. The results of content uniformity test of dosage units for two batches of MCGs 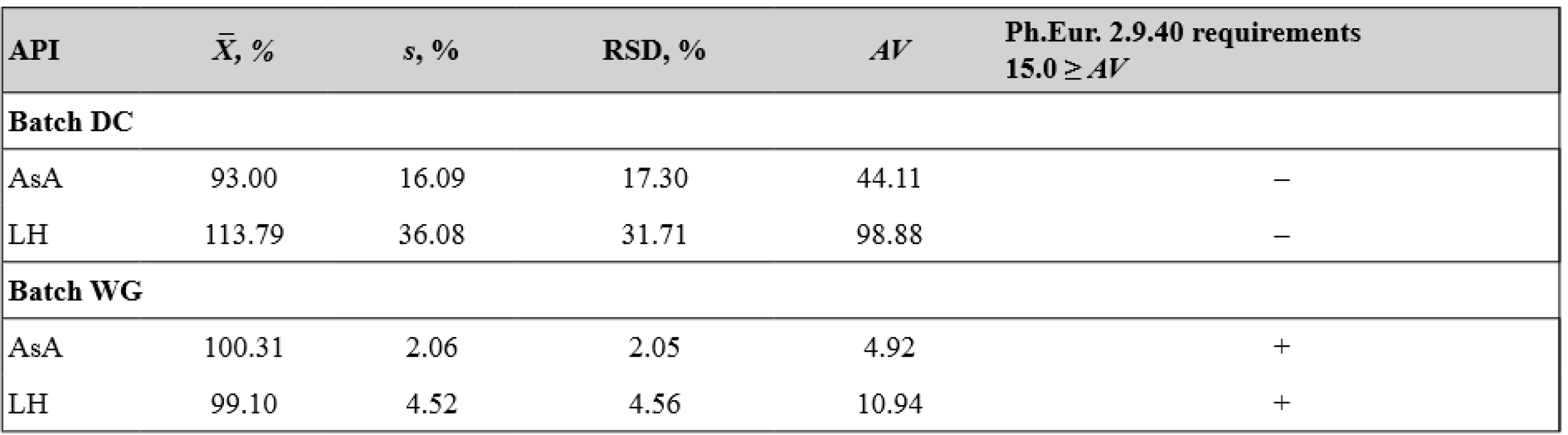
(+) passed the test, (–) failed the test Since for batch DC MCGs acceptance values (AV) calculated for both APIs are significantly higher than L1 values, it was decided that further testing of 20 units (e.g., requirements of Ph.Eur. chapter 2.9.40) for this batch would not be appropriate.
Based on RSD and AV calculated for each API in MCGs of two different batches, it can be concluded that only batch WG dosage units meet requirements of Ph.Eur. 2.9.40 L1 = 15.0 ≥ AV. So, further investigations were performed only for this batch of MCGs.
It is obvious that content uniformity is integral to mass uniformity of dosage units and critical deviations in drug content are often related to weight variations. In turn, weight variations can occur because of inadequate flow of the blend for compression. Therefore, we have evaluated mass uniformity of batch WG MCGs in accordance with Ph.Eur. Chapter 2.9.5 “Uniformity of mass of single-dose preparations”. The acceptance criterion in this test is a condition that not more than 2 of the individual MCG masses deviate from the average mass by more than ± 5% and none deviates by more than ± 10%. To ensure that each individual mass will be within established limits we also calculated RSD for this batch of MCGs and compared it with RSDmax, which was determined as mentioned above. The results of mass uniformity test are given in Table 6.
