-
Medical journals
- Career
Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
Authors: Kateřina Sadilová; Milada Halačová 1; Dalibor Černý 2
Authors‘ workplace: Institute of Pharmacology, 2nd Medical Faculty, Charles University, Prague, Czech Republic 1; Institute of Pharmacology, 1st Medical Faculty, Charles University, Prague, Czech Republic 2
Published in: Čes. slov. Farm., 2020; 69, 17-23
Category: Review Articles
Overview
Early and appropriate antibiotic therapy remains the key intervention for successful treatment of infection in critically ill patients, particularly in the current era of increasing antibiotic resistance. Optimization of the antimicrobial dosing regimens to achieve therapeutic plasma concentrations and concentrations at the site of infection is crucial for maximizing the therapeutic response and minimizing the risk of organ toxicity and is also an important tool to avoid the resistance emergence. Beta-lactam antibiotics have been considered relatively safe and, as opposed to aminoglycosides, therapeutic drug monitoring as a tool conventionally used primarily to minimize toxicity in drugs with narrow therapeutic window or complex pharmacokinetics, has not been provided routinely yet. However, emerging data suggest that optimal antibiotic exposure may not be achieved with traditional dosing strategies in a significant number of critically ill patients and, on the contrary, concerns about insufficient plasma concentrations leading to microbiological and clinical failure are warranted. The treatment of infections in the intensive care unit (ICU) patients is often challenging because of disease complexity, pathophysiologic alterations they undergo and reduced susceptibility of nosocomial pathogens. Therefore, it is of paramount importance to update current recommendations on dosing of beta-lactam antibiotics in severe infections and therapeutic drug monitoring may be regarded as the only exact method to ensure pharmacodynamics target achievement. Na Homolce Hospital is one of the first medical institutions in the Czech Republic where the practice of routine TDM of beta-lactam antibiotics in ICU-patients has been established. In this paper, we introduce our experience and first case reports.
Keywords:
pharmacokinetics – beta-lactam antibiotics – dose optimization – pathophysiologic changes – intensive care unit (ICU) – therapeutic drug monitoring (TDM)
Introduction
Optimization of antibiotic dosage regimens requires deep knowledge of the mechanisms involving the effect of the antibiotics (pharmacodynamics, PD) and the evolution of plasma and tissue drug concentrations over time (pharmacokinetics, PK). Given that in antimicrobial therapy the effect is produced on the bacterial pathogen, the main indicator of the pharmacodynamic efficacy of antibiotics is the minimum inhibitory concentration of the pathogen (MIC), which is defined as the lowest concentration of an antimicrobial agent that is able to prevent the visible growth of bacteria1, 2). Only the free (unbound) fraction of the drug exhibits an antimicrobial effect3). Pharmacokinetics involves processes such as absorption, distribution, metabolism and elimination which determine the concentration-time profile of the drug and may be characterized by the primary and secondary pharmacokinetic parameters. Changes in primary PKparameters (for instance, the volume of distribution Vd and clearance Cl) may be explained due to the changes in physiological variables. The elimination half-life (t1/2), area under the concentration-time curve (AUC) and bioavailability (F) could be considered as secondary PK-parameters because they are derived from primary parameters.
The quantitative relationship between a pharmacokinetic and a microbiological parameter known as a PK/PD index is the basis on which antibiotics can be classified into three categories reflecting the mechanism of bactericidal effect4, 5).
Intravenously administrated broad-spectrum β-lactam antibiotics are the most frequently prescribed antimicrobials in the ICU both in monotherapy and combination therapy with aminoglycosides and glycopeptides6, 7). Because of its favourable safety profile and spectrum of antimicrobial activity, including gram-negative bacteria, β-lactams still represent first choice therapy in both empirical and targeted treatment of serious infections8, 9).These drugs are characterised as small hydrophilic molecules with predominantly time-dependent activity defined as the cumulative percentage of time (%) over a dosing interval (T) in which the free drug concentration (F) exceeds the minimum inhibitory concentration (MIC) of an offending pathogen (%fT > MIC)10).
