-
Medical journals
- Career
Double-coated pellets with semipermeable ethylcellulose coating for detection of cholinesterase inhibitors
Authors: Jiří Zeman 1; Sylvie Pavloková 1; David Vetchý 1; Vladimír Pitschmann 2
Authors‘ workplace: Department of Pharmaceutics, Faculty of Pharmacy University of Veterinary and Pharmaceutical Sciences Palackého 1946/1, 612 42 Brno, Czech Republic 1; Oritest Ltd., Prague, Czech Republic Faculty of Biomedical Engineering, Czech Technical University in Prague, Kladno, Czech Republic 2
Published in: Čes. slov. Farm., 2020; 69, 24-32
Category: Original article
Overview
Currently, nerve agents are often used in terrorist attacks or assassinations. In such cases, it is necessary to detect them quickly, accurately and easily right in the field. Detection tubes, which are small devices containing pellets with immobilized cholinesterase and detection reagents, meet these conditions. Their detection mechanism is based on a highly sensitive enzymatic Ellman reaction, when in the absence of cholinesterase inhibitors the pellets develop a visible yellow color, whereas in their presence the carriers retain the original color. The rate of reaction, its sensitivity and the distinct color transition are the key points of the research. In this experiment, double-coated pellets were prepared. The first coating contained the butyrylcholinesterase immobilized in hypromellose, while the second coating consisted of ethylcellulose and triethyl citrate. Based on the properties of such carriers, samples containing lactose dispersed in the ethylcellulose coating were also prepared, which was expected to have an effect on increasing the permeability of the coating and hence the detection rate and color intensity. In addition to selected physicochemical properties, carriers were evaluated for enzyme activity, sensitivity and color transition intensity. Samples showing the best properties were subjected to a 24-months stability test at three different temperatures and humidity.
Keywords:
ethylcellulose – pellets – cholinesterase – detection tube – nerve agent
Introduction
Cholinesterase inhibitors are a group of substances among which belongs pharmaceuticals (donepezil, tacrine, rivastigmine, etc.)1–4), pesticides (organophosphorus – parathion, malathion, dichlorvos or carbamate – carbofuran, aldicarb)5, 6) and most importantly nerve agents (G agents – soman, sarin, tabun, cyklosarin; V agents – VX, R-33 or Novichok type substances)7, 8). These substances inhibit reversibly or irreversibly acetylcholinesterase (eventually butyrylcholinesterase). Acetylcholinesterase (AChE) is an enzyme responsible for hydrolytic inactivation of neurotransmitter acetylcholine. In case of its inhibition, acetylcholine accumulates at nervous synapses and stimulate nicotinic and muscarinic receptors. This may lead to a cholinergic crisis associated with generalized seizures and respiratory insufficiency leading to multi-organ failure and death9–12). In order to reduce the health risks associated with exposure to cholinesterase inhibitors, an adequate treatment should be given to affected persons as soon as possible13). This requires rapid detection of substances responsible for poisoning.
Detectors are usually based on enzyme reaction (e.g. detection tubes or strips) or various physicochemical methods (e.g. GC-MS, IMS, FTIR spectroscopy) with enzyme-based detectors having the highest sensitivity. As mentioned above, detection tubes are such type of detector whose other advantages are low cost, easy manufacture and handling14). The current design of the tubes and used detection reagents make it possible to achieve a sensitivity of up to 0.005 mg · m–3. The tube consist of two ampoules with buffer and two layers, the first contains detection reagents adsorbed on crushed glass and the second is made of carriers in the form of spherical pellets with immobilized enzyme. The detection itself is based upon Ellman colorimetric reaction, during which a visible yellow color develops on the carriers in the absence of cholinesterase inhibitors, otherwise the carriers retain their original color15, 16). The main advantages of this reaction are speed and simplicity. The major drawback is then an indistinct color transition from white to yellow, which is the subject of current research17–20). There is also risk of interferences with other substances, which can lead to bias in the results21, 22). Besides Ellman reaction, there are other colorimetric reactions with a more pronounced color transition, such as white to blue (indoxyl acetate) or yellow to dark violet (indophenol acetate), but these reactions are up to ten times slower21, 23).
During the preparation of the carriers, immobilization of the enzyme is one of the most important steps since the enzymes in the free form tend to lose their activity rapidly24). The oldest and commonly used method is adsorption to an insoluble carrier due to its simplicity and low cost. Such a method has been utilized in recent studies in which matrix carriers had an enzyme immobilized on their surface17, 18, 20, 25). The main disadvantage of these carriers was the leaching of the enzyme into the solution, which made it difficult to observe the color transition on the pellets. To avoid that, a new composition of carriers was developed and recently published19). Immobilization of the enzyme in the hypromellose layer, which was sprayed onto inactive pellet cores, reduced costs by reducing the amount of enzyme required. In addition, the semipermeable second layer of Eudragit® RL prevented enzyme leaching and intensified the color transition on the surface of the pellets19, 26).
Apart from copolymers of acrylic and methacrylic acid, such as Eudragit® RL and RS, ethylcellulose (EC) is often used as a semipermeable film-forming agent27). For this reason, EC (with MW = 75,000 and viscosity of 8–11 mPa · s) was utilized as the main component of the secondary layer with triethyl citrate as suitable plasticizer28). Five samples with different thickness of EC coating were prepared and evaluated for their activity and inhibition. Based on the results, lactose was added into the EC coating, as it was expected to facilitate pore formation, thereby increasing activity. After all samples were evaluated, four samples with sufficient activity were selected and subjected to a 24-months stability test at three different temperatures and humidity levels as recommended by WHO29). Results were statistically evaluated by means of analysis of variance (ANOVA) or paired t-test.