6. The results of mass uniformity of dosage units for MCGs of batch WG 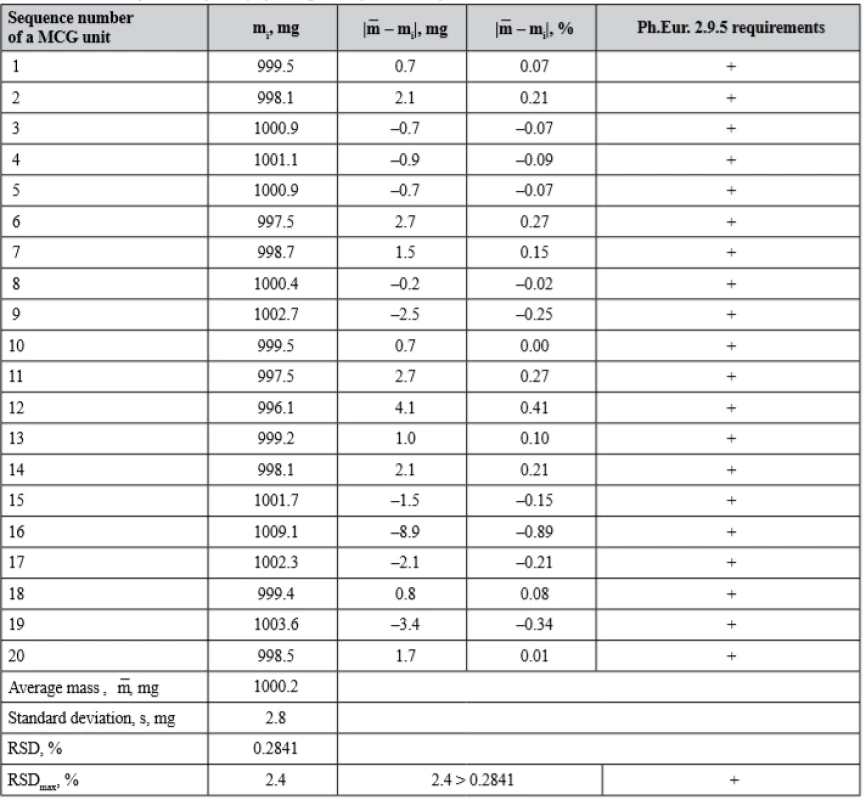
(+) passed the test, (–) failed the test Taking into account the data of Table 6, MCGs of the batch WG meet the requirements of Ph.Eur. Chapter 2.9.5 “Uniformity of mass of single-dose preparations”, since the deviation from the average mass for each of 20 dosage units does not exceed the acceptable value. Also, RSD calculated for this batch is less than RSDmax value.
The test of content uniformity of single-dose preparations (Ph.Eur. chapter 2.9.6) is also very important for single-dose preparations with a low content of API(s). This test is based on the assay of API(s) contents in a number of individual dosage units in order to determine whether the individual contents are within the limits set with reference to the average content of the sample21). The nominal contents for LH and AsA are 10.0 and 20.0 mg per gum weighing 1000 mg, respectively, that is less than 2% of the total dosage unit mass for both APIs. The batch was considered to comply with the test if each individual content was between 85 and 115% of the average content21).
The results of the test for uniformity of AsA content and LH content in 10 MCGs units of the batch WG are given in Tables 7 and 8, respectively.
7. The results for the test of ascorbic acid content uniformity in 10 MCGs units of batch WG according to Ph.Eur. Chapter 2.9.6 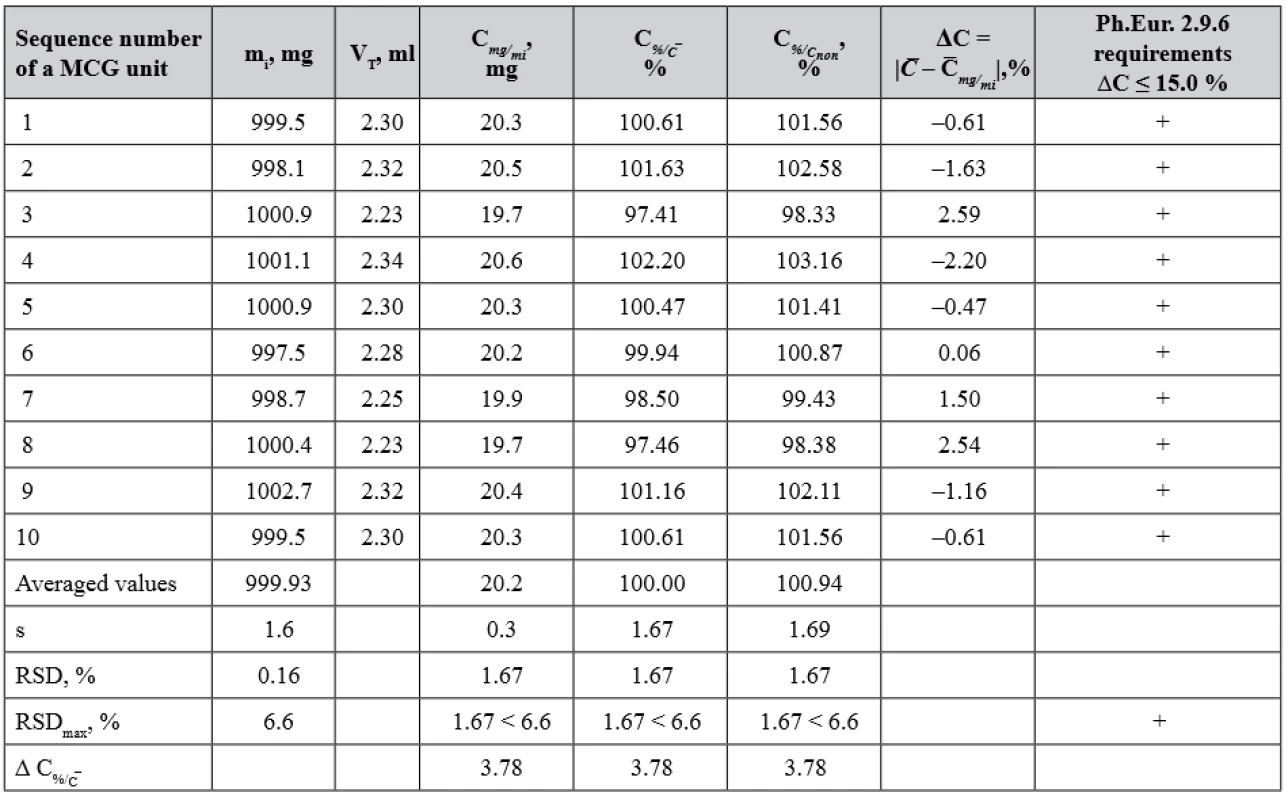
(+) passed the test, (–) failed the test 8. The results for the test of lysozyme hydrochloride content uniformity in 10 MCGs units of batch WG according to Ph.Eur. Chapter 2.9.6 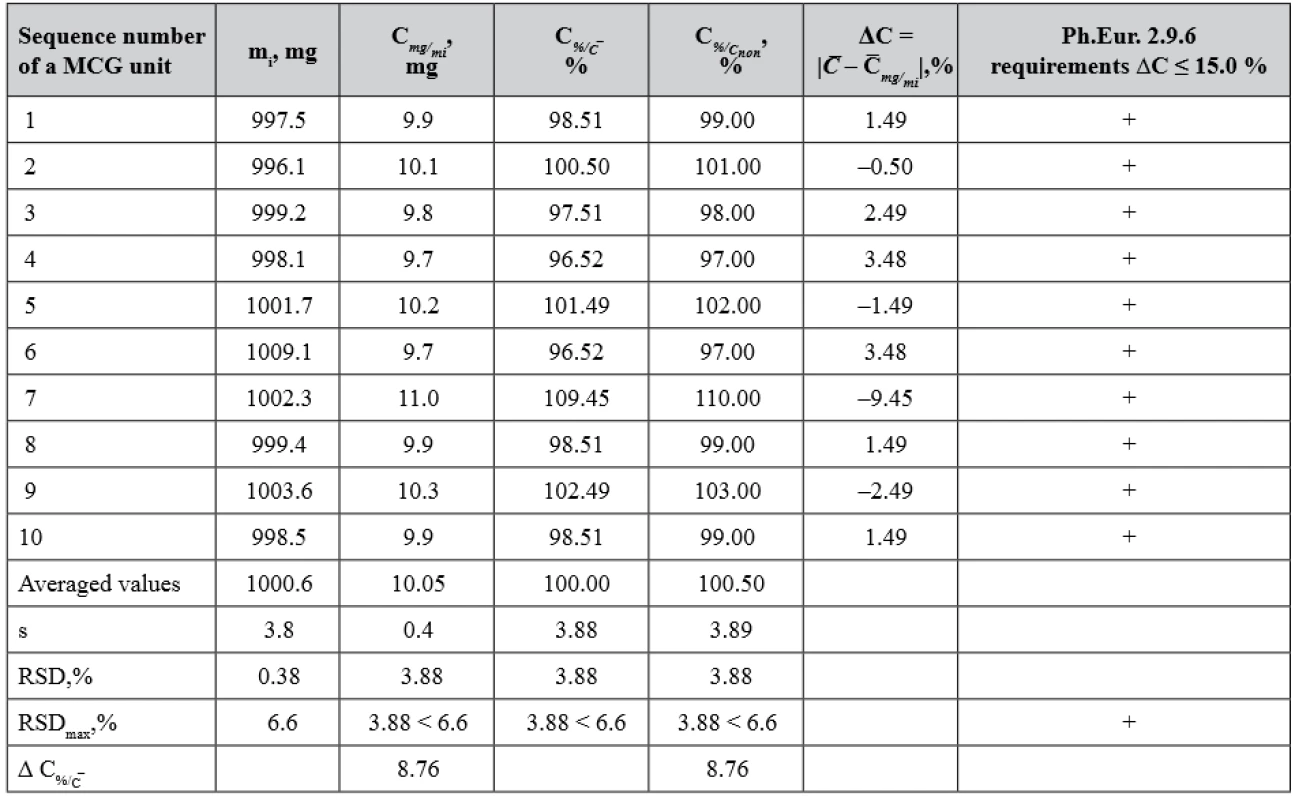