According to recent studies, to date acknowledged and generally accepted PK/PD target of 40–70%fT > MIC is not sufficient to achieve maximal bactericidal activity in intensive care setting, being derived from experimental trials using neutropenic animal models and relatively sensitive bacterial strains11, 12). This conservative target is likely to be efficient in the treatment of less severe infections. However, in critically ill patients with serious infections, which are often caused by resistant organisms with high bacterial burden, immunological limits of the patient and reduced β-lactam penetration at the site of infection, maintaining at least 100%fT > MIC may be necessary13), whereas some authors suggest that maximum killing of bacteria occurs when serum concentrations are maintained above the MIC of the causative pathogen for four to five times the MIC (fT > 4–5 × MIC)14–16). A consensus regarding the %fT > MIC value that should be chosen as the pharmacodynamics target for antimicrobial therapy in critically ill patients has not been reached. However, the maintaining antibiotic serum concentration above the MIC of the pathogen for extended periods of 100%fT > MIC is clearly associated with better bacteriological eradication and therapeutic outcomes than fT > MIC < 100%8, 13, 17).
The in vitro susceptibility of a causal pathogen has been considered a cornerstone of antibiotic prescription. Nevertheless, choosing a molecule and dosing schedule according to susceptibility of the infectious microorganism is only one component of optimal antibiotic therapy and many other factors must be considered in clinical practice. Due to the critical illness in combination with therapeutic interventions in the ICU, essential pathophysiologic changes occur leading to significant alterations in both the primary and secondary pharmacokinetic parameters18, 19). Especially in case of hydrophilic antibiotics largely distributed into the interstitial fluid and with predominantly renal elimination, there are two important factors influencing the achieved antibiotic concentrations in plasma and subsequently at the infection site, namely alteration of renal function (affecting clearance) and increased volume of distribution19).
Alterations of volume of distribution
A characteristic feature described in critically ill patients, particularly in patients with severe inflammatory disease (e.g. pancreatitis, extensive burns) is endothelial dysfunction and increased vascular permeability as a result of systemic inflammatory response syndrome (SIRS). Capillary leakage leads to fluid extravasation into the interstitial space and consequently to the systemic hypotension requiring aggressive fluid resuscitation. In addition, mechanical ventilation, extracorporeal circulation and the presence of surgical drains contributes to further increase of the volume of distribution in critically ill patients. Finally, there is an enormous expansion of Vd for hydrophilic β-lactam antibiotics in a relatively short period of time in the initial phase of antimicrobial therapy. As a result, achieved plasma concentrations and the concentrations at the site of infection may be substantially lower when standard dosage regimens are used in the early phase of the critical illness, which is associated with serious clinical consequences9, 20, 21).
Another factor contributing to the increase in Vd of some β-lactam antibiotics is hypoalbuminemia, defined as a serum albumin concentration < 25 g/l, which is present in 40–50% of critically ill patients9, 22). Hypoalbuminemia increases the unbound fraction of beta-lactam antibiotics, which is available for renal clearance. Moreover, it increases fluid shift into the interstitial space leading to further expansion of the volume of distribution. As a result, transient (about 5 elimination half-lives) increase in serum concentration of β-lactam antibiotics may be observed, especially in case of highly protein bound antibiotics (e.g. ceftriaxone, oxacillin), with a sequential decrease in the free drug fraction as a consequence of an augmented clearance22, 23).
• Case report
Obesity
An acute surgery was indicated in a 51 years old morbidly obese man (BMI 42.8, serum creatinine 76 µmol/l, GFR 2.16 ml/s) with finding of perforated appendicitis with circumscript peritonitis. Empiric antimicrobial therapy was initiated immediately with piperacillin/tazobactam (PIP/TAZO) at a dose of 4.5 g each 4 hours (27 g a day) together with a single dose of gentamicin 480 mg. 24 hours after the antimicrobial treatment initiation, control trough levels were determined. The measured concentration PIP/TAZO of 1.2 mg/l was far below this needed to manage a community-acquired intra-abdominal infection, where non-resistant Enterobacteriaceae and anaerobes (MIC value for PIP/TAZO 8 mg/l) may be expected, undoubtedly because of extreme obesity and augmented renal clearance. Nevertheless, perioperative samples were negative, the infectious focus was not confirmed. Five-day antibiotic therapy remained in medication rather from clinical embarrassment. The patient remained afebrile, without clinical signs of infection, intestinal passage was restored. On day 5, it was decided to discontinue antimicrobial therapy and on day 10 after the surgery, the patient was released in good condition.
