-
Medical journals
- Career
Merkel Cell Carcinoma of the Eyelids (Clinical-Histological Study)
Authors: J. Krásný 1; J. Šach 2; V. Kolín 2; J. Zikmund 3
Authors‘ workplace: Oční klinika FN Královské Vinohrady, Praha, přednosta: prof. MUDr. Pavel Kuchynka, CSc. 1; Ústav patologické anatomie FN Královské Vinohrady a 3. LF UK, Praha, přednosta: doc. MUDr. Tomáš Jirásek, PhD. 2; Klinika dětí a dorostu FN Královské Vinohrady, Praha, přednosta: doc. MUDr. Felix Votava, PhD. 3
Published in: Čes. a slov. Oftal., 74, 2018, No. 5, p. 198-205
Category: Original Article
doi: https://doi.org/10.31348/2018/5/5Overview
Aim:
To get acquainted with the issues of Merkel cell carcinoma (MCC) when the eyelid is affected and with its surgical solution.
Materials:
MCC of the right upper eyelid was at the Department of Ophthalmology in the Faculty Hospital Královské Vinohrady in Prague (Czech Republic, EU) in 1998 woman 78 year. Another cases of the MCC on the left upper eyelid were observed in two women aged 48 and 67 years and they were pick up in a retrospective study covered 1033 operated tumors eyelids years 2007 – 2015. 47,5% of operated tumors were benign and 52,5% malignant. Most common malignant processes were basal cell carcinoma in 77,3% and squamous cell carcinoma at 15,7%.
Results:
The oldest patient died after three years of cardiopulmonary failure. 48 year old patient (age of diagnosis MCC) has been monitoring for five years without proven recurrence or metastasis dissemination. Oncological initial staging was negative regarding. An ultrasound examination of the lymphatic system of the neck was followed every six months. Another 67 year old patient (age of diagnosis MCC) was followed for 2,5 years. There was a suspicion of a metastatic process in the same side salivary gland and lungs, therefore chemotherapy was performed. Definitely, this process has not been proven. Now there is continuing follow up without sings of local recurrence or metastatic dissemination.
Conclusion:
The authors confirmed a rare occurrence of MCC, and only 0,37% among malignant eyelids tumors. Essential importance for successful treatment is a sufficiently radical excision supported by histological verification and a subsequent plastic surgery of the eyelid is also necessary.
Key words:
Merkel cell carcinoma, tumor of eyelid, ultrasound examination of nodes
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare primary neuroendocrine skin carcinoma with a high level of recurrence, with subsequent excisions and a tendency toward metastatic spreading via the regional lymph nodes. One half of tumours are localised on the head and neck. Ocular localisation is primarily on the upper eyelid. Clinically the pathology presents itself as purple vascularised skin nodules. In 1875 Friedrich Sigmund Merkel described oval, non-dendritic, epidermal clear cells (28). Histologically they were characterised practically one hundred years later as cells with a clear cytoplasm rich in various organelles within a narrow band around the oval nucleus. This characteristic became the basis of the clinical description of MCC. With regard to the rare incidence primarily in the area of the eyelids and as yet what is the only case report of affliction of the eyelids, together with another orbital observation published to date in the basic journal of the Czech and Slovak Ophthalmology Society in 2018 (5), we decided to present a breakdown of a number of clinical observations. We documented the identification of MCC in relation to other skin tumours in the area of the eyelids. We supplemented the report with a literary analysis of the current observations on the etiology, histological verification and treatment of MCC.
Material
In 1998 a 78 year old patient was sent to the Department of Ophthalmology at the Královské Vinohrady University Hospital with a recurrence of MCC on the upper eyelid of the right eye. The initial excision of the tumour and its histological verification was performed at the department of ophthalmology of the district hospital, when there was a recurrence of the tumour within a year, with a subsequent new excision. Upon a further recurrence the patient was sent to our clinic. Upon the third surgical procedure of radical extirpation with plastic surgery of the eyelid in May 1998, the initial diagnosis of MCC was confirmed. After this operation the patient did not manifest any further recurrence or spread of the tumour into the local area of the lymph nodes. After three years the patient died of cardiopulmonary failure not connected with the tumour, no autopsy was performed.
A retrospective study was compiled for the period of September 2007 to September 2015 within the framework of an attestation study from the field of pathology regarding the issue of evaluating histological findings of tumours of the eyelids in patients observed and operated on at the Department of Ophthalmology at the Královské Vinohrady University Hospital or in co-operation with the Department of Plastic Surgery at the same hospital, aged between 3 and 97 years, with a median age of 61 years. In total 1033 tumours were evaluated, of which 47.5% were benign and 52.5% malignant. Of the benign tumours the most widely represented were epithelial tumours, with 247 findings, which predominantly concerned seborrheic keratosis (69%), followed by squamous cell papillomas (16%) and skin adnexal tumours (6%). Neuroectodemal lesions were represented in 191 patients, and in the absolute majority (97%) consisted of nevi. The least represented were mesenchymal tumours in 52 patients, which were primarily haemangiomas (70%), in rare cases lipomatous lesions or histocytoma.
Table 1 presents the diagnostic division of malignant tumours, in which basal cell carcinoma predominated. Among the other isolated tumours there also appeared 4x sebaceous carcinoma, 3x lymphoma, 2x DLB-CL (diffuse large B cell lymphoma) and one CLL (chronic lymphatic leukaemia) or de-differentiated sarcoma. The table also shows the relative percentage representation with simultaneous classification also of benign tumours. In addition to genuine tumours, pseudotumours were also histologically described in 195 lesions, which constituted 13% of the total number of 1228 excisions from eyelids. Etiologically this concerned chronic inflammations (45x chalazion and 15x pyogenic granuloma) or cystic formations or degenerative changes. The observed diagnoses also included two cases of MCC, and as a result we classified them as two separate case reports in addition to the initial observation from 1998.