Experimental part
Materials
Avicel® PH 101 microcrystalline cellulose (MCC, FMC Biopolymer, Ireland), Pharmacoat® 606 hypromellose (HPMC, Shin-Etsu, Japan), butyrylcholinesterase (BuChE, Sigma-Aldrich, USA), AqualonTM N10 PHARM ethylcellulose (EC, Ashland, USA), triethyl citrate (TEC, Sigma-Aldrich, USA), Pharmatose® 200 M lactose (DFE Pharma, Germany), ethanol 96% (Dr. Kulich Pharma, Czech Republic) and purified water (Ph. Eur. 9) were used for the preparation of double-coated spherical pellets. Disodium phosphate dodecahydrate, potassium dihydrogen phosphate (Sigma-Aldrich, USA), sodium hydrogen carbonate, ethanol 96%, isopropyl alcohol (Dr. Kulich Pharma, Czech Republic), S-butyrylthiocholine iodide (Sigma-Aldrich, Switzerland), 5,5’-dithiobis-2-nitrobenzoic acid (Sigma-Aldrich, USA), physostigmine (Sigma-Aldrich, USA) and purified water (Ph. Eur. 9) were used for the activity and inhibition tests. All ingredients were of analytical grade.
Sample preparation
The preparation of samples was based on previous experiment, which utilized Eudragit® RL as a semipermeable film-forming agent19, 26). In the present study, an influence of ethylcellulose coating on pellets properties was examined. At first, microcrystalline cellulose and purified water were mixed in a 1 : 1.1 ratio in Stephan UMC 5 mixer (A. Stephan u. Söhne GmbH & Co., Germany). Moistened MCC was then extruded using a single-screw axial extruder/spheronizer PharmTex 35 T (Wyss & Probst Engineering, Switzerland) through an extrusion membrane with 1.25-mm pores at a screw speed of 60 rpm. Thereafter spheronization was performed at 1000 rpm for 5 min. Prepared inactive pellet cores were dried in a hot air oven Horo 038 A (Dr. Ing. A. Hoffman GmbH, Germany) at 60 °C for 24 h. The dried pellet cores underwent sieve analysis (AS200 Basic, Retsch GmbH & Co., Germany) and the size fraction of 0.8–1.25 mm was used for the coating to ensure optimal physical properties of the carriers17).
In the next step, the inactive cores were coated with aqueous dispersion of a hypromellose and butyrylcholinesterase. The amount of HPMC was calculated for 5% weight gain with 200 IU of butyrylcholinesterase per 100 g of pellet cores. The coating process was performed at 40 °C in the coating device M-100 (MEDIPO, Czech Republic) using a Wurster column. Pellets were subsequently dried at 25 °C for 24 h in the hot air oven.
At last, using the same coating procedure and conditions as mentioned above, pellets with hypromellose layer with immobilized BuChE were coated with an ethanol solution of ethylcellulose and triethyl citrate in a 4 : 1 ratio (samples EC1.5 – EC10; n = 1). Similar composition was used for samples EC1.5–5, EC1.5–10, EC1.5–15 and EC2.5–5, EC2.5–10, EC2.5–15 (n = 1) but with an additional content of dispersed lactose. The exact composition of the ethylcellulose coating is summarized in Table 1. Dried samples were sieved again to obtain the size fraction of 0.8–1.25 mm.
1. Composition of semipermeable coating (calculated for 100 g of pellets) 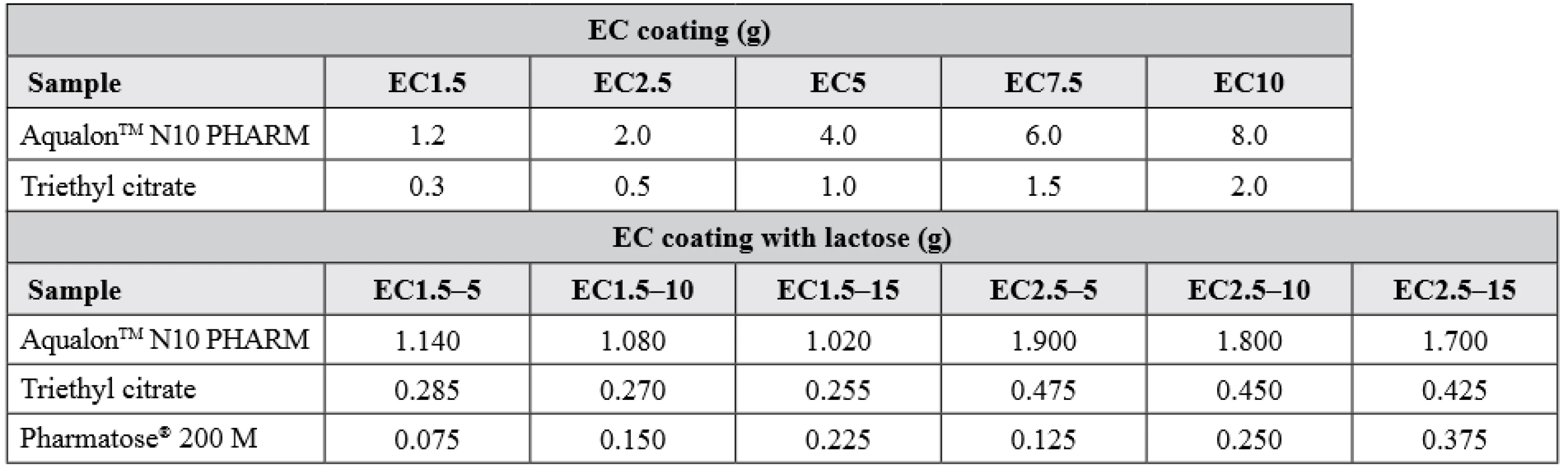
EC stands for ethylcellulose; 1.5, 2.5, 5, 7.5 and 10 is the percent increase in dry weight after coating; 5, 10 and 15 is the percentage of lactose in the coating composition Stability test
Twenty-five grams of newly prepared samples EC1.5, EC1.5–5, EC1.5–10 and EC1.5–15 (n = 3) were placed in three 100ml amber glass wide-neck bottles with PP cap (Rixius AG, Germany). The samples were stored for 24 months in three KBF stability boxes (Binder, Germany) differing in set temperature and relative humidity (RH): 25 ± 2 °C/60 ± 5% RH, 30 ± 2 °C/65 ± 5% RH and 40 ± 2 °C/75 ± 5% RH. The properties of the samples were measured at time 0 (before stability test), 3, 6, 12 and 24 months. The samples were evaluated for their enzyme activity, sensitivity, hardness, pycnometric density and sphericity. Color intensity was measured only at time 0, 12 and 24 months, while moisture loss at time 0 and 24 months. Hausner ratio, friability and interparticular porosity were due to the small amount of samples measured only at time 0.
