-
Medical journals
- Career
ABSTRACTS
Published in: Čes-slov Pediat 2010; 65 (9): 531-555.
RENAL TRANSPLANTATION IN ATYPICAL HUS
Riedl M., Hofer J., Rosales A., Yeutukhova Y., Jungraithmayr T., Zimmerhackl B. L. for the HUS Study Group
1st Department of Paediatrics, Medical University, Innsbruck, AustriaObjectives: Renal transplantation in atypical hemolytic uremic syndrome (aHUS) is characterized by a high rate of disease recurrence and subsequent graft loss.
Methods: Since 2001 the European Paediatric Research Group for HUS and related disorders (HUSnet) (www.hus-online.at) has been recruiting data and performing complement analysis in patients with atypical STEC negative HUS. In 25 out of 135 patients of the registry 47 renal transplantations (1–4 per patient) were performed, including 5 patients with heterozygous factor H (fH), 1 homozygous MCP, 2 heterozygous factor I (fI) mutations, 1 combined fH and fI mutation and 1 patient with fH antibodies and CFHR1 deletion.
Results: Graft loss occurred in 33/46 transplants (72%), in median after 7 months (1 day – 11.4 years). The main reasons for graft loss were HUS recurrence in 76% (25/33), followed by rejection in 5 cases (15%). Loss of function was reported in 8 of 28 grafts (29%) in the first month after transplantation, in 6 cases due to disease recurrence. 16 of 28 (57%) grafts were lost within the first year after renal transplantation. In 7 out of 9 patients with graft loss due to disease recurrence this was also the reason for graft loss in a further transplantation.
The follow-up time of the 13 grafts with preserved renal function is about 15 months in median (4.3 months – 8 years).
Conclusions: The data of our registry show that graft loss due to disease recurrence is very common in patients with atypical HUS. Graft loss was reported in more than 50% in the first year of transplantation. However, new treatment options like Eculizumab give hope for the future to perform renal transplantations in aHUS patients with better long term outcome and graft survival.
Supported by ÖNB grant.
CARDIAC MAGNETIC RESONANCE IMAGING (MR) IN CHILDREN WITH CHRONIC KIDNEY DISEASE AND RENAL TRANSPLANTATION
Schäfer B., Rusai K., Tóth A.1, Krekó M., Horváth E., Sallay P., Reusz Gy. S., Merkely B.1, Tulassay T., Szabó A. J.
First Department of Paediatrics, Semmelweis University, Budapest, Hungary
1Heart Center, Semmelweis University, Budapest, HungaryIntroduction: Cardiovascular alterations are the major cause of morbidity and mortality in patients with end-stage renal disease (ESRD) significantly reduced by renal transplantation (NTx). Uremic cardiomyopathy is associated with changes in the heart structure and function, such as left ventricular dilatation, systolic and diastolic dysfunction, and concentric left ventricular (LV) hypertrophy with increased LV mass. Cardiac assessment by 2D echocardiography is sensitive to the degree of cardiac filling, but accurate LVM measurements require a full long-axis apical view. Other disadvantages include subjective interpretation, and semi-quantitative evaluation of regional systolic LV contraction.
Cardiac MR (CMR) imaging is emerging as a gold standard method, however, data on cardiac assessment of pediatric renal patients before and after NTx using CMR are missing.
The aim of this study was to determine the accurate cardiac morphology and function of children with ESRD and after NTx using CMR.
Methods: We analyzed the data of 14 CKD (age: 16 years, 2 CKD, 5 PD, 7 HD) and 24 NTx (age: 16.5 years) children. CMR was performed 4.3 years after NTx. 6 NTx children had MR imagining 3 months before and 6 months after transplantation.
Results: Left and right ventricular ejection fraction values were higher in the NTx group; end diastolic and systolic left ventricular volume index and left ventricular total mass index were lower after NTx compared to patients with CKD (all p<0.05). All examined parameters tended to be improved in 6 children after NTx compared to the assessed values before NTx.
Conclusion: In summary, we are among the first who used CMR to assess cardiac morphology in CKD and NTx pediatric patients. Our results suggest marked improvement of the cardiac function and morphology after NTx. We conclude that CMR provided accurate data on all assessed cardiac parameters.
TÁMOP-4.2.2-08/1/KMR-2008-0004, NNF 78846 (OTKA), OTKA T071730, ETT 435/200.
THE CORRELATION BETWEEN GENOTYPE AND PHENOTYPE IN AUTOSOMAL DOMINANT POLYCYSTIC KIDNEY DISEASE (ADPKD) IN CHILDREN
Fencl F., Štekrová J.*, Bláhová K., Janda J., Seeman T.
Department of Paediatrics, Charles University in Prague, 2nd Faculty of Medicine, University Hospital Motol, Czech Republic
*Institute of Biology and Department of Medical Genetics, Charles University in Prague, 1st Faculty of Medicine, Czech RepublicIntroduction: ADPKD is the most frequent inherited renal disorder (incidence 1 : 500–1000). It is characterized by cyst development, which leads to renal failure in adults. Complications include macrohaematuria, urinary tract infections (UTI), hypertension, cardiac valve abnormalities, cerebral aneurysms, hepatic/pancreatic cysts. ADPKD is caused by mutation in PKD1 (85%)/PKD2 (14%)/PKD3 (probably 1%) gene.
The aim of study was to compare disease progression and complications in children with genetically confirmed PKD1/PKD2 mutation.
Patients: 60 children (50 PKD1, 10 PKD2) were investigated. All patients had positive family history, positive direct molecular genetic analysis or indirect genetic analysis with positive ultrasound (US) finding of renal cysts.
Anamnestic data. Subjective symptoms/UTI/ macrohaematuria/hypertension were present in 9 (18%)/6 (12%)/3 (6%)/7 (14%) PKD1 and in 0/1 (10%)/0/0 PKD 2 patients respectively.
Biochemistry. Pathological proteinuria (PU) had 20 (48%)/13 (65%) PKD1 and 4 (50%)/4 (67%) PKD2 children, mean PU in PKD1 group was higher by 38%/102% according to [mg/m2 per day]/[mg/mmol of creatinine]. 1 PKD1 patient had pathological GFR, no one had microhaematuria.
Blood pressure (BP). Occasional BP: No differences were proved. ABPM: PKD1 patients had significantly higher B/P index of day and night systolic BP, 9 PKD1 (27%) and 0 PKD2 patient were hypertensive.
US findings. Highly significant relationships were between total number of cysts/diameter of greatest cyst/kidney length in SDS and genotype – all higher in PKD1 group and between bilateral cysts/enlarged kidneys and genotype – 43 (86%)/16 (32%) PKD1 and 3 (30%)/0 PKD2 patients. Prenatal US finding of cysts is highly specific for PKD1 (7 PKD1, 0 PKD2 patient). No one had extrarenal cysts.
Many studies prove better prognosis of PKD2 patients (renal survival, BP, cyst number/volume), but only in adults. We demonstrate significant differences between PKD1/PKD2 disease developments in children, esp. in US findings.
DIFFERENTIATION OF PROTEINURIA IN FOCAL SEGMENTAL GLOMERULOSCLEROSIS USING PROTEOMICS-ANALYSIS
Lackner S.1, Zimmerhackl L. B.1, Mischak H.2, Toenshoff B.3, Bjerre A.4, Seeman T.5, Dötsch J.6, Jungraithmayr C. T.1
11st Department of Paediatrics, Medical University of Innsbruck, Austria
2Mosaiques Diagnostics GmbH, Hannover, Germany
3Department of Paediatrics, University of Heidelberg, Germany
4Department of Paediatrics, Oslo, Norway
5Department of Paediatrics, University Prague, Motol, Czech Republic
6Department of Paediatrics, University Hospital Erlangen, GermanyIntroduction: Focal Segmental Glomerulosclerosis (FSGS) is in 10–15% the primary disease for paediatric end stage renal failure. Moreover, FSGS relapses after kidney transplantation in over 80% depending on the genetic constellation. Different Biomarkers, which might give information about the course of disease before and after transplantation can be found in the urine of patients with FSGS. Proteomics is a non invasive method to detect these Biomarkers.
Patients and methods: Urine of 15 transplanted FSGS-patients and 9 non-transplanted FSGS-patients (all biopsy-proven and screened for NPHS2) was analyzed using Proteomics. Patients were divided into groups according to their genetic disposition regarding NPHS2 (Podocin) mutations and with regard to recurrence in case of transplantation. Based on existing Proteomics Biomarker patterns accordance for i.e. chronic kidney disease, FSGS and other renal diseases was calculated. Results around +1 and above are indicative for a disease, whereas results of -1 and below militate against the respective disease.
Results: The overall accordance with the typical FSGS pattern was 0,9 +/ - 0,16 (mean +/ - SEM) in our study population. Only transplanted patients showed a conformance of 0,7 +/-0,2 compared to before transplantation with 1,2 +/-0,3 (p=0,24). Due to non-recurrence of NPHS2-positive patients we only report on the 10 NPHS2-negative transplanted patients regarding relapse. 4 patients recurred and presented with an accordance of 1,2 +/-0,2 as against 0,9 +/-0,3 in patients without recurrence (n=6, n.s.). Comparing the 5 transplanted patients with NPHS2 mutations to those 10 without mutation the accordance was 0,02 +/-0,1 versus 1,02 +/-0,2, respectively (p=0,005).
Discussion: Urine of transplanted and non-transplanted FSGS patients showed a good accordance with the FSGS Biomarker pattern. Transplanted patients without mutation of NPHS2 had a significant higher conformance to the FSGS pattern compared to patients with NPHS2 mutations. Relapsing patients tend to show a higher accordance compared to non-relapsing patients. This important issue remains to be proven in a larger study population.
*Supported by GPN and Medical University Innsbruck, Austria.
MTHFR GENE POLYMORPHISM C677T ASSOCIATED WITH URINARY TRACT ANOMALIES IN GIRLS – SEX DIFFERENCES RELATED TO METHYLATION?
Behunová J.1, Klimčáková L.2, Podracká Ľ.1
11st Department of Paediatric, PJ Safarik University School of Medicine and University Children Hospital, Kosice, Slovakia
2Institute of Medical Biology, PJ Safarik University School of Medicine, Kosice, SlovakiaThe aim of the study: Periconceptional folate was referred to have a preventive effect not only to neural tube defects (NTD), but also to some other birth defects as urinary tract anomalies (UTA), orofacial clefts and conotruncal heart defects. Folate metabolism genes´ variants are therefore being investigated as susceptibility factors for these anomalies. Our aim was to determine the association of MTHFR gene polymorphisms C677T and A1298C with UTA.
Methods: We genotyped 132 UTA patients and 290 control newborns (by PCR followed by enzyme restriction or by real time PCR), also confronting the results with previously evaluated 93 NTD patients (copmparing the whole groups, as well as with respect to sex).
Results: We found significantly higher incidence of T allele resp. TT genotype of C677T polymorphism in UTA patients compared to the controls (p=0,019 resp. p=0,044). When we analysed the sexes separately, T allele resp. TT genotype frequencies in the UTA girls vs. control girls were 42,55% vs. 21,68%, p<0,0001 (OR=2,676; 95%CI [1,629–4,396]) resp. 19,15% vs. 5,59% p=0,008 (OR 3,9967; 95%CI [1,2615–12,6931]), while there was no difference between the boys´ groups. Opposite this, we did not find any sex differences either within the NTD group, or within the controls.
Conclusion: Unexpectedly high incidence of C677T MTHFR gene polymorphism in girls with UTA could point to developmental sex-differences independent on hormonal status. These differences might be related to sexual dimorphism in methylation, that could be limited in lower activity of MTHFR and expressed mainly in the system with the highest sexual dimorphism – urogenital system. Naturally, this hypothesis should be further tested.
TOLL-LIKE RECEPTOR 4 POLYMORPHISM IS ASSOCIATED WITH INCREASED RISK OF URINARY TRACT INFECTION IN GIRLS ABOVE AGE 2
Jankó V.1, Pozsgayová S.1, Podracká Ľ.2, Kovács L.1
12nd Department of Paediatrics, Comenius University Medical School, Bratislava, Slovakia
21st Department of Paediatrics, PJ Safarik University School of Medicine, Košice, SlovakiaIntroduction: Innate immunity and urinary tract response play a central role in the development of urinary tract infection (UTI). Although several studies suggest a possible role for genetics in human UTI susceptibility, the genes involved remain largely unknown.
The aim of the study was to test the hypothesis that toll-like receptor (TLR) 4, a key element of innate defense mechanisms, is associated with susceptibility to UTIs in children.
Methods: We determined TLR4 A(896)G polymorphism by means of polymerase chain reaction in 113 children treated for upper UTI. Of them, 63 aged <2 years (34 girls, 29 boys) and 50 were >2 years (43 girls, 7 boys). Allelic prevalence was compared with reference values of 230 healthy controls. Clinical data were also statistically evaluated.
Results: Urinary microbiological analyses have detected E. coli (95 cases), Enterococcus faecalis (12 cases), P. mirabilis (5 cases) and Citrobacter (1 patient). CRP values (in mg/l) did not differ significantly between patients <2 years (mean: 81,7, median: 58) and >2 years (mean: 93,4, median: 56,7). In the whole group of UTI patients, the prevalence of the TLR4 (896)AG genotype and TLR4 (896)G allele did not statistically differ from that in healthy controls (98/15 and 218/17, p=0,104). However, their prevalence was significantly higher in patients >2 years as compared to patients <2 years (50/11 versus 63/4, p<0.03) or to the control group (50/11 versus 218/17, p<0.041). The association with UTI was even more significant in the older age group of patients (p<0,001), partly because of significantly higher proportion of girls in this subgroup.
