-
Medical journals
- Career
Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
Authors: Maksym Almakaiev 1; Olga Vashchenko 2; Dmitry Sofronov 3; Liliia Budianska 2; Longin Lisetski 2
Authors‘ workplace: National University of Pharmacy, Valentynivska str. 4, 61168 Kharkiv, Ukraine 1; Institute for Scintillation Materials of NAS of Ukraine, Kharkiv, Ukraine 2; State Scientific Institution «Institute for Single Crystals», NAS of Ukraine, Kharkiv, Ukraine 3
Published in: Čes. slov. Farm., 2020; 69, 43-47
Category: Original Articles
Overview
To identify possible interactions of components in dosage forms, studies are usually carried out at the stage of pharmaceutical development. Such studies can predict compatibility of active pharmaceutical ingredients and excipients in order to optimize drug formulation and certain parameters of technological process. Compatibility of some components of a newly developed neuroprotective medicinal product ‘Neuronucleos’, namely, thioctic acid, pyridoxine hydrochloride, magnesium stearate and magnesium lactate, was studied by means of differential scanning calorimetry (DSC) and Fourier-transform infrared spectroscopy (FTIR). No interactions were observed between thioctic acid and pyridoxine hydrochloride. Formation of an intermolecular complex between thioctic acid and magnesium stearate was established, in which this acid substitutes the crystalline water of magnesium stearate. No significant interactions were found for magnesium lactate with thioctic acid or magnesium stearate. Thus, pharmaceutical compatibility of the most of the tested ‘Neuronucleos’ components was studied and established, with the only exception (thioctic acid with magnesium stearate). Moreover, the present study provides valuable information about thermal effects in a certain temperature range, which is important for setting the technological process parameters.
Keywords:
pharmaceutical compatibility – thioctic acid – pyridoxine hydrochloride – magnesium stearate – differential scanning calorimetry – IR-spectroscopy
Introduction
Studies of components compatibility in combined dosage forms are an important stage in pharmaceutical development because physico-chemical interactions are possible between some components. As a result of incompatibility, changes in a medicinal product appearance and increase of impurities level may take place. Therefore, the compatibility study at the stage of pharmaceutical development can reveal significant interactions between the components of a dosage form that could adversely affect stability and efficacy of this dosage form.
Calorimetric techniques are considered to be among the basic in the field of pharmaceutical compatibility investigations1). Deformations or disappearance of characteristic calorimetric peaks in a mechanical mixture of substances serves as incompatibility markers. Molar ratios of components in such mixtures are generally chosen as 1 : 1 or as the corresponding mass ratio in the dosage form1, 2).
The compatibility studies were carried out during pharmaceutical development of a new combined medicinal product in capsules, which was notionally named ‘Neuronucleos’. The medicinal product formulation was developed on the basis of literature data and our own experimental experience. The developed medicinal product is recommended for treatment of disorders of the peripheral nervous system that are symptoms of osteoarticular, metabolical and infectious diseases. The medicinal product formulation includes uridine-5-monophosphate disodium salt and cytidine-5-monophosphate disodium salt, as well as other active pharmaceutical ingredients, viz pyridoxine hydrochloride, thioctic acid and magnesium lactate dihydrate, which enhance the effect of the former ones. Silica and magnesium stearate were chosen as excipients. It should be noted that a medicinal product with a similar performance under the name Nucleo CMP forte (Ferrer International S.A., Spain) is widely administered in Europe.
In order to obtain a medicinal product of appropriate quality containing thioctic acid, compatibility data were required for this acid as the most sensitive active substance of the dosage form. In the present work the experimental data are collected concerning the compatibility of thioctic acid (TA), pyridoxine hydrochloride (PH), magnesium stearate (MgSt) and magnesium lactate dihydrate (MgLac).
Materials and methods
Several components of the capsules ‘Neuronucleos’ were examined. Among these components were thioctic acid (TA) (Shanghai Modern Pharmaceuticals, China, batch 1104001 А); pyridoxine hydrochloride (PH) (DSM Nutritional Products GmbH, Germany, batch UQ 11113337); magnesium lactate dihydrate (MgLac) (Моес Cantabtia, Spain, batch 2327), and magnesium stearate (MgSt) (Calmags GmbH, Germany, batch 02021011). MgSt (Magnesia, Germany, batch 15000959/0) was additionally used to obtain the binary phase diagram of MgSt : TA. All the components were of pharmacopeia grade.
The compatibility studies were performed by means of differential scanning calorimetry (DSC) using a Mettler DSC 1 microcalorimeter (Mettler Toledo). To obtain DSC thermograms, 4 to 7 mg of a sample were placed into a standard 40 μl aluminum crucible with a perforated lid (additionally, a 0.3 mg sample of MgSt was used, which corresponds to the MgSt amount in the sample of TA : MgSt mixture). Thermal scanning was carried out in the air atmosphere within the temperature range of 0 to 150 °C at the scanning rate of 5 °C/min. Original DSC thermograms were plotted as heat flow (mW) vs. sample temperature (°C). Normalized DSC thermograms are also used, where the original values of heat flow are normalized by the TA mass (W/g). The processing of the obtained DSC thermograms (determination of DSC peaks maxima, peak normalization, etc.) was performed using the original STARe SW 11.00 software of the microcalorimeter. Experimental error of the determination of peak temperatures was 0.3 °C.