Evaluation of physicochemical properties
Activity was tested as the time (in seconds) required to develop an observable yellow color on the pellets after the addition of Ellman’s test solution. Pellets were filled to a 1.0 cm height into glass tubes of 0.5 cm diameter. Subsequently, 0.2 ml of purified water was added and 3 minutes later an equal amount of test solution (containing Ellman’s reagent and substrate) was added. The speed of transition from white to yellow was evaluated visually for another 2 minutes and documented by photos using digital reflex camera (D5500, Nikon, Japan). Samples were considered active if a yellow color developed within this time limit.
Color intensity was evaluated from captured photos (0 and 120 s) during the activity test. Adobe Photoshop CS5 (Adobe Systems Inc., USA) with the eyedropper tool (101 × 101 pixels) was used to obtain and define color in 10 different areas with pellets on each photo. Using a statistical software R (version 3.6.1, The R Foundation, Austria), obtained RGB coordinates were converted to CIELAB color space and subsequently parameter ΔE2000 was calculated to evaluate the color intensity. The above mentioned methodology was already validated17) and was further improved based on previous research18).
Inhibition (sensitivity) was assessed similarly to test of activity, but instead of purified water a physostigmine test solution was used to moisten the samples. After a 10-minute incubation period, the Ellman’s test solution was added and the sample was visually observed for 2 minutes and documented by photos. Samples were considered inhibited if they remained its original color for at least 2 minutes. Based on previous experiments17–19), various predefined concentrations of physostigmine were used until the minimal inhibition concentration (MIC) was determined. All test solutions were prepared according to the standard methodology17, 19).
Hardness was evaluated on ten randomly selected pellets of each sample using a C50 Tablet Hardness Tester (Engineering Systems, UK) equipped with a C5 cell for the pellet measurement.
Friability was assessed on the TAR10 testing device (Erweka GmbH, Germany) at 20 rpm for 10 min using approximately 10 g of sample, which was put inside a steel discs together with 200 glass spheres. Subsequently, the content of the steel disc was sieved to separate glass spheres, pellets and dust. Each sample was evaluated three times and the result was expressed as a percentage of weight loss.
Moisture loss during drying of the samples was analyzed using the Moisture Analyzer HX204 (Mettler Toledo, Switzerland) at 105 °C. Approximately 1.0 g of pellets was pulverized in a mortar and used for the test. Each sample was evaluated three times and the result was expressed as a percentage of weight loss.
Pycnometric density (ρp) was assessed on a helium pycnometer Pycnomatic-ATC (Porotec GmbH, Germany) according to Ph. Eur. 9.
Interparticular porosity was then calculated from bulk and pycnometric density according to the following formula:
where IP is interparticular porosity (%), ρ0 is bulk density (g · cm–3) and ρp is pycnometric density (g · cm–3).
Hausner ratio characterizes flow properties and it was calculated from bulk (ρ0) and tapped (ρ1250) density according to Ph. Eur. 9 (the sample amount was 50 g). The tapping was performed on a SVM 102 tapping device (Erweka GmbH, Germany) and samples were tapped 1250 times. Bulk and tapped density was measured three times.
Sphericity was assessed using a stereoscopic microscope NIKON SMZ 1500 (Nikon, Japan) equipped with a 70AUC02 USB camera (The Imaging Source, Germany) and NIS-Elements AR 4.0 software (Nikon, Japan). Using this software a sphericity of 200 randomly selected pellets of each sample was calculated according to the following formula:
where S is the sphericity, A is the surface area (mm2) and p is the perimeter (mm)30).
Scanning electron microscopy (SEM)
The morphology of pellet surface and cross-section of coatings were documented by means of scanning electron microscopy (MIRA3, Tescan Orsay Holding, Czech Republic). The fixed samples (carbon conductive double-faced adhesive tapes, Agar Scientific, UK) were coated with a 20 nm layer of gold using the ion sputtering coating method with argon atmosphere (Q150R ES Rotary-Pumped Sputter Coater/Carbon Coater, Quorum Technologies, UK). A secondary electron detector and an accelerating voltage of 3 kV were used for the measurements.
Data analysis
Influence of lactose presence and its concentration in connection with stability conditions (temperature/humidity and time) on the properties of the carriers were evaluated by means of analysis of variance (ANOVA) or t-test based on the number of compared groups. During statistical testing, specific studied parameter is considered significant if the calculated p-value is equal to or smaller than α (the level of significance was set to the value of α = 0.05). The p-values (especially in cases of variable significance) are given in parentheses for the discussed carrier characteristics in the section Results and Discussion. For statistical computations, software R (version 3.6.1) and QC.Expert 3.2 were used.
Results and discussion
In current study, a new type of double-coated pellets for the detection of cholinesterase inhibitors in liquids was prepared and evaluated. Ethylcellulose was selected based on its suitable properties, such as semipermeability and insolubility in aqueous media. Triethyl citrate was utilized as plasticizer based on other studies28, 31). The composition of the double-coated pellets allows better detection of cholinesterase inhibitors in liquids since the semipermeable coating prevents enzyme leaching and pellet cracking due to water uptake. This should also lead to a more intense color transition on the pellets as was observed in recent study19). The aim of this study was to find out how the ethylcellulose coating affect the activity, color intensity and sensitivity of the carriers as well as other physicochemical properties.