1. Percentage division of malignant tumours of eyelid region 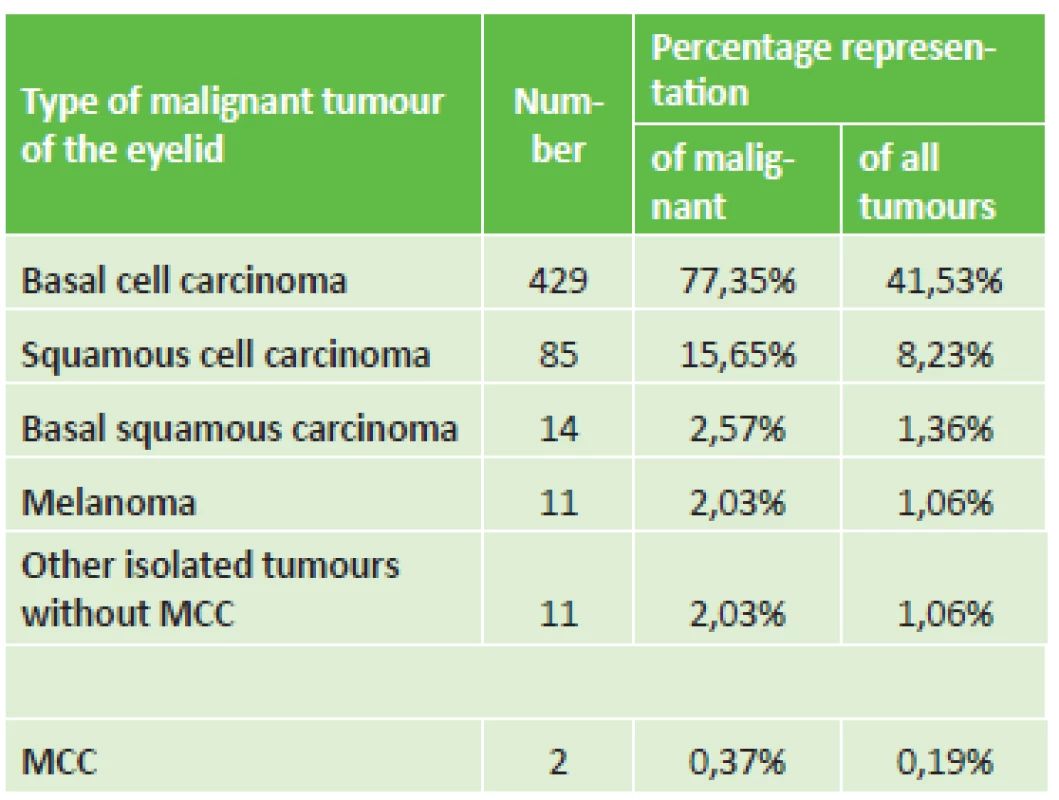
Case report no. 1
At the end of July 2012 a 48 year old woman reported to the general outpatient clinic of the Department of Ophthalmology at the Královské Vinohrady University Hospital in Prague, with reddening of the upper left eyelid persisting for seven weeks, in the form of slightly painful resistance above the edge of the eyelid. Suspicion of chalazion was stated, which was extirpated two weeks later (without histological verification), but the condition did not improve. After an interval of six weeks probatory excision was performed under local anaesthesia in order to obtain histological verification of the process, which revealed MCC. At the beginning of October the same year the patient was hospitalised for total extirpation of the upper left eyelid tumour under general anaesthesia. The clinical picture showed livid, rigid and solid resistance at least 6 mm above the level with numerous superficial capillaries, immobile as against the base. Its size was 13 x 10 mm (fig. 1A). The incision was made in the place of the original probative excision, there followed preparation to the edge of the tumour (fig. 1B) above beneath the skin, also to depth toward the edge of the tarsus, from which it was progressively separated and resected. With regard to the fact that the tissue in front of the margin is persistently overgrown with a tumour, there followed resection of the edge of the eyelids also with eyelashes (fig. 1C). The procedure was accompanied with profuse bleeding. Control samples of the skin tissue were taken in a direction toward the tarsus, from the nasal and temporal edge of the skin by the tarsus. There followed plastic surgery, incorporating a shift of the skin upward and at the same time cutting of the tarsus. Both tissues were shifted in order to create a continuity of the edge of the eyelid. The wound was closed with individual embedded resorbable sutures, namely Vicryl 7-0 (Ethicon, USA): tarsus to skin - “end to end” (fig. 1D).
1. Clinical finding of solid MCC of upper eyelid in 48 year old patient (A). Surgical procedure: removal of eyelid (B, C) with subsequent plastic surgery (D) 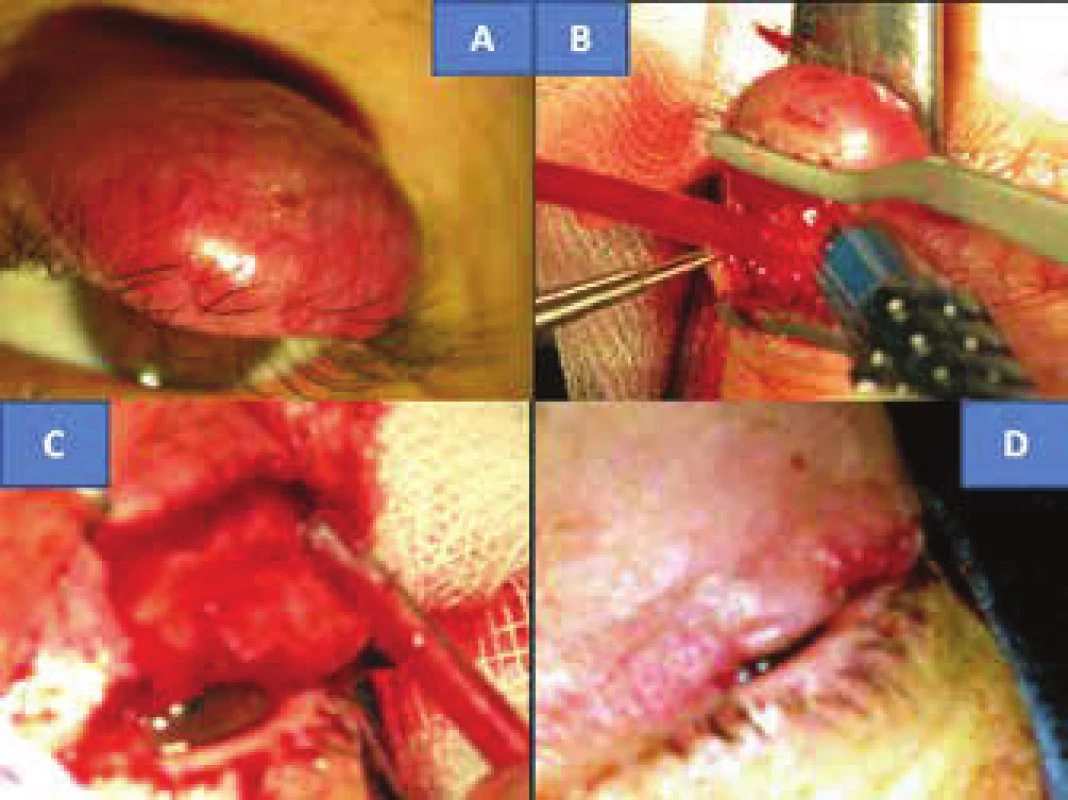
Histological verification demonstrated a tumour with a microscopic image characteristic of MCC. The radical nature of the procedure in the form of non-tumorous tissue on the edges of the main sample was confirmed (fig. 2A), as was the correctness of the decision on resection of the edge of the eyelid with the tarsus, since the tumour encircled the follicles of the eyelashes (fig. 2B). Although the borderline sample from the subcutaneous tissue documents infiltration of MCC, its outer edges are without tumorous manifestations. The nasal and temporal skin samples were without tumorous infiltration. This also confirmed that the procedure was sufficiently radical. The diagnosis was also confirmed by the range of immunohistochemical examinations. At the same time both the epithelial markers (cytokeratin CK116 and AE1-3) and the neuroendocrine markers chromogranin (fig. 2C) were positive, and NSEs were markedly positive in the tumour. The proliferation marker Ki67 (MIB1) was positive in fluctuating intensity in as much as approx. 98% of cells (fig. 2D). Infiltration with T-lymphocytes (CD3 positive) and B-lymphocytes (CD20 positive) was not found in the tumour. The only positive was in the reactive lymphocyte infiltrate in the surrounding area of the tumour – control samples, with pronounced predominance of T-lymphocytes CD3. This could attest to the onset of the patient's own immune defensive capacities against the tumour.
