-
Medical journals
- Career
Fatal myocarditis after the first dose of nivolumab
Authors: E. Zomborska 1; S. Kasperova 2; J. Slopovsky 1; N. Pazderová 1; B. Kasperova 3; P. Penz 4; O. Nyitrayová 5; T. Salek 1; S. Porsok 1; B. Mladosievicova 6; M. Mego 1
Authors‘ workplace: National Cancer Institute, Bratislava, Slovak Republic ; 2nd Department of Oncology, Comenius University, Faculty of Medicine and National Cancer Institute, Bratislava, Slovak Republic 1; 1st Department of Internal Medicine, Faculty of Medicine, Comenius University, Bratislava, Slovak Republic 2; Department of Oncohematology, Faculty of Medicine, Comenius University, Slovakia 3; Department of Dia gnostic and Interventional Radiology, National Heart Institute, Bratislava, Slovak Republic 4; Biopsy centrum Cytophatos, Bratislava, Slovak Republic 5; Institute of Pathological Physiology, Faculty of Medicine, Comenius University, Bratislava, Slovak Republic 6
Published in: Klin Onkol 2022; 35(6): 486-492
Category: Case Reports
doi: https://doi.org/10.48095/ccko2022486Overview
Background: Thymic carcinoma (TC) is a rare subtype of thymic epithelial malignancy. Surgical resection is a mainstay in the treatment of TC, while radiotherapy and chemotherapy are modalities used in adjuvant or palliative setting. Immune checkpoint inhibitors (ICI) including anti-PD-1 (programmed cell death 1) antibodies represent an emerging treatment modality in TC; however, their administration could be associated with life-threatening toxicity. Case: We present a case of a 59-year-old female with grade III TC, who had received neoadjuvant chemotherapy followed by surgery and subsequent adjuvant radio-immunotherapy with an ICI, nivolumab. We provide our experience with the toxicity of an administered treatment. Results: Fourteen days after the first dose of nivolumab and on 21st day after starting of radiotherapy (total dose of 40 Gy), the patient developed fulminant myocarditis with subsequent heart failure. Despite immunosuppressive therapy with high-dose glucocorticoids and mycophenolate mofetil and intensive support, the patient died within 6 days after the onset of first symptoms. Conclusion: Physicians should be aware of these extremely rare, but potentially fatal complications of immunotherapy.
Keywords:
nivolumab – immunotherapy – cardiotoxicity – immune checkpoint inhibitors – thymic carcinoma
Introduction
Currently, immune checkpoint inhibitors (ICI) are emerging treatment modalities with effectivity in broad spectrum of tumors [1]. They are mainstay in the treatment of cancers in monotherapy or in combination with chemotherapy or radiation therapy [2]. However, substantial proportion of patients experience immune-related adverse events including fatal complications [2]. Most common immune-related adverse events include hepatitis, colitis, pneumonitis, hypophysitis, myocarditis, nephritis, hematologic adverse effects, and others [3].
Herein, for the first-time we report a patient with a rare type of cancer, thymic carcinoma, primarily refractory to chemotherapy, with high expression of programmed cell death 1 (PD-1) protein, treated with adjuvant combination of radio-immunotherapy due to R1 tumor resection. After first administration of anti-PD-1 inhibitor, nivolumab, severe cardiac toxicity was observed. The patient developed severe fulminant myocarditis with fatal course despite complex supportive care.
Case analysis
We report the case of a 59-year-old female patient presented herself with a history of fever of unknown origin, which persisted one month despite repeated antibiotic treatments. The patient had no previous history of cardiac disease and the rest of physical exam was within normal limits. She underwent diagnostic procedures in the University Hospital in Martin in May 2019. The tumor size 69 × 79 × 56 mm was found in the anterior mediastinum on CT scan. Subsequently, tumor biopsy was performed, which showed a squamous cell thymic carcinoma. The tumor was borderline resectable; therefore, in July 2019, the patient underwent two cycles of neoadjuvant chemotherapy consisted of cisplatin 75 mg/m2 on 1st day and gemcitabine 1 000 mg/m2 on 1st and 8th days. However, CT scan after two cycles of therapy revealed no tumor regression. In August 2019, the tumor was resected with concurrent brachiocephalic plastic vein replacement, in a specialized center for thoracic surgery in Bratislava. The postoperative period was complicated by a formation of an anterior mediastinal abscess, with the need for drainage. Definitive histology confirmed a thymic carcinoma, grade 3, pT1bN1, with PD-L1 expression > 50%, R1 resection, and microscopic infiltration of resection borders. Subsequently, the tumor board suggested adjuvant treatment consisted of nivolumab with concomitant external radiotherapy to the mediastinal area. In November 2019, external radiotherapy was started using three direct fields 18MVX technique at 2 Gray (Gy) to a planned total dose of 50 Gy with concomitant nivolumab, 240 mg bi-weekly.