(+) passed the test, (–) failed the test The evaluation of AsA content uniformity in the MCG dosage units was carried out in two ways. Firstly, according to the requirements of Ph.Eur. 2.9.6, the percentage deviations of AsA content in the individual MCG units from its average content were compared with acceptable limits of 85–115% (ΔC ≤ 15.0%). It should be noted that when testing according to Ph.Eur. 2.9.6 requirements, the value of the deviation from the nominal content is not taken into account. Secondly, the statistical parameters of the obtained results were compared with the critical values for these metrological characteristics (Table 7). The content of AsA in the MCG units is within 20.2 mg ± 3.78% relative to its average content and 20.0 mg ± 3.78% relative to its nominal content, i.e. the deviation of the average content from the nominal content in the tested batch (100.94 – 100.00 = 0.94%) does not significantly affect the distribution of individual contents in single dose units and is within the acceptable limits in accordance with the requirements of Ph.Eur.
As it is seen from Table 8, the deviation of the average content of LH from its nominal content Δ = |10.0 – 10.05| = 0.05 mg or |100.00 – 100.50| = 0.5 %.
Therefore, MCG batch WG meets the requirements of Ph.Eur. chapter 2.9.6 (Test A) – the content of APIs in each MCG unit is within the limits of 85–115% of the average content.
Content uniformity is one of the critical quality attributes of compressed solid dosage forms (tablets, MCGs, etc.) containing low doses of APIs. A number of factors, such as the concentration of individual components in the mixture for compression, particle shape and size distribution, bulk and flow properties, cohesive and adhesive characteristics of substances have a potential effect on this attribute. In addition, parameters of the technological process also have a certain impact on uniformity content: the intensity and duration of mixing, the shape and degree of loading of the mixer, etc.22, 23). For instance, the effect of mixing time and different low API concentrations on the content uniformity of directly compressed warfarin tablets was examined in the works of J. Muselík and colleagues22, 23), and