Conclusions: Our findings demonstrate that carrier status of TLR4 (896)G is associated with altered ability of girls >2 years to respond to urinary pathogens. It is suggested, that this polymorphism act in concert with other genetic changes or acquired factors to influence the complex physiological defense against urinary tract.
DIFFERENTIAL METHYLATION IN CORD BLOOD T-LYMPHOCYTES OF CHILDREN PRENATALLY EXPOSED TO TOBACCO SMOKE COMPARED TO CONTROLS AND RISK FOR ASTHMA
Grychtol R.1, Heinzmann A.1, Kreutz C.2
1Centre of Paediatrics and Adolescent Medicine, University of Freiburg, Germany
2Center for Data Analysis and Modeling (FDM) of the Physics Department, University of Freiburg, GermanyThe aim of the study: More than 100 candidate genes for asthma have already been identified. Still, a lot of open questions remain due to the polygenic character of asthma and the additional influence of environmental factors. Epigenetic studies focus on the inheritance of phenotypes which are not transmitted due to changes in the DNA sequence itself. Epigenetic modification is induced by environmental stimuli. Thus, epigenetic is a promising new field to study the relationship between environmental factors and genetics in asthma development. Epidemiology data already gave proof to this concept: For example, smoking during pregnancy does not only increase the risk of asthma in the child but also in the grandchild. The basic principle of epigenetic gene regulation is histone modification and cytosine-methylation. Hypermethylation of cytosine usually induces down regulation of gene transcription. So far, most studies of epigenetics have been performed in cancer. We examine the differential DNA-methylation in lymphocytes of neonates with different risk for asthma due to prenatal environmental influences.
Methods: Newborns from mothers who smoked during pregnancy, who are asthmatics themselves or – as a potential protective factor – were exposed to farming environment, were compared to controls (10 subjects per group). For this purpose, CD 34 positive T - lymphocytes were purified from cord blood and the DNA isolated. The methylated DNA was separated by immunprecipitation with specific antibodies against methylated DNA, amplified and finally hybridized to tiling microarrays covering chromosomes 5, 7 and 16. Regions with differential methylation pattern between the groups are identified and will be examined in our asthma cohort of 500 children using methylation-specific genotyping methods. The methods applied and the results of differential methylation between children prenatally exposed to tobacco smoke and controls will be presented.
LYMPHOCYTE CALCIUM INFLUX CHARACTERISTICS AND THEIR MODULATION BY Kv1.3 AND IKCa1 CHANNEL INHIBITORS IN THE NEONATE
Toldi G., Mészáros G., Treszl A., Tulassay T., Vásárhelyi B.
1st Department of Paediatrics, Semmelweis University, Budapest, HungaryAim: Cytokine production in T lymphocytes of the term neonate is reduced compared to adults. Since Kv1.3 and IKCa1 lymphocyte potassium channels influence calcium influx and short-term lymphocyte activation, and thus cytokine production of lymphocytes, we tested their functionality and expression in the neonate.
Methods: We isolated lymphocytes from peripheral blood of 9 adults and cord blood of 9 term neonates. We measured the alteration of calcium influx and Kv1.3 channel expression with flow cytometry in the Th1, Th2, CD4 and CD8 subsets upon treatment of samples with specific potassium channel inhibitors (margatoxin and TRAM-34, respectively). Samples were activated with phytohemagglutinin.
Results: Calcium influx kinetics is decreased upon activation in neonatal lymphocytes compared to adults. The basal intracellular calcium level is elevated in lymphocytes isolated from neonates. Neonatal lymphocytes were found to be less sensitive to the specific inhibition of Kv1.3 and IKCa1 potassium channels. The expression of Kv1.3 channels is increased on lymphocytes of newborns.
Conclusion: Our findings suggest that the characteristics of short-term activation of major neonatal T-lymphocyte subsets are altered compared to adults. The altered function of neonatal lymphocyte potassium channels may contribute to this phenomenon. These results may also partly explain why neonatal lymphocytes are less responsive to activating stimuli, and why their cytokine production is decreased upon activation.
COUGH CHARACTERISTICS IN ASTHMATIC CHILDREN
Jesenak M., Banovcin P.
Department of Paediatrics, Center of Experimental and Clinical Respirology, Comenius University in Bratislava, Jessenius School of Medicine, Martin, SlovakiaThe aim of the study: Bronchial asthma is the most frequent chronic respiratory disease in children clinically presented by wheeze, dyspnoea, chest tightness and cough, which is major symptom in some children. Recently, several objective methods for its assessment were developed. Cough reflex sensitivity (CRS) test with capsaicin is one of the most important tool for studying cough. We aimed to study the CRS sensitivity (expressed by C2 parameter = the lowest concentration of capsaicin in aerosol that evoked ≥2 coughs) in various phenotypes of asthma.
Methods: We studied a population of 101 children (52.5% boys): 86 asthmatic children (aged 13.34±3.09 years) and 15 healthy control subjects of comparable age.
Results: Comparing CRS between asthmatic population of 86 children and 15 healthy subjects, we observed significant lower values in asthmatics (53.56±18.98 vs. 115.96±58.14 μmol/l, p=0.017). Asthmatic children during acute exacerbation showed increase of CRS in comparison with stable asthmatics (5.05±2.73 vs. 63.33±22.56 μmol/l, p=0.002). Children with isolated asthma showed nearly the same values of C2 parameter than healthy controls (93.94±45.87 vs. 115.96±58.14 μmol/l, p=0.066), but conversely, children suffering from concomitant allergic rhinitis revealed increased CRS (28.73±11.86 vs. 115.96±58.14 μmol/l, p=0.014). Atopic bronchial asthma was associated with enhanced CRS comparing to controls (17.22±3.45 vs. 115.96±58.14 μmol/l, p=0.005). Capsaicin hypersensitivity was also slightly increased in non-atopic asthmatics in comparison with healthy subjects (34.27±23.73 vs. 115.96±58.14 μmol/l, p=0.019).
Conclusions: Various characteristics of asthmatic children influence the cough sensitivity to different extent. Cough reflex sensitivity measurement can add valuable information besides commonly used spirometric and inflammometric methods in management of asthmatic children and in differential diagnosis of chronic persisting cough.
PROPHYLAXIS AGAINST BIRCH POLLEN ALLERGY BY NEONATAL COLONIZATION WITH A LACTOBACILLUS PLANTARUM PRODUCING THE MAJOR BIRCH POLLEN ALLERGEN BET V 1
Repa A.1,3*, Schwarzer M.2*, Schabussova I.1, Daniel C.4, Hrnčíř T.2, Pot B.4, Štěpánková R.2, Hudcovic T.2, Pollak A.3, Tlaskalová-Hogenová H.2, Wiedermann U.1, Kozáková H.21Department of Specific Prophylaxis and Tropical Medicine, Medical University of Vienna, Austria
2Institute of Microbiology, Academy of Sciences of the Czech Republic
3Department of Paediatrics and Adolescent Medicine, Division of General Pediatrics and Neonatology, Medical University of Vienna, Austria
4Laboratoire de Bactériologie des Ecosystèmes, Institut Pasteur de Lille, Lille Cedex, FranceBackground: The use of non-invasive and non-pathogenic recombinant lactic acid bacteria (LAB) as vehicles for mucosal delivery of vaccine antigens is an attractive concept in allergy research. The interventions with LAB are likely to be of great relevance in early life when immune programming is initiated. Here we investigated the effect of neonatal colonization with recombinant LAB producing the major birch pollen allergen Bet v 1 on the development of allergic responses in mice.
Methods: We constructed a recombinant Lactobacillus plantarum NCIMB 8826 constitutively producing Bet v 1 and used this strain for perinatal mono-colonisation of germ-free mice via their mothers. Allergen-specific immunomodulatory effects of this colonization on humoral and cellular immune responses were investigated in adult mice with or without Bet v 1-sensitization.
Results: Neonatal colonization with recombinant L. plantarum led to induction of Bet v 1-specific IFN-alfa in re-stimulated spleen cells, indicating a shift towards non-allergic Th1 phenotype. Neonatal mono-colonization with the Bet v 1 producing L. plantarum, but not with the control strain prevented the induction of allergen-specific antibodies in serum of mice subsequently sensitized with Bet v 1. Successful immunomodulation by the recombinant strain but not by the control was further demonstrated by reduced Th2 and increased Th1 allergen-specific cellular responses. Moreover, colonization with recombinant L. plantarum increased expression of regulatory markers on mRNA in the spleen cells.
Conclusion: We conclude that mucosal intervention with live recombinant L. plantarum producing a clinically relevant allergen in combination with application early in life might become an effective strategy in primary prevention of type I allergy.
*both authors contributed equally to the work
USE OF THE NEW AMPLATZER DUCT OCCLUDER II IN COMPARISON WITH THE AMPLATZER DUCT OCCLUDER – SINGLE CENTRE EXPERIENCE
Venczelová Z., Tittel P., Masura J.
National Institute of Heart Diseases – Pediatric Cardiac Centre, Bratislava, SlovakiaObjectives: To determine the safety and efficacy using the Amplatzer duct occluder (ADO) or Amplatzer duct occluder II (ADO II) in different types of arterial ducts.
Methods: All patients with a device ductal closure between September 2005 and February 2010 were included. Retrospectively, we analyzed the catheterization and follow-up data of these patients.
Results: Between September 2005 and February 2010, 44 ducts were closed with ADO (group ADO) and 52 ducts were closed with ADO II (group ADO II). In the group ADO, the mean age was 3 years 4 months, the mean weight was 14,8 kg. The closure with ADO was successfully performed in all patients. Complete closure at 24 hours was attained in 42 of 44 patients (95,45%). No major complications occurred. In the group ADO II, the mean age was 6 years, the mean weight was 24,2 kg. The closure with ADO II was successfully performed in all patients excepting two patients, in these patients the occluder protruded significantly into the aortic isthmus and was replaced by ADO. Complete closure at 24 hours was attained in 51 of 52 patients (98,08%). There were no major complications.
Conclusion: In our experience, the duct closure with the ADO II is a safe and effective method. The advantages of using this device are smaller sheath sizes and a softer shape of the device. A confirmation of our results in a larger number of patients is necessary.
EPIDEMIOLOGY OF END-STAGE RENAL DISEASE IN SLOVAK CHILDREN
Koľvek G., Reijneveld A. S., Podracká Ľ., Rosenberger J., Van Dijk J. P.
1st Department of Paediatric, PJ Safarik University School of Medicine, Kosice, Slovakia
Department of Social Medicine, University of Groningen, The NetherlandsThe aim of the study: The paediatric population suffering from end-stage renal disease (ESRD) in Europe is growing, but reliable epidemiological information on ESRD in children is lacking especially in Central and Eastern European countries. The aim of this study was to examine the occurrence of ESRD in Slovak children, to compare it with earlier data on Slovakia and with data from other European countries and finally to explore aetiology and treatment modes.
Methods: Over the years 2005–2008 data on the incidence and prevalence of cases of ESRD in children from all four tertiary paediatric centers in Slovakia were collected. Incidence and prevalence rates were calculated per million age-related population (pmarp) and per million total population (pmtp). The data were compared with two earlier Slovak studies and with European data from European Society of Paediatric Nephrology datasets.
Results: The mean annual incidence rate of ESRD in Slovak children under 15 years of age observed in the study period was 5.8 pmarp (0.9 pmtp). The prevalence rate on 31st December 2008 was 23.9 pmarp (3.7 pmtp). Differences between 2008 and 2002 (18.6 pmarp, 3.2 pmtp) were not statistically significant. Incidence and prevalence rates of ESRD in Slovak children are comparable to surrounding European countries. Aetiology mainly concerned congenital anomalies (42.9%) and hereditary nephropaties (21.4%).
Conclusion: During the last decade the incidence and prevalence rates of ESRD in Slovak children have remained stable and compared to neighbouring countries they are for the most part similar.
Supported by grant: APVV No. 20-038305.
SHIGATOXIN AND COMPLEMENT ACTIVATION ARE TOXIC TO HUMAN PODOCYTES
Dettmar A.1, Binder E.1, Feifel E.2, Gstraunthaler G.2, Karch H.3, Zimmerhackl L. B.1
1Abteilung für Kinder - und Jugendheilkunde, Medizinische Universität, Innsbruck, Austria
2Abteilung für Physiologie, Medizinische Universität, Innsbruck, Austria
3Institut für Hygiene, Westfälische Wilhelms Universität, Münster, GermanyObjectives: Resulting in similar clinical presentation different pathomechanisms are responsible for typical and atypical hemolytic uremic syndrome (tHUS, aHUS). Shigatoxin (Stx), produced by EHEC 0157:H7, is responsible for renal damage in tHUS, activation of complement for thrombotic microangiopathy and renal failure in aHUS. The aim of our studies is to show the influence of these toxins to podocytes and to identify their role in both diseases.
Methods: Cultured human podocytes were incubated with Stx, complement activated human serum (CAS), or both together. Cells were stained with Hoechst and lysates were tested using assays for caspase 1,4 and 3 activity, and LDH - release after incubation.
Results: Podocytes changed their general shape after treatment, but Hoechst staining showed no difference in number of apoptotic nuclei, compared to controls.