On 14th day of administration of the first nivolumab dose and on the 21st day of external radiotherapy (40 Gy), the patient started to complain of mild dyspnea. She felt a mild chest pain and palpitation lasting a few minutes. ECG revealed atypical complete LBBB, with QS complexes in V1–V5, ST depressions in II, III, aVF, and elevations in I, and aVL in the positive QRS complex (Fig. 1). Laboratory findings showed an elevation in troponin T (3.08 ng/mL), pro-brain natriuretic peptide (2 509 ng/L), C-reactive protein (17 mg/L), and white blood cells 6.12 g/L (Tab. 1). Subsequently, the patient reported worsening of palpitations, and ECG showed ventricular tachycardia at a heart rate of 150 beats per minute.
1. Laboratory test results of a 59-year-old female patient with myocarditis. 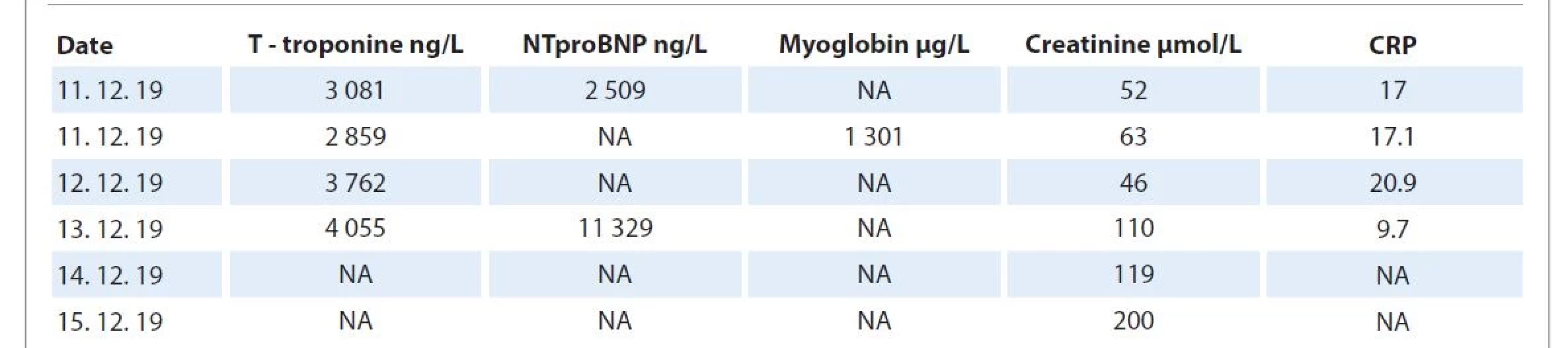
CRP – C-reactive protein, NA – not available, NTproBNP – N-terminal pro-brain natriuretic peptide Acute myocardial infarction was initially suspected; therefore, the patient was transported to the intensive care unit of National Institute of Cardiovascular Diseases, Bratislava. Urgent coronary angiography ruled out acute coronary syndrome (Fig. 3, 4). On the day of admission, transthoracic cardiac ultrasound examination showed hypertrophy of the left ventricle with preserved function and ejection fraction (EF) 60%. Frequent ventricular ectopy has progression to hemodynamically significant persistent ventricular tachycardia. There was no effect of amiodarone treatment and due to concerns of its proarrhythmogenic action, direct current electrical cardioversion was performed with acute success, but with early recurrence of ventricular tachycardia. Due to high levels of troponin T and exclusion of acute coronary syndrome using coronary angiography, ICI-associated myocarditis was suspected.