In the first step, five samples (EC1.5, EC2.5, EC5, EC7.5 and EC10) with different coating thicknesses were prepared and evaluated for their activity and sensitivity. It was found that only sample EC1.5 (which developed observable yellow color after 80 s) and sample EC2.5 (after 90 s) had sufficient activity. The remaining samples (EC5, EC7.5 and EC10) did not develop any visible yellow color within 2 minutes due to excessive coating thickness. In terms of sensitivity, both active samples were inhibited with physostigmine concentration of 0.5 µg · ml–1, which is sufficient for this type of detectors17, 18). When the activity results were compared to samples coated with Eudragit® RL, it was apparent that the EC coating had a much lower permeability.
In the second step, water-soluble sugar (lactose) was dispersed in the EC coating at three different concentrations (5, 10 and 15% w/w). When immersed in an aqueous test solution, lactose was expected to dissolve, creating pores in the coating and thus increasing the EC coating permeability. A total of six samples were prepared with three concentrations of lactose for 1.5% and 2.5% coating thickness (EC1.5–5/10/15 and EC2.5–5/10/15). While the EC1.5–5/10/15 samples had activity about 60 s, an improvement over the EC1.5 sample without lactose, the EC2.5–5/10/15 samples were considered inactive (an observable yellow color developed after the required 2 minutes). All three EC1.5–5/10/15 samples were inhibited by the physostigmine concentration of 1.0 µg · ml–1. An increase in the required MIC was associated with an increase in activity. Based on these results, only EC1.5 and EC1.5–5/10/15 samples were selected for stability test.
New batches were prepared and evaluated (i.e. time 0) before loading into stability boxes. As before, significantly (p < 0.001) slower development of yellow color was observed for samples EC1.5 with activity 80 ± 5 s, whereas samples containing lactose (EC1.5–5/10/15) were visibly yellow in 60 ± 0 s, 55 ± 5 s and 60 ± 5 s, respectively. The development of the activity during the stability test is shown in Table 2. During storage, the activity of all samples decreased significantly (p < 0.001) over time. A statistically significant effect of lactose on carrier activity during stability testing was not confirmed (p = 0.294). It also turned out that the storage condition of 40 °C/75% RH was critical for all samples as they had insufficient activity after 6 months. This was related to the color change of carriers, which began to lose their originally white color and get brown. At 25 °C/60% RH (Fig. 1) and 30 °C/65% RH, all samples remained active and differed insignificantly (p = 0.497) over a 24-months stability study with minimal (but noticeable) browning. Similar results were observed and explained in the recently published article concerning uncoated matrix pellets18). It was discussed that the browning is probably caused by the recrystallization and degradation of sugars, such as lactose or even cellulose18). In the case of this study, the heat degradation of the ethylcellulose coating should also be considered32). The stability study of double-coated pellets with Eudragit® RL has not been published yet19).
1. Activity test of samples stored at 25 °C/60% RH for 24 months (photos were taken 120 s after the addition of Ellman’s test solution) 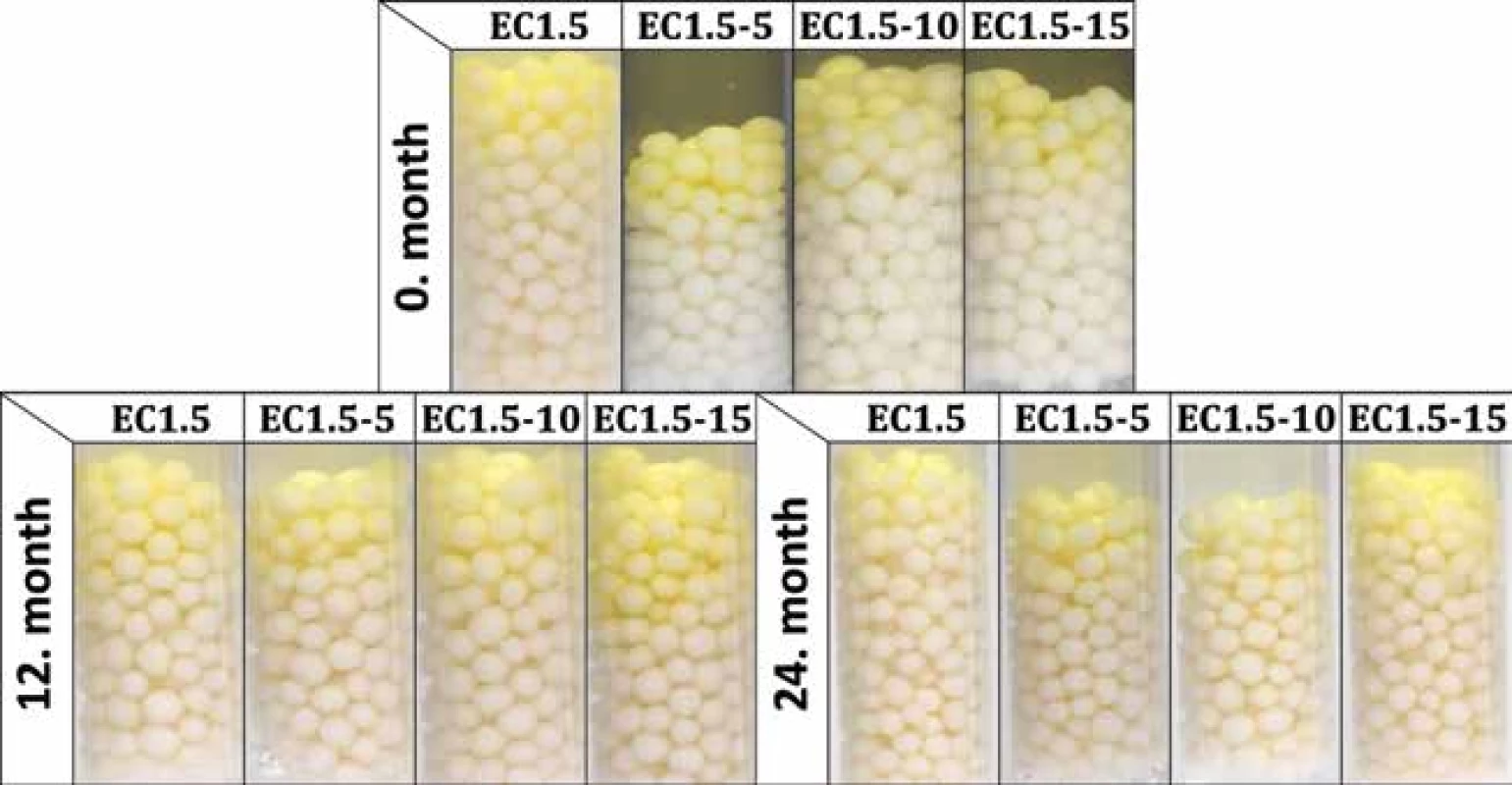
The intensity of the developed yellow color was evaluated using the parameter ΔE2000 after the conversion of the recorded RGB values into the CIELAB coordinate system. Advantage of this method over RGB-based evaluation is that it takes into account that the human eye perceives different colors with different intensities. ΔE2000 values range from 0 (same colors) to 100 (opposite colors), which means that it is desirable to achieve ΔE2000 values as high as possible. The results of the color intensity evaluation are summarized in Table 2. Compared to the results of matrix or double-coated pellets18, 19), it is evident that the EC coating distinctly reduced the intensity of the developed yellow color. In addition, a statistically significant (p < 0.001) effect of lactose was observed only in samples stored at 25 °C/60% RH. As in the previous study18), the intensity of all samples decreased significantly with storage time and increasing temperature/humidity (both p < 0.001). The ΔE2000 values of most inactive samples corresponded (p > 0.05) with the values of the fully inhibited samples, which had an average ΔE2000 of 7.45 ± 1.29 without significant lactose effect (p = 0.757). Although one inactive sample (EC1.5–5 at 40 °C after 12 months) differed significantly (p = 0.025) from the ΔE2000 of inhibited samples, the difference was very small and indistinguishable to the human eye33).