Thermal stability studies of TA were performed by thermogravimetric analysis (TGA) using the TG 10 A block of the Mettler TA 3000 thermoanalytical system. For TGA studies, about 7 mg of the sample were placed into a 70 μl aluminum oxide crucible without a lid. Thermal scanning was carried out in the air atmosphere within the temperature range of 30 to 250 °C at the scanning rate of 5 °C/min. TGA curves were plotted as sample mass (percents of the initial value) vs. sample temperature. Experimental error of sample mass determination was 0.1 mg.
Fourier-transform infrared (FTIR) spectra were obtained using a Spectrum One spectrophotometer (Perkin-Elmer). The samples were placed between ZnSe plates by ‘crushed drop’ technique and examined in the range of 4000 to 400 cm–1 at room temperature. The original FTIR spectra were obtained in the values of optical transmission and then they were recalculated into the values of absorbance with subtraction of the baseline (absorption of the ZnSe plates). Experimental error of wavelength values was 1 cm–1.
Results and discussion
The results of the TA and MgSt compatibility studies are shown in Figure 1. As it can be seen, the DSC thermogram of TA contains a single endothermic peak with the maximum about 60.9 °C, which originates from TA melting3). The DSC thermogram of MgSt contains two characteristic endothermic peaks. The first one (98.0 °C) corresponds to elimination of crystallization water, and the second one (112.7 °C) is related to MgSt melting4). It should be underlined that both peaks are clearly distinguished in DSC thermograms of MgSt samples of 0.3 mg weight, which corresponds to MgSt amount in the sample of TA : MgSt mixture (Fig. 1, upper curve). The weight loss of the MgSt samples after heating was about 6 % w/w. Basing on this value, crystallization water content was estimated as 2.0 mol/mol. This indicates that the tested substance is MgSt dihydrate, which is considered to be the optimal for the use as an excipient5).
1. Original DSC thermograms of individual TA, MgSt and mixture TA : MgSt 20 : 1 by mass 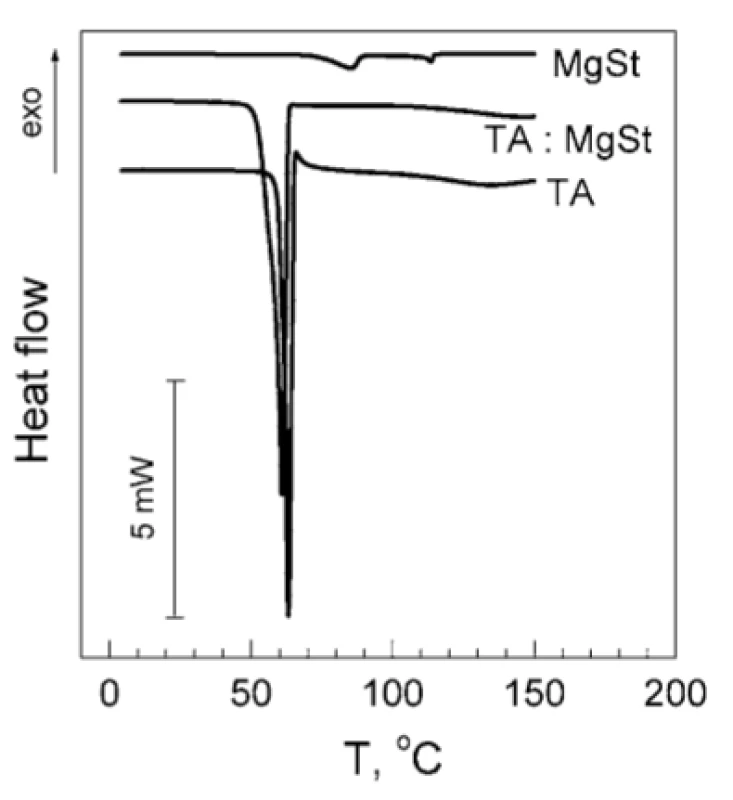
In the mixture TA : MgSt (Fig. 1, middle curve) the peak of TA melting decreases by 2.2 °C and becomes smeared, whereas both characteristic MgSt peaks are absent. It could indicate incompatibility of TA and MgSt1). A possible reason of such incompatibility is the formation of a complex between these components, which involves both the polar and non-polar parts of the MgSt molecule.
