-
Medical journals
- Career
A New Approach in DCE MRI Data Analysis for Differentiating Benign and Malignant Breast Lesions
Authors: P. Hnilicova; T. Jaunky; E. Baranovicova; E. Heckova; D. Dobrota
Authors‘ workplace: Department of Medical Biochemistry, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Slovak Republic
Published in: Klin Onkol 2015; 28(1): 44-50
Category: Original Articles
doi: https://doi.org/10.14735/amko201544Overview
Background:
Dynamic contrast enhanced MRI (DCE MRI) is able to reflect changes in vascularity, vessel permeability and extracellular diffusion space of tissues. The goal of this study was to investigate the use of DCE MRI to differentiate benign and malignant breast lesions.Patients and Methods:
From a database, five patients with malignant and five patients with benign lesions were randomly chosen. All patients underwent measurement in a 3T MR scanner using a breast coil. A series of T1 - weighted MRI were performed using an intravenously delivered contrast agent. Then, 17 post‑contrast sets were acquired within a timeframe of 13 seconds. All DCE MRI data were evaluated using the JIM image analysis package. We observed changes in signal intensity over the acquisition time – curves of dynamic contrast enhancement.Conclusion:
We investigated parts of the curves with the largest increase in signal intensity during the timeframe. For further comparison, we used values of the highest signal intensity increases between the timeframes. Analysis of these results led to the proposal that the threshold between benign and malignant lesion had a relative value of 100. Furthermore, there was a significant difference between these two types of lesions.Key words:
breast neoplasms – magnetic resonance imaging – contrast mediaBackground
Breast cancer is a major cause of cancer‑related deaths among women in most western countries. According to recent statistics, mortality and morbidity rates of breast cancer are the highest of all cancers in women all over the world. Therefore, early detection and treatment of breast cancer is necessary to save more lives [1 – 4].
The use of MRI as a diagnostic tool for analysis of breast cancer began in the 1970s [2,5]. Development of new imaging protocols was possible with the use of contrast agents and advances in surface coil technology. MRI has emerged as a promising modality for detection, diagnosis and staging of breast cancer [5 – 9]. MRI also enables a helpful and an investigative method known as the dynamic contrast enhanced MRI (DCE MRI) [10 – 12].
DCE MRI yields appropriate pharmacokinetic data of physiological parameters that relate to tissue perfusion, microvascular vessel wall permeability and extracellular volume fraction [12 – 15]. Its technological process includes a serial of T1 - weighted 3D MRI of a tissue acquired before and repetitively after the administration of a contrast agent. This agent, usually gadolinium based (gadolinium – diethylenetriamine penta acetic acid; Gd – DTPA [10,11]), is a paramagnetic substance which generates its own magnetic field [7,14]. This magnetic field decreases relaxation times (T1, T2) and thus enables differences to be distinguished between tissues [14,16]. Changes in the post‑contrast signal intensity help to distinguish lesions according to characteristic enhanced accumulation of the contrast agent that can be related to higher tissue microvascularity [13,15,17,18]. Tumor growth is dependent on angiogenesis, which provides the tumor with oxygen and nutrients. Therefore, the microvessel density is much higher in cancers when compared to healthy tissue [14,16,19].
Subsequently, a small region of interest (ROI) is drawn over the region that appears to be the most enhanced in the lesion, and the enhancement values at different pre ‑ and post‑contrast time points are calculated over this to form the kinetic DCE MRI curve [10,20,21]. There are two methods used to describe lesion enhancement kinetics. The most common practice is to observe the signal intensity behavior in intermediate and late post‑contrast phases of the DCE MRI curves [9,22,23]. These parts of the kinetic curve depend on their shapes and are categorised into three types as follows [10,11,13]:
- a persistent curve with continuous increase in enhancement for benign lesions;
- a washout curve with decreasing signal intensity after peak enhancement for malignant lesions;
- a plateau curve which signal intensity remains constant after reaching the maximum. This type is complicated because it can be observed in both benign and malignant lesions.
A rarely used method for investigating DCE MRI curves is to analyze the behavior of signal intensity in the early phase after the administration of contrast material, which is referred to as a wash ‑ in rate. The analysis of the following parameters has been described previously [1,7,11,21] – the early ‑ phase enhancement rate, the enhancement velocity, the percentage of increase in signal intensity or the curve slope. These always demonstrated a maximum signal intensity, which the DCE MRI curve reaches.