Stx application results in an increase of caspase 3 activity and LDH - release, but there was no change after incubation with complement activated serum.
The increase of caspase activity and LDH - release was smaller, when cells were incubated with Stx and CAS together, or Stx and normal human serum.
Conclusions: Podocytes, as an important part of the glomerular filter, are propably damaged in tHUS and aHUS. In vitro they reacted to both, Stx and complement activation, but in different ways. Human serum, no matter if complement activated or not, seem to protect from damage through Stx application.
Further studies will be done to detect the difference of the influence of Stx or complement activation and the protecting agent in human serum against shigatoxin.
HEMOLYTIC UREMIC SYNDROME ASSOCIATED TO SORBITOL FERMENTING O157:H- SHIGA TOXIN PRODUCING ESCHERICHIA COLI
Rosales A.1, Hofer J.1, Riedl M.1, Jungraithmayr T. C.1, Würzner R.2, Karch H.3, Zimmerhackl L. B.1
1Department of Paediatrics, Medical University of Innsbruck, Austria
2Division of Hygiene and Medical Microbiology, Innsbruck Medical University, Austria
3Zentrum für Kinderheilkunde und Jugendmedizin, Universität Freiburg, Germany
4Kinderklinik Memmingen, Memmingen, Germany
5Robert Koch Institut, Berlin Germany
6Department für Medizinische Statistik, Informatik und Gesundheitsökonomie, Medizinische Universität Innsbruck, Austria
7Institut für Hygiene, Universität Münster, GermanyInfections with Shiga toxin producing Escherichia coli (STEC) are the main cause of hemolytic uremic syndrome, which represents the most common cause of acute renal failure in children. The most prevalent E. coli serotype worldwide is O157:H7, but other serotypes are emerging. Recently, a new STEC strain, which unlike O157:H7 ferments sorbitol and has been found only in humans, was associated to HUS cases in Europe. The clinical presentation of HUS due to this sorbitol fermenting (SF) O157:H- is not well described.
From January 1st 1997 to December 31st 2002, 628 patients <21 years of age with the clinical diagnosis of HUS were registered in a prospective multicenter study in Austria and Germany. This study was performed to evaluate the influence of the different STEC serotypes in the acute presentation and long term clinical course of disease.
SF O157:H- STEC were found in 44 cases out of 298 cases in which STEC serotype could be determined. In contrast to other serotypes SF O157:H- did not show a seasonal distribution. These patients had a severe acute illness presenting with hypertension (22.6%) and neurological symptoms (41.5%) more frequently than O157:H7 cases. 38 of 44 cases presented with diarrhea, which was bloody in 22 of them. Among the patients with SF O157:H- STEC, 62.2% needed hemodialysis and 27.3% plasmapheresis, both significantly more frequently than in O157:H7 patients (p<0.05, x2). Regarding the long term outcome, a significant association between SF O157:H- STEC and the presence of symptoms after one year was found. This findings reveal that SF O157:H- STEC are at least as aggressive as other STEC serotypes.
SF O157:H- STEC associated HUS is a critical disease. The detection of STEC should not be limited to the O157:H7 serotypes, since SF O157:H- and other non-O157:H7 serotypes are highly virulent and are associated to severe course of disease.
PULSE WAVE VELOCITY AND INTIMA-MEDIA THICKNESS IN RENAL TRANSPLANT CHILDREN
Kerti A., Kis É., Cseprekál O., Horváth T., Szabó A. J., Kollai M., Tulassay T., Reusz Gy.
1st Department of Pediatrics and Institute of Human Physiology and Clinical Experimental Research, Semmelweis University, Budapest, HungaryBackground: Vascular calcification, accelerated by uraemia, could be characterized by pulse wave velocity (PWV), arterial distensibility (D) and intima-media thickness (IMT). These parameters can individually predict CV mortality in adults, but data is sparse in renal transplant (tx) children.
Aims: To describe functional (PWV, D) and structural (IMT) vascular disorders and to analyse vascular risk factors in children after tx.
Subjects, methods: In this cross-sectional study vascular parameters (PWV, IMT, D) of 24 tx patients aged (mean (SD)) 16,6 (4,9) years, were measured by applanation tonometry and sonographic evaluation of carotid artery 4,5 (3,1) years after tx. Results were expressed as standard deviation scores (SDS). Routine laboratory parameters were analysed.
Results: Carotid artery distensibility of tx children was in the normal range (D SDS:-0,01 (0,98)). PWV SDS was increased (0,97 (0,71)) and mean IMT SDS was around the 95th percentile (1,64 (1,36)). There was a positive correlation between PWV SDS and creatinine, P and CaxP (r=0,51; r=0,40; r=0,46). In the subgroup of tx children with significantly increased IMT we found elevated P level and CaxP product at first year control following tx (IMT SDS:0,13 vs 2,61; P:1,24 vs 1,63 mmol/l; CaxP:3,19 vs 4,18 mmol/l).
Conclusion: There are structural (IMT) and functional (PWV) vascular alterations in tx children 4,5 years after tx. Our results suggest that decreased graft function and disturbed Ca-P metabolism might have a role in posttx vascular disorders.
OTKA 71730, TÁMOP-4.2.2-08/1/KMR-2008-0004, OTKA 49690.
THE ROLE OF BIOIMPEDANCE ANALYSIS IN THE DIAGNOSIS OF MALNUTRITION IN CHRONICALLY ILL CHILDREN
Svekusova M., Podracka Ľ.
1st Department of Paediatric, PJ Safarik University School of Medicine and University Children Hospital, Kosice, SlovakiaIntroduction: Malnutrition in chronic diseases impairs growth and development and is an independent prognostic factor of morbidity and mortality. Early recognition of nutritional deterioration is therefore essential.
The aim of the study: To evaluate the sensitivity of bioimpedance analysis in the diagnosis of malnutrition compared to standard anthropometric parameters.
Methods: Seventy-nine children were enrolled in the study (42 cystic fibrosis (CF) patients, 12 patients with end-stage-renal-disease (ESRD) and 25 healthy controls). Anthropometric measurements (height-for-age, BMI) expressed as standard deviation scores and percentile values were evaluated according to reference data for Slovak population. Whole-body bioimpedance analysis (BIA) was performed using the multifrequency device BioScan 920-2. Obtained BIA parameters (total fat mass in kilograms (TFM), total percentage body fat (PBF), total fat free mass in kilograms (TFFM)) were expressed as percentile values according to CDC NHANES III. body composition data. The percentage of children with values of anthropometric and BIA-derived indices below the 5th and between the 5th and 25th percentiles was calculated in order to detect, respectively, patients with severe or moderate nutrition derangement.
Results: Chronically ill children (CF + ESRD) had significantly lower height-for-age SD values compared to healthy controls (-1,35 vs. 0,28, p<0,05). In CF patients linear growth remains preserved, PBF is significantly decreased (15,98 vs. 22,28, p<0,05. In ESRD patients the mean height-for-age SD is significantly decreased (-3,11 vs. 0,28, p<0,001), indices of fat stores are less sensitive in diagnosis of malnutrition. BIA identified higher percentage of children with nutritional compromise compared to anthropometric indices (CF: 93% vs. 57%, ESRD: 92% vs. 84%).
Conclusion: BIA is more sensitive than anthropometry in the diagnosis of malnutrition. In specific disease populations different BIA parameters may be used.
Supported by grant VEGA No.: 1/0805/08.
PRETREATMENT PLASMA FOLATE MODULATES THE PHARMA CODYNAMICS EFFECT OF SYSTEMIC MTX IN CHILDREN WITH NHL AND ALL: („FOLATE OVER RESCUE“) CONCEPT REVISITED
Bernatikova H., Valik D., Dubska L., Ruzickova P., Domansky J., Krenova Z., Mendelova D., Sterba J.
Paediatric Oncology Department, Masaryk University, University Hospital, Brno, Czech Republic
Masaryk Memorial Cancer Institute, Brno, Czech RepublicIntroduction: Optimal MTX dose, and the need for folinic acid rescue for children with ALL/NHL remain an issue. Recent study showed HD MTX no better than low dose oral MTX.
Objectives: Time profiles of plasma MTX levels, homocysteine and folate were analyzed.
Methods: We analyzed 100 HD MTX courses and 50 courses of low dose MTX without leukovorin rescue. The study endpoints were to determine how methotrexate exposure is translated to homocysteine accumulation and whether it is influenced by pretreatment plasma folate.
Results: HD MTX courses showed decreasing course-to-course tendencies for the area under the curve (AUC) for plasma homocysteine, indicating decreased whole body homocysteine accumulation in response to unchanged exposure of consecutive HD MTX courses. Therapeutic courses with low initial folate concentrations (<10 nmol/L) gave significantly higher responses in homocysteine accumulation than did courses with initial low folate. Escalating low doses of Capizzi MTX without leukovorin rescue showed antifolate effect measured by homocystein AUC comparable with HD MTX.
Conclusion: Endogenous pretreatment plasma folate modulates the magnitude of the MTX effect, providing support for a „folate over rescue” concept. The optimal dosing of systemic MTX for children with NHL and ALL still remains to be established.
CLINICAL RELEVANCE OF GGH-401C>T AND THE RFC1 A(80)G POLYMORPHISM IN TREATMENT OF OSTEOSARCOMA IN CHILDHOOD
Hegyi M. Z.1, Semsei Á. F.2, Cságoly E.2, Erdélyi D.2, Szalai C.2, Kovacs G.1
12nd Department of Paediatrics, Semmelweis University, Budapest, Hungary
2Department of Genetics, Cell - and Immunobiology, Semmelweis University, Budapest, HungaryBackground: The gamma-glutamyl hydrolase (GGH) plays an important role in the antifolate-resistance in tumour cells. Presence of the-401T allele in the promoter of the GGH gene causes increased gene expression in leukemic cell lines. G(80)A polymorphism has been described in the reduced folate carrier (RFC1) gene which encodes the major methotrexate (MTX) transporter. Children with acute lymphoblastic leukemia homozygous for A(80) had worse prognoses and higher levels of MTX than the others with G allele. We examined the association of the GGH-401C>T and the RFC1 G(80)A polymorphism with respect to toxicity and pharmacokinetics of MTX treatment in children with osteosarcoma.
Materials and methods: We analysed the data of 571MTX blocks administered to 72 patients (COSS86 or 96 protocol, 1987–2004). From medical records we examined serum drug levels 6, 24, 36, 48 hours after MTX infusion; the highest serum GPT, GGT, bilirubin values and the lowest serum protein levels in the 1st week after MTX treatment. The polymorphisms were determined by a PCR-RFLP method using DNA extracted from peripheral blood.
Results: The incidence of serious acute hepatotoxicity was less frequent (p=0.0033) and drug serum levels at 48 hours were significantly lower (p=0.0003) in patients with GGH-401TT genotype than in those who carried the C allele. Plasma clearance was significantly lower (p=0.0029) and the frequency of serious acute hepatotoxicity was significantly higher (p=0.001) in patients with RFC1 80AA genotype than in those who carried the G allele. This difference was even higher between patients with RFC1 80AA plus GGH-401CC+CT genotypes and patients with other genotypes (p=0.00005).
Conclusions: Patients with GGH-401TT genotype had less hepatotoxicity and faster MTX elimination compared to those with -401CC+CT genotype. Hepatotoxicity was more frequent in patients with RFC1 80AA genotype than in those who carried the G allele and the difference was intensified without the protective effect of GGH-401TT genotype.
TRANSIENT MYELOPROLIFERATIVE DISEASE IN CHILDREN WITH DOWN SYNDROME MOSAIC
Kolenova A., Reinhardt K., Nathrat M., Rössig C., Pekrun A., Neuhoff C. V., Reinhardt D.
Department of Pediatric Oncology , Comenius University Medical School and University Children’s Hospital, Bratislava, SlovakiaChildren with Down Syndrome have a 5 to 10% risk of transient leukemia (TL) and a ~2% risk of myeloid leukemia (ML-DS). Both, TL and ML-DS, could occur in infants with trisomy 21 mosaic. However, in children with no or rare clinical signs of DS, it can be difficult to diagnose TL or ML-DS. Further, the treatment strategies are not defined.
Patients/Material: Between 2002–2009, 11 newborns and infants were diagnosed with DS mosaic and TL (n=7, 2 of them developed ML-DS) or ML-DS (n=6). GATA1 mutation was confirmed in all patients.
Results: All newborns with TL achieve remission, in 2 children low-dose cytarabine (1.5 mg/kg BW/1 week) was applied because of clinical symptoms caused by the leukemic blasts. One patient died because of cardiovascular failure. Minimal residual disease by qPCR (mutation-specific probes) or immunophenotyping (IP) revealed negativity in 3 out of 3 children monitored. Two of the 7 children (not monitored) experienced ML-DS.
Infants with ML-DS were initially treated according the AML-BFM protocol. After ML-DS was confirmed, therapy was continued with the intensity reduced schedule according to the AML-BFM ML-DS guidelines or ML-DS 2006 registry, respectively. MRD-monitoring showed negativity for ML-DS in all children after two treatment elements (qPCR <10-4 n=3; IP <10-3 n=6). In one child another refractory myeloid leukemia population (GATAmut negative) arised after 1st induction and allogenic stem cell transplantation was applied. To date, all children are in complete remission.