The disappearance of MgSt peaks in TA : MgSt mixtures with various MgSt content is shown in Figure 2a. The whole binary phase diagram of TA : MgSt melting is presented in Figure 2b. The shape of the diagram clearly suggests the formation of eutectic TA : MgSt mixture with 1 : 1 stoichiometry.
2. a) Normalized DSC thermograms of TA-MgSt mixtures (vertical lines are plotted to trace the characteristic peaks of TA and MgSt); b) binary phase diagram of TA-MgSt melting. MgSt : TA molar ratios are indicated beneath the experimental points 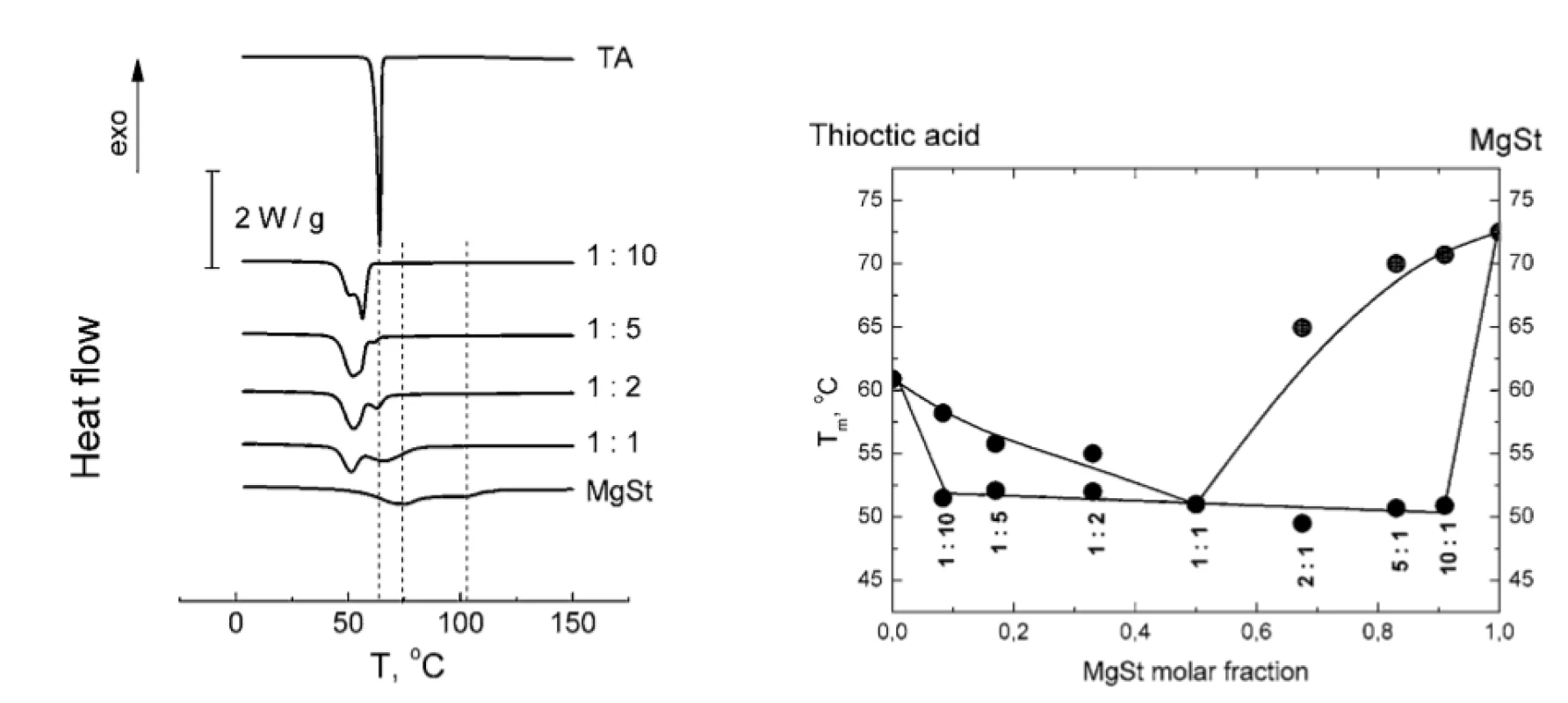
The absence of the DSC peak of MgSt dehydration in TA : MgSt mixture (Fig. 1) may result from two possible mechanisms: (1) substitution of crystalline water in MgSt by TA; (2) formation of water bridges between TA and MgSt. In order to clarify this matter, FTIR spectra were obtained for the individual substances and for their mixture (Fig. 3). The absorption band at ~3400 cm–1 corresponding to valence vibrations of OH groups in water molecules served as a characteristic marker of water content. This band is inherent to the MgSt spectrum and evidently corresponds to the crystalline water. The band disappears in the mixture, so it can be assumed that dehydration of MgSt occurs in the presence of TA. This hypothesis is supported by comparing the MgSt spectra in the initial state and after drying (70 °C, 30 min). After MgSt drying, the sample mass was decreased by 4% w/w, and the characteristic band at ~ 3400 cm–1 almost disappeared (Fig. 4). Similar changes were observed for another characteristic water absorption band, about 1640 cm–1, which corresponds to the librational vibrations of OH groups. Thus, FTIR data prove the complex formation of TA with MgSt, where TA substitutes the MgSt crystalline water. It should be noted that MgSt is generally characterized by high ability for complex formation with a number of active pharmaceutical ingredients, but this fact is usually neglected considering a low MgSt content in dosage forms1, 6–8).