Therefore, we decided to investigate the largest increase in signal intensity per timeframes as a different entity to the maximum signal intensity. The results therefore bring a new insight into analysis of DCE MRI curves. The main goal of this study was to investigate the use of DCE MRI in differentiating between benign and malignant breast lesions.
Patient Group and Methods
Case Description
MRI data of 10 patients with a total of 10 lesions were chosen from a large database of patients who had undergone MRI for breast examination and subsequently, biopsy and histological confirmation. Out of the patient database we randomly chose five patients with malignant and five patients with benign lesions. DCE MRI were obtained using a T1 - weighted 3D spoiled gradient echo sequence with the following parameters – repetition time “TR“ = 3.61 ms, echo time “TE“ = 1.21 ms, flip angle = 6 °. Fat suppression was not employed. The patients were scanned using a standard double‑breast coil on a 3 Tesla whole ‑ body MR scanner Siemens (MAGNETOM Trio, A Tim System, Erlangen, Germany). After the acquisition of the pre‑contrast series, gadobenic acid contrast agent (Multihance) was delivered intravenously by power injection (dose: 0.2 ml per kilogram of body weight) [8,9,13]. Seventeen post‑contrast sets were then acquired within a timeframe of 13 seconds. Each set contained 72 coronal slices, 2 mm thick. Ensuing sets were not acquired as the goal of this study was to investigate the behavior of signal intensity only in the early phase after the administration of the contrast agent. The experimental protocol and informed consent procedure were in compliance with the Helsinki Convention and were approved by the relevant local ethics committee.
Data Analysis
All DCE MRI data were evaluated using the JIM image analysis package (Version 5.0. Xinapse Systems Ltd., Northants, UK) [24]. Among the series of MRI, we first selected slices showing the highest enhanced lesion. Subsequently, a small ROI outside the necrotic or surrounding tissue was drawn over this lesion. The enhancement values of signal intensity at different pre ‑ and post‑contrast time points were calculated over the ROI to form the kinetic DCE MRI curve [10,20,21]. For all data sets, we evaluated a wash ‑ in rate of DCE MRI curves and investigated their features and differences.
All statistical analyses were performed by using a software program known as GraphPad InStat® (GraphPad Software, Inc.; version 3.01). The significance in difference between benign and malignant lesion groups was tested with a two‑tailed unpaired t‑test and the significance in the threshold value between these two lesion groups with a two‑tailed one sample t‑test, p < 0.05 was considered to be a statistically significant difference.
Results
The first step was to evaluate the DCE MRI curves for all lesions (Graph 1). The signal intensity values over time acquisition from all these curves are shown in Tab. 1. These demonstrated that timeframes after 65 seconds appear to be the wash ‑ in rate part of the DCE MRI curves. There were different signal intensity increases between timeframes of acquisition in the wash ‑ in rate of the DCE MRI curves (Graph 2). Our interest was to find the highest one – in other words, finding the maximum difference in signal intensity between two consecutive timeframes (Tab. 2). Because of a relative value of signal intensity increase, it was possible to compare all signal intensity increases with regard to a common baseline (for these purposes it was given a value of 0). As shown in our analysis, the largest signal intensity increase was observed between 78 and 104 seconds (highlighted in Tab. 2). In our study, all malignant lesions had higher enhancement maximum when compared to benign lesions (Graph 3). We performed a statistical analysis of differences between relative values of the largest signal intensity increases in the malignant and the benign group. Thus, we found that the mean values in the malignant group (mean ± SD = 189.0 ± 57.7) and the benign group (mean ± SD = 57.0 ± 22.6) differ very significantly (p = 0.0014) (Graph 4). Furthermore, we observed that the relative values of the largest signal intensity increases for malignant lesions exceeded 100, whereas those for benign lesions remained below this value. We analyzed that the means values of the largest signal intensity increases in the malignant lesion group differ significantly (p = 0.0261) from threshold value of 100, as well as the mean values in the benign lesion group (p = 0.0131).
1. Signal intensity values. The table shows the signal intensity values over acquisition time from the DCE MRI curve of each lesion. Timeframes after 65 seconds appeared to be a wash-in rates part of the curves. The largest signal intensity increases were observed between 78–104 seconds (blue numbers). 