In obese patients whose body weight exceeds the ideal body weight (IBW) over more than 30%, the increase in Vd not only lipophilic antibiotics but also some hydrophilic antibiotics occurs due to the extracellular fluid contained in the adipose tissue. The clinical significance of increasing Vd of hydrophilic time-dependent antibiotics in obese patients is also related to their half-life. According to the current state of knowledge, for antibiotics with ultrashort t½ (e.g. PIP/TAZO 0.7–1.2 hour) and the characteristic of T > MIC it may not be automatically necessary to exceed the recommended maximum daily dose (e.g. PIP/TAZO 27 g/day), nevertheless, this case report shows that even the recommended dose for morbidly obese patients may result in inadequate antibiotic levels in the context of the MIC values of the suspected pathogen, when augmented renal clearance (ARC) is present. Taking into account that this patient did not display any signs of sepsis, serum concentration above the MIC for four to five times the MIC (fT > 4–5 × MIC) may not be required. However, for the maximal bacterial killing associated with successful antimicrobial therapy in critically ill patients, the target of 100%fT > MIC needs to be achieved, while this must be based on the MIC of the least sensitive bacterial pathogen according to clinical breakpoints determined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST), till the cultivation result is obtained. Therefore, the dosage of β-lactam antibiotics in critically ill morbidly obese patients represents a controversial issue, as current recommendations appear to lead to insufficient serum drug levels attainment and risk of antimicrobial therapy failure. Only the establishment of routine monitoring of β-lactam antibiotics will provide the answer to this systemic question.
Changes of drug clearance
While expansion of the volume of distribution may play a key role in decreasing antibiotic concentration at the start of therapy, drug clearance is considered a major determinant of steady-state drug concentration. Alterations in renal function may lead, on the one hand, to the prolonged elimination half-life with risk of accumulation of the drug with systemic toxicity symptoms. On the other hand, patients with altered renal function within the meaning of hyperfiltration may be put at significant risk of underdosing. Systemic inflammation, increased cardiac output and intensive fluid resuscitation results in increased renal perfusion and subsequently in increased renal clearance. According to recent studies, 50–60% of critically ill patients experience ARC with creatinine clearance values ≥ 130 ml/min during their ICU-stay, particularly in the initial phase of sepsis. The ARC presence facilitates solute elimination (including β-lactam antibiotics) potentially resulting in pharmacodynamics targets non-achievement resulting in poor clinical outcome as well as the risk of development of antibiotic resistance24–29).
• Case report
Augmented renal clearance
A 63 years old woman (163 cm, 57 kg) underwent a subtotal gastrectomy for gastric cancer. On the day 8 after surgery, signs of wound infection including fever, elevated inflammatory markers and elevated renal parameters were observed. Empiric antimicrobial therapy with piperacillin/tazobactam (PIP/TAZO) at a dose of 4.5 g each 6 hours was initiated immediately with control trough levels determination 24 hours after the first dose. Based on the serum PIP/TAZO trough level of 4.6 mg/l and microbiological results with findings of Klebsiella in secretion samples, antibiotic dosing was increased to the maximal recommended dose of 4.5 g each 4 hours (27 g/day), which led to microbiological and clinical cure (Fig. 1).