Conclusions: The identification of GATA mutation in infants with DS mosaic implicates the necessity of GATA1s diagnostics in all newborn and infants with megakaryoblastic leukemia. Treatment with reduced intensity protocol like ML-DS 2006 seems to be possible in children trisomy 21 mosaic and ML-DS.
1. Patient´s characteristics. 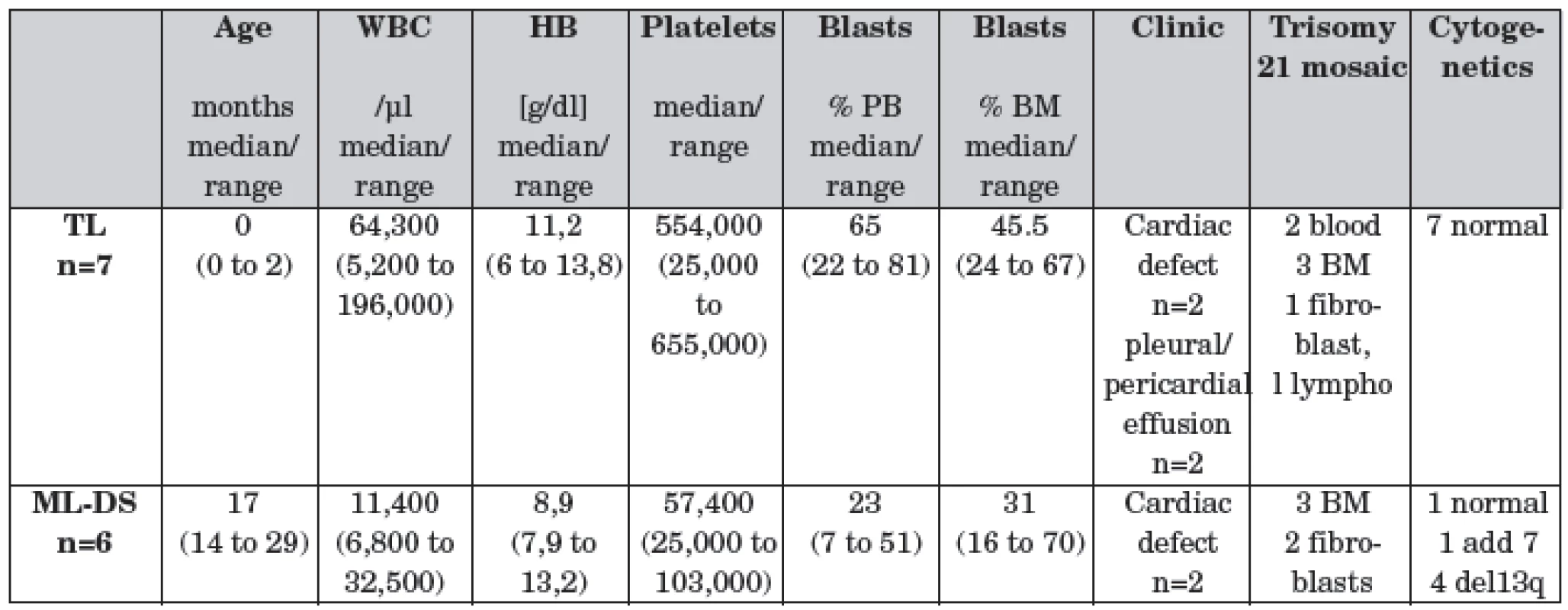
PB: peripheral blood, BM: bone marrow PARALLEL DEVELOPMENT OF DOXORUBICIN AND PACLITAXEL RESISTANT CELL LINES TO ESTABLISH A MODEL FOR INDEPENDENTLY EVOLVING RESISTANCE
Tegze B., Munkácsy Gy., Pénzváltó Zs., Győrffy B.
Joint Research Laboratory, Semmelweis University and Hungarian Academy of Sciences, Budapest, HungaryAim: Chemotherapy is one of the most common treatments in metastatic cancer. Treatment efficacy is hampered by intrinsic and acquired resistance. Current hypotheses suggest independent evolution of resistance in the primary tumor and the metastases. However, recent microarray data suggest that the same mechanism might cause resistance in the primary tumor and the metastases. Our aim was to establish a robust experimental model enabling to investigate the mechanisms of independently evolving resistance.
Methods: MCF7 and MDA-MB-231 cell lines were cultured as a monolayer in L-15 medium. The cultures were maintained in a humified atmosphere of 95% air and 5% CO2 at 37 °C. Cells were treated with continuously increasing concentration of doxorubicin (0,0002–0,06 µg/ml) and paclitaxel (0,00025–0,075 µg/ml). The development of 10 cell line/drug was launched. MTT cell proliferation assay was used to determine the level of resistance. IC50 values were determined using GraphPad Prism software.
Results: As a result of 15 months treatment 27 cell lines became resistant (average level of resistance is 7.6 (range: 2–96) fold compared to the parental cell lines). The remaining 13 cell lines reduced proliferation rate which did not enable to develop resistant derivatives. The cell lines also exhibited cross-resistance to the other agent.
Conclusion: The established set of cell lines is appropriate to examine independently evolving resistance. We are currently examining different mechanisms of resistance of these cell lines using FACS, IHC, RT-PCR, microarray, CGH and sequencing.
MOLECULAR ANALYSIS OF THE NF1 GENE IN A GROUP OF SLOVAK PATIENTS
Rybarova A.1, Nemethova M.2, Ilencikova D.3, Hlinkova K.3, Hlavata A.1, Kovacs L.1, Kadasi L.4,2, Zatkova A.4,2
12nd Department of Paediatric, Faculty of Medicine, Comenius University and University Children’s Hospital, Bratislava, Slovakia
2Department of Molecular Biology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia
3Department of Cancer Genetics, National Cancer Institute, Bratislava, Slovakia
4Laboratory of Genetics, Institute of Molecular Physiology and Genetics, Slovak Academy of Sciences, Bratislava, SlovakiaIntroduction: Neurofibromatosis type 1 is one of the most common autosomal dominant disorders (NF1; 1 : 3500) The clinical manifestation varies from mild forms with skin lesions to severe with life thretening complications. It is caused by mutations in RAS negative regulator – neurofibromine 1. Molecular diagnostics requires analyses of the entire NF1 gene.
The aim of the study mRNA-bases screening protocols are preferred since approximately one third of the pathological NF1 mutations affect mRNA splicing, out of which 30% would remain undetected when only methods based on the analysis of DNA are used. Complex analysis of the NF1 gene has not been routinely available in Slovakia yet. We introduced and employed diagnostic protocol based on the sequencing analysis of complete coding region of NF1 as developed by Messiaen and Wimmer (2008), as well as MLPA analysis employing P122, P081 and P082 sets (MRC-Holland).
Results: Screening in 37 Slovak patients with suspected NF1 was completed at the time of abstract submission. Out of 33 patients meeting clinical criterias 97% had café-au-lait spots, 61% had freckling in axilas and ingvinas; 30% had Lisch nodules of the iris, 27% had optic glioma, 58% neurofibromas, 6% had typical sceletal disorders and 48% patients had family members with NF1. cDNA sequencing uncovered the presence of small NF1 mutations in 29 patients: 18 frameshift, 4 misssense, 3 stop mutations, 3 typical splicing changes, and one in-frame deletion in exon 39. No mutation was found in 8 patients. MLPA analysis was performed in 4 patients that lack small NF1 mutations. Type I deletion of the entire NF1 gene and surrounding region was identified in 2 of them (6,3%), and the intragenic deletion of 5 continuous NF1 exons was uncovered in one additional patient (3,1%).
Conclusion: Our protocol enabled us to identify disease causing mutations in 32 out of 33 clinically confirmed NF1 patients (97%). We found out that cDNA sequencing is not a sufficient method for identifying causal mutations. MPLA method discloses whole gene mutations and is a necessary diagnostic tool for the completion of genetic diagnostics. The majority of patients presented with café-au-lait spots. Deletion of the whole NF1 gene doesn’t predict severe clinical picture. Genotype-fenotype correlations has not yet been found.
EFFECT OF ANTHRACYCLINE THERAPY ON BAROREFLEX SENSITIVITY IN CHILDREN AND ADOLESCENTS BETWEEN 11 TO 20 YEARS
Šťastná J.1, Hrstková H.1, Nováková Z.2, Honzíková N.2, Fišer B.2, Závodná E.2
1Department of Paediatrics, Faculty of Medicine, Masaryk University and Faculty Hospital, Brno, Czech Republic
2Department of Physiology, Faculty of Medicine, Masaryk University, Brno, Czech RepublicIntroduction: The baroreflex sensitivity is an index of the autonomic control of the heart. Anthracycline are established antineoplastic agents for various tumors (lymphomas, leukemias). On the other hand is well-known their cardiotoxic effect. The influence of anthracycline therapy on blood pressure we presented in our previous study.
The aim of our study is to determine the effect of anthracycline therapy on baroreflex sensitivity in children and adolescents aged 11 to 20.
Methods: We use our previous results in 415 healthy persons (group HP, 11–20 years old) as a standard values of our population. The 45 patients as group AP (age-matched) with acute lymphoblastic leaukemia were treated by anthracycline drugs. Continuous non-invasive blood pressure measurement by Peňaz method were taken in all subjects for 5 min. Measurements were made in a sitting, rested position with respiratory frequency of 20 breaths per minute. Beat to beat values of systolic and diastolic blood pressure (SBP, DBP) and inter-beat intervals (IBI) were record. Baroreflex sensitivity was determined by spectral method and expressed as BRS in ms/mmHg (dependent on IBI) and in BRSf in mHz/mmHg (IBI independent).
Results: We found statistical significance in the difference between the BRS (which is age-independent index for children and adolescents) between groups HP and AP (group HP versus AP: mean ±standard deviation;10.1±5.2 vs 7.6±4.8 ms/mmHg). The relationship between BRSf and age in HP corresponds to the regression line with a negative slope. The corresponding line in AP group was below the median line (p<0.01) and negative slope was missing.
Conclusion: Decrease of baroreflex sensitivity detected an impairment of the autonomic control of the heart by anthracycline therapy.
Supported by grant MSM No.0021622402.
CLINICAL CHARACTERISTICS AND MOLECULAR ANALYSIS OF 37 CENTRAL AND EAST EUROPEAN PATIENTS WITH CHRONIC GRANULOMATOUS DISEASE
Markelj G., Debeljak M., Pašić S., Čižnár P., Janda A., Poloučková A., Freiberger T., Šedivá A., Avčin T.
Department of Allergology, Rheumatology and Clinical Immunology, University Children’s Hospital, University Medical Center, Ljubljana, SloveniaBackground: Chronic granulomatous disease (CGD) is a rare primary immunodeficiency characterized by the failure of phagocytic cells to produce superoxide due to mutations in one of the four components of the phagocyte-specific NADPH oxidase. Mutations in the CYBB gene, which encodes the 91-kD glycoprotein gp91-phox, cause the X-linked form of the disease that affects about 65% of all CGD patients. No data of mutations in CGD patients from Central and Eastern Europe have yet been published.
Objective: To analyze epidemiological, clinical and genetic characteristics in patients with CGD from Central and Eastern Europe.
Methods: Clinical information and DNA samples from CGD patients and their mothers from Slovenia, Czech Republic, Slovakia and Serbia were collected. Detailed clinical questionnaires were completed by their clinicians. Thirteen exons and the exon/intron boundaries of the CYBB gene were individually PCR amplified, directly sequenced using the Big Dye terminator cycle sequencing kit and ABI PRISM 310 automated sequencer and compared to the normal CYBB gene sequence. The novel mutations were identified and named starting numbering from AUG codon (Gene Bank Access No. AF469757), and as recommended by den Dunnen and Antonarakis, 2001.
Results: Thirty-seven CGD patients (all boys) from 35 unrelated families were identified. Patients included in our study have similar frequencies of infections and infecting microorganisms as patients described in other series. Twenty-seven (73%) patients had mutations in the CYBB gene and ten patients had novel mutations. Mothers from 3 of our patients have normal homozygous CYBB state and the mutations in their children occurred de novo.
Conclusions: We found a high percentage of mutations in the CYBB gene (73%) in patients with CGD from Central and Eastern European countries. Ten of 27 (37%) CGD patients with mutations in the CYBB gene had novel mutations, emphasizing the specific molecular genetic features.
MONOGENIC DIABETES TYPE MODY IN SLOVAKIA: RESULTS OF A NATION-WIDE SURVEY
Staník J.2,1, Gašpieriková D.1, Hučková M.1, Valentínová L.1, Barákň Ľ.2, Kúseková M.3, Šandríková V.4, Javorková J.5, Mašindová I.1, Michálek J.6, Slovak Minogenic Diabetes Group and Klimeš I.1
1DIABGENE, Institute of Experimental Endocrinology, Bratislava, Slovakia
2Children Diabetes Center, First Department of Paediatrics, Comenius University, Bratislava, Slovakia
3First Dept. of Paediatrics, Safarik University, Kosice, Slovakia
4Outpatient Children Endocrinology Clinic, Prievidza, Slovakia
5Dept. of Paediatrics, Jessenius Medical School, Comenius University, Martin, Slovakia
6National Institute of Diabetes and Endocrinology, Lubochna, SlovakiaIntroduction: Maturity Onset Diabetes of the Young (MODY) is a heterogeneous group of monogenic diabetes with early onset, familiar appearance, and autosomal dominant inheritance. MODY could be caused by a mutation in one of the nine MODY genes, or genes causing predominantly neonatal diabetes. Nevertheless, still large number of MODY families does not have any mutation in the so far identified genes (MODY-X).