Cardiac MR revealed severe hypokinesis of the anterior wall, interventricular septum, EF 40–42% and akinesis of the greater part of the right ventricle, EF 20% (Fig. 5). Subsequently, endomyocardial biopsy under ultrasonographic navigation was performed. It revealed myocardial infiltration of inflammatory cells, and T cell-dominant lymphocyte infiltration, which were consistent with acute lymphocytic myocarditis. Viral PCR testing using myocardial specimens for coronavirus, influenza and parainfluenza virus, adenovirus, respiratory syncytial virus, bocavirus, echovirus, coxsackie virus and enterovirus were all negative. In an electron microscope without clear evidence of a replicated virus, there was a genetically persistent parvovirus B19 RNA, which is clinically insignificant. Serum antibodies measured in the convalescent phase against coxsackie virus, enterovirus, echovirus, adenovirus, influenza and parainfluenza virus, and respiratory syncytial virus were not elevated. Samples of endomyocardial biopsy for transmission electron microscopy (TEM) investigation were fixed with 3% solution of glutaraldehyde, postfixed with 1% solution of OsO4, embedded into Durcupan AMC and cut by ultramicrotome. Thin sections were contrasted by uranyl acetate and lead citrate. The documentation was made by transmission microscope Tesla BS 540. TEM examinations showed nonspecific changes in cardiomyocytes, like hypercontractions, myofibrillar lysis, autophagic vacuoles, myelin-like structures and alteration of mitochondria shape and size (Fig. 6–8). The finding of TEM is without conclusive evidence of replicated viral particles.
Fig. 5. Cardiac MRI scan. 
In the upper anterior mediastinum, parasagittally left diffusely increased adipose tissue density (40 HU), v.s. is a correlate of postoperative and post-radiation fibrosis, the nearby pericardium is partially involved in these changes. Postradiation fibrotic changes also in the parenchyma of the adjacent upper lobe of the lung on the left. No oppression of the large vessels of the mediastinum, no oppression of the cardiac compartments. Fig. 6. Autophagic membrane bound vacuole with glycogen content (near the nucleus), × 12 000. 
Fig. 7. Hypercontracted myofibrils and myelin-like figures, × 15 000. 
Fig. 8. Alteration of mitochondria – degenerated mitochondria with a dissolved membrane and abnormal size and shape, × 30 000. 
Immunosuppressive therapy was started on the 3rd day after the onset of first cardiac symptoms. The patient received high-dose glucocorticoids (intravenous methylprednisolone administered at 1 mg/kg/day for 3 days and then the patient continued on prednisone treatment (1 mg/kg). Echocardiography on 4th day showed biventricular heart failure with left ventricular ejection function 35%. Due to the development of fulminant heart failure, mycophenolate mofetil, a reversible, non-competitive inhibitor of inosine-5’-monophosphate dehydrogenase, was added in the dose 1 000 mg twice daily, to further intensify immunosuppression. Extracorporeal membrane oxygenation (ECMO) was not indicated because of concerns of its safe implantation after multiple thoracic surgery. Despite complex supportive therapy and early administration of immunosuppressants, multi organ failure and persistent ventricular tachycardia (Fig. 9) with progression to fatal acute heart failure developed and patients died 6 days after the onset of first symptoms.
Fig. 9. Progressive ventricular arrhythmia. 
Discussion
Standard treatment options for patients with operable thymic carcinoma include the surgery for Masaoka stage I with adjuvant radiation therapy in stage II. In III and IVa Masaoka stages, the tumors are primarily inoperable, so multimodal approach with neoadjuvant chemotherapy and radical resection followed by adjuvant chemoradiation is suggested [4].
Chemotherapy is the primary treatment modality for patients with IVb thymic carcinoma. Most regimens involve a platinum composite with or without an anthracycline (PAC – cisplatin, doxorubicin, cyclophosphamide, VIP – etoposide, ifosfamide, and cisplatin, ADOC – doxorubicin, cisplatin, vincristine, cyclophosphamide) [5]. A gemcitabine and cisplatin combination offers a tolerable and effective treatment option. In retrospective analysis, 13 patients with untreated or unresectable recurrent thymic squamous cell carcinomas were treated with gemcitabine and cisplatin. Eight patients achieved a partial response, four patients had a stable disease, and one had a progressive disease. The overall response rate (ORR) and disease control rate were 61.5 and 92.3%, respectively [6].
Anti PD-1/L1 inhibitors are approved for the treatment of multiple malignancies including melanoma, non–small‐cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck squamous cell cancer, urothelial carcinoma, colorectal cancer and many others. Recently, ICI showed effectivity in epithelial thymic tumors as well [7]. Yang et al report a case of a patient with thymic carcinoma, who achieved a partial response with nivolumab after standard care [8]. Single-arm, multicenter, phase II trial (PRIMER) assessed effectiveness and safety of nivolumab for previously treated thymic carcinoma patients. While nivolumab produced no responders among the 15 patients accrued during the first stage, the disease control rate 73% suggested clinical benefit [9].