1. The time course of plasma concentrations after two PIPERACILLIN-TAZOBACTAM dosing regimens 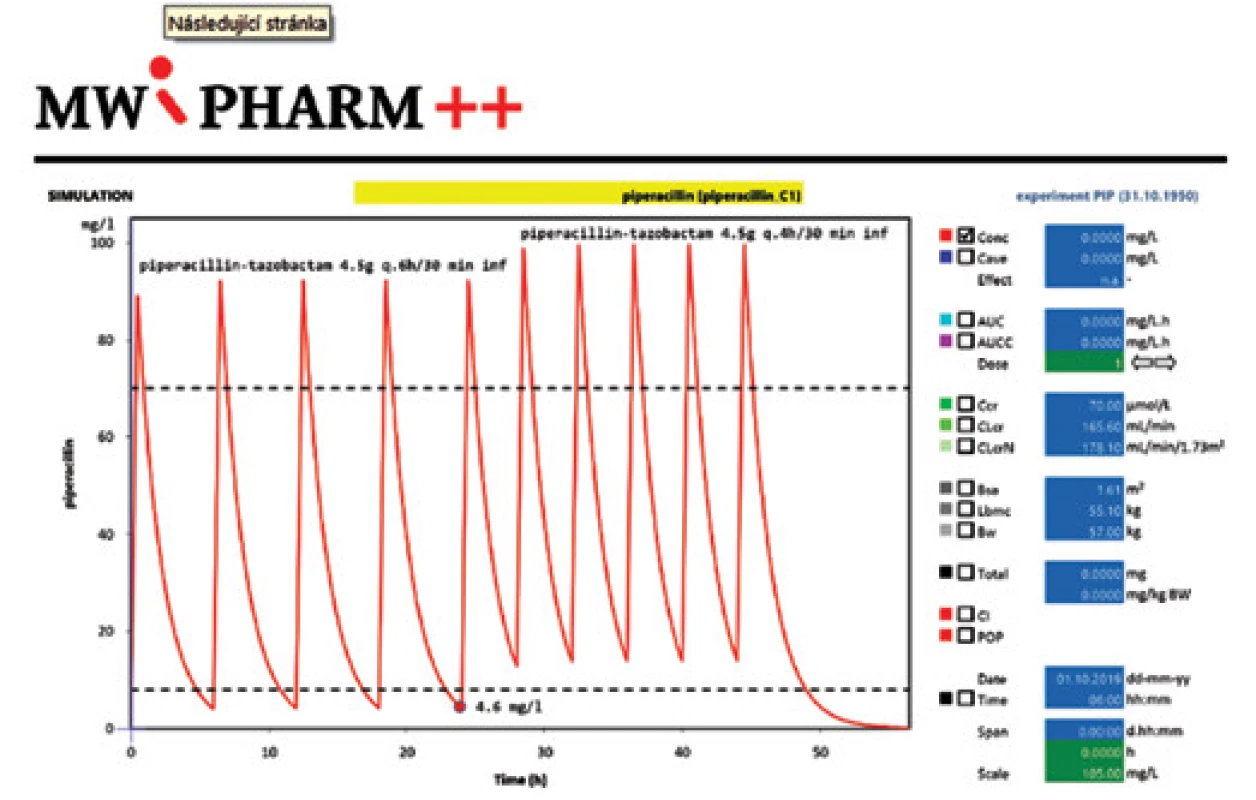
The desired serum trough level of PIP/TAZO necessary to manage an infection due to Klebsiella pneumoniae (MIC value for PIP/TAZO 8 mg/l) had not been reached in convenient dosing schedule, presumably due to extremely augmented renal clearance of the patient (GFR 2.76 ml/s). In this case, TDM made it possible to reveal subtherapeutic concentrations early and answer rapidly with dosage adjustment, which was essential for treatment success. Otherwise, inadequate antibiotic dosing in relation to ARC may have resulted in insufficient serum antibiotic levels and consecutively the failure of antimicrobial therapy.
After the initial phase of sepsis associated with expansion of distribution volume and subsequently with the risk of underdosing, the later stage of sepsis may arise as the compensatory mechanisms are exhausted and progressive organ damage, including acute renal and hepatic failure, develops. On the contrary to the above mentioned situation of ARC, the reduced removal capacity of an eliminating organ emerges as significant risk factor for drug accumulation and development of systemic side effects (e.g. carbapenems-induced neurotoxicity, tetracyclines-induced hepatotoxicity, nephrotoxicity associated with aminoglycoside administration)30). However, warranted fear of overdosing and side effect is often a cause of choosing inappropriately low β-lactam doses in critically ill patients with renal insufficiency, especially in the early phase of infection. Clearance of β-lactam antibiotics eliminated predominantly by glomerular filtration (e.g. cefepime, meropenem) correlates well with creatinine clearance (ClCr), nonetheless, the estimation of glomerular filtration rate (GFR) from serum creatinine according to the equation may be misleading and its use to predict real ClCr in ICU-patients is markedly limited31). Although determining creatinine clearance from urine collection may present a more convenient practice, monitoring of serum antibiotic concentrations in patients with renal or hepatic impairment is the only exact method to consider the optimal dosage regimen.