2. Extensive infiltration of eyelid with MCC with non-tumorous tissue in edges, colouring HE – enlargement 100x (A) infiltration with tumour in surrounding area of eyelash follicles, colouring HE – enlargement 200x (B), neuroendocrine marker – chromogranin, enlargement 400x (C), proliferation marker Ki67, enlargement 400x (D) 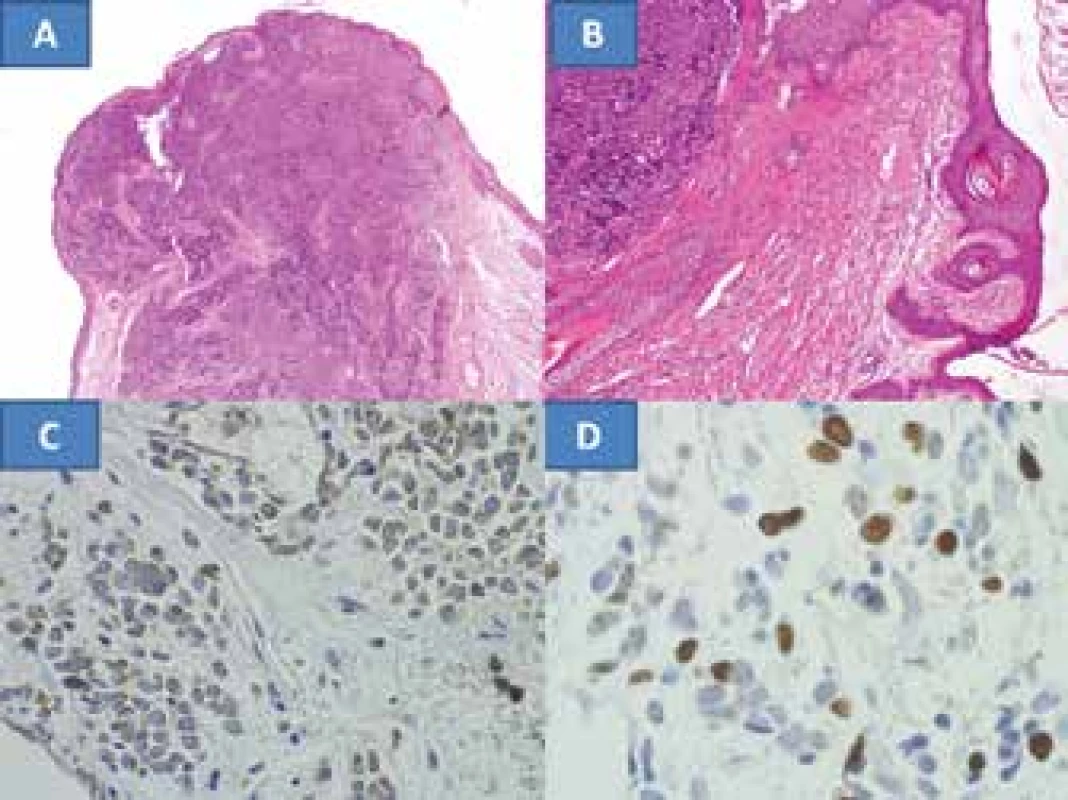
The postoperative course took place without complications, local recurrence did not appear up to the last follow-up examination in September 2017. The upper eyelid contoured the ocular aperture upward well even despite a slightly irregular edge, the eyelashes remained only at both ends, without manifestations of trichiasis (fig. 3A). The eyelid adhered well to the eyeball, and the ocular aperture was spontaneously closed (fig. 3B). The intraocular finding was bilaterally symmetrical and physiological, IOP = IOL 5/5 nat.
3. Clinical finding of upper eyelid five years after surgery for MCC on now 53 year old patient (A), spontaneous closure of ocular aperture (B), typical flat benign reactive node in area of musculus sternokleidomastoideus in right eye in our patient (C) and for comparison example of bundle of malignant predominantly almost spherical nodes with clear echogenic stroma (archive of MUDr. J. Zikmund, CSc.) in same region (D) 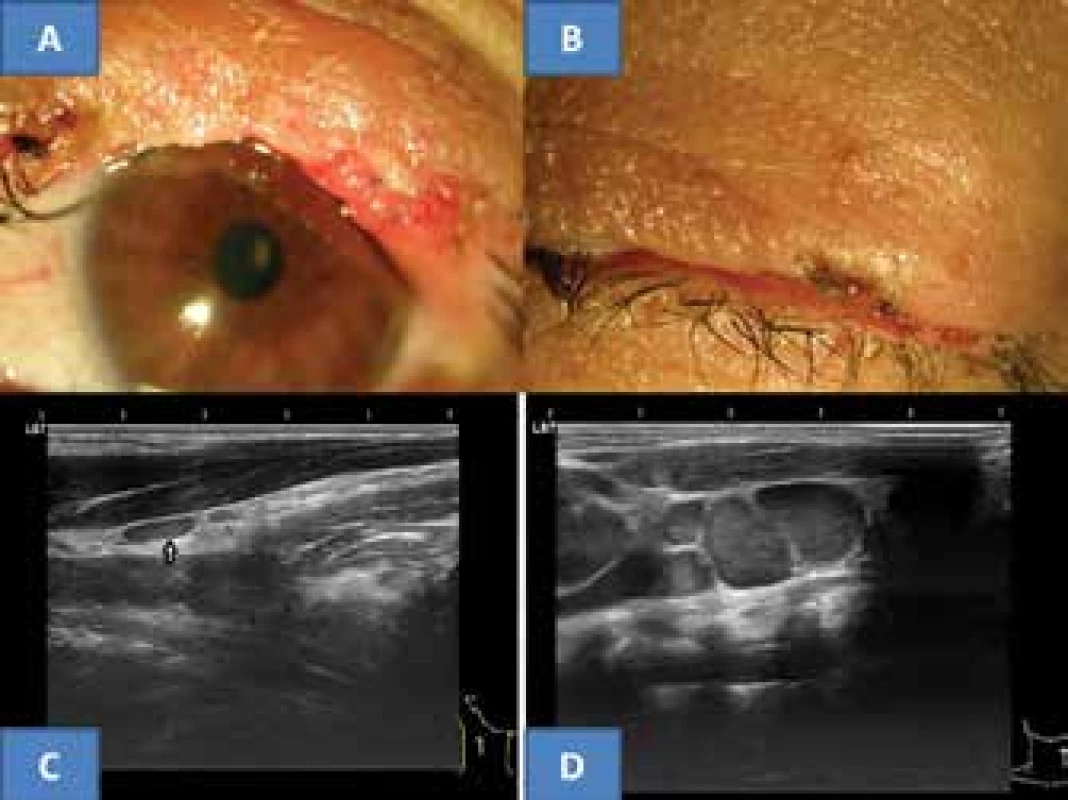
The recommended oncological staging following the histological verification was composed of an X-ray of the lungs, sonography of the liver, blood count with detailed differential image and liver tests. All the examinations were negative from an oncological perspective. Ultrasound examination of the neck nodes (instruments Sonoline Versa Plus, linear probe 7.5 MHz, and since 2015 ultrasound instrument Login S7 from the GE company) did not demonstrate suspicion of malignancy. Only a small, flat anechogenic reactive node was determined in a tail-like structure from the right salivary gland, with a size of 8 x 3 mm in lengthwise cross-section (fig. 3C). The thyroid gland and salivary glands were without pathology. The oncological conclusion was determined only by further ultrasound examinations of the neck and the above-mentioned blood samples. Upon further observations the blood tests continued to be negative and the ultrasound finding on the neck did not record progression in the appearance of nodes in the given area. The above-described node persisted without changes. The last examination in September 2017 did not demonstrate malignant affliction of the lymph nodes of the neck.