M – malignant lesion, B – benign lesion 2. Signal intensity increases between timeframes. The table contains the values of signal intensity increases between timeframes for each patient. The highest maximum signal intensity increase per timeframes for each DCE MRI curve (blue numbers). 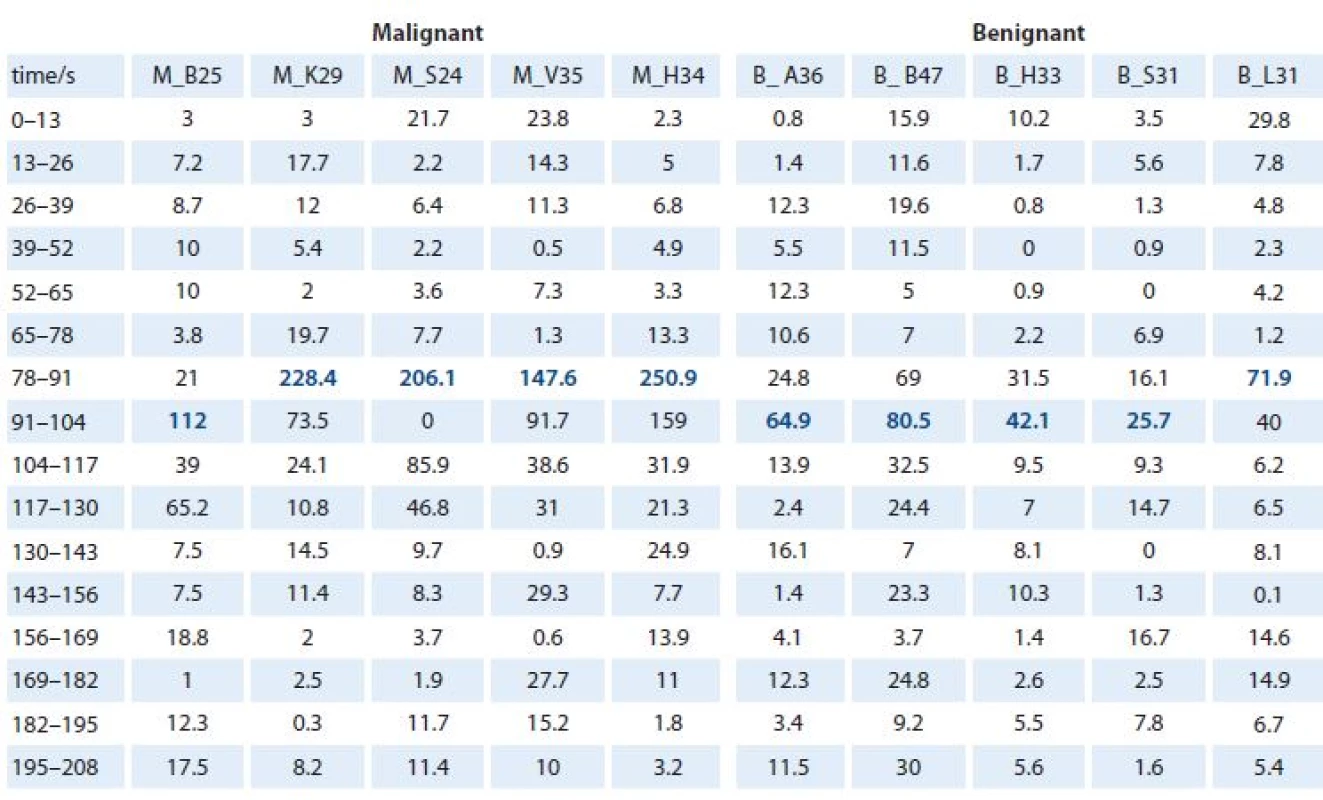
M – malignant lesion, B – benign lesion 1. Signal intensity values over acquisition time. 
The graph represents signal intensity values over acquisition time from wash-in rates of the DCE MRI curve for each lesion. M – malignant lesion, B – benign lesion 2. Signal intensity increases between timeframes. 
The graph below represents the signal intensity increases between timeframes for each lesion. We compared only parts with the highest maximum signal intensity increase per timeframes. M – malignant lesion, B – benign lesion 3. Maximum signal intensity increases over acquisition time. 