2. Activity and ΔE2000 (color intensity) values during 24-months stability test 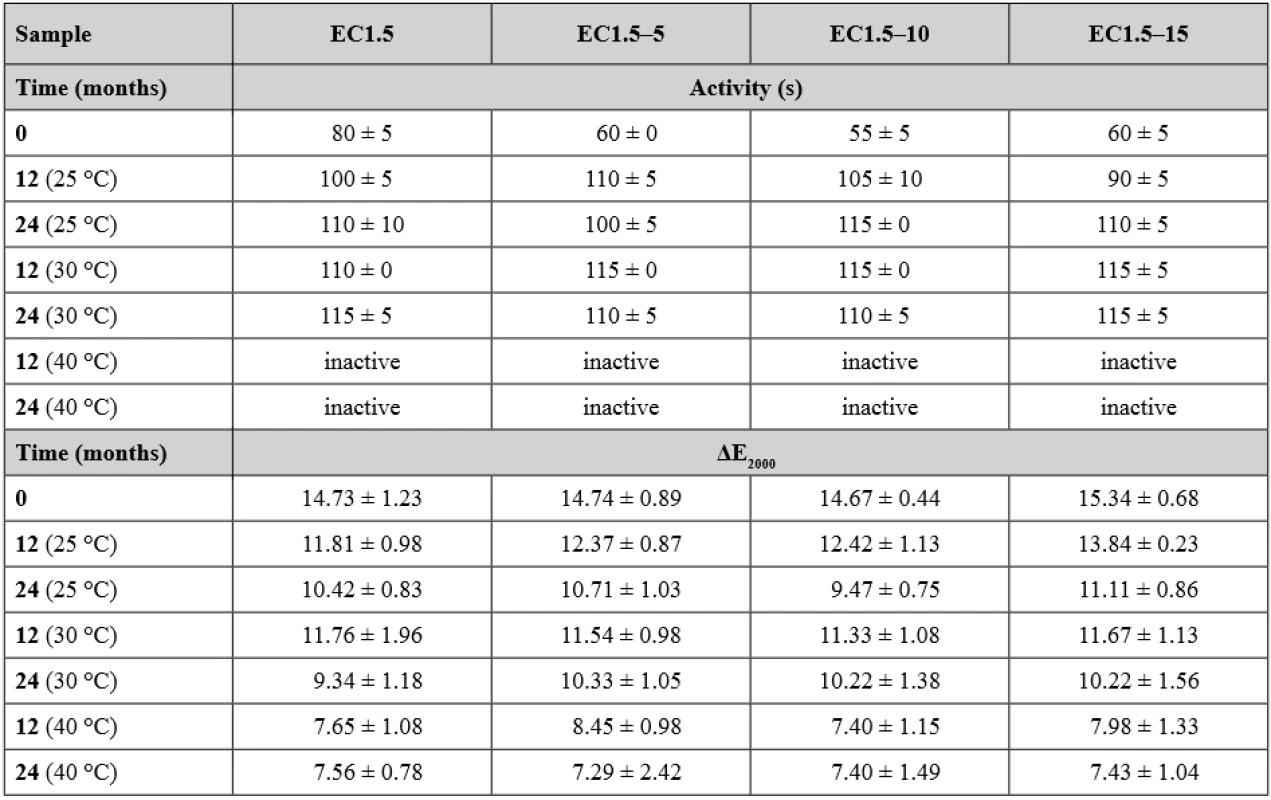
Inactive pellets did not develop observable yellow color within 120 s after the addition of Ellman’s test solution. ΔE2000 values of inactive samples were caused by the original Ellman’s solution color and were similar to values measured for fully inhibited samples. Samples were at time 0 again inhibited with a physostigmine concentration of 0.5 µg·ml–1 for EC1.5 and 1.0 µg·ml–1 for EC1.5–5/10/15. After the first 6 months, sensitivity of samples stored at 25 °C and 30 °C significantly decreased (p < 0.001) as they were inhibited by 4.0 µg · ml–1 and remained unchanged for the remainder of the stability test, while samples stored at 40 °C were already inactive and therefore their sensitivity could not be tested. No significant effect of lactose on sensitivity was observed (p = 0.999). The decrease in sensitivity was associated with a decrease in activity and this could probably be explained by a change in the EC and/or hypromellose coating permeability or other properties caused by the coating ageing34, 35).