3. FTIR spectra of MgSt, TA and their mixtures 20 : 1 (w/w) 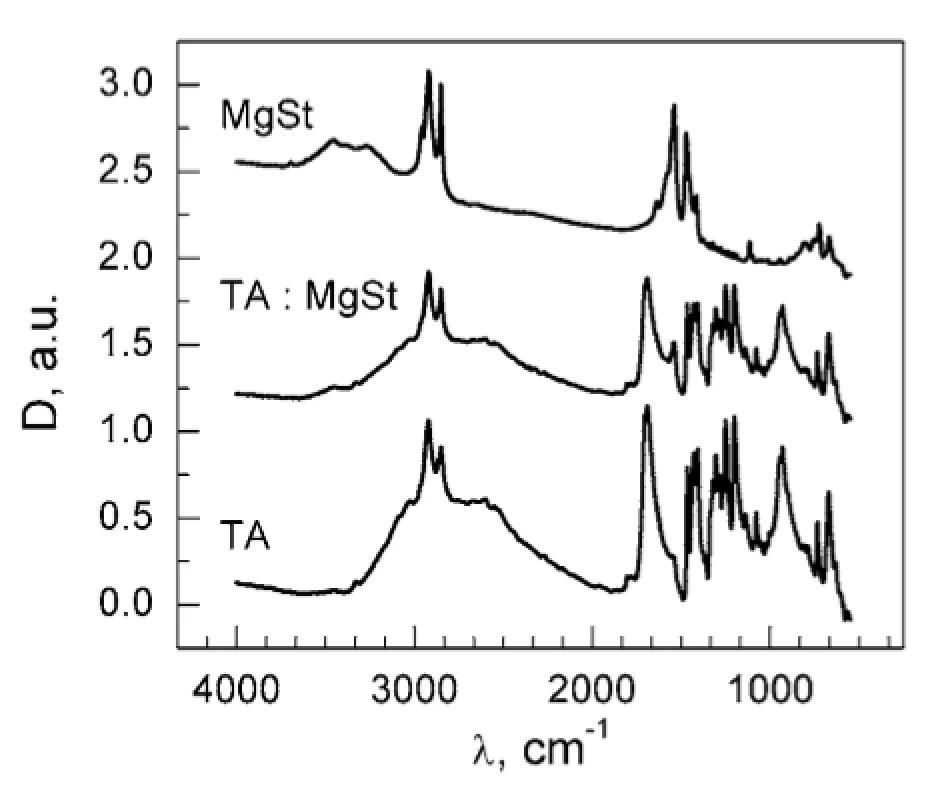
4. FTIR spectra of MgSt in the initial state (solid line) and after drying (dashed line) 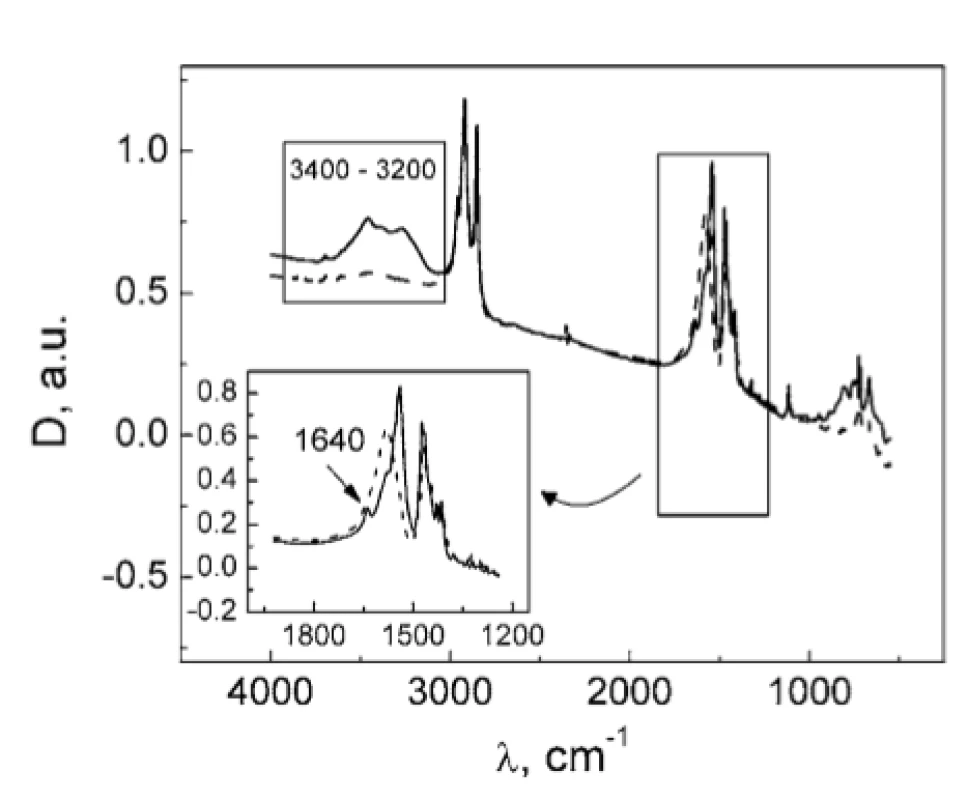
The investigation of TA and PH compatibility requested a preliminary examination of TA thermal stability because the melting point of PH is 207 °C. As it was established by the TGA technique, TA is stable up to 150 °C under the given thermal regime (Fig. 5). Above 150 °C, a rapid weight loss of the sample takes place indicating thermal destruction of TA. Therefore, heating of the TA : PH mixture to the temperature of PH melting point seems inappropriate.