The graph below shows the maximum signal intensity increases over acquisition time from all wash-in rates of the DCE MRI curves with respect to a baseline value of 0. The threshold between benign and malignant lesions had a relative value of 100 (horizontal line). Both lesion type groups signifi cantly diff er from this threshold (for the benign lesion group: p = 0.0131; for the malignant lesion group: p = 0.0261). M – malignant lesion, B – benign lesion 4. Difference between benign and malignant groups. 
The graph below shows statistical differences (unpaired t-test) between relative values of the largest signal intensity increases in the malignant and the benign lesion group. The mean values in the malignant group (mean ± ± SD = 189.0 ± 57.7) and the benign group (mean ± SD = 57.0 ± 22.6) diff er very signifi - cantly (p = 0.0014; **). M – malignant lesion, B – benign lesion Discussion
After establishing the existence of a lesion in the breast, it is critical to determine whether this lesion is benign or malignant. The sensitivity of breast cancer detection by mammography is 69 – 90%. Sonographic classification of benign and malignant tumors is of low specificity as well‑approximately 30% [25 – 27]. The sensitivity reported for diagnosis of breast cancer using MRI is larger than 90%, and using DCE MRI is in the range of 90% to 100%. The specificity in both methods varies considerably and may be substantially lower (between 20% and 90%) [28 – 31]. The biggest advantage of both MRI methods is the lack of ionizing radiation and non‑invasivity [20]. Despite these facts, MRI and its modality are still not so frequently used in comparison to mammography and breast ultrasound. From current consensus, there are particularly suited for specific cases, such as patients who have undergone breast ‑ conserving therapy, patients who have a high risk of developing breast cancer, patients with implants, plus postoperative scars, or clinical evidence of breast cancer that could not be detected by other diagnostic methods [1,5,12,15,23].
Whereas it is still not standardized, the best evaluation method is to use the DCE MRI curves. We decided to try a new development approach of breast DCE MRI analysis. We investigated the rarely studied early phase after the administration of contrast material. A breast tumor leads to angiogenesis, i.e. the formation of new vessels and/ or the sprouting of existing capillaries. Moreover, the vessels, as a result of the tumor activity, become highly permeable. These yield early contrast agent enhancement, and therefore, strong contrast agent wash ‑ in would be expected in the tumorous tissue [2,9,16,19]. This is the essence of wash ‑ in rate DCE MRI curves. The usual analysis includes parameters describing enhancement rate or steepness of increase in signal intensity [13,14,21]. These always displayed the maximum of signal intensity, which the DCE MRI curve reaches.
The aim of this analysis was to find the largest increase of signal intensity per timeframes. It is important to realize that the largest increase of signal intensity in wash ‑ in rate DCE MRI curves not to be the same as the maximum value of signal intensity. The signal intensity increase occurs over all timeframes, but does not show similar big differences between time intervals (Graph 2). The signal intensity increases are relative values which represent changes related to the previous value of signal intensity increase. In other words, each value of signal intensity increase is obtained by adding the difference in signal intensity values of the two subsequent timeframes to the previous value of signal intensity increase. Therefore we can observe a gradual increase of signal intensity. We were interested in the maximal values of signal intensity increase in these gradual curves, which were for benign lesions 57.0 ± 22.6 (mean ± SD) and for malignant lesions 189.0 ± 57. We found a very significant (p = 0.0014, unpaired t‑test) difference between these two types of lesions. According to these findings, a relative value of 100 appeared to be a possible threshold between these types of lesions. Furthermore, the mean values in both groups significantly differ from this threshold. Although in our study significant differences were observed, we realize that only a small sample group of patients were examined, which should be extended to validate the findings. This group was chosen for the initial research and testing methodology. Our results were consistent with basic theoretical findings that malignant tissue is characterized by a faster and stronger signal enhancement than that of benign lesions with relation to their neoangiogenesis [5,14,20].
Conclusion
In this study, we demonstrated that DCE MRI helps to differentiate lesions in healthy breast tissue according to characteristically enhanced accumulation of contrast agent that can be related to higher tissue microvascularity [17 – 19,22]. Thus, we showed that in this field of interest it is possible and important to continue developing new methods of data evaluation. Finally, we considered it important to highlight the major disadvantage of using DCE MRI analysis. It uses contrast agents, which are contra ‑ indicated in some cases. The Committee for Medicinal Products for Human Use (CHMP) agency does not recommend their use in patients with a high‑risk of nephrogenic systemic fibrosis or kidney problems, in patients receiving liver transplant, in neonates or infants, in the elderly, in pregnant or breastfeeding women [10,15,32].