Case report no. 2
In November 2015 a 67 year old woman was sent to the Department of Ophthalmology at the Královské Vinohrady University Hospital in Prague, suffering from MCC which had been verified in October the same year from a probative excision at the local regional hospital. In the same month we performed a total extirpation of a nodular formation of reddish-purple colour measuring 6 x 8 mm on the edge of the upper left eyelid (fig. 4B) with subsequent plastic surgery treatment. The tumour was sent for histological verification. Borderline tissues were taken as samples in an upward direction into the hypodermis, into the tarsus and skin samples nasally and temporally from the tumour. In all the borderline samples the presence of a tumour was no longer demonstrated, only in the borderline tissue nasally in the direction toward the extirpated tumour the infiltration was demonstrated. The excision here was very close, but the further content of the sample was without tumorous tissue. We were able to conclude the condition as total removal of MCC of the eyelid.
4. 4. MCC of the eyelid in a 78 year old patient (A), MCC of the eyelid in a 67 year old patient (B), the characteristic histological finding of MCC is formed by oval, non-dendritic, epidermal clear cells with mitoses – indicated with a circle (C), HE colouring, enlargement 300x 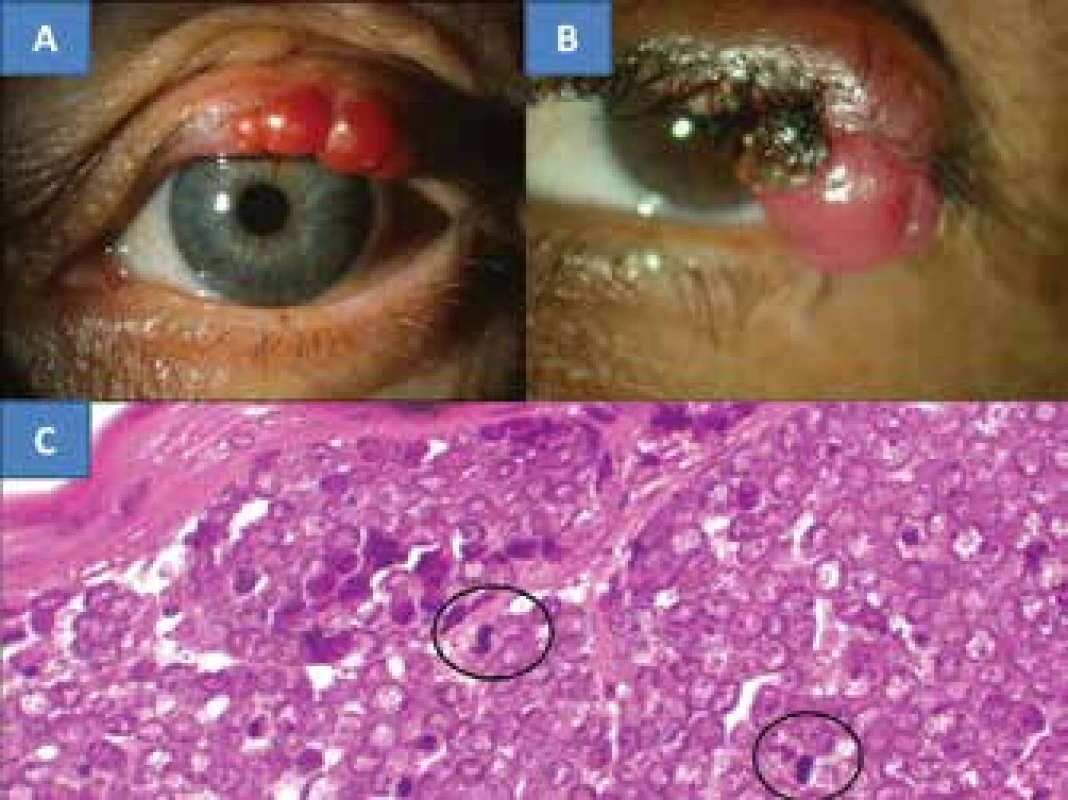
The histological examination again demonstrated MCC. The tumour cells had voluminous rounded to oval nuclei, with finely dispersed chromatin and evident small nucleoli. The actual cytoplasm of the tumour cells was not abundant, and formed only a fine lining around the cell nuclei (fig. 4C). Mitotic activity was present in the nuclei. The diagnosis was supported with a range of immunohistochemical examinations. The epithelial markers incorporated cytokeratins CK20, CAM 5.2 and AE1-3 – in all cases they were strongly positive. The neuroendocrine markers, namely chromogranin and NSE were also positive. The proliferation marker Ki67 (MIB1) was positive in 25% of the cells.