5. TGA curve of TA sample. The dotted lines are plotted to show the limit of thermal stability of the TA. The values of initial and final sample mass (m0 and mfin, respectively) are indicated. 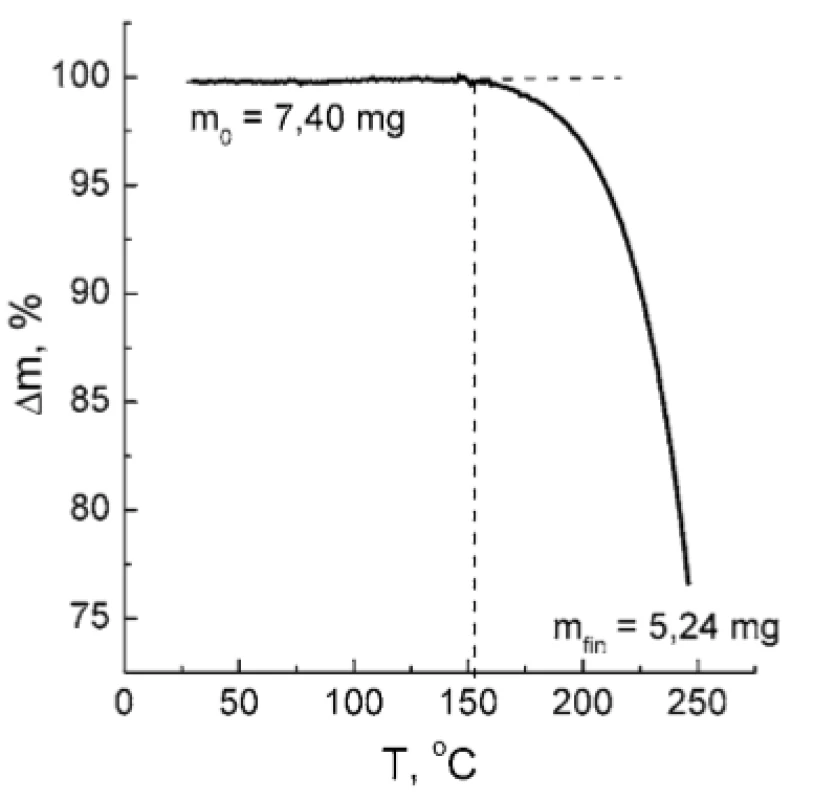
Taking into account the above finding, the investigation of TA : PH mixture was carried out in the temperature range of 0 to 90 °C, which only contained the melting peak of TA. DSC thermograms for TA and TA : PH mixture coincides within the experimental error (Fig. 6), thereby the absence of TA and PH interaction in the studied mixture could be asserted.