The aim of this study was to identify the etiology of the Slovakian families fulfilling the MODY criteria and calculate the frequency of selected MODY subtypes.
Methods: 239 patients from 97 families fulfilling the MODY clinical diagnostic criteria (Ellard et al, 2008) were actively searched in the diabetes outpatient clinics throughout Slovakia. The relevant genes responsible for most common MODY subtypes (i.e. GCK, HNF1A, HNF4A, HNF1B, KCNJ11 and insulin genes) were analyzed using the direct sequencing and by MLPA technique.
Results: In 152 patients from 57 families had a mutation in one of the target genes: 33 probands and 65 family relatives had a mutation in GCK gene; 22 probands and 29 their family relatives had a mutation in HNF1A gene and one family (2 pts) had a mutation in the HNF4A gene. Among 40 families without a mutation in the tree most prevalent MODY genes, one proband with a HNF1B whole gene deletion and one proband with a heterozygous insulin gene mutation were identified. No KCNJ11 gene mutation carriers were found. In 39 families no mutations in the analyzed genes were found.
Conclusions: Among the MODY subtypes, the most prevalent are GCK mutations (34% of MODY), followed by HNF1A (23%), HNF4A (1%), HNF1B (1%) and insulin (1%) gene mutations. Despite of DNA analysis of the six MODY genes, the MODY-X families account up to 40%. This is still a great challenge for identification of other genes causing the MODY diabetes.
Supported in part by research grants MZ 2005/15-NEDU-01, APVV-51-014205 and CENDO.
ALTERATIONS OF BONE METABOLIC PARAMETERS IN CHILDREN AND ADOLESCENTS WITH CHRONIC MYELOID LEUKEMIA (CML) UNDER PROLONGED TREATMENT WITH THE TYROSINE KINASE INHIBITOR IMATINIB
Tauer J. T., Jaeger B. A. S., Suttorp M.
Department of Paediatrics, Division Paediatric Haematology and Oncology, University Hospital Carl Gustav Carus, Dresden, GermanyBackground: CML is rare in childhood constituting only 2% of pediatric leukemias. The disease is characterized by a BCR-ABL fusion protein representing a constitutively active, oncogenic tyrosine kinase (TK) that causes cell transformation. The TK inhibitor imatinib (IMA; Glivec®, Novartis) has dramatically improved therapy of CML. However, first reports described disturbances in bone metabolism in adult patients (pts) and growth retardation in single pediatric pts while on IMA treatment. To further elucidate IMA effects on bone metabolism we analyzed prospectively bone biological markers in pediatric pts with CML undergoing IMA therapy.
Methods: Samples from 16 pts were analyzed with respect to specific bone metabolic markers within a time frame of 0-148 weeks after initiation of IMA therapy. Bone formation was analyzed by measuring serum osteocalcin (OC) and PINP, while CTX-I and DPD were determined as bone resorption markers. In addition, plasma levels of vitamin D metabolites and parathyroid hormone (PTH) were monitored.
Results: During IMA therapy 25-OH-vit.-D3 levels were lowered while 1,25-(OH)-vit.-D3 levels were stable with a non-significant tendency of decrease. Bone formation marker PINP showed no significant difference to normal range but we found an increased OC level at the beginning with significant decline to normal range during ongoing IMA therapy. In contrast, bone resorption marker CTX-I displayed increased levels compared to normal range over the entire period of IMA therapy in 60% of the pts. DPD measurement revealed an increased level at the beginning of IMA therapy followed by a significant decline.
Conclusion: Keeping in mind the small number of pts studied, we could demonstrate alterations of bone metabolic parameters in the juvenile still growing bone contrasting partially with data published in adults. However, long-term side effects of IMA on the juvenile and adult skeleton still remain to be determined in ongoing studies.
KNOCKDOWN OF CYTOCHROME C OXIDASE STRUCTURAL SUBUNITS IN HEK293 CELLS
Fornuskova D., Zeman J.
Department of Paediatrics, 1st Faculty of Medicine, Charles University in Prague, Czech RepublicBackground: Cytochrome c oxidase (CcO) is the multiprotein complex composed of 13 subunits sequentially assembled within the inner mitochondrial membrane. CcO deficiency in childhood represents heterogeneous group of disorders which predominantly affects tissues with high energetic demand including brain, muscle and heart. We diagnosed CcO deficiency on biochemical level in 105 children, diagnostics on molecular level is much more difficult because many nuclear and mitochondrial genes are involved in CcO biogenesis.
The aim of our study was to analyze an impact of downregulation of structural CcO subunits on CcO assembly, especially Cox4 and Cox5a subunits, both of which enter the initial stage of the process and Cox6a subunit taking a part in a terminal step of CcO holocomplex assembly.
Methods: The downregulation of selected subunits was performed using RNA interference. We constructed 33 derivatives of pCMV-GIN-ZEO plasmid coding for hairpins targeted to different positions of COX4I1, COX5A and COX6A1 mRNA, respectively, and stable HEK293 cell lines were prepared.
Results: The depletion of Cox4, Cox5a and Cox6a was confirmed by SDS-PAGE immunoblot analyses. The depletion of Cox4, Cox5a and Cox6a subunits in HEK293 cells was accompanied by diminished amount of CcO holoenzyme and an altered assembly pattern. Lower content of CcO correlated with decreased CcO activity. Isolated CcO deficiency also manifested at the level of supercomplexes. The high-resolution respirometry showed normal normoxic maximally stimulated respiration after FCCP treatment (state 3u) in knockdown cells with Cox5a and Cox6a1 depletion but increased P50 values.
Conclusion: Our results argue for importance of Cox4, Cox5a and Cox6a subunits for CcO biogenesis. Obtained knockdown-specific CcO assembly patterns could facilitate diagnostics of CcO deficiency in children with CcO deficiency and improve the genetic counseling in their families.
Supported by IGA MZ NS 10581/3 and MSM 0021620806.
GENE MANIPULATIONS IN DECIPHERING PATHOGENESIS OF BIRTH DEFECTS
Dudáš M.
Department of Biological and Ecological Sciences, PJ Safarik University School of Medicine in Kosice, SlovakiaBirth defects represent a major burden to the society, while genetic counselling is still quite inefficient in prevention of genetic disorders. Whole-genome sequencing has opened a new era in genetic research. In the field of birth defects, it is currently possible to model human diseases by selective abrogation or mutation of one or more selected genes in vivo, including regulated spatiotemporal alterations in gene expression. In addition to clinically used diagnostic techniques, animal models created by gene manipulations offer unprecedented opportunities to exploit unique diagnostic approaches that are impossible in clinical practice. For example, these techniques include cell fate tracking and cell migration mapping inside a living organism, monitoring of in vivo gene expression patterns, whole-body histological analysis, and many other futuristic techniques. This way, accumulation of mutation-specific data ranging from intravital histology up to metabolic and psychological analyses is feasible to be accomplished within a few years. Thus, providing genuine clues to diagnosis, prevention, therapy, and possibly also to intrauterine therapy of familiar as well as of de novo arising birth anomalies.
TLR2 AND TLR4 POLYMORPHISMS IN KAWASAKI DISEASE
Kitzmüller E., Sadhegi K., Dangl A., Herkner K., Förster-Waldl E., Greber-Platzer S.
Department of Paediatrics and Adolescent Medicine, Division of Paediatric Cardiology, Division of General Paediatrics and Neonatology, Medical University Vienna, AustriaKawasaki disease (KD) is a febrile acute systemic disease in early childhood affecting small and medium-sized vessels involving coronary arteries. The cause is unknown, but similarities to toxic shock syndromes without detection of a pathogen emphasize the involvement of infectious agents, cofactors, and genetic predisposition. We tested the hypothesis that polymorphisms of TL-2 and TLR4, key mediators of the innate immune system influence coronary artery involvement in KD patients. The study included 15 young adult patients with KD in childhood and compared them to an age - and sex related control group. Both groups were clinically evaluated and analysis of TLR2 and TLR4 regarding expression pattern, stimulation effects and single nucleotide polymorphisms (SNP) were performed.
Heterozygote Genotype for TLR2 SNP was found in two KD patients, one also positive for TLR4 SNP. Both patients had no acute or late cardiac symptoms, meaning no clinical involvement of coronary arteries. The expression pattern of TLR2 and TLR4 showed no difference between the KD and control group, nor in the KD patients with or without cardiac pathology. Stimulation via TLR2 and TLR4 also showed no significant influence on p38 and ERK phosphorylation and cytokine induction.
We can conclude that there is no disturbance in the innate immune system of previous patients with KD, but a modest positive effect of TLR2 and TLR4 SNPs on coronary involvement may be discussed.
LACK OF SPECIFICITY OF IMMUNOGLOBULIN D FOR HYPER-IGD SYNDROME
Dallos T.1, Hlavatá A.1, Paulovičová E.2, Baldovič M.3, Fehérvízyová Z.1, Kovács L.1
12nd Department of Paediatrics, Comenius University Medical School, Bratislava, Slovakia
2Department of Molecular Genetics, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia
3Institute of Chemistry, Slovak Academy of Sciences, Bratislava, SlovakiaIntroduction: PFAPA (periodic fever, aphtous stomatitis, pharyngitis, adenopathy) syndrome is the most prevalent of periodic fever syndromes. There is a significant overlap of clinical pictures of PFAPA and Hyper-IgD syndrome (HIDS). For HIDS diagnosis, the fulfilment of clinical criteria and the presence of IgD are considered sufficient. In this study, we aimed to explore the specificity of IgD elevation for HIDS.
Patients and methods: We searched our database of patients followed-up for recurrent fever episodes (n=214) for individuals who fulfilled diagnostic criteria of PFAPA syndrome. Clinical data, laboratory studies including IgD levels and mevalonic aciduria were revised retrospectively. Mevalonate kinase (MVK) gene mutations were determined by sequencing in all patients.
Results: 14 unrelated patients (7 male, 7 female) diagnosed with PFAPA syndrome at a mean age (± SD) of 42.7±11.7 months were included in the study. IgD elevation >100 U/l was determined in all patients. At the same time, neither mevalonic aciduria nor MVK gene mutations were found in any of the patients.
Conclusion: IgD elevation is not specific for HIDS and is readily observed in PFAPA syndrome patients. Genetic testing for MVK gene mutations is indicated to differentiate HIDS from PFAPA if clinical criteria for both are fulfilled and appropriate therapeutic measures are to be taken.
CARDIOVASCULAR DISEASE IN TURNER SYNDROME
Klásková E., Zapletalová J., Wiedermann J., Sobek A. Jr.
Department of Paediatrics, Palacky University and University Hospital, Olomouc, Czech RepublicThe aim of study: To evaluate the prevalence of cardiovascular abnormalities in Turner syndrome (TS).
Background: TS is the most common chromosomal abnormality, affecting 1/2000 live female birth. Congenital heart disease (CHD) affects approximately 50% of TS individuals and it is the major cause of premature morbidity and mortality in TS adults. Recent data report an association of TS with a generalised vasculopathy and aortic abnormalities such as aortic dilatation (AoDil) and dissection. The higher risk of CHD is associated with severe dysmorphic features. Bicuspid aortic valve (BAV), aortic coarctation (CoA), aortic root dilatation (ARD) and arterial hypertension (AH) are the risk factors for aortic dissection. Risk of the aortic dissection is increased in young women particularly during pregnancy. Accurate data on prevalence of aortic valve abnormalities, AH and ARD are not available yet.
Patients and methods: Forty four individuals with TS (18 with karyotype 45,X) age 0.1 to 19.0 years underwent complete cardiologic examination including ECG, echocardiography, 24 hours monitoring of arterial blood pressure and 38 of them also magnetic resonance imaging (MRI).
Results: BAV or dysplastic aortic valve was found in 29% of subjects, CoA was found in 2% of patients, aortic stenosis in 2% of subjects, AoDil in 18% and AH in 11% of subjects. 33 % of patients had at least one risk factor for aortic dissection, but in the 45,X group 44.5% of individuals.
Conclusion: Our data confirm the high risk for cardiovascular diseases in TS. The patients should be carefully examined just after diagnosis of TS to detect the cardiovascular abnormalities and to identify many asymptomatic individuals with risk factors. Regularly follow-up by experienced cardiologist is necessary for early detection of cardiac abnormalities. MRI is helpful for early detection of AoDil in all TS over 15 years of age to prevent cardiovascular morbidity and mortality.
JOINT INFLUENCES OF INTERLEUKIN 4 AND 13 WITH ACIDIC-MAMMALIAN-CHITINASE IN THE GENETICS OF ASTHMA
Heinzmann A., Brugger M., Grychtol R., Strauch K.
Centre of Paediatrics and Adolescent Medicine, University of Freiburg, GermanyThe aim of the study: Single genetic polymorphisms confer only a quite small individual risk factor in the genetics of complex diseases. Performing haplotype-based association analyses, including joint analyses of several candidate genes, might therefore yield more convincing results than single-region statistics. We were interested in possible joint influences of different candidate genes in the genetics of pediatric bronchial asthma. For this purpose we included all asthma genes previously identified in our study population, i.e., acidic mammalian chitinase (AMCase), Toll-like receptor (TLR)-10 and the interleukins IL-4, IL-13, IL-8, and IL-15.