• Case report
Bronchopneumonia
A polymorbid elderly patient (82 years, 68 kg) underwent surgical resection for acute aortic dissection. The postoperative course was characterized by worsening of chronic obstructive pulmonary disease with the need of more frequent non-invasive ventilation and consistent respiratory rehabilitation, SpO2 values ranged around 70%. On the chest X-ray, signs of bronchopneumonia were detected and antimicrobial therapy with cefepime at loading dose of 2 g every 8 hours (Krea 239, ClCr 0.35 ml/s) was initiated. 24 hours after the first dose, the serum cefepime trough level was found to be 114 mg/l (!). In accordance with progression of renal impairment (ClCr 0.21 ml/s), cefepime dosing was deescalated to 500 mg each 8 hours. Because of further progression of renal insufficiency, CVVHD must be started and cefepime dosing was increased to 1 g every 6 h due to the changes in elimination parameters. In sputum samples, positive culture for Klebsiella pneumoniae susceptible to fourth-generation cephalosporins was referred by microbiologist. Antibiotic treatment was successfully completed on day 7.
Although toxic serum concentrations of cefepime are not defined in the current literature, higher plasma levels are clearly associated with a significantly higher risk of neurotoxicity, attributed to the ability of the drug to cross the blood-brain barrier and display a dose-dependent gamma-aminobutyric acid (GABA) antagonistic effect. Neurotoxic symptoms may be manifested as alteration of consciousness, encephalopathy, aphasia, myoclonus, seizures and coma. Risk factors include pre-existing brain condition, high antibiotic doses and renal insufficiency potentially leading to drug accumulation. In an attempt to reach rapid bactericidal effect, aggressive initiation of antimicrobial therapy with full-dose cefepime regimen was chosen. This led, however, to a prompt increase in plasma concentrations up to more than 10 times the MIC of the least sensitive bacterial pathogen (Pseudomonas aeruginosa, MIC 8 mg/l). Only using early therapeutic monitoring with real-time adaptive feedback permits the clinician to answer immediately by reducing the dose of cefepime and thus prevent the development of epileptic seizures or emergence of other neurotoxic manifestations.
Continuous elimination methods
Acute kidney injury requiring the use of extracorporeal removal techniques (RRT – renal replacement therapy) makes the situation even more complex. The changes in drug removal induced by CRRT initiation depend on the physiochemical properties of a molecule (molecular weight, lipophilicity/hydrophilicity), volume of distribution and protein binding. Variability in the mode and setting of renal replacement therapy (flow rate, pre - and post-dilution) increases the complexity of antibiotic dosing in dialysed patients. In addition, differential extrarenal drug removal and varying patient parameters (residual renal function and non-renal clearance) limit the utilizing of standard doses. Significant extracorporeal drug removal may be expected particularly in drugs with predominantly renal elimination, volumes of distribution about equal to extracellular fluid volume and low protein binding32).
The efficacy of elimination techniques generally increases in order from intermittent haemodialysis (iHD) through continuous venovenous haemodialysis (CVVHD) and continuous venovenous hemofiltration (CVVHF) to continuous venovenous hemodiafiltration (CVVHDF), however, the final removal of solutes (drugs) by CRRT (CLCRRT) may vary greatly depending on the physiochemical properties of the molecule and characteristics of the renal replacement therapy device30, 33). In terms of substitutional fluid administration, approximately 20% lower drug removal will be obtained by using predilution in comparison to postdilution30). In other words, in postdilution hemofiltration the drug clearance equals the ultrafiltration rate, while in predilution hemofiltration, the dilution of the blood prior to filtration needs to be considered when calculating clearance34). In general, CRRT functions at a drug filtration rate of 10–50 ml/min, depending on the blood flow rate, the membrane flux and the use of dialysate. In addition to the CLCRRT, the residual clearance of the patient must be taken into account35).