also J. Elbl and colleagues24). According to their results, concentrations of API smaller than 2% per tablet did not allow to obtain, by the direct compression method, the products meeting the requirements of the Pharmacopoeia 2.9.6 and 2.9.40 even when using the optimized preparation and mixing technology.
Another study by M. T. am Ende and colleagues was devoted to the application of the granulation process (roller compaction) in the preparation of low-dose tablets. The use of this step was shown to minimize the segregation potential by improving content uniformity due to more homogeneous particle size distribution25).
D. G. Morris and colleagues examined the influence of the excipient type and method of producing on the content uniformity of low-dose tablets. According to their conclusions, direct compression may be applicable for low-dose tablets (e.g. < 1 mg) when API has a suitable particle size and potency loss to equipment is negligible26).
It should be noted that a principal difference between medicated chewing gums obtained by compression and common tablets is the presence of chewing gum base in the formulation, such as directly compressible HiG®. The possibility for obtaining MCGs by direct compression using this gum base is well described in other works27–29), however, in those studies MCGs contain either one API that is easier to distribute in the blend for compression, or a higher content of API in the formulation. In the present study the principal ingredient of low-dose combination – HiG® PWD 01 – significantly differs from the APIs in particle and technological properties, which do not allow obtaining by direct compression a uniform API content distribution in the final MCG batch. The solution proposed by us for this problem – preliminary wet granulation of the API with the finest particles – improved flow properties of the blend for compression and has led to MCGs which fully meet Ph.Eur. 2.9.6 and 2.9.40 requirements.