Methods: A total of 26 polymorphisms within these genes were genotyped on 321 asthmatic children and 270 controls using restriction fragment length polymorphism. Haplotype-based association analyses were performed by the program FAMHAP. Single-, two - and three-gene analyses were conducted as well as conditional analyses for pairs of genes.
Results: In the two-region analyses, the best results were found for a joint effect on asthma for AMCase and IL-4 (praw<5x10-7) as well as AMCase and IL-13 (praw=5x10-7). Besides, IL-13 and TLR-10 showed a stronger two-gene result (praw=0.001607) than the respective single-gene analyses. Conditional analyses yielded similar results for these two-gene combinations.
Conclusion: In this study, best evidence was found for a joint effect of AMCase with IL-4 or IL-13 on the trait. The corresponding pathways might therefore be of particular importance in the genetics of asthma. Different studies, mainly in mice, have already demonstrated that IL-13 act downstream of AMCase in the asthmatic pathway. Now further studies in humans are needed to elucidate the functional importance of these gene-gene effects and their precise role in asthma pathogenesis.
COMPARING THE QUALITY OF NEURALLY ADJUSTED VENTILATION ASSIST VERSUS CONVENTIONAL VENTILATION SUPPORT IN CHILDREN WITH ACUTE LUNG INJURY
Fremuth J., Sasek L., Pizingerová K., Vrzalova J., Topolcan O., Kobr J.
Department of Paediatrics, Faculty of Medicine, Charles University, Pilsen, Czech RepublicObjective: To comparison the efficiency and biological impact of pneumatically versus neurally triggered ventilation (NAVA) lung-injured children.
Design and setting: Prospective, randomised and comparison study in Paediatric Intensive Care Unit.
Material and methods: Total of 16 children were enrolled in the study and divided into two groups. In the group NAVA were 8 children (male 3, female 5), median age 3.7 years (4 weeks – 12 years), weight 13.0 kg (3.1 to 60 kg), PRISM= 15.3±5.75 points and LIS=1.6±0.77 points. The conventionally ventilated group (CV) included 8 children (male 4, female 4), median age of 3.4 years (3 weeks – 11 years), weight 12.4 kg (2.8 to 28.6 kg), PRISM 16.8±3.27 points and LIS 1.9±0.53 points. Ventilation indices and the biomarkers of immunoassays for blood and tracheal aspirate have been collected in one hour after the start of ventilation (time -1) and in the intervals 12, 24 and 48 hours (time -2, -3 and -4).
Results: In the group NAVA were shorter length of ventilation (P<0.001), lower alveolar-arterial oxygen tension difference (P<0.01), dead space to tidal volume ratio (P<0.05), tracheal aspirate levels of interleukine-6 (P<0.01), intercellular (P<0.05) and vascular (P<0.01) cell adhesion molecule-1, and conversely higher hypoxemic index (P<0.05), plasma levels of matrix metaloproteinase-9 (P<0.01), and aspirate levels of fractalkine (P<0.001) compared with group CV.
Conclusion: Neurally adjusted ventilatory assist increase efficiency and quality of ventilation, accelerated conversion to spontaneous breathing and minimize the inflammatory response. Children in study tolerated this mode well and perfect synchronized with the device NAVA.
THE USE OF aEEG MONITORING IN ASSESSMENT OF SHORT–TERM AND LONG-TERM NEUROLOGICAL OUTCOME IN NEONATES WITH PERINATAL ASPHYXIA
Lukášková J., Kokštein Z., Kubinová H.
Department of Neonatology, University Hospital, Hradec Králové, Czech RepublicAim: Hypoxic-ischaemic encephalopathy (HIE) as a result of perinatal asphyxia is still severe problem of current neonatology and subsequent care of children with severe form of cerebral palsy. The aim of this study was to confirm the importance of aEEG monitoring in the first hours of life after perinatal asphyxia in prediction of neurological outcome.
Patients and method: The aEEG was recorded in 88 neonates suffering from perinatal asphyxia. Background activity of the aEEG traces was assessed according to Hellstrom-Westas classification. Short term neurologic outcome was assessesd according to Sarnat–Sarnat classification of HIE. Follow up was evaluated in 12–24 months of age.
Results: We recorded continuous normal voltage trace in 33% of neonates, discontinuous normal voltage trace in 4%, burst-suppression trace in 16% and severe depression of background activity (low voltage trace and flat trace) had 27% of patients. HIE was not developed in 26% of neonates, 16% newborns had HIE grade I, HIE grade II developed in 31% and 27% patients had HIE grade III.
All of the neonates without development of HIE or with HIE grade I had trace type continuous normal voltage or discontinuous normal voltage. All types of traces from continuous normal voltage to flat trace were recorded in neonates with HIE grade II. There were recorded patologic types of traces in group of HIE grade III.
94% of children with normal aEEG trace had normal outcome, 6% had mild psychomotoric retardation. 67% of children in group with moderately abnormal trace had normal outcome and 33% had severe psychomotoric retardation. 83% of children with burst-suppression trace died in in the group with severe depression of background activity.
Conclusion: Normal outcome was evaluated in most of children with normal aEEG trace. Patients with patological trace had severe sequellae in development or died. Our results confirm usefullness of aEEG monitoring in prediction of neurological outcome.
ROLE OF AUTOANTIBODIES AND CYTOKINES IN PREDICTION OF DIABETES TYPE 1
Petruzelkova L., Stechova K., Siftancova J., Hubackova M., Stavikova V., Lebl J., Kolouskova S.
Department of Paediatrics, Second Faculty of Medicine, Charles University, Prague, Czech RepublicType 1 diabetes (T1D) is a T cell-dependent autoimmune process leading to selective destruction of the beta cells of the pancreatic islets. The Czech Republic has an intermediate incidence of T1D (18.3/100,000 children/year in 2003).
Prediction programme for T1D is available for the first degree relatives of diabetic patients (siblings or offspring) in the Czech Republic since 2001. Children undergo HLA genotyping, estimation of GAD65 and IA2 autoantibodies (frequency depend on HLA genotype) and, in children who are autoantibodies positive, intravenous glucose tolerance testing to asses the first phase insulin response. ZnT8A autoantibodies were added recently to improve the stratification of T1D risk. For better understanding of the mechanisms of driving the activation and differentiation of proinflammatory T cells we examined the response of patient´s immunocompetent cells to diabetes associated autoantigens by using Elisa kits (Bender System) and gene microarrays (Phalanx or NimbleGen platform).
Within period of follow-up, 1.8% of tested children developed solely GAD65 autoantibodies, 7.5% solely IA2 autoantibodies and 3.7% were positive for both GAD65 and IA2 autoantibodies. 1.7% of autoantibodies positive children progressed to overt T1D. By using Elisa kits, we identified new potential protein biomarkers represented by interleukines IL-9, IL-10, TGF-beta, IL-17 and IL-23. Analysis of gene expression data of 76 patients with various autoantobody profile proved that the estimation of IL-10 and TGF-beta production rate has the highest power to predict progression to overt diabetes (p<0.001). Additionally, gene expression of IL-9 and IL-27 pathways and of genes belonging to toll-like receptor signalling cascades seems very promising, too (p<0.01). These biomarkers need further studies to estimate their usefulness in clinical prediction of T1D and their potential for future prevention strategies.
The programme is supported by projects 00064203 and NPVII 2B06019.
PROMETHEUS IN HEPATAL FAILURE
Nowaková M., Hladík M., Zaoral T.
Department of Paediatrics, University Hospital Ostrava, Czech RepublicPrometheus stands for a device which substitutes renal as well as liver functions. Using a combination of two elimination methods: albuminopheresis (with the consecutive treatment of albumin by adsorption) and hemodialysis. The „artificial liver“ treatment falls into the group of so called „bridging therapy“ which enables either liver regeneration or liver transplantation in favourable conditions of stable metabolic homeostasis. It is not any reparative treatment for a severly damaged liver, the cornerstone of which still largely remains within the limits of supportive conservative approach, but with the unexceptionable participation of the liver – self autoreparative ability.
The decision for the commence of the elimination in hepatic failure is based on clinical signs and symptoms of encephalopathy and laboratory markers. The King´s College Criteria is the auxiliary method to allow for a prognosis estimation in acute liver failure; this scoring system has been also used as a criterion for urgent liver transplantation.
So far two critically ill children – aged 7 years and 15 months – were treated with Prometheus. Though, unfortunately, both our patients died of brain oedema, we evidenced a good elimination efficiency of the system, for which the well-timed initiation is the crucial moment.
The Prometheus treatment should be restricted to a few centers around the country, also for the fact that the one-day expenses for this method is approximately 80 000 CZK (2860 Euros).
DECREASED EXPRESSION OF INTESTINAL ALKALINE PHOSPHATASE (iAP) IN THE COLONIC MUCOSA OF PAEDIATRIC IBD PATIENTS
Molnar K., Vannay A., Szebeni B., Arato A., Gyorffy H., Sziksz E., Nagy-Szakal D., Cseh A., Fanni Banki N., Tulassay T., Dezsofi A., Veres G.
1st Department of Paediatrics, Semmelweis University, Budapest, HungaryObjectives: In inflammatory bowel disease (IBD) the tolerance towards the resident intestinal flora is diminished. Lipopolysaccharide (LPS), the bacterial outer membrane component, binding to Toll like receptor 4 (TLR4) participates in damaging the gut mucosa. Intestinal Alkaline Phosphatise (iAP) plays a remarkable part in intestinal barrier maintenance by detoxification of lipopolysaccharide (LPS).
Aim: Our aim was to investigate iAP in paediatric IBD.
Patients and methods: Colonic biopsy samples were taken from macroscopically inflamed and non-inflamed regions of the mucosa of 10 children freshly diagnosed with Crohn’s disease (CD) and from the inflamed mucosa of 5 children with ulcerative colitis (UC). Ten control biopsy specimens were enrolled in this study. The iAP protein levels were determined by Western blot analysis. Co-localisation of iAP and TLR4 protein in the colonic and terminal ileal mucosa was allocated by immunofluorescent staining.
Results: The iAP protein levels were found in the inflamed tissue of children with UC with 38% and with 45% lower in CD compared to healthy controls and samples from uninvolved (non-inflamed) regions of paediatric CD patients. In children with inflamed colonic mucosa was a 32% protein level decrease compared to uninflamed mucosa in CD. In freshly diagnosed CD, UC and control patients the iAP and TLR4 staining was restricted to the epithelial surface of colon and terminal ileum.
Conclusion: The decreased levels of iAP in the inflamed colonic mucosa of children with IBD suggest the theory that the intestinal barrier maintenance plays an important role in the pathogenesis of IBD. Our findings try to reveal the molecular background of promising iAP therapy in IBD.
AZATHIOPRINE TREATMENT AND TPMT POLYMORPHISMS IN PEDIATRIC PATIENTS WITH INFLAMMATORY BOWEL DISEASE
Dzurenkova A.1, Soltysova A.2, Cierna I.1, Minarik G.2, Kovacs L.1, Mladosievicova B.3
12nd Department of Paediatrics, Medical Faculty, Comenius University, and Children´s Hospital, Bratislava, Slovakia
2Department of Molecular Biology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia
3Department of Normal and Pathological Physiology, Medical Faculty, Comenius University, Bratislava, SlovakiaIntroduction: Azathioprine is used as immunosupressive agent in treatment of inflammatory bowel disease (IBD). Thiopurine methyltransferase (TPMT) is the key enzyme in degradation of active metabolites. Some polymorphisms for TPMT are associated with decreased enzyme activity resulting in severe adverse reaction, especially myelosupression.
The aim of the study was to analyze the relationship between adverse effects of azathioprine administration and polymorphisms for TPMT in pediatric patients with inflammatory bowel disease (IBD). To this date we completed retrospective analysis and genotyping in 35 out of 90 IBD patients.
Methods: We performed the retrospective analysis of 35 patients (21 boys and 14 girls) treated with azathioprine for IBD at 2nd Department of Pediatrics of Comenius University and Children´s Hospital in Bratislava. The median age was 16.9 years (4.9–21.4 years). For genotyping we used PCR (Polymerase Chain Reaction) and sequencing of the gene for TPMT. We focused on 3 major polymorphisms in Caucasian population – TPMT*1, TPMT*2, TPMT*3.
Results: Out of 35 patients, 5 have adverse effects, which resulted to decrease or stop of azathioprime therapy. Leucopoenia was present in 3 patients, elevation of pancreatic enzymes but without clinical correlation was present in 2 patients. After genotyping it was found, that 2 of 3 patients with severe leucopoenia were heterozygotes for TPMT2*. The most prevalent polymorphism associated with low activity of TPMT in Caucasians TPMT*3 was not present in our group.
Conclusion: Patients with polymorphisms associated with low activity of TPMT are at higher risk of adverse effects, but TPMT genotype is not the only cause of adverse effects of treatment. Previous determination of TPMT activity detects patients at higher risk of toxicity of azathioprine treatment.
Project was supported by GUK 421/2009 and GUK 254/2010.
HAPTOGLOBIN POLYMORPHISM IN CHILDREN WITH TYPE 1 DIABETES MELLITUS AND WITH DIABETES AND COELIAC DISEASE
Nagy Szakál D., Papp M., Körner A., Pályu E., Molnár K., Dezsőfi A., Arató A., Veres G.