6. Normalized DSC thermograms of TA melting: individual TA (solid line); mixture TA : PH 2 : 1 (w/w) (dashed line) 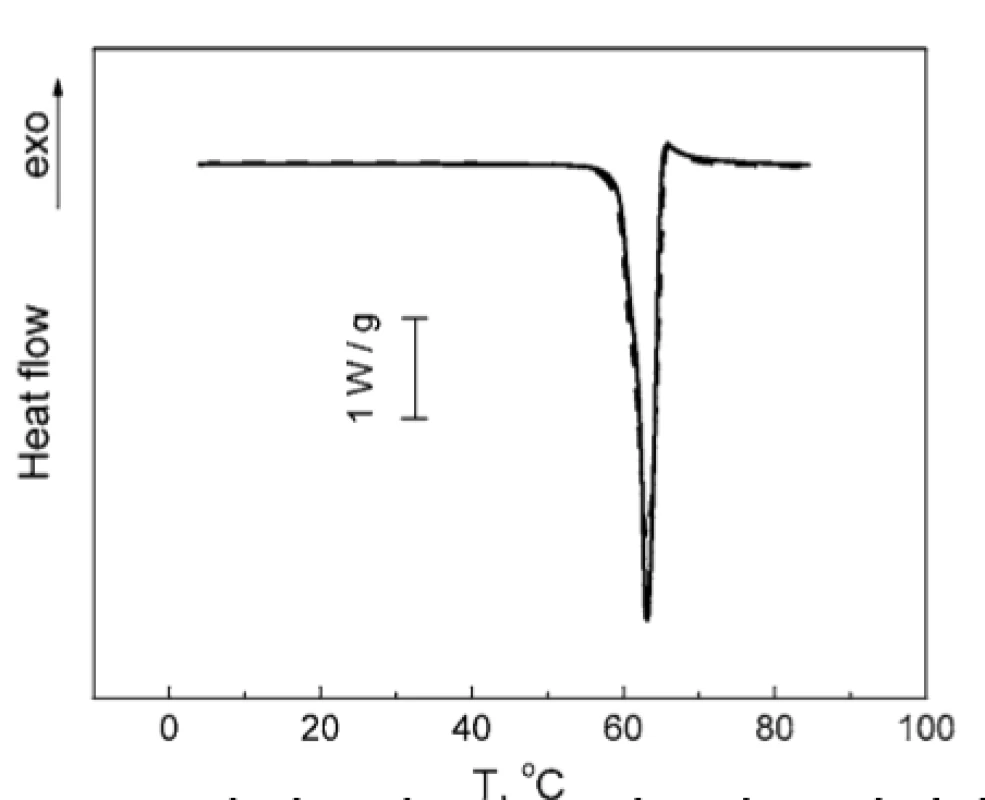
No notable interactions could be observed for other combinations of the compounds studied. As can be seen from Figure 7, the melting peak of TA is decreased by 0.5 °C in the TA : MgLac mixture. In the mixture of MgSt : MgLac, the dehydration peak of MgSt decreases by 1.5 °C, while the melting MgSt peak remains unchanged (Fig. 8). To our mind, it rather reflects minor restructuring of hydrogen bonds of MgSt crystallization water than significant intermolecular interactions between the components. The temperatures of the calorimetric peaks observed in all the tested systems are presented in Table 1.
7. Normalized DSC thermogram of TA melting: individual TA (solid line); mixture TA : MgLac 1 : 4 (w/w) (dashed line) 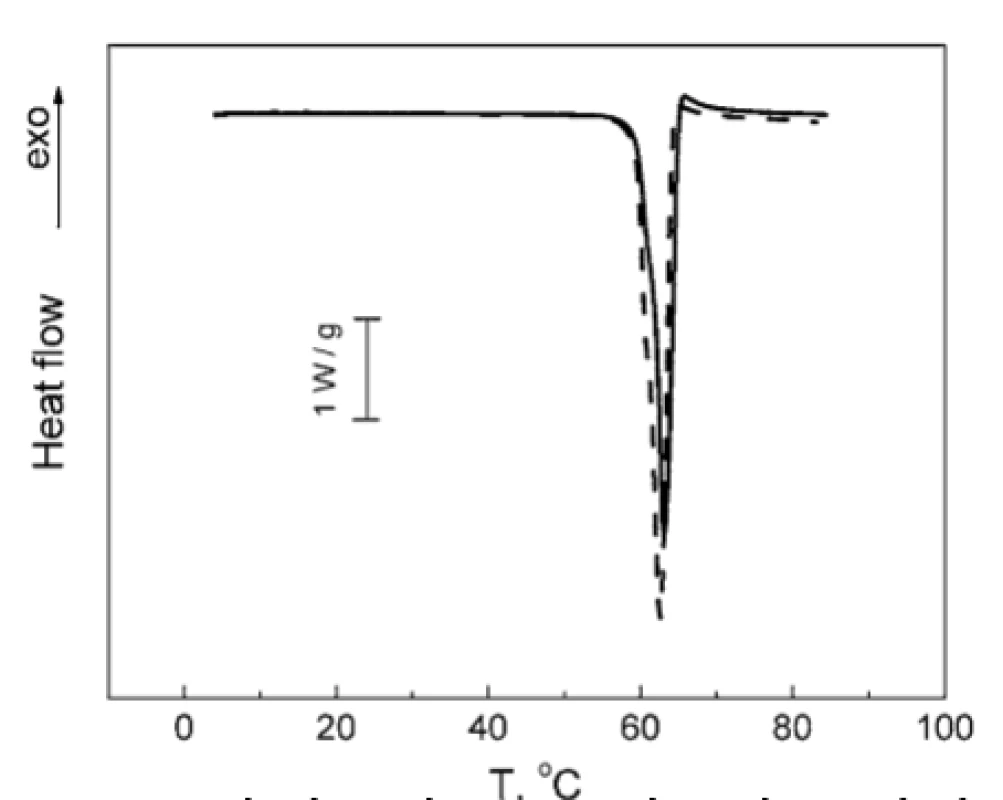
8. Normalized DSC thermograms of MgSt (solid line) and MgSt : MgLac (dashed line). The vertical lines indicate the dehydration temperature (Tdh) and the melting temperature (Tm) of MgSt. 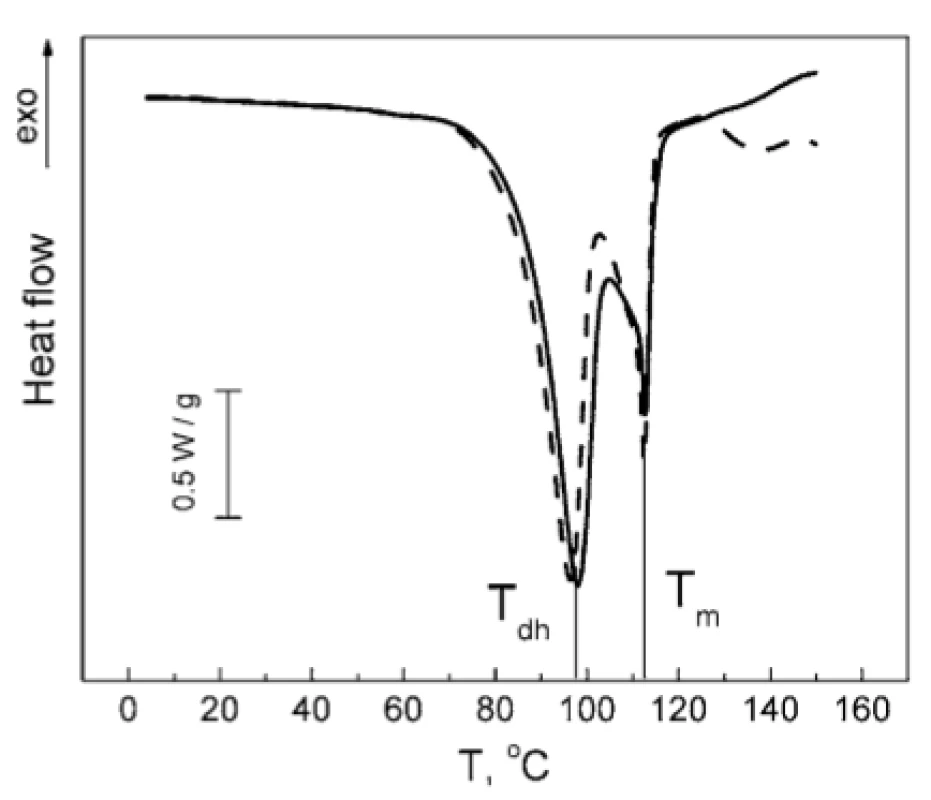