Before the patient was discharged, ultrasound examination of the neck was performed (Sonoline Versa Plus, linear probe 7.5 MHz) with the following conclusion: nodes along the sternocleidomastoid muscles anechogenic, homogeneous and not enlarged, thyroid gland with calcification, on the surface of the left salivary gland an anechogenic cyst was detected with dimensions of 5x3x3 mm. The patient was discharged to return to the place of residency, and oncological follow-up examinations conducted within the framework of home care did not record recurrence in the region of the eyelids. With regard to suspicion of the possibility of metastases in the left salivary gland according to PET-CT, as well as in the lungs in the form of three small nodes in the middle lobe of the right lung and due to increased focus of cumulation in the left lobe of the liver, chemotherapy was commenced in January 2016 (seven cycles of cisplatin and etoposide). In February 2017 it was stated that the accumulation in the liver need not be in correlation with progressing metastasis, but with cirrhosis. The finding in the lungs was stable over the long term, i.e. small nodules of a benign and post-infectious nature, since they had a fibrous character and the deposit in the left salivary gland was without progression. This finding was confirmed also in October 2017, when cervical, axillary and hilar lymphadenopathy were not demonstrated. The patient remained under oncological supervisory care. The ocular finding in October 2017 conducted close to the patient's place of residence also did not demonstrate local recurrence, on the upper left eyelid there was only a shallow trough coloboma on the border of the outer and medium third of the margin, with isolated trichiasis of the eyelashes. The intraocular finding was bilaterally symmetrical and corresponded to the norm for the patient's age, IOP = IOL 5/5 with +0.75.
DISCUSSION
MCC ranks among rarely occurring skin tumours, although the level of its incidence is increasing. The incidence in 1999 was 0.23/100 000 per year (29), rising to 0.6/100 000 in 2007 (3). It therefore has a rapidly rising trend with an inter-year increase of 8% (2), which is also confirmed by the most recent studies (39). MCC was first described in 1972 by Cyril Toker as trabecular carcinoma of the skin in five patients (44). Since then it has also been referred to as cutaneous APUDom, neuroendocrine carcinoma of the skin, squamous cell carcinoma, anaplastic squamous cell carcinoma and primary undifferentiated carcinoma of the skin. At present the term Merkel cell carcinoma is predominantly used (32). The most common localisation of MCC is parts of the body exposed to the sun, of which one half are localised on the head and neck in 44% of cases (41), within the range of 40-70% (11). Detailed processing of the literary source materials states 86 afflictions of eyelids out of a total number of 1200 observations of MCC, which represents 7% (33). Further locations of MCC may be the limbs, upper limb in 23% and lower limb in 13% (41), also the torso within the range of 10-20 (11). With regard to the low incidence of eyelid manifestations of MCC, initially in the literature there appear only individual case reports of affliction of the upper eyelids from individual centres from the 1980s (21, 23). At the turn of the century these reports are more numerous, in groups of two patients, three times the upper eyelid and once
the lower eyelid (10, 16), or in groups of three patients (30, 37) on six upper eyelids as in our report. Simultaneous occurrence of MCC on both eyelids of the same eye has been reported (26). There is therefore a clear predominance of affliction of the upper eyelid, which is linked to its greater exposure to sunlight (30). Evaluation of a larger number of patients with eyelid form of MCC was performed with the aid of a multicentric study from seven centres (33), in which 14 patients were identified, of whom nine were women and five men, 11 upper eyelids were afflicted, two lower eyelids and one canthus. The age of the patients was within the range of 48 to 96 years, on average 72 years. The most numerous study from the period of 1988 to 2011 incorporates 375 patients with MCC of all types, with an average age of 75 years, of whom 70% were women (41). The higher representation of women in 64% of cases is confirmed also by a multicentric study of affliction of the eyelids specifically (33). The absolute numbers of women with MCC of the eyelids is stated in a publication on a small number of patients (16, 21, 23) in accordance with our observation. Patients younger than 50 years with MCC are represented in only 5% of cases (2). Entirely rare cases of MCC in individuals younger than 18 years of age have been described, in the English speaking literature it was possible to find 9 case reports as of 2011 (2). As a result, it is necessary to emphasise the necessity of systematic paediatric oncological care and its screening programme in the Czech Republic, since two observations of MCC in children have been recorded. Prague paediatric oncologists describe the youngest patient with MCC ever, a two year old girl (31). Brno paediatric oncologists observed a nine year old girl with MCC of the head with long-term survival for over 10 years after undergoing comprehensive therapy (excision of tumour, radiotherapy 40 Gy, dissection of local lymph nodes with chemotherapy) (2).
The clinical picture of MCC in our three patients differed. In the two older patients (fig. 4A and 4B) it concerned a classic nodular, semi-spherical, painless and rigid reddish-purple tumour (34), whereas in the younger patient the finding was similar to a chronic chalazion. It was only its progression and the adverse response to excochleation that led to the performance of probative excision. Chalazion pertains to the differential diagnosis of MCC, it has been stated as the primary diagnosis in two patients (10). The memento is a Chinese case report, in which pulmonary complaints caused by metastases and ulcerous changes in the orbital region appeared on the previously affected side three years after the removal of a “chalazion” of the upper eyelid without histological verification (7). A similar observation was recorded also by Slovak authors, in which initial electro-cauterisation was followed six months later by excochleation of a “chalazion”. After a further three months diprophos was applied into the lesion. One year later there followed total removal of the now histologically diagnosed MCC (15). Despite comprehensive oncological therapy, both cases ended in death. Chalazion ranked in the first place in differential diagnosis of the multicentric study of eyelid manifestations of MCC, in 36% of cases (33). Rapid recurrence of any chalazion in a patient of middle age or older patients should be an indication for histological verification in order to exclude the possibility of the appearance of a malignant tumour (10). We confirmed the necessity of histological verification of unclear lesions in 60 patients with inflammatory pseudotumour in a retrospective study. Benign tumours of the eyelids in differential diagnosis include cavernous haemangioma and haemangiolymphangioma (2), which rank among vascular hamartomas (34). In malignant processes of the eyelids this is above all malignant lymphoma, amelanotic melanoma, squamous cell carcinoma, basal cell carcinoma (34, 35) and sebaceous carcinoma (43). These tumours were also represented in our cohort of more than one thousand individuals.
MCC is also referred to as neuroendocrine carcinoma, since the cytoplasma contains a large amount of free ribosomes, fibres containing cytoplasmic “tips”, which are usually linked with nerve endings. They are characterised by granules with a bond to a membrane, considered neurosecretory. The presence of neurosecretory granules led to analogies between MCC and the system incorporating amine precursor uptake decarboxylation (APUD) (38), which gave rise to what is now the historical title of cutaneous APUDom. Toker described trabecular skin carcinoma as a non-differentiated lesion manifesting frequent mitoses, composed of anastomosing “trabeculae” and nests of cells (44). Verification of MCC at present, in addition to the microscopic image, relies also on immunohistochemical examinations, which include positivity for epithelial and neuroectodermal markers (17, 34), which are becoming the main diagnostic criterion. The Slovak authors do not state these in their two observations (15). With regard to the fact that diagnosis of MCC may be dependent on the immunophenotype of tumour cells, new markers are constantly being sought. The most recent of these include: Insulinoma-associated 1, which is a transcription factor manifesting itself in tissues undergoing terminal skin neuroendocrine neoplasmatic differentiation (35). Pathologists from the Královské Vinohrady University Hospital also included in the range of markers a hitherto unused type for the early phase of neuronal differentiation in neuroendocrine MCC, namely III beta-tubulin with a 91% yield (18). Furthermore, in the case of recurrent tumours they confirmed the possibility of change of expression of the individual markers (18, 19). We followed on from their study, and to the range of epithelial and neuroectodermal markers (19) we added the proliferation marker Ki67.