1st Department of Paediatrics, Semmelweis University, Budapest, Hungary
2nd Department of Medicine, University of Debrecen, HungaryBackground: The type 1 diabetes mellitus (T1DM) and coeliac disease (CD) are both frequent immune-mediated diseases in childhood. The altered phenotypes of haptoglobin have various immunomodulatory properties. Hp 2-2 phenotype can modify the prevalence and the clinical presentation of diseases.
Aim: The aim of our study was to investigate the distribution of haptoglobin polymorphism in T1DM and in patients with T1DM+CD.
Patients and methods: 129 T1DM and 44 T1DM+CD children (mean age: 11.6 years, range 1.5–18 years) were examined. 384 healthy people served as control. Hp phenotypes were determined by SDS-polyacrylamide gel electrophoresis and immunoblotting of the sera, which clearly identifies the genotypes.
Results: In T1DM the prevalence of Hp1-1 was 16/129 (12.4%), Hp2-1 was 65/129 (50.4%) and Hp2-2 was 48/129 (37.2%). In T1DM+CD the distribution of Hp phenotype was different, Hp1-1 : 5/44 (11.4%), Hp2-1 : 17/44 38.6%, Hp2-2 : 22/44 (50%). In controls the prevalence of Hp1-1 was 44/384 (11.5%), Hp2-1 was 177/384 (46.1%) and Hp2-2 was 163/384 (42.4%). As compared with a recent large-scale study, the frequency of Hp2-2 phenotypes was significantly higher in patients with T1DM+CD compared to the coeliac patients (p<0.05).
Conclusion: Our results suggest dominancy of Hp2-2 phenotype can play a central role in the pathomechanism of T1DM and evolving CD. The phenotype-dependent effect of Hp may result from the molecule’s structural and functional properties with different antioxidant, scavenging and immunomodulatory properties.
MUTATIONAL ANALYSIS OF MC4R GENE IN OBESE CHILDREN IN SLOVAKIA
Vitáriušová E.1, Polák E.2, Kúseková M.3, Pribilincová Z.1, Košťálová Ľ.1, Hlavatá A.1, Tichá Ľ.4, Kádaši Ľ.2,5, Kovács L.1
12nd Department of Paediatrics, Medical Faculty, Comenius University and University Children´s Hospital, Bratislava, Slovakia
2Department of Molecular Biology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia
31st Department of Paediatrics, Faculty of Medicine of P. J. Safarik University, Kosice, Slovakia
41st Department of Paediatrics, Medical Faculty, Comenius University and University Children´s Hospital, Bratislava, Slovakia
5Institute of Molecular Physiology and Genetics, Slovak Academy of Sciences, Bratislava, SlovakiaSubject: Mutations of the melanocortin 4 receptor (MC4R) are the common causes of monogenic obesity. The aim of our study was to estimate the prevalence of carriers of MC4R mutations in Slovak obese children and evaluate their phenotype.
Methods: The study presents data from 92 children with exogenous obesity and 4 family members. All children were enrolled to the study according to following criteria: a.) the onset of obesity before 11 years of age, b.) body mass index above 97th percentile for certain age and gender. We counted BMI and examined the blood sugar and lipids in every subject of cohort. The analysis of DNA was done by using PCR and direct sequencing method.
Results: There were 3 heterozygous variants of MC4R coding region identified in cohort. While I251L and V103I, previously reported variants, are not associated with monogenic obesity, variant W174X is a nonsense mutation for which no evidence exists in the available literature. Patient carrying this mutation is 6.5 years old male with severe obesity, but no history of binge eating. He is normoglycemic with normal levels of total cholesterol and elevated blood triglycerides. Although his mother and her mother are carriers of the same mutation, they are not obese at present time.
Conclusions: The clinical features of MC4R mutation are not specific. The binge eating is not present in every carrier. We confirm this statement, child carrying W174X mutation has no history of binge eating but the obesity was present in his very early childhood. His lifestyle may influence the course of illness. The variable expression and penetrance of gene may be the reason of related adult carriers phenotype. Although W174X variant was not reported previously according to the latest data, W174 is the residue with strong prediction and should have high priority for experimental vetting to increase understanding of function relationship for this receptor.
MICRORNA EXPRESSION ANALYSIS IN HUMAN LYMPHOMA/LEUKEMIA CELLS
Nemes K.1,2, Márk Á.2, Hajdu M.2, Csorba G.2, Kopper L.2, Sebestyén A.2, Csóka M.1
12nd Department of Paediatrics, Semmelweis University, Budapest, Hungary
21st Department of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, HungaryBackground: MiRNAs are small non-coding RNAs that regulate post-transcriptional gene expression, supposedly by inhibiting protein translation. In recent years more and more studies have described changes of miRNA expression levels in different types of human cancer and their role in cancer development, differentiation and progression. The aim of our study was to determine the expression of different miRNAs in human lymphomas and leukemias.
Methods: Human lymphoma/leukemia cell lines (BHD1, Nalm6, Mn60, KMH2, Jurkat, HL60, Raji, Ramos, BJAB, Daudi) were cultured according to standard methods. Acute lymphoblastic leukemia (ALL) cells were isolated from the bone marrow of pediatric ALL patients by Ficoll gradient centrifugation. MiRNAs were isolated by miR Vana TM miRNA Isolation Kit, and cDNA was reverse transcribed with the TaqMan MicroRNA Reverse Transcription Kit. MiRNA expression was determined with real-time PCR using TaqMAn micro-RNA Assays (miR21, miR24, miR155, miR16, miR128b, miR142-3p, miR29b, miR223). Values were normalized to normal B - and T-cells.
Results: In the present study, the expression level of different miRNAs was analyzed in human lymphoma/leukemia cell lines, T - and B-cells, and in childhood ALL bone marrow cells. MiR21 – known to be oncogenic (onco-miR) – was expressed in nearly all examined lymphoma cell lines. The onco-miR 155 was overexpressed in 20% of lymphoma/leukemia cell lines. MiRNA-128b was overexpressed in all cell lines, but extremely high values were measured in ALL (Jurkat – T-ALL, Nalm6 – ALL) cell lines and isolated ALL cells.
Conclusion: In this study, the presence of several miRNAs was confirmed in human lymphoma/ leukemia cell lines and in ALL cells. Our results suggest that different hematological malignancies have distinct miRNA expression profiles. Increasing knowledge of miRNA expression signatures may help characterize tumor subtypes, predict prognosis, and identify their regulatory role in cellular processes.
Supported by: OTKA F048380, OTKA 81624, OTKA 68341 T68341 projects of Hungarian Academy of Sciences.
MOLECULAR PRINCIPLE OF CORTICOID´S EFFECT IN CHILDHOOD ACUTE LYMPHOBLASTIC LEUKEMIA
Mihal V., Spenerova M., Koudelakova V., Benedikova A., Ruzkova V., Srovnal J., Hajduch M.
Department of Paediatrics, Palacky University and Faculty Hospital in Olomouc, Czech Republic
Laboratory of Experimental Medicine, Palacky University and Faculty Hospital in Olomouc, Czech RepublicIntroduction: Acute lymphoblastic leukemia (ALL) represents most common pediatric hematological malignancy. Although cure rates have improved over past decades, there is still a group of patients who are irresponsive to the treatment. The child patients with diagnosis of ALL are treated using of BFM international protocols. Therapy is initiated by one week corticosteroid (Prednisone) monotherapy. The ability of corticoids to inhibit growth of immature lymphoid cells and induce apoptosis determine them as one as most significant drugs in treatment of children´s ALL. Corticoid resistance is poor prognostic factor in stratificated risk-groups over the treatment. Mechanism of resistance affects expression of many genes that regulate different cell pathways include proliferation and differentiation, cell-cycle regulation, apoptosis, DNA reparation, etc.
Aim: Identification of predictive biomarkers of corticoid resistance represent the main goal of our study. Using DNA microarrays methods enable analyzing level of thousands genes in short time. Detection of genetic changes in whole genome is crucial knowledgement to predict resistance to the therapy. Although using of current therapeutic protocols for children’s ALL is strictly determine, there is still effort to optimize therapy to the individual patient.
Methods: The investigation is performed by Laboratory of Experimental Medicine and Pediatric Department Faculty Hospital Olomouc and cooperative oncological pediatric workplaces across Czech Republic. The study runs on leukemic cells obtained by bone marrow aspiration. Gradient centrifugation is standard using to separate the mononuclear cell fraction. In vitro analyzing of chemosensitivity/ chemoresistance to corticoids is realized by MTT assay. Crucial principe of the method is reduction of soluble tetrazoliumbromide (MTT) added to leukemic cells after 3 day cultivation with cytostatics to insoluble formazan. The reaction run on mitochondrial membrane of viable cells, absorbancy is measured spectrophotometrically and correspond with number of viable cells. The results put out the information about the concentration of given cytostatic that kill 50% of leukemic cells. Preparation of the samples for microarrays analysis using Trizol method according to the protocol to extract RNA from leukemic cells. Using microarrays technologies are based on Affymetrix platform.
Conclusion: During past 10 months we tested 28 child patients with ALL. Preliminary results of in vitro chemosensitivity monitoring report considerable individual variability. Samples were prepared to microarrays analysis. Microarrays data will be processing by biostatistic methods.
TYROSINE KINASE RECEPTORS IN PEDIATRIC BRAIN TUMORS
Virág J., Tímár J., Hegedűs B., Garami M.
2nd Department of Paediatrics and Pathology, Semmelweis University, Budapest, HungaryIntroduction: The most frequently encountered solid tumors in children are brain tumors, namely medulloblastoma, astrocytoma and ependymoma. Even though the expression of some tyrosine kinase receptors has already been demonstrated in some of these tumors, PDGFRα, PDGFRβ and c-Kit have not been thoroughly studied yet. Since the inhibition of growth factors receptors found a place in therapy of certain tumors in adults (GIST, NSCLC), the characterization of growth factor receptors in pediatric brain tumors might provide a rationale for new targeted therareutic approaches.
Methods: Retrospective examination of 46 histopathological specimens from children operated at the National Institute of Neurosurgery (15 ependymoma, 18 astrocytoma, 13 medulloblastoma) was performed. Immunohistochemistry for PDGFRα, PDGFRβ, and c-Kit was conducted on paraffin embedded, formalin fixed sections. The expression was estimated in both the tumor and stromal cells of the tissue.
Results: We have frequently found a strong expression of PDGFRα in all three types of tumors. c-Kit was frequently expressed in the endothelial cells present in the vascular network of tumors both in astrocytoma and ependymoma but not in medulloblastoma.
Interestingly, a few c-Kit positive tumor cells were found in a significant number of meduloblastoma. The PDGFRβ protein was weakly present in only a few specimens, where it was restricted to the vascular network of tumor.
Conclusions: These results indicate that PDGFRα and c-Kit inhibitors may have a therapeutic role in the treatment of pediatric brain tumors.
DEFINED MARKERS FOR DETECTION OF GERM CELLS AT RISK FOR MALIGNANT TRANSFORMATION IN PATIENTS WITH DISORDERS OF SEX DEVELOPMENT
Pleskacova J.1, Stoop H.2, Oosterhuis J. W.2, Cools M.3, Lau Y.-F. Ch.4, Drop S. L. S.5, Snajderova M.1, Lebl J.1, Looijenga L. H. J.2
1Department of Paediatrics, Second Faculty of Medicine, Charles University in Prague, Czech Republic
2Department of Pathology, Erasmus MC – University Medical Center Rotterdam, Daniel den Hoed Cancer Center, Josephine Nefkens Institute, Rotterdam, The Netherlands
3Department of Paediatrics, Division of Paediatric Endocrinology, University Hospital Ghent, Ghent University, Belgium
4Division of Cell and Developmental Genetics, Department of Medicine, VA Medical Center, University of California, San Francisco, California, USA
5Department of Paediatric Endocrinology, Erasmus MC – Sophia Children’s Hospital, Rotterdam, The NetherlandsCertain group of patients with Disorders of Sex Development (DSD) with Y chromosome material in their karyotype are at higher risk for development of type II germ cell tumors (GCT). Therefore, it is important to assess properly the presence of (potentially) malignant cells in gonadal tissue of DSD patients. In many centers routine histological examination still encompasses assessment of samples stained by hematoxylin and eosin (HE) only. To prove the usefulness of additional immunohistochemical staining in identifying gonads at risk for GCT development, 75 gonadal tissue samples from 42 DSD patients (karyotype 45,X/46,XY, 46,XX/46,XY and 46,XY, mean age at the time of biopsy 10.9±8.8 years) were examined. The gonads were taken out for prophylactic reasons or biopsied for diagnostic purpose, and based on HE staining, no neoplastic or dysplastic changes or the presence of immature germ cells had been reported in them previously. Additional immunohistochemistry using antibodies against OCT3/4 (Octamer binding transcription factor) identified (together with gonadal morphology and location of positive germ cells within the seminiferous tubules) dysplastic germ cells in 13.3 % of gonads. In another 8 % of gonads immature germ cells were found. Identity of the cells was further supported by examination of SCF (Stem Cell Factor) expression and by double-staining for OCT3/4 and TSPY (Testis-Specific Protein Y-encoded). Based on current knowledge, gonads containing dysplastic germ cells are at high risk for future GCT development, while the presence of immature germ cells might indicate a substantial risk, influenced by various parameters, including age. In conclusion, in contrast to routine HE staining, immunohistochemistry identified germ cells at risk for malignant transformation in an important number of reassessed gonads (21.3%) and therefore it is of high relevance to apply this technique, especially when examining biopsy material from DSD gonads left in situ.