Acknowledgements
The authors would like to acknowledge Stephan Gruber, Mag. Dr. rer. nat., Assistant Professor at the Centre of Excellence „High‑Field MR“ in Medical University of Vienna for help with this study.
This study was supported by Ministry of Health of the Slovak Republic under the project registration number 2012/31-UKMA-8 and by project ’Increasing Opportunities for Career Growth in Research and Development in the Field of Medi-cal Sciences’, code IMTS 26110230067, co-funded from EU sources and European Social Fund.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Ing. Petra Hnilicova, PhD.
Department of Medical Biochemistry
Jessenius Faculty of Medicine in Martin
Comenius University in Bratislava
Malá hora 4
036 01 Martin
Slovak Republic
e-mail: petra.hnilicova@jfmed.uniba.skSubmitted: 12. 9. 2014
Accepted: 20. 10. 2014
Sources
1. Gilhujs KG, Gigeret ML, Bick U. Computerized analysis of breast lesions in three dimensions using dynamic magnetic ‑ resonance imaging. Med Phys 1998; 25(9): 1647 – 1654.
2. Glaßer S, Schäfer S, Oeltze S et al. A visual analytics approach to diagnosis of breast DCE ‑ MRI data. Computers & Graphics 2010; 34(5): 602 – 611.
3. Nishiura M, Yasuhiro T, Murase K. Evaluation of time ‑ intensity curves in ductal carcinoma in situ (DCIS) and mastopathy obtained using dynamic contrast enhanced magnetic resonance imaging. J Magn Reson Imaging 2011; 29(1): 99 – 105. doi: 10.1016/ j.mri.2010.07.011.
4. Fait V, Chrenko V, Schneiderová M et al. Changes in breast surgery spectrum after the introduction of breast screening. Klin Onkol 2007; 20(1): 38 – 41.
5. Orel SG, Schnall MD, LiVolsi VA et al. Suspicious breast lesions: MR imaging with radiologic ‑ pathologic correlation. Radiology 1994; 190(2): 485 – 493.
6. Petráková K. Precursors of breast cancer. Klin Onkol 2013; 26 (Suppl): S7 – S12.
7. Tozaki M. Interpretation of breast MRI: correlation of kinetic and morphological parameters with pathological findings. Magn Reson Med Sci 2004; 3(4): 189 – 197.
8. Orel SG, Schnall MD. MR Imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology 2001; 220(1): 13 – 30.
9. Kurz KD, Roy S, Mödder U et al. Typical atypical findings on dynamic MRI of the breast. Eur J Radiol 2010; 76(2): 195 – 210. doi: 10.1016/ j.ejrad.2009.07.036.
10. Chen W, Gigeret ML, Bickal U et al. Automatic identification and classification of characteristic kinetic curves of breast lesions on DCE ‑ MRI. Med Phys 2006; 33(8): 2878 – 2887.
11. Kuhl CK, Mielcareck P, Klaschik S et al. Dynamic breast MR imaging: are signal intensity time course data useful for defferential diagnosis of enhancing lesions? Radiology 1999; 211(1): 101 – 110.
12. Yankeelov TE, Lepage M, Chakravarthy A et al. Integration of quantitative DCE ‑ MRI and ADC mapping to monitor treatment response in human breast cancer: initial results. Magn Reson Imaging 2007; 25(1): 1 – 13.
13. Sinha S, Lucas ‑ Quesada FA, Sinha U et al. In vivo diffusion ‑ weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging 2002; 15(6): 693 – 704.
14. Galiè M, Farace P, Merigo F et al. Washout of small molecular contrast agent in carcinoma ‑ derived experimental tumors. Microvasc Res 2009; 78(3): 370 – 378. doi: 10.1016/ j.mvr.2009.09.004.
15. Lehotská V. Význam a možnosti magnetickej rezonancie (MR ‑ MAMOGRAFIE) v diagnostike prsníkových lézií. Onkológia 2007; 2(4): 211 – 214.
16. Castellani U, Cristani M, Daducci A et al. DCE ‑ MRI data analysis for cancer area classification. Methods Inf Med 2009; 48(3): 248 – 253. doi: 10.3414/ ME9224.