Excessive exposure to solar or ultraviolet radiation, together with immune suppression and advanced age, are the main risk factors for the occurrence of MCC (39). In the etiology of the process, the significant influence of the polyomarival infection of Merkel cells is considered. The Merkel Cell Polyomavirus (MCPyV or MCP) is a small DNA virus with a circular and two-fibre DNA genome. MCP is a virus frequently found on healthy human skin, which indicates that its infection is widespread in the general population, and has become a lifelong component of skin flora. Primary infection appears in childhood (24). Serological studies demonstrate up to 88% presence of antibodies for MCP in adults and more than 40% presence in children younger than five years. The discovery of MCP in 80% of MCC supports the hypothesis of viral etiology (8, 24), whereas the remaining 20% of tumours have a large amount of mutations linked with UV radiation. It is therefore considered that MCP may be responsible for malignant transformation, and ultraviolet radiation causing damage to DNA contributes to the generation of MCP negative tumours (8). An important factor is that UV-induced antigen in virus-negative tumours and the MCP oncogenetic antigen are necessary for the growth of the tumour, and are therefore immunogenetic. Defensive T-lymphocytes specific for these antigens are often exhausted in patients, and inhibiting molecules PD-1 remain present in tumours (8). The connection with the development of MCC tumours indicates a retrospective viral causal linkage, since there is a higher incidence of MCC in patients with immunosuppressive therapy and in organ transplantations (18). With the influence of organ transplantations and B-lymphocyte malignities there is an increase in the incidence of MCC in these patients, but a higher number of MCC was not demonstrated in a cohort of 300 000 AIDS patients. The risk of occurrence in the case of this disease corresponds with that in the healthy population (14). Secondary malignities also accompany MCC, these are squamous cell carcinomas elsewhere on the skin, or adenocarcinoma of the ovaries and breasts (6), or haematological malignities (6, 42). Both of these studies relate to the Israeli population. Upon a comparison of patients with MCC and a healthy control group, an MCP-derived subpopulation of CD 8 with T-lymphocyte response against 35 different peptide sequences was identified. One epitope bound to HLA-A24 was also identified (25). A morphological evaluation demonstrated that Merkel cells with negative MCP have irregular nuclei and richer composition of cytoplasm, whereas in Merkel cells with positive MCP there are uniformly oval nuclei and weaker composition of cytoplasm (17). The immunological phenomenon of spontaneous regression of the metastatic process indicates the importance of the immune system in this disease (9). Since 1997 six further observations have been recorded in the literature. In the lymph nodes, fibrosis and cumulation of macrophages and other chronic inflammatory cells was subsequently demonstrated, which led to a hypothesis concerning immune reaction generated by T-lymphocytes (46).
MCC is an aggressive tumour with a high tendency toward local recurrence and remote spreading, which influences the character of treatment (27). Therapy of MCC as a whole takes place in a number of stages, according to the clinical guideline for treatment and management of MCC. The first and most fundamental is total removal of the tumour with confirmation that the tumour has not overrun on the edges of the surgical excision. Block dissection of the lymph nodes is indicated only upon a proven finding of tumorous infiltration (32). Radiotherapy has an increasing role, representing an alternative method for lymphatic dissection in patients with an incomplete surgical procedure that cannot be further supplemented, and as a palliative procedure in inoperable conditions (32), and reduces the risk of local recurrences (27). The change of quality of life caused by the disease and by the treatment can often not be precisely separated. In particular it is important for patients who could avoid adverse side effects in connection with radiotherapy. The operation itself could provide a similar benefit as operations with adjuvant radiotherapy rather than aggressive irradiation, which could be more appropriate in the case of incurable conditions (36). Systemic chemotherapy often supplements a surgical and radiotherapeutic approach, and follows lymphatic dissection. Chemotherapy is also indicated for disseminated form in remote metastases (32), despite the fact that the clinical benefit for overall survival is unclear (39). There is a wide selection of cytostatic agents available: e.g. eposide and cisplatina, which were used on our patient, and doxorubicin and paclitaxen are also recommended. All the above substances are also mutually combined in the therapeutic protocols (4). A new therapeutic strategy utilises inhibitions and stimulations of the immune reaction against tumour cells infected by MCP with the aid of immunostimulatory cytokines, including interferon and interleukin-2 (48). Immunocompromised patients with tumorous PD-1 were approved by FDA for testing of immunotherapy with the aid of an anti-PD-1 antibody: avelumab (5). In 88 patients not responding favourably to chemotherapy, there was an objective response to avelumab in 28, of whom 8 recorded a complete therapeutic effect (20).
The therapy of eyelid manifestations of MCC rests upon sufficient resection of the tumour into the healthy tissue (30, 33), which we confirmed also in our patients. The necessity of adjuvant therapy was demonstrated by 7 observed studies (10, 16, 21, 23, 30, 33, 37), and in our own patients was required in 24% of 29 patients. This concerned separate radiotherapy in two patients before the year 2000 (13, 16). In two patients, including one patient we observed, separate chemotherapy was applied (33), in which in our patient this concerned a palliative form in order to prevent growth, without the aim of liquidating the tumour, which was shown to be the correct procedure. It rather concerned prevention, since ultimately genuine metastatic dissemination was not demonstrated with certainty. This procedure thus prevented potential pronounced side effects of cytostatic agents upon classic use of aggressive chemotherapy with the aim of liquidating tumour cells dispersed throughout the organism. Three patients underwent combined therapy, namely radiotherapy with chemotherapy in two cases and one with nodular dissection which was safeguarded by radiotherapy (33). Remote metastases of MCC may be manifested in bones, the brain, liver and lungs via blood, the early phase of their spread is represented by metastases by via a lymphatic route to the regional nodes (30, 33, 41). Entirely isolated intraocular metastases of MCC into the uveal tissue have been described (22, 40, 45), thus via blood.
Classic imaging methods: X-ray of lungs and sonography of liver are a component of every oncological staging, which serves for identifying applicable pathological changes in the given organs. We always performed this examination on our patients. Staging of MCC incorporates complete examination of the skin and lymphatic system, and if stated may also include special imaging methods (CT, MR or PET-CT) (32). For the lymphatic system this is primarily scintigraphy, which upon periocular primary localisation may detect a pathological process in the preauricular and submandibular nodes (12). For the detection of potential pathological changes of the lymphocytic system and salivary glands, we used ultrasound examination with the use of a probe with a frequency of 7.5-10 MHz. Current modern instruments enable the detection of changes from a size of 0.1 mm. On the basis of an evaluation of the character of the nodes (size, shape, surface, ecogenicity, structure and vascularisation) it is possible to express suspicion of possible malignity or metastatic affliction (1, 13, 47). At the same time the photo documentation taken serves for a comparison of the findings over time. The examination is not burdensome and can be performed at any time by an experienced professional.