Thus, these findings confirm that unlike simple mixing the use of granulation technique for API having the finest particles, which are significantly smaller than the gum base HiG® particles, allows obtaining compressed MCGs with acceptable mass and drug content uniformity.
Conclusions
Compression method for the production of MCGs offers many advantages; however, some crucial issues similar to the tablet manufacture should be considered in this case. One of the most important of them is the achievement of weight and drug content uniformity in dosage units, directly affected by the blend for compression – its proper flow and absence of segregation caused by particle inhomogeneity. Our studies have demonstrated that the use of direct compression method for the production of low-dose combination MCGs does not allow obtaining dosage units with acceptable drug content uniformity. However, the use of granulation technique for API with the finest particles and insufficient flow not only improves flow properties of the blend for compression but also contributes to homogeneous drug content for both APIs in compressed MCGs. The propriety for the MCG preparation method that has been chosen is also confirmed by mass and drug content uniformity tests according to Ph.Eur.
Conflict of interest: none.
Yuliia Maslii • О. Ruban • T. Kolisnyk
Department of Industrial Technology of Drugs, National University of Pharmacy Valentynіvska str. 4, 61168 Kharkiv, Ukraine
e-mail: julia.masliy@gmail.com
O. Yevtifieieva • V. Hrudko
Department of Pharmaceutical Chemistry, National University of Pharmacy, Kharkiv, Ukraine
S. Gureyeva • A. GoyJSC Farmak, Kyiv, Ukraine
Sources
1. Al Hagbani T., Nazzal S. Medicated Chewing Gums (MCGs): Composition, Production, and Mechanical Testing. AAPS PharmSciTech. 2018; 19(7): 2908–2920.
2. Aslani A., Rostami F. Medicated chewing gum, a novel drug delivery system. J. Res. Med. Sci. 2015; 20(4): 403–411.
3. Chaudhary S. A., Shahiwala A. F. Medicated chewing gum – a potential drug delivery system. Expert Opin. Drug Deliv. 2010; 7(7): 871–885.
4. Dodds M. W. The oral health benefits of chewing gum. J. Ir. Dent. Assoc. 2012; 58(5): 253–261.
5. Itthagarun A, Wei S. H. Chewing gum and saliva in oral health. J. Clin. Dent. 1997; 8(6): 159–162.
6. Wessel S. W., van der Mei H. C., Maitra A., Dodds M. W., Busscher H. J. Potential benefits of chewing gum for the delivery of oral therapeutics and its possible role in oral healthcare. Expert Opin. Drug Deliv. 2016; 13(10): 1421–1431.
7. Ogundele M. O. A novel anti-inflammatory activity of lysozyme: modulation of serum complement activation. Mediators Inflamm. 1998; 7(5): 363–365.
8. Ragland S. A., Criss A. K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017; 13(9): e1006512.
9. Zhang X., Wu F. X., Sun M., Wang Q. Y., Wang Y. J., Yang X. K. Study on antimicrobial and antiviral activities of lysozyme from marine strain S-12-86 in vitro. Agric. Sci. China 2008; 7(1): 112–116.
10. Krzyściak W., Jurczak A., Piątkowski J., Kościelniak D., Gregorczyk-Maga I., Kołodziej I., Papież M. A., Olczak-Kowalczyk D. Effect of histatin-5 and lysozyme on the ability of Streptococcus mutans to form biofilms in vitro conditions. Postepy Hig. Med. Dosw. 2015; 69 : 1056–1066.
11. Moslemi M., Sattari M., Kooshki F., Fotuhi F., Modarresi N., Khalili Sadrabad Z., Shadkar M. S. Relationship of salivary lactoferrin and lysozyme concentrations with early childhood caries. J. Dent. Res. Dent. Clin. Dent. Prospects 2015; 9(2): 109–114.
12. Karami-Nogourani M., Kowsari-Isfahan R., Hosseini-Beheshti M. The effect of chewing gum’s flavor on salivary flow rate and pH. Dent. Res. J. (Isfahan) 2011; 8(Suppl 1): S71–S75.