DEVELOPING A BIOINFORMATIC TOOL FOR THE PRELIMINARY ASSESMENT OF CANCER BIOMARKERS USING PUBLICLY AVAILABLE MICROARRAY DATASETS
Györffy B.1, Lanczky A.1, Szallasi Z.2
11st Department of Paediatrics, Joint Research Laboratory, Semmelweis University and Hungarian Academy of Sciences, Budapest, Hungary
2Children’s Hospital Informatics Program at the Harvard-MIT Division of Health Sciences and Technology (CHIP@HST), Harvard Medical School, Boston, USAFollowing the identification of new gene expression based cancer progression biomarkers various steps of independent validations must be completed. While direct measurement of gene expression levels (RT-PCR), is the most reliable method to do this, it is often desirable to test few candidate genes without major further investment in order to choose the most promising candidates and eliminate those that are most likely to fail. Microarray cohorts combined with appropriate clinical data offer exactly such a cost effective tool to prescreen potential new biomarkers. We developed an online tool to draw survival plots, which can be used to assess the relevance of the expression levels of various genes on the clinical outcome. A background database is established using gene expression data and survival information downloaded from GEO (Affymetrix HGU133A and HGU133+2 arrays). After quality control and normalization only probes present on both platforms are retained (n=22,277).
To analyze the prognostic value of a particular gene, the cohorts are divided into two groups according to the median (or upper/lower quartile) expression of the gene. The two groups can be compared in terms of relapse free survival, overall survival and distant metastasis free survival. A survival curve is displayed, and the hazard ratio with 95% confidence intervals and logrank P value are calculated and displayed. Additionally, subgroups based on treatment groups and patients with a distribution of clinical characteristics representative of those seen in general clinical practice in the US can be assessed. Homepage: www.kmplot.com. At present the tool is already available for adult breast cancer (here was most of the microarray data published), we are currently working to include lung and colon and childhood central nervous system tumors. Our tool is highly valuable for the preliminary assessment of biomarkers, especially for research groups with limited bioinformatic resources.
List of Registered Participants
- Avcin Tadej, MD., PhD., University Children’s Hospital Ljubljana, Slovenia, tadej.avcin@kclj.si
- Banovčin Peter, Prof., MD., PhD., Department of Paediatrics, Jessenius Medical School, Comenius University, Martin, Slovakia, banovcin@jfmed.uniba.sk
- Behunová Jana, MD., PhD., Faculty of Medicine, P. J. Šafárik University, Children University Hospital, Košice, Slovakia, barbjane1@yahoo.com
- Bernatíková Hana, Department of Paediatric Oncology, University Hospital Brno, Brno, Czech Republic, hana.bernatikova@fnbrno.cz
- Boršková Kamila, MUDr., Hospital České Budějovice a.s. Paediatric Department, České Budějovice, Czech Republic, kamila.borskova@seznam.cz
- Csóka Monika, MD., PhD., 2nd Department of Paediatrics, Semmelweis University, Budapest, Hungary, csokam@t-online.hu
- Dallos Tomáš, MUDr., PhD., 2nd Department of Paediatrics, Comenius University Medical School, Bratislava, Slovakia, dallos@dfnsp.sk
- Dettmar Anne, Medical University of Innsbruck, Innsbruck, Austria
- Dudáš Marek, Doc., MUDr., PhD., P. J. Šafarik University, Košice, Slovakia
- Dzurenková Alica, MUDr., 2nd Department of Paediatrics, Comenius University Medical School, Bratislava, Slovakia, dzurenkova@dfnsp.sk
- Fencl Filip, MD., PhD., Department of Paediatrics, 2nd Faculty of Medicine, Charles University and University Hospital Motol, Prague, Czech Republic, filip.fencl@gmail.com
- Fornuskova Daniela, Mgr., Department of Paediatrics, 1st Faculty of Medicine, Charles University, Prague , Czech Republic, danielafornuskova@seznam.cz
- Fremuth Jiří, MD., Department of Paediatrics, Faculty of Medicine in Pilsen, Charles University, Pilsen, Czech Republic, fremuthj@gmail.com
- Greber-Platzer Susanne, Prof., Dr., Department of Paediatrics and Adolescent Medicine, Medical University, Wien, Austria, paediatrie@meduniwien.ac.at
- Grychtol Ruth, MD., Centre for Paediatrics and Adolescent Medicine, University of Freiburg, Freiburg, Germany, ruth.grychtol@uniklinik-freiburg.de
- Györffy Balazs, Dr., 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary, zsalab2@yahoo.com
- Hegyi Márta Z., 2nd Department of Paediatrics, Semmelweis University, Budapest, Hungary, marta.hegyi@gmail.com
- Heinzmann Andrea, PD., Dr., Centre for Paediatrics and Adolescent Medicine, University of Freiburg, Freiburg, Germany, andrea.heinzmann@uniklinik-freiburg.de
- Hladík Michal, Doc., MUDr., PhD., University Hospital Ostrava, Czech Republic
- Hrstková Hana, Prof., MUDr., CSc. Paediatric Clinic, Faculty of Medicine, Masaryk University and Faculty Hospital, Brno, Czech Republic, hrstkova@fnbrno.cz
- Janda Jan, Prof., MD., CSc., Department of Paediatrics, 2nd Faculty of Medicine, Charles University and University Hospital Motol, Prague, Czech Republic, jandajan1@seznam.cz
- Jankó Viktor, MUDr., 2nd Department of Paediatrics, Comenius University Medical School, Bratislava, Slovakia, janko@dfnsp.sk
- Jeseňák Miloš, MD., PhD., MBA., Department of Paediatrics, Jessenius Faculty of Medicine, Comenius University, Martin, Slovakia, jesenak@gmail.com
- Jurko Alexander, Prof., MD., DrSc., Department of Paediatrics, Jessenius Faculty of Medicine, Comenius University, Martin, Slovakia, jurko@jfmed.uniba.sk
- Kerti Andrea, First Department of Pediatrics, Semmelweis University, Budapest Hungary, kertiandi@gmail.com
- Kitzmüller Erwin, Department of Paediatrics and Adolescent Medicine, Medical University, Wien, Austria
- Klásková Eva, MUDr., Department of Paediatrics, Palacky University and University Hospital, Olomouc, Czech Republic, e.klaskova@seznam.cz
- Kobr Jiří, Assoc. Prof., MD., PhD., Faculty of Medicine, Charles University in Prague, Pilsen, Czech Republic, kobr@fnplzen.cz
- Kolenová Alexandra, MD., Depatrtment of Pediatric Oncology, Comenius University Medical School and University Children’s Hospital, Bratislava, Slovakia, sasa.kolenova@gmail.com
- Koľvek Gabriel, MD., 1st Department of Paediatrics, Faculty of Medicine, P. J. Šafarik University, Kosice, Slovakia, gabrielkolvek@yahoo.com
- Kovács Gábor, Med. habil, PhD., 2nd Department of Paediatrics, Semmelweis University, Budapest, Hungary, kovi@gyer2.sote.hu
- Kovács László, Prof., MUDr., DrSc., MPH, 2nd Department of Paediatrics, Comenius University Medical School and University Children’s Hospital, Bratislava, Slovakia, kovacs@dfnsp.sk
- Lackner Sandra, Medical University of Innsbruck, Innsbruck, Austria, sandwich07@gmx.at
- Lukášková Jana, MUDr., Department of Neonatology, University Hospital, Hradec Králové, Czech Republic, j.lukaskova@centrum.cz
- Markelj Gašper, MD., University Children’s Hospital, University Medical Centre, Ljubljana, Slovenia, gmarkelj@gmail.com
- Mihál Vladimír, Prof., MD., PhD., Department of Paediatrics, Palacky University and Faculty Hospital, Olomouc, Czech Republic, mihalv@fnol.cz
- Molnár Kriszta, MD., 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary, molnarkriszta@hotmail.com
- Nagy Szakál Dorottya, MD., PhD., 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary, nagyszaky@hotmail.com
- Nemes Karolina, MD., 2nd Department of Paediatrics and 1st Department of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, Hungary, karolina.nemes@ gmail.com
- Nowaková Markéta, MUDr. Department of Pediatrics, University Hospital, Ostrava, Czech Republic, mummi@email.cz
- Petruželková Lenka, MD., Department of Paediatrics, 2nd Faculty of Medicine, Charles University, Prague, Czech Republic, lenkapetruzelkova@gmail.com
- Pleskačová Jana, MUDr., Department of Paediatrics, 2nd Faculty of Medicine, Charles University, University Hospital Motol, Prague, Czech Republic, pleskac.jana@seznam.cz
- Podracká Ľudmila, Prof., MD., PhD., 1st Department of Paediatrics, Faculty of Medicine, P. J. Šafarik University, Košice, Slovakia, podracka12@yahoo.com
- Repa Andreas, Dr., Department of Paediatrics and Adolescent Medicine, Medical University, Wien, Austria, andreas.repa@meduniwien.ac.at
- Reusz György, Prof., MD., PhD., DSc., 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary, reusz@gyer1.sote.hu
- Riedl Magdalena, Medical University of Innsbruck, Innsbruck, Austria, magdalena.riedl@i-med.ac.at
- Rosales Alejandra, Dr., Medical University of Innsbruck, Innsbruck, Austria, alejandra.rosales@i-med.ac.at
- Rusai Krisztina, Dr., 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary, rusai@gyer1.sote.hu
- Rybárová Anna, MUDr., 2nd Department of Paediatrics, Comenius University Medical School and University Children’s Hospital, Bratislava, Slovakia, rybarova@dfnsp.sk
- Schäfer Betti, 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary
- Skálová Sylva, MD., Department of Paediatrics, University Hospital, Hradec Kralove, Czech Republic, skalova.s@seznam.cz
- Špenerová Michaela, MUDr., Laboratory of Experimental Medicine, Palacky University and Faculty Hospital, Olomouc, Czech Republic, michaela.spenerova@gmail.com
- Staník Juraj, MUDr., PhD., 1st Department of Paediatrics, Comenius University Medical School, Bratislava, Slovakia, stanik@dfnsp.sk
- Šťastná Jana, MUDr., Paediatric Clinic, Faculty of Medicine, Masaryk University and Faculty Hospital, Brno, Czech Republic, happyjana@centrum.cz
- Švekušová Martina, MD., 1st Department of Paediatrics, Faculty of Medicine, P. J. Šafárik University, Košice, Slovakia, msvekusova@zoznam.sk
- Szabó András, Prof., Dr., 2nd Department of Paediatrics, Semmelweis University, Budapest, Hungary, merzsi@gyer2.sote.hu
- Tauer Josephine, M.Sc., B.Sc., Department of Paediatrics, Division Paediatric Haematology and Oncology, University Hospital Carl Gustav Carus, Dresden, Germany, josephinetabea.tauer@uniklinikum-dresden.de
- Tegze Bálint, Dr., Joint Research Laboratory of the Hungarian Academy of Sciences and the Semmelweis University, Budapest, Hungary, balint@tegze.net
- Toldi Gergely, Mr., 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary, toldigergely@yahoo.com
- Tulassay Tivadar, Prof., Dr., 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary, tulas@gyer1.sote.hu
- Urbanek Radvan, Prof., Dr., Austria/Germany, rurb@gmx.at
- Virág József, Dr., 2nd Department of Paediatrics, Semmelweis University, Budapest, Hungary, virag.jozsef@freemail.hu
- Vitáriušová Eva, MUDr., 2nd Department of Paediatrics, Comenius University Medical School and University Children’s Hospital, Bratislava, Slovakia, vitariusova@dfnsp.sk
- Zeman Jiří, Prof., MUDr., DrSc., Department of Paediatrics, 1st Faculty of Medicine, Charles University, Prague, Czech Republic, jzem@lf1.cuni.cz
- Zimmerhackl Lothar Bernd, Prof., Dr., Medical University of Innsbruck, Innsbruck, Austria, lothar-bernd.zimmerhackl@uklibk.ac.at
Labels
Neonatology Paediatrics General practitioner for children and adolescents
Article was published inCzech-Slovak Pediatrics

2010 Issue 9-
All articles in this issue
- Expression Pattern of Homeodomain Genes Does Not Define the Known Subgroups of Childhood Acute Lymphoblastic Leukemias
- The Effect of Initial Management of Ventilation on the Incidence of Bronchopulmonary Dysplasia and Other Morbidities in Neonates Born at 24th–27th Weeks of Gestation at Clinic of Neonatology Slovak University Hospital Nove Zamky
- Neonatal Hydrocephalus – The Value of Evaluation of the Brain by Means of Sonography
- Congenital Hyperinsulinism – Most Frequent Cause of Persistent Hypoglycemia in Newborns and Infants
- 19th Annual Meeting of the European Society for Pediatric Clinical Research (ES-PCR)
- ABSTRACTS
- Czech-Slovak Pediatrics
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Congenital Hyperinsulinism – Most Frequent Cause of Persistent Hypoglycemia in Newborns and Infants
- Neonatal Hydrocephalus – The Value of Evaluation of the Brain by Means of Sonography
- The Effect of Initial Management of Ventilation on the Incidence of Bronchopulmonary Dysplasia and Other Morbidities in Neonates Born at 24th–27th Weeks of Gestation at Clinic of Neonatology Slovak University Hospital Nove Zamky
- Expression Pattern of Homeodomain Genes Does Not Define the Known Subgroups of Childhood Acute Lymphoblastic Leukemias
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