Hardness and friability were measured to assess the physical resistance of the pellets to disintegration, as they should withstand filling into detection tubes, transport and storage. The hardness of the samples at the beginning and after the stability test is given in Table 3. Due to the layered structure of the carriers (Fig. 2), its values were higher than in the case of matrix pellets18). However, a similar phenomenon was observed as the hardness decreased significantly (p < 0.001) over time and with increasing storage temperature, while the effect of lactose presence on hardness during the stability test was insignificant (p = 0.190). For matrix pellets, this was mostly due to an increase in residual moisture. As can be seen in Table 3, the residual moisture of all samples was significantly higher after 24 months (p < 0.001), but without the influence of lactose (p = 0.927), indicating the effect of another factor, probably the aging of the coating, which then becomes more brittle35). The friability of the samples at time 0 varied insignificantly (p = 0.580) and was less than 0.3%.
2. Cross-section SEM photos of double-coating of samples EC1.5 (A) and EC1.5–15 (B) EC – ethylcellulose, HPMC – hypromellose, BuChE – butyrylcholinesterase 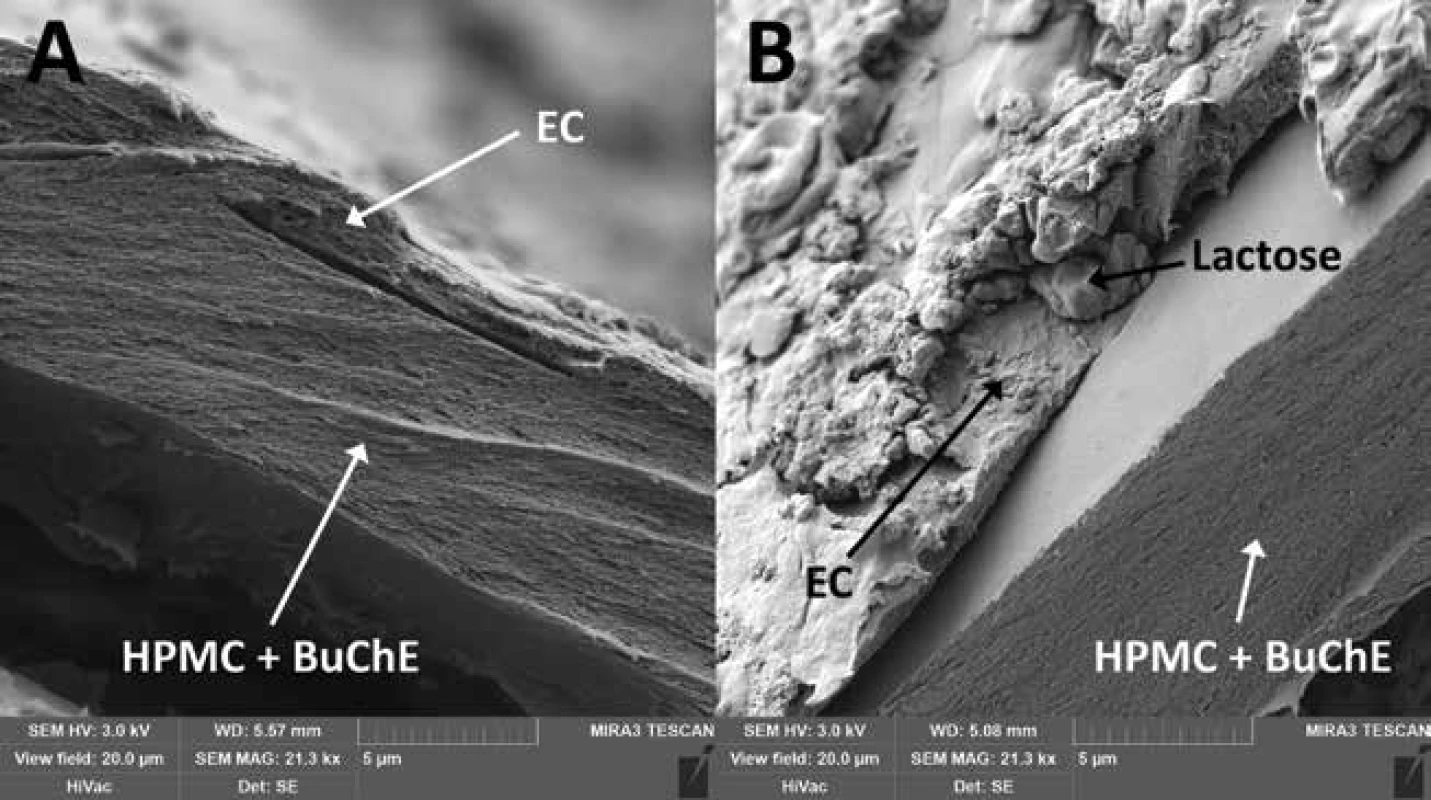
3. Selected physicochemical properties of samples after 24-months stability test 
Pycnometric density (Table 3), affecting both hardness and interparticular porosity, decreased significantly over time (p < 0.001) independent of storage conditions (p = 0.992) or lactose concentration (p = 0.061), as there were no significant differences in residual moisture between samples, which were reported to have the major influence on pycnometric density values18).
The good flow properties of the carriers are another important parameter as they affect the loading into the detection tubes as well as the detection itself17, 18). According to Ph. Eur. 9, the Hausner ratio was evaluated at time 0, differed insignificantly between samples (p = 0.177) and ranged from 1.018 ± 0.018 to 1.039 ± 0.014 indicating excellent flow properties. The interparticular porosity in the range of 26 to 48% is considered ideal and also indicates good flow properties as the air gaps between pellets are small36). Samples EC1.5 and EC1.5–5/10/15 had interparticular porosity of 40.04 ± 0.63%, 38.10 ± 0.65%, 40.18 ± 0.60 and 39.00 ± 0.62%, respectively. Although intraparticular porosity differed significantly (p = 0.011) between samples, no relationship with lactose concentration was evident. In terms of sphericity, values above 0.8 are considered sufficiently spherical37). Since the sphericity of all samples during the stability test was greater than 0.943, all samples were considered spherical, while no statistically significant (p = 0.702) effect of lactose on sphericity was observed. This conclusion was also supported by SEM photos (Fig. 3), where a smooth pellet surface was also observed. The surface was slightly rougher for the EC1.5–5/10/15 samples due to the presence of lactose crystals (as can be seen in Fig. 2).