13. Padayatty S. J., Levine M. Vitamin C: the known and the unknown and Goldilocks. Oral Dis. 2016; 22(6): 463–493.
14. Lingström P., Fure S., Dinitzen B., Fritzne C., Klefbom C., Birkhed D. The release of vitamin C from chewing gum and its effects on supragingival calculus formation. Eur. J. Oral Sci. 2005; 113(1): 20–27.
15. Kása P., Jójárt I., Kelemen A., Pintye-Hódi K. Formulation study of directly compressible chewable polymers containing ascorbic acid. Pharm. Dev. Technol. 2013; 18(2): 384–389.
16. Meeus L. Direct compression versus granulation. Pharm. Technol. Eur. 2011; 23 : 21–22.
17. Gryzodub O. I., Leontiev D. A., Levin M. G., Asmolova N. N., Vyrova H. V. Realization of “Content uniformity” and “Dissolution” tests by chromatographic methods under production quality control. J. Org. Pharm. Chem. 2004; 2(1): 24–34. Russian.
18. Hoag S. W. Capsules dosage form: Formulation and manufacturing considerations. In: Developing Solid Oral Dosage Forms, 2nd ed. Qiu Y., Chen Y., Zhang G. G. Z., et al. (eds.) Academic Press 2017; 723–747.
19. Jójárt I., Kása P. Jr, Kelemen A., Pintye-Hódi K. Study of the compressibility of chewing gum and its applicability as an oral drug delivery system. Pharm. Dev. Technol. 2016; 21(3): 321–327.
20. Pavlovska G, Tanevska S. Influence of temperature and humidity on the degradation process of ascorbic acid in vitamin C chewable tablets. J. Therm. Anal. Calorim. 2013; 111(3): 1971–1977.
21. Council of Europe. European Pharmacopoeia, 9th edition, Strasbourg: Council of Europe 2016.
22. Muselík J., Franc A., Štarková J., Matějková Z. Optimization of technological processes for the preparation of tablets with a low content of warfarin by direct compression. Čes. slov. Farm. 2014; 63 : 217–221.
23. Alyami H., Dahmash E., Bowen J., Mohammed A. R. An investigation into the effects of excipient particle size, blending techniques and processing parameters on the homogeneity and content uniformity of a blend containing low-dose model drug. Pharm. Dev. Technol. 2009; 14(5): 451–460.
24. Elbl J., Muselík J., Franc A., Mikušová J., Dolejší Z., Vetchý D. Influence of drug concentration and blending technology on the content uniformity of mixture for low dose warfarin tablets. Čes. slov. Farm. 2016; 65(6): 211–215.
25. am Ende M. T., Moses S. K., Carella A. J., Gadkari R. A., Graul T. W., Otano A. L., et al. Improving the content uniformity of a low-dose tablet formulation through roller compaction optimization. Pharm. Dev. Technol. 2007; 12(4): 391–404.
26. Morris D. G., Truitt B. F., Kong A., Leyva N., Luner P. E. Influence of formulation composition and processing on the content uniformity of low-dose tablets manufactured at kilogram scale. Pharm. Dev. Technol. 2009; 14(5): 451–460.
27. Özlem Akbal, Erdal Cevher, Ahmet Oğul Araman. The development and in vitro evaluation of benzydamine hydrochloride medicated chewing gum formulations. Istanbul J. Pharm. 2017; 47(2): 45–51.
28. Ranmale J. S., Thombre N. A., Kshirsagar S. J., Aher A. S. Formulation development and evaluation of amoxicillin based medicated chewing gum. Indian Journal of Novel Drug Delivery 2015; 7(3): 108–115.
29. Mansi Paradkar, Balaram Gajra, Bhautik Patel. Formulation development and evaluation of medicated chewing gum of anti--emetic drug. Saudi Pharm. J. 2016; 24 : 153–164.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 1-
All articles in this issue
- Metal complexes in medicine and pharmacy – the past and the present II
- K životnému jubileu pani doc. RNDr. Zuzany Vitkovej, CSc.
- Životné jubileum prof. RNDr. Daniela Grančaia, CSc.
- Zomrel Alois Borovanský
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Double-coated pellets with semipermeable ethylcellulose coating for detection of cholinesterase inhibitors
- Development and uniformity evaluation of low-dose medicated chewing gums prepared by compression method
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- The amino acid and carbohydrate composition of the herb and roots of Smallanthus sonchifolius
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Metal complexes in medicine and pharmacy – the past and the present II
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- Zomrel Alois Borovanský
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career