The use of CRRT may translate into alteration in pharmacokinetics, namely of hydrophilic drugs with low binding to tissue structures, small volume of distribution limited to plasma and extracellular space, and primarily renal elimination, thus, dose adjustment may be necessary. There are mathematical formulas and CLCRRT calculation algorithms, but their description and interpretation go beyond the scope of this paper. For empirically optimized dosing, a loading dose in order to compensate the increased volume of distribution may be required with following administration of maintenance dose, which must be adjusted to RRT setting and patient’s own variables. Although many recommendations regarding drug dosing in patients undergoing renal replacement therapy have been published, there is considerable discrepancy between the sources. With respect to the number of variables which must be considered when calculating drug dosing in RRT, all current best practices are inapplicable and inappropriate for an individual patient’s needs. Therefore, TDM of antibiotic levels is the only exact method allowing to predict clinical effectiveness and safety of antimicrobial therapy in critically ill haemodialysed patients and it becomes a useful tool to daily individualize dosing and to ensure maintaining antibiotic levels within the therapeutic range30, 32, 35).
• Case report
Staphylococcal mediastinitis
A 74 years old man (100 kg, 183 cm) after biological aortic valve replacement and quadruple aortocoronary bypass surgery was rehospitalized on day 18 postoperatively for an increased pain and redness around the surgical wound, abdominal pain, nausea, vomiting, and general fatigue. On admission to the hospital, he already showed signs of sepsis with the development of acute renal injury and anuria with the need of CRRT initiation. The crepitation of sternum was observed, and on the CT, liquid collection along the sternum and suspected forming abscess was described. CRP levels raised over 300 mg/l and elevated white blood cell count (12.6 × 109/l) was found. Acute surgical revision was performed. After the blood cultures sampling, meropenem (2 g every 8 hours) in combination with vancomycin (1.5 g every 12 hours) was administered as empirical antimicrobial therapy. 24 hours after the initial dose, control trough level of meropenem was measured with the founding of 2.8 mg/ml. In haemocultivation and peroperative samples, Staphylococcus aureus with good susceptibility has been identified, and according to this finding, antibiotic therapy was modified to oxacillin 3 g every 4 hours with a good clinical outcome.
Regarding the patient’s low to null residual renal function, only CLCRRT 30–50 ml/min was considered when estimating the initial dose of meropenem using CVVHDF in the pre-dilution mode. Thus, full-dose regimen was chosen. However, the serum level of 2.8 mg/l is still insufficient in the context of the MIC of the least susceptible pathogen (e.g. 8 mg/l for Pseudomonas aeruginosa), which must be accounted, till the cultivation results are obtained. Based on this case report we suggest that the use of renal replacement therapy does not always justify dose adjustment in the meaning of reduction. The patient’s residual renal function, dialysis flow rates (2–4 l/h) and CLCRRT (10–50 ml/min depending on the CRRT modality and intensity) should be considered when prescribing β-lactams in critically ill patients receiving CRRT. Nonetheless, even a qualified estimate may not lead to therapeutic serum concentrations achievement and without TDM of β-lactam antibiotics, we are moving on the level of crystal ball prophecy.
Prolonged infusions vs. intermittent bolus dosing
β-lactam antibiotics have been conventionally administered almost exclusively by intermittent bolus dosing36). However, challenging global rates of microbial resistance and declined development pipeline for new antibiotics over recent decades have increased the interest in alternative antimicrobial dosing strategies in an attempt to improve attainment of PK/PD targets and clinical outcomes of severe infections in critically ill patients. Maximization the effect of time-dependent antibiotics can be theoretically accomplished in three possible ways reflecting the mechanism of action. As the most important determinant of bacterial killing is the duration that the pathogen is exposed to the β-lactam concentrations exceeding the MIC, optimization of dosing regimens could be on theoretical basis provided by increasing the dose (single or daily), shortening the dosing interval or prolonging the infusion time. Prolonged infusion as opposed to standard intermittent bolus dosing (IB) may include either extended infusion (EI) over 2 to 4 hours or continuous infusion (CI) over the entire dosing interval. Available data obtained from observational or non-randomized prospective studies based on pharmacodynamics principles suggest that prolonged infusions of β-lactam antibiotics are at least equally effective as, and in certain circumstances (such as severe infection in critically ill), even more effective than traditional intermittent bolus dosing37).