For non-ocular MMC it applies that it returns in 66% of cases, and the fatality rate is 33%. As yet no pronounced difference has been recorded in the prognosis of metastatic MCCs with positive or negative MCP (17). The future shall show how significant immunomodulation therapy is, as well as treatment by other anti-PD1 antibodies pembrolizumab and nivolumab in addition to the already permitted FAD avelumab for studies (9). Thorough observation of these patients is recommended due to the potential high incidence of recurrences, with possible infiltration of the lymphatic system (30). In very rare cases MCCs occur in regions protected against sunlight, such as the genitalia and mouth, where the starting point is the mucous membrane. These cases are characterised by an especially unfavourable prognosis (17). The danger of death is represented by the increasing size of the tumour, primarily with a positive finding of the presence of MCC in the regional nodes, as confirmed by a study of 375 patients (41). The prognosis was determined in long-term observed studies of MCC tumours of the eyelids in three patients, in whom the average observation period was 50 months (30), or 2 years, 4.6 and 6 years (37), similarly as in our sample. Another 14-member study had an average length of observation of 33 months (33). Only one death was recorded on a background of metastasis into the salivary gland, even despite combined radiochemotherapy (33), which demonstrates only 4% fatality.
CONCLUSION
We confirmed the rare occurrence of MCC in ophthalmological localisation. In our eight-year analysis of all tumours of the eyelids, it was represented only in 0.19% of cases, of which in the part of the cohort with malignant tumours in 0.37%. Of fundamental importance for the successful treatment of MCC is a sufficiently radical procedure, confirmed by histological verification, and at the same time subsequent plastic surgery of the eyelid is essential. The oncological approach differed fundamentally in both patients. Upon negative staging in the younger of the patients there followed only control examinations, including ultrasound examination of the cervical nodes, which was important for the potential timely identification of metastases. The observation period was five years, which from an oncological perspective means a cured patient. In the older patient, chemotherapy was used due to the suspicion of metastases. Fortunately metastases were ultimately not confirmed, and as a result after two years this treatment could be discontinued. The patient will be further observed, with an expected good prognosis.
Presented at the 21st annual congress of the Czech Ophthalmology Society in Brno, 2013.
The authors of the study declare that no conflict of interest exists in the compilation, theme and subsequent publication of this professional communication, and that it is not supported by any pharmaceuticals company.
Received: 25. 7. 2018
Accepted: 24. 10. 2018
Available on-line: 27. 3. 2019
MUDr. Jan Krásný
Škrobárova 50, 10 034 Praha 10
Sources
1. Ahuja, AT., Ying, M., Ho, SY. et al.: Ultrasound of malignant cervical lymph nodes. Cancer Imaging, 8; 2008 : 48–56.
2. Bajčuiová, V.: Merkelův karcinom u mladé dívky. Somatuline bulletin 3; 2012 : 9–13.
3. Bichakjian, CK., Lowe, L., Lao, CD. et al.: Merkel cell carcinoma: critical review with the guidelines for multidisciplinary management. Cancer, 110; 2007 : 1–12.
4. Becker, JC., Lorenz, E., Ugurel, S., et al.: Evalution of real-world treatment outcome in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget, 8; 2017 Jul, doi: 10.18632/oncoterget.19218
5. Bommareddy, PK., Kaufman, HK.: Avelumab and other recent advances in Merkel cell carcinoma. Future Onkol, 13; 2017 Dec, doi: 10.2217/fon-2017-0305
6. Brenner, B., Sulkes, A., Rakowsky, E., et al.: Second neoplasma in patient with Merkel cell carcinoma. Cancer, 91, 2001 : 1358–1362.
7. Chen, L., Zhu, L., Wu, J., et al.: Giant Merkel cell carcinoma of eyelid: a case report and review of the literature., Word J Surg Oncol, 9; 2011 May, doi: 10.186/1477-7819-9-58.
8. Colunga, A., Pulliam, T., Nghiem, P.: Merkel cell carcinoma in the age of immunotherapy: afacts and hopes. Clin Cancer Res, 1, 2017 Dec, doi: 10.1158/ccr-17-0439.
9. Connelly, TJ., Kowalcyk, AP.: Another case of spontaneous regression of Merkel cell (neuroendocrine) carcinoma. Dermatol Srg, 23; 1997 : 588–590.
10. Di Maria, A., Carnevali, L., Redaelli, C., et al.: Primary neuroendocrine carcinoma (Merkel cell tumor) of the eyelid: a report of two cases. Orbit, 19, 2000 : 171–177.
11. Duprat, JP., Landman, G., Salvajoli, JV., et al.: A review of the epidemiology and treatment of Merkel cell carcinoma. Clinics, 66; 2011 : 1817–1823.
12. Echegoyen, JC., Hirabavashi, KE., Lin, KY., et al.: Imaging of eyelid lymphatic drainage. Saudi J Ophthalmol, 4; 2012 : 441–443.
13. Eisenmenger, LB., Wigging, RH. 3rd.: Imaging of head and neck lymph nodes. Radio Clin North A, 53; 2015 : 115–132.
14. Engel, EA., Frisch, M., Goedert, JJ., et al.: Merkel cell carcinoma and HIV infection. Lancet, 359, 2002 : 497 – 498.
15. Furdová, A., Michálková, M., Javorská, L.: Karcinóm z Merkelových buniek mihalnice a očnice. Ces Slov Oftal, 74, 2018 : 37–43.
16. Gäckle, HC., Spraul, CW., Wagner, P. et al.: Merkel cell tumor of the eyelids: review of the literature and report of 2 patients. Klin Monbl Augenheilkd, 216; 2000 : 10–16.
17. Jaeger, T., Ring, J., Andres, C.: Histological, immunohistological, and clinical features of Merkel cell carcinoma in correlation to Merkel cell polyomavirus status. J Skin Cancer, 7; 2012 May, doi: 10.1155/2012/983421
18. Jirásek, T., Mandys, V., Viklický, V.: Expression of class III beta-tubulin in neuro-endocrine tumors of gastrointestinal tract. Folia Histochem Cytobiol, 40, 2002 : 305–310.
19. Jirásek, T., Matěj, R., Pock, L. et al.: Karcinom z Merkelových buněk - imunohistochemická studie v souboru 11 pacientů. Ces Slov Patol, 45, 2009 : 9–13.
20. Kaufman, HL., Russell, J., Hamod, O. et al.: Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol, 17; 2016 : 1374–385.
21. Kirkham, N., Cole, MD.: Merkel cell carcinoma: a malignant neuroendocrine tumour of the eyelid. Brit J Ophthalmol, 67; 1983 : 600–603.