3. SEM photos of samples EC1.5 and EC1.5–15 stored at 25 °C/60% RH (A and B) and at 40 °C/75% RH (C and D) for 24 months. A detail of the surface is in the right upper corner of each SEM photo. 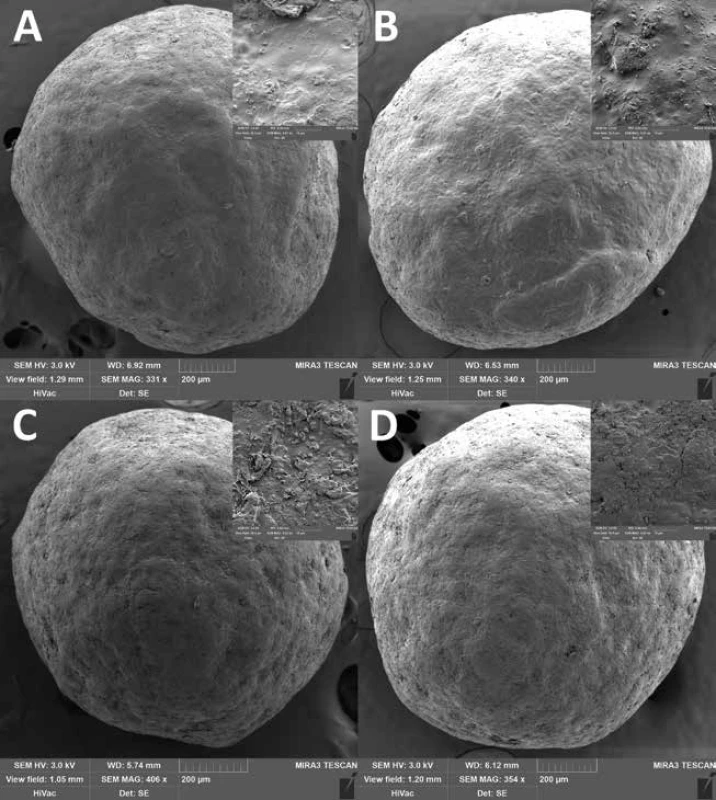
Conclusion
In the present study, the effect of ethylcellulose coating with or without dispersed lactose on the properties of pellets with immobilized butyrylcholinesterase was investigated. It was found that ethylcellulose coating substantially reduced the reaction rate and the intensity of the developed color compared to a similar coating of Eudragit® RL or uncoated matrix pellets. However, samples EC1.5 and EC1.5–5/10/15 remained active during 24-months stability test at 25 °C/60% RH and 30 °C/65% RH. The harshest condition of 40 °C/75% RH occurred to be critical when after 6 months of storage the samples started to brown and were insufficiently active, which was a similar problem as in the case of matrix pellets. This was probably due to ageing of the coating or thermal degradation of the cellulose. As for the other properties, all samples showed sufficient sensitivity to physostigmine, excellent flow properties and good physical resistance.
Acknowledgements
This work was supported by ITA FaF/Vetchý/ITA2019 VFU Brno.
Conflict of interest: none.
J. Zeman • S. Pavloková • Assoc. prof. PharmDr. et Mgr. David Vetchý, Ph.D. (∗)
Department of Pharmaceutics, Faculty of Pharmacy
University of Veterinary and Pharmaceutical Sciences
Palackého 1946/1, 612 42 Brno, Czech Republic
e-mail: vetchy@email.cz
V. Pitschmann
Oritest Ltd., Prague, Czech Republic
Faculty of Biomedical Engineering, Czech Technical University
in Prague, Kladno, Czech Republic
Sources
1. Kaduszkiewicz H., Zimmermann T., Beck-Bornholdt H.-P., van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. BMJ 2005; 331, 321–327.
2. Berrih-Aknin S., Frenkian-Cuvelier M., Eymard B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J. Autoimmun. 2014; 48, 143–148.
3. Pagano G., Rengo G., Pasqualetti G., Femminella G. D., Monzani F., Ferrara N., Tagliati M. Cholinesterase inhibitors for Parkinson’s disease: a systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2015; 86, 767–773.
4. Almasieh M., MacIntyre J. N., Pouliot M., Casanova C., Vaucher E., Kelly M. E. M., di Polo A. Acetylcholinesterase inhibition promotes retinal vasoprotection and increases ocular blood flow in experimental glaucoma. Investig. Opthalmology Vis. Sci. 2013; 54, 3171–3183.
5. Colović M. B., Krstić D. Z., Lazarević-Pašti T. D., Bondžić A. M., Vasić V. M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 2013; 11, 315–335.
6. Teixeira H., Proença P., Alvarenga M., Oliveira M., Marques E. P., Vieira D. N. Pesticide intoxications in the centre of Portugal: three years analysis. Forensic Sci. Int. 2004; 143, 199–204.
7. Chauhan S., Chauhan S., D’Cruz R., Faruqi S., Singh K. K., Varma S., Singh M., Karthik V. Chemical warfare agents. Environ. Toxicol. Pharmacol. 2008; 26, 113–122.
8. Vale J. A., Marrs T. C., Maynard R. L. Novichok: a murderous nerve agent attack in the UK. Clin. Toxicol. 2018; 56, 1093–1097.
9. Petroianu G., Toomes L. M., Petroianu A., Bergler W., Rüfer R. Control of blood pressure, heart rate and haematocrit during high-dose intravenous paraoxon exposure in mini pigs. J. Appl. Toxicol. 1998; 18, 293–298.
10. Petroianu G. Organophosphate poisoning: the lesser-known face of a toxidrome. Eur. J. Emerg. Med. 2005; 12, 102–103.
11. Balali-Mood M., Balali-Mood K. Neurotoxic disorders of organophosphorus compounds and their managements. Arch. Iran. Med. 2008; 11, 65–89.