Limited clinical data suggest a comparable incidence of β-lactam antibiotic adverse events with prolonged infusions compared to intermittent bolus dosing38). However, well-designed and adequately powered randomized controlled trials are needed to provide definitive evidence of clinical advantages of prolonged (extended/continuous) infusion of β-lactam antibiotics.
Conclusion
As opposed to aminoglycosides and glycopeptides, serum concentrations of β-lactam antibiotics have not been determined routinely yet, considering the TDM as a tool used primarily to minimize toxicity in drugs with narrow therapeutic window or complex pharmacokinetics. However, recent studies have demonstrated that standard β-lactam doses do not reach therapeutic concentration in a significant number of critically ill patients. Thus, there is an increasing interest in the TDM use for antibiotic dose optimization in an attempt to improve attainment of PK/PD targets and clinical outcomes. Despite the global growth in practice of antibiotic TDM, current state of knowledge does not provide any definitive evidence of β-lactam dosing in critically ill patients and dose adjustments have been made on the basis of an expert estimation only. As highlighted on the examples from clinical practice, critically ill patients exhibit extreme inter-individual pharmacokinetic variability underlying dynamic changes in the course of the infection process, which makes antibiotic levels very difficult (if not impossible) to predict. Thus, ICU-patients are jeopardized by insufficient β-lactam concentrations resulting in treatment failure or, on the other side, are at significant risk of high-level antibiotic exposure associated with potential systemic toxicity. Exact determination of pharmacokinetic changes in critically ill patients is becoming a prerequisite for optimizing of today’s inconvenient “one-size-fits-all” model dose regimens. The establishment of a validated analytical method for measurement of serum concentrations of β-lactam antibiotics and routine implementation of TDM using the PK-software provides maximizing clinical efficiency and minimizing the risks of both under - and overdosing. Evaluation of the clinical advantage of TDM praxis is still an area for future study, though we hope our experience may build a basis for further development.
Supported by Ministry of Health, Czech Republic – conceptual development of research organization (NHH, 00023884) IG 168601, IG 168602.
K. Sadilová • PharmDr. Milada Halačová, Ph.D. • D. Černý
Na Homolce Hospital,
Department of Clinical Pharmacy, Prague,
Czech Republic Roentgenova 2, 150 30 Praha 5
e-mail: Milada.halacova@homolka.cz
Sources
1. Asín-Prieto E., Rodríguez-Gascón A., et al. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015; 319–329.
2. Andrews J. M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001; 49(Suppl 1), 5–16.
3. Zeitlinger M. A., Derendorf H., et al. Protein binding: do we ever learn? Antimicrob. Agents Chemother. 2011; 55, 3067–3074.
4. Mouton J. W., Dudley M. N., et al. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J. Antimicrob. Chemother. 2005; 55, 601–607.
5. Lux L. J, Posey RE, et al. Pharmacokinetic/Pharmacodynamic Measures for Guiding Antibiotic Treatment for Hospital-Acquired Pneumonia. Comparative Effectiveness Reviews. 2014; 11, 136.
6. Rello J., Ulldemolins M., et al. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur. Respir. J. 2011; 37, 1332–1339.
7. Dellinger R. P., Levy M. M., et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2008; 36, 296–327.
8. Taccone F. S., Laterre P. F., et. al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010; 14(4), R126.
9. Osthoff M., Siegemund M., et al. Prolonged administration of β-lactam antibiotics – a comprehensive review and critical appraisal. Swiss Med. Wkly. 2016; 10, 146.
10. Craig W. A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998; 26, 1–10.
11. Drusano G. L. Antimicrobial pharmacodynamics: critical interactions of ‚bug and drug‘. Nat. Rev. Microbiol. 2004; 2, 289–300.
12. Roberts J. A., Paul S. K., et al. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014; 58(8), 1072–1083.
13. McKinnon P. S., Paladino J. A., Schentag J. J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents. 2008; 31, 345–351.
14. Sinnollareddy M. J., Roberts M. S., et al. Beta-lactam pharmacokinetics and pharmacodynamics in critically ill patients and strategies for dose optimization: A structured review. Clin. Exp. Pharmacol. Physiol. 2012; 39, 489–496.
15. Pea F., Viale P., Furlanut M. Antimicrobial therapy in critically ill patients. A review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 2005; 44, 1009–1034.