1. The maxima of calorimetric peaks of the individual components and their mixtures : melting temperatures (Tm) and dehydration temperature (Tdh) of the corresponding substances 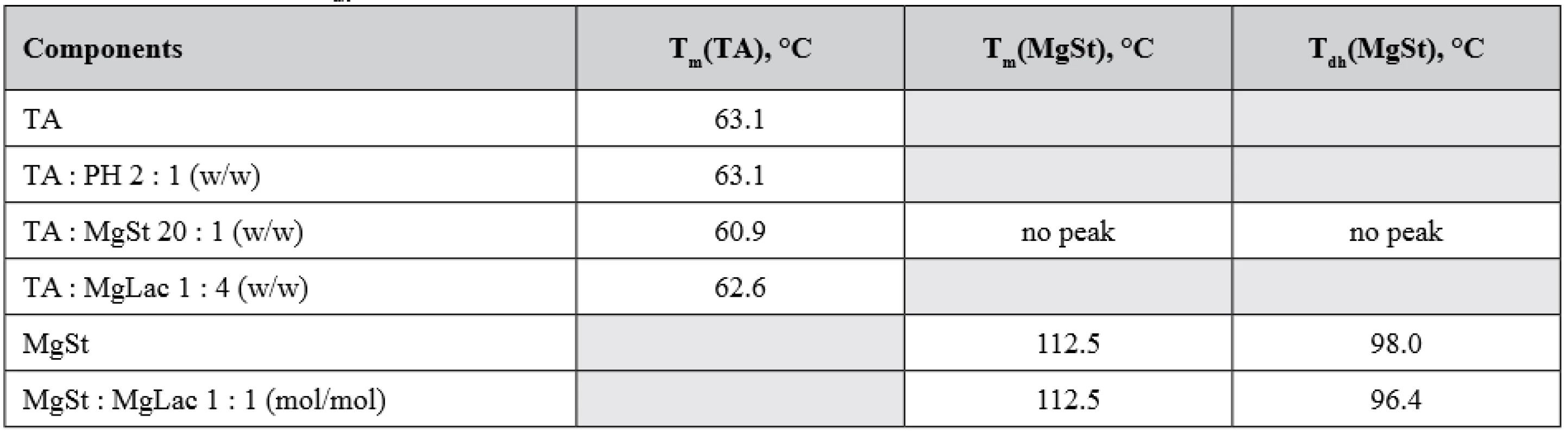
Conclusions
The pharmaceutical compatibility between some components of the capsules ‘Neuronucleos’ (thioctic acid, pyridoxine hydrochloride, magnesium stearate dihydrate and magnesium lactate dihydrate) was studied using differential scanning calorimetry, thermogravimetry, and Fourier-IR spectroscopy techniques. No interactions were found between thioctic acid and pyridoxine hydrochloride. A complex formation between thioctic acid and magnesium stearate was established, in which thioctic acid substitutes the crystalline water of magnesium stearate. No significant interactions were found for magnesium lactate with thioctic acid and magnesium stearate. Thus, pharmaceutical compatibility of the tested ‘Neuronucleos’ components was established, with exception of thioctic acid interaction with magnesium stearate. The obtained results were used in the technology development of the combined medicinal product in the capsules ‘Neuronucleos’.