22. Kirwan, C., Carney, D., O´Keefe, M.: Merkel cell carcinoma metastasis ot the iris in a 23 year old female. Ir Med J, 102; 2009 : 53–54.
23. Lamping, K., Fischer, MJ., Vareska, G., et al.: A Merkel cell carcinoma of the eyelid. Ophthalmology, 90; 1983 : 1399–1402.
24. Liu, W., MacDonald, M., You, J.: Merkel cell polyomavirus infection and Merkel cell carcinoma. Curr Opin Virol, 20; 2016 : 20–27.
25. Lyngaa, R., Pedersen, NW., Schrama, D., et al.: T-cell responses to ontogenic Merkel cell polyomavirus proteins distinguish Merkel cell carcinoma patients from healthy donors. Clin Cancer Res, 20; 2017 : 1768–1778.
26. Marshmann, WE., McNab, AA.: Merkel cell tumour occurring simultaneously in the upper and lower eyelids. Austral N Z J Ophthalmol, 24; 1996 : 377–380.
27. Medina-Franco, H., Urist, MM., Fiveash, J. et al.: Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol, 8, 2001 : 204–208.
28. Merkel, F.: Tastzellen und Tastkorperchen bei den Haustieren und Menschen. Arkiv für Mikroskopische Anatomie und Entwicklungsmechasnik, 11; 1875 : 636–652.
29. Miller, RW., Rabkin, CS.: Merkel cell carcinoma and melanoma. ethiological similarities and differences. Cancer Epidemiol Biomarkers Prev, 8; 1999 : 153–158.
30. Missotten, GS., de Wolff-Rouendaal, D., de Keizr, RJW.: Merkel cell carcinoma of the Eyelid. Review of the Literature and Report of Patients with Merkel Cell Carcinoma Showing Spontaneos Regression. Ophthalmology, 115; 2008 : 195–201.
31. Mottl, H., Abrhámová, J.: Nádor z Merklových buněk u dvouleté dívky – kasuistika. Klin Onkol, 3; 1990 : 19–21.
32. NCCN Merkel Cell Carcinoma Panel Members. NCCN Clinical Practice Guidelines: Merkel cell carcinoma V.1.2010. National Comptrehesive Cancer Network, PA, USA, 2010 ww.nccn.org/index.asp.
33. Peters GB., Meyer, DR., Shields JA., et al.: Management and prognosis of Merkel cell carcinoma of the eyelid. Ophthalmology, 108; 2001 : 1575–1579.
34. Rodoers, R., Jakobiec, FA., Hidayat, AA.: Eyelid tumor of apocrine, endocrine and pilar origins. In Albert, D.M., Jakobiec, F.S.: Principes and Practice of Ophthalmiology (vol. 3), W. Saunder Comp., Philadelphia, 1994, p. 1771–1798.
35. Rush, PS., Rosenbaum, JN., Roy, M., et al.: Insulinoma-associated 1: a novel nuclear marker in Merkel cell carcinoma (cutaneous neuroendorine carcinoma). J Cutan Pathol, 1, 2017 Nov, doi: 10.1111/cup. 13079.
36. Rish, Z., Fields, RC., Lee, N., et al.: Radiation therapy in the management of Merkel cell carcinoma current perspectives. Excerpt Rev Dermatol, 6, 2011 : 395–404.
37. Saadi, AK., Danks, JJ., Cree, IA., et al.: Merkel cell tumor: case report and review. Orbit, 18; 1999 : 45–52.
38. Sassani, JW., Jakobiec, FA., Hidayat, AA.: Usual eyelid tumors. In Albert, D.M., Jakobiec, F.S.: Principes and Practice of Ophthalmiology (vol. 3), W. Saunder Comp., Philadelphia, 1994, p. 1812–1823.
39. Schadendorf, D., Lebbé, C., Zur Hausen, A. et al.: Merkel cell carcinoma. epidemiology, prognosis, therapy and unmed medical needs. Eur J Cancer, 71, 2017 : 53–69.
40. Smal, KW., Rosenwasser, GO., Alexander, E. et al.: Presumed chorioidal metastasis of Merkel cell carcinoma. Ann Ophthalmol, 22; 1990 : 187–190.
41. Smith, FO., Yue, B., Marzban, SS., et al.: Both tumor depth and diameter predictive of sentinel lymph nodules. Status and survival in Merkle cell carcinomas. Cancer, 15; 2015 : 3252–3260.
42. Tadmor, T., Liphshitz, I., Aviv, A. et al.: Increased incidence of chronic lymphocytic leukaemia and lymphomas in patients with Merkel cell carcinoma – a population bases study of 335 cases with neuroendocrine skin tumour. Br J Haematol, 157, 2912 : 457–462.
43. Tanahashi, J., Kashima, K., Daa, T. et al.: Merkel cell carcinoma co-existent with sebaceous carcinoma of the eyelid. J Cutan Pathol, 36; 2009 : 383–386.
44. Toker, C.: Trabecular carcinoma of the skin: an ultrastructural study. Arch Dermatol, 105; 1972 : 107–110.
45. Trichopoulos, N., Augsburger, JJ.: Neuroendocrine tumors metastatic to the uvea: diagnosis by fine needle aspiration biopsy. Graefes Arch Clin Exp Ophthalmol, 244; 2006 : 524–528.
46. Wooff, JC., Tritnes, JR., Walsh, NM., et al.: Complete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literature. Am J Dermatopathol, 32, 2010 : 614–617.
47. Ying, M., Bhatia, KSS., Lee, YP, et al: Review of ultrasonography of malignant neck nodes. Cancer Imaging, 13; 2013 : 658–669.
48. Zanetti, I., Coati, I., Alaibac, M. Interaction between Merkel cell carcinoma and the immune system: pathogenetic and terapeutic aplications. Mol Clin Oncol, 7; 2017, doi: 10.3892/mco. 2017.1406
Labels
Ophthalmology
Article was published inCzech and Slovak Ophthalmology

2018 Issue 5-
All articles in this issue
- Ocular Manifestations of Granulomatosis with Polyangiitis
- Yield of Display Modules of Corneal Tomography for Early Diagnosis of Corneal Ectasia
- Changes of Central Corneal Thickness in Normotensive and Hypertensive Glaucoma
- Pilot Results of Implantation of the New Hydrophobic Intraocular Lens Zeiss LUCIA 611P in the Czech Republic
- Merkel Cell Carcinoma of the Eyelids (Clinical-Histological Study)
- Pachychoroid Disease of the Macula – Case Report
- Czech and Slovak Ophthalmology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Ocular Manifestations of Granulomatosis with Polyangiitis
- Merkel Cell Carcinoma of the Eyelids (Clinical-Histological Study)
- Pachychoroid Disease of the Macula – Case Report
- Pilot Results of Implantation of the New Hydrophobic Intraocular Lens Zeiss LUCIA 611P in the Czech Republic
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