16. Levison M. E. Pharmacodynamics of antimicrobial drugs. Infect. Dis. Clin. N. Am. 2004; 18, 451–465.
17. Nicolau D. P., Onyeji C. O., et al. Pharmacodynamic assessment of cefprozil against Streptococcus pneumoniae: implications for breakpoint determinations. Antimicrob. Agents Chemother. 2000; 44, 1291–1295.
18. Ulldemolins M., Vaquer S., et al. Beta-lactam dosing in critically ill patients with septic shock and continuous renal replacement therapy. Crit. Care 2014; 18, 227.
19. Roberts J. A., Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009; 37, 840–851.
20. Roberts J. A., Abdul-Aziz M. H., et al. International Society of Anti-Infective Pharmacology and the Pharmacokinetics and Pharmacodynamics Study Group of the ESCMID. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect. Dis. 2014; 14(6), 498–509.
21. Blot S. I., Pea F., Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient – concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 2014; 77, 3–11.
22. Ulldemolins M., Roberts J. A., et al. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 2011; 50, 99–110.
23. Joynt G. M., Lipman J., et al. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 2001; 47(4), 421–429.
24. Udy A. A., Roberts J. A., Lipman J. Implications of augmented renal clearance in critically ill patients. Nat. Rev. Nephrol. 2011; 7, 539–543.
25. Udy A. A., Baptista J. P., et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit. Care Med. 2014; 42, 520–527.
26. Claus B. O., Hoste E. A., et al. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J. Crit. Care 2013; 28, 695–700.
27. Huttner A., von Dach E., et al. Augmented renal clearance, low β-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int. J. Antimicrob. Agents. 2015; 45, 385–392.
28. Udy A. A., Varghese J. M., et al. Subtherapeutic initial β-lactam concentrations in select critically ill patients: association between augmented renal clearance and low trough drug concentrations. Chest 2012; 142, 30–39.
29. Udy A. A., Lipman J., et al. Are standard doses of piperacillin sufficient for critically ill patients with augmented creatinine clearance? Crit. Care 2015; 19, 28.
30. Halačová M. Farmakologická charakteristika antibiotik. (author of the book) Jindrák V., Hedlová D., et al. Antibiotická politika a prevence infekcí v nemocnici. Praha: Mladá fronta 2014; 49; 52.
31. Martin J. H., Fay M. F., et al. Pitfalls of using estimations of glomerular filtration rate in an intensive care population. Intern. Med. J. 2011; 41(7), 537–543.
32. Heintz B. H., Matzke G. R., et al. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy. Pharmacotherapy 2009; 29(5), 562–577.
33. Pea F., Viale P., et al. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin. Pharmacokinet. 2007; 46(12), 997–1038.
34. Kes P. Continuous renal replacement therapy. Acta Clin. Croat. 2000; 39(2).
35. Aronoff G. R., Bennett W. M., et al. Drug prescribing in renal failure. Dosing guidelines for adults and children. American Colledge of Physicians 2007; 5–9.
36. Abdul-Aziz M. H. Continuous infusion vs. bolus dosing: implications for beta-lactam antibiotics. Minerva Anestesiol. 2011; 77, 1–2.
37. Moehring R., Sarubbi C. Prolonged infusion of beta-lactam antibiotics. UpToDate (Online) 17. 5 2019. (Citation: 23. 7 2019). www.uptodate.com
38. Dulhunty J. M., Roberts J. A., et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clinical Infectious Diseases 2013; 56(2), 236–244.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 1-
All articles in this issue
- Metal complexes in medicine and pharmacy – the past and the present II
- K životnému jubileu pani doc. RNDr. Zuzany Vitkovej, CSc.
- Životné jubileum prof. RNDr. Daniela Grančaia, CSc.
- Zomrel Alois Borovanský
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Double-coated pellets with semipermeable ethylcellulose coating for detection of cholinesterase inhibitors
- Development and uniformity evaluation of low-dose medicated chewing gums prepared by compression method
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- The amino acid and carbohydrate composition of the herb and roots of Smallanthus sonchifolius
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Metal complexes in medicine and pharmacy – the past and the present II
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- Zomrel Alois Borovanský
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