Conflict of interest: none.
Maksym S. Almakaiev PhD
National University of Pharmacy Valentynivska str. 4,
61168 Kharkiv, Ukraine
e-mail: almakaeva@ukr.net
O. Vashchenko • L. Budianska • L. Lisetski
Institute for Scintillation Materials of NAS of Ukraine,
Kharkiv, Ukraine
D. Sofronov
State Scientific Institution «Institute for Single Crystals»,
NAS of Ukraine, Kharkiv, Ukraine
Sources
1. Bharate S. S., Bharate S. B., Bajaj A. N. Interactions and incompatibilities of pharmaceutical excipients with active pharmaceutical ingredients – a comprehensive review. J. Excipients and Food Chem. 2010; 1(3), 3–27.
2. Serajuddin A. T. M., Thakur A. B., Ghoshal R. N., Fakes M. G., Ranadive S. A., Morris K. R., Varia S. A. Selection of Solid dosage form composition through drug-excipient compatibility testing. J. Pharmaceut. Sci. 1999; 88(7), 696–704.
3. Електронний ресурс: https://pubchem.ncbi.nlm.nih.gov/compound/6112.
4. Delaney S. P., Nethercott M. J., Mays C. J., Winquist N. T., Arthur D., Calahan J. L., Sethi M., Pardue D. S., Kim J., Amidon G., Munson E. J. Characterization of synthesized and commercial forms of magnesium stearate using differential scanning calorimetry, thermogravimetric analysis, powder X-ray diffraction, and solid-state NMR spectroscopy. J. Pharmaceut. Sci. 2017; 106(1), 338–347.
5. Li J., Wu Y. Lubricants in pharmaceutical solid dosage forms. Lubricants 2014; 2, 21.
6. da Silva E. P., Pereira M. A. V., de Barros Lima I. P., Barros Lima N. G. P., Barbosa E. G., Soares Aragão C. F., Barreto Gomes A. P. Compatibility study between atorvastatin and excipients using DSC and FTIR. J. Therm. Anal. Calorim. 2016; 123(2), 933–939. doi:10.1007/s10973-015-5077-z
7. Fernandes F. H. A., de Almeida V. E., de Medeiros F. D., da Silva P. C. D, da Simões M. O. S., Veras G., Medeiros A. C. D. Evaluation of compatibility between Schinopsis brasiliensis Engler extract and pharmaceutical excipients using analytical techniques associated with chemometric tools. J. Therm. Anal. Calorim. 2016; 123(3), 2531–2542. doi:10.1007/s10973-016-5241-0
8. Santana C. P., Fernandes F. H. A., Brandão D. O., da Silva P. C. D., Correia L. P., Nóbrega F. P., de Medeiros F. D., Diniz P. H. G. D., Véras G., Medeiros A. C. D. Compatibility study of dry extract of Ximenia americana L. and pharmaceutical excipients used in solid state. J. Therm. Anal. Calorim. 2018; 133(1), 603–617. doi:10.1007/s10973-017-6764-8
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 1-
All articles in this issue
- Metal complexes in medicine and pharmacy – the past and the present II
- K životnému jubileu pani doc. RNDr. Zuzany Vitkovej, CSc.
- Životné jubileum prof. RNDr. Daniela Grančaia, CSc.
- Zomrel Alois Borovanský
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Double-coated pellets with semipermeable ethylcellulose coating for detection of cholinesterase inhibitors
- Development and uniformity evaluation of low-dose medicated chewing gums prepared by compression method
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- The amino acid and carbohydrate composition of the herb and roots of Smallanthus sonchifolius
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Metal complexes in medicine and pharmacy – the past and the present II
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- Zomrel Alois Borovanský
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