12. Lincová D., Farghali H. Základní a aplikovaná farmakologie. Praha: Galén 2007.
13. Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004; 38, 151–216.
14. Zeman J., Vetchý D., Franc A., Pitschmann V. Methods of enzymes immobilization and their use for optical (colorimetric) detection of cholinesterase inhibitors. Chem. Listy 2018; 112, 434–439.
15. Ellman G. L., Courtney K. D., Andres V., Featherstone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961; 7, 88–95.
16. Oritest. Oritest Ltd.: http://www.oritest.cz/wp-content/uploads/2017/12/Tubes-CWA-brochure-2012.pdf, cited 26 September, 2019.
17. Zeman J., Vetchý D., Franc A., Pavloková S., Pitschmann V. Matějovský L. The development of a butyrylcholinesterase porous pellet for innovative detection of cholinesterase inhibitors. Eur. J. Pharm. Sci. 2017; 109, 548–555.
18. Zeman J., Vetchý D., Pavloková S., Franc A., Pitschmann V., Dominik M., Urbanová M., Šeděnková I. Tubes for detection of cholinesterase inhibitors – unique effects of Neusilin on the stability of butyrylcholinesterase-impregnated carriers. Enzyme Microb. Technol. 2019; 128, 26–33.
19. Vysloužil J., Vetchý D., Zeman J., Farsa O., Franc A., Gajdziok J., Vysloužil J., Ficeriová K., Kulich P., Kobliha Z., Pitschmann V. Pellet patented technology for fast and distinct visual detection of cholinesterase inhibitors in liquids. J. Pharm. Biomed. Anal. 2018; 161, 206–213.
20. Vetchý D., Leštinová H., Tušarová I. Methods of pharmaceutical technology in preparation of pellets for detection of acetylcholinesterase inhibitors. Čes. slov. Farm. 2012; 61, 234–239.
21. Pohanka M., Vlček V., Žďárová-Karasová J., Kuča K., Cabal J. Colorimetric detectors based on acetylcholinesterase and its construction. Vojen. Zdrav. Listy 2010; 79, 9–14.
22. Pitschmann V., Matějovský L., Vetchý D., Kobliha Z. Enzymatic determination of anticholinesterases using a composite carrier. Anal. Lett. 2016; 49, 2418–2426.
23. Gelman C, Kramer D. N. US3049411 (A) 1962.
24. Nisha S., Arun K. S., Gobi N. A review on methods, application and properties of immobilized enzyme. Chem. Sci. Rev. Lett. 2012; 1, 148–155.
25. Vetchý D., Pitschmann V., Vetchá M., Kašparovský T., Matějovský L. Preparation and evaluation of carriers for detection of cholinesterase inhibitors. Neuroendocrinol. Lett. 2015; 36, 95–99.
26. Vetchý D., Franc A., Gajdziok J., Vysloužil J., Pitschmann V., Matějovský L. CZ306803 (B6) 2017.
27. Kato T., Unno K., Goto A. Ethylcellulose microcapsules for selective drug delivery. Methods Enzymol. 1985; 112, 139–150.
28. Siepmann F., Siepmann J., Walther M., MacRae R. J., Bodmeier R. Polymer blends for controlled release coatings. J. Control. Release 2008; 125, 1–15.
29. WHO. Stability testing of active pharmaceutical ingredients and finished pharmaceutical products. WHO Tech. Rep. Ser. 2009; 87–130.
30. Sienkiewicz G., Pereira R., Rudnic E. M., Lausier J. M., Rhodes C. T. Spheronization of Theophylline-Avicel combinations using a fluidized-bed rotogranulation technique. Drug Dev. Ind. Pharm. 1997; 23, 173–182.
31. Lecomte F., Siepmann J., Walther M., MacRae R. J., Bod-meier R. Polymer blends used for the aqueous coating of solid dosage forms: importance of the type of plasticizer. J. Control. Release 2004; 99, 1–13.
32. Rekhi G. S., Jambhekar S. S. Ethylcellulose – a polymer review. Drug Dev. Ind. Pharm. 1995; 21, 61–77.
33. Mokrzycki W. S., Tatol M. Color difference Delta E – a survey. Mach. Graph. Vis. 2011; 20, 383–411.
34. Perera D. Y. Physical ageing of organic coatings. Prog. Org. Coatings 2003; 47, 61–76.
35. Heng P. W. S., Chan L. W., Ong K. T. Influence of storage conditions and type of plasticizers on ethylcellulose and acrylate films from aqueous dispersions. J. Pharm. Pharm. Sci. 2003; 7 (6), 334–344.
36. Rabišková M. Technological parameters of drug microforms, their importance and methods of their determination. Čes. slov. Farm. 1996; 45, 177–179.
37. Deasy P. B, Law M. F. L. Use of extrusion-spheronization to develop an improved oral dosage form of indomethacin. Int. J. Pharm. 1997; 148, 201–209.
Labels
Pharmacy Clinical pharmacology
Article was published inCzech and Slovak Pharmacy

2020 Issue 1-
All articles in this issue
- Metal complexes in medicine and pharmacy – the past and the present II
- K životnému jubileu pani doc. RNDr. Zuzany Vitkovej, CSc.
- Životné jubileum prof. RNDr. Daniela Grančaia, CSc.
- Zomrel Alois Borovanský
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Double-coated pellets with semipermeable ethylcellulose coating for detection of cholinesterase inhibitors
- Development and uniformity evaluation of low-dose medicated chewing gums prepared by compression method
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- The amino acid and carbohydrate composition of the herb and roots of Smallanthus sonchifolius
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Metal complexes in medicine and pharmacy – the past and the present II
- Pharmacokinetic aspects of beta-lactam antibiotic therapy in intensive care unit patients: A one-center experience with TDM
- Investigation of thioctic acid, magnesium stearate and pyridoxine hydrochloride compatibility
- Zomrel Alois Borovanský
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career