17. Riedl ChC, Pfarl G, Helbrich TH. [homepage on the Internet] American College of Radiology, Breast imagng reporting and data system [updated 2011 January 17; cited 2014 January 18]. Available from: http:/ / www.birads.at/ info.html.
18. Lucht RE, Delorme S, Hei J et al. Classification of signal ‑ time curves obtained by dynamic magnetic resonance mammography: statistical comparison of quantitative methods. Invest Radiol 2005; 40(7): 442 – 447.
19. Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res 2007; 10 : 1186 – 1796.
20. Jackson A, O’Connor JP, Parker GJ et al. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast enhanced magnetic resonance imaging. Clin Cancer Res 2007; 13(12): 3449 – 3459.
21. Twellmann T, Saalbach A, Gerstung O et al. Image fusion for dynamic contrast enhanced magnetic resonance imaging. Biomed Eng Online 2004; 3(1): 35.
22. Elmore JG, Armstrong K, Lehman CD et al. Screening for breast cancer. JAMA 2005; 293(10): 1245 – 1256.
23. Siegmann KC, Müller ‑ Schimpfle M, Schick F et al. MR imaging ‑ detected breast lesions: histopathologic correlation of lesion characteristics and signal intensity data. Am J Roentgenol 2002; 178(6): 1403 – 1409.
24. Xinapse Systems Ltd [homepage on the Internet]. West Bergholt, Colchester, UK, c2014 [updated 2014 September 12; cited 2014 September 18]. Available from: http:/ / www.xinapse.com .
25. Morris EA. Review of breast MRI: indications and limitations. Semin Roentgenol 2001; 36(3): 226 – 237.
26. Barker PB, Bizzi A, Stefano ND et al. MRS in breast cancer. In: Clinical MR Spectroscopy, Techniques and Applications. Cambridge University Press 2010 : 229 – 242.
27. Yoshikawa MI, Ohsumi S, Sugata S et al. Comparison of breast cancer detection by diffusion ‑ weighted magnetic resonance imaging and mammography. Radiat Med 2007; 25(5): 218 – 223.
28. Katz ‑ Brull R, Lavin PT, Lenkinski RE. Clinical utility of proton magnetic resonance spectroscopy in characterizing breast lesions. J Nati Cancer Inst 2002; 94 : 1197 – 1203.
29. Buchberger W, Niehoff A, Obrist P et al. Clinically and mammographically occult breast lesions: detection and classification with high‑resolution sonography. Semin Ultrasound CT MR 2000; 21(4): 325 – 336.
30. Guo Y, Cai YQ, Cai ZL et al. Differentiation of clinicallybenign and malignant breast lesions using diffusion ‑ -weighted imaging. J Magn Reson Imaging 2002; 16(2): 172 – 178.
31. Nass SJ, Henderson IC, Lashof JC (eds). Mammography and beyond: developing technologies for the early detection of breast cancer. Washington, DC: Institute of Medicine, National Academy Press 2001.
32. European Medicines Agency (EMEA) [homepage on the Internet]. Questions and answers on the review of gadolinium ‑ containing contrast agents. c2014 [updated 2014 September 12; cited 2014 September 18]. Available from: http:/ / www.emea.europa.eu.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2015 Issue 1-
All articles in this issue
- Assessment of the Spiritual Needs of Patients in Palliative Care
- Dental Abnormalities after Treatment for Childhood Cancer
- Management of Patients with Castration‑resistant Metastatic Prostate Cancer
- The Possibility of the Serum Concentration of Osteocalcin Determination in Lung Cancer Patients with Suspected Bone Metastases
- Domácí parenterální výživa v onkologii
- Efekt akcelerované radioterapie u plicního adenokarcinomu
- Estimating Cancer Incidence, Prevalence, and the Number of Cancer Patients Treated with Anti‑tumor Therapy in 2015 and 2020 – Analysis of the Czech National Cancer Registry
- A New Approach in DCE MRI Data Analysis for Differentiating Benign and Malignant Breast Lesions
- Sarcomatoid Carcinoma of the Lung – a Case Report
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Sarcomatoid Carcinoma of the Lung – a Case Report
- Assessment of the Spiritual Needs of Patients in Palliative Care
- Management of Patients with Castration‑resistant Metastatic Prostate Cancer
- The Possibility of the Serum Concentration of Osteocalcin Determination in Lung Cancer Patients with Suspected Bone Metastases
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