A single arm, phase II study performed by Giaccone et al assessed the efficacy of pembrolizumab in 40 patients with recurrent TC (rTC). The overall response rate was 22.5%. The response lasted for 22.4 months. The median progression-free survival was 4.2 months and the median overall survival was 24.9 months [10]. Cho et al evaluated pembrolizumab in 26 patients with rTC and 7 patients with recurrent thymoma. The ORR was 19.2% in patients with thymic carcinoma and 28.6% in patients with thymoma [11]. In both trials, high PD-L1 expression was associated with a response to therapy [12]. Rajan et al evaluated avelumab in 8 TET patients (7 thymoma and 1 TC) with no history of autoimmune disease. Four of seven patients with thymoma had an objective response including a confirmed partial response in 2 (29%) patients. Noteworthy tumor shrinkage was observed [13].
ICI are associated with multiple immune related adverse events including gastrointestinal, endocrine toxicity and dermatologic toxicity as most common side effects. Neurotoxicity and pulmonary toxicity are relatively rare but can be fatal [14]. Serious adverse events include also cardiotoxicity, such as myocarditis, rhythm disorders (atrioventricular blocks, atrial and ventricular arrhythmias), myocardial infarction, pericardial disease, left ventricular dysfunction, dilated cardiomyopathy, heart failure, cardiogenic shock and sudden cardiac death [15–19]. Based on recently presented findings from a large meta-analysis of randomized clinical trials, ICI related cardiac adverse events are uncommon with occurrence ≤ 4% of patients [20]. Immunotherapy in patients with preexisting conditions for cardiac damage, diabetes mellitus, underlying autoimmune disease and some other factors could increase the risk of cardiotoxicity as well [21]. The risk of ICI-associated cardiotoxicity might be increased also in combination with other cancer treatments [22]. The role of radiation therapy in the development and progression of these cardiovascular events should be also considered [23]. However, according to the largest report of adverse effects of combined treatment with radiotherapy and ICI, there was no higher risk of myocarditis in patients receiving ICI treatment with radiotherapy compared to patients without radiotherapy [24]. The most common cardiac event is ICI‐related myocarditis. It has a reported incidence of 0.04–1.14%. Compared with other adverse events, it has a significantly higher associated mortality of 25–50% [25].
The variability in time diagnosis of ICI-related myocarditis after the initiation of ICI is known from a small number of individual case reports. The study by Mahmood et al reported evaluated time of onset of ICI associated myocarditis after initiating of immunotherapy in 35 cases. They observed the median 34 days (interquartile range 21–75 days) to the onset of myocarditis from the start of ICI administration [26]. Another cohort presented by Escudier et al revealed the median 65 days (range 2–454 days) to a diagnosis of cardiotoxicity after stating of ICI. There was an average of three infusions administered before cardiotoxicity developed [27]. Data from the VigiBase (which is World Health Organization‘s global database of individual case safety reports), that included 33 cases with ICI related myocarditis suggest that 75% of them developed myocarditis in the first 6 weeks of treatment, (median onset 27 days). Almost two thirds of these patients had received only one or two doses of therapy before the onset of myocarditis [16]. Cardiac magnetic resonance (CMR) has become nowadays a cornerstone of the diagnosis of myocarditis and its high sensitivity and specificity is valuable especially in less severe forms and in cases of uncertainty. In our case of rapid onset of fulminant myocarditis, CMR was not performed due to hemodynamic instability of the patient.
In our patient, myocarditis developed on 14th day after first dose of nivolumab. Consistently, with previous data, our case indicates, that nivolumab-induced myocarditis can develop even one dose after its administration. In case presented, patient was pre-treated with neoadjuvant chemotherapy and subsequent adjuvant radioimmunotherapy. While patient had negative medical history for other diseases including cardiovascular or autoimmune, concomitant radiation therapy could increase risk of myocarditis in our case. Previous data suggests that the mechanism associated with the development of myasthenia gravis in patients with thymic epithelial tumor (TET) may be a result of immature, TET derived thymocytes that have escaped self-tolerance and become auto-reactive [28]. ICI therapy further activates T lymphocytes, exacerbating the autoimmune reactivity of the cells and most likely resulting in the increased rates of immune-related adverse events (irAEs) compared to other cancers [29]. However, in the PRIMER trial (see above), only 2 of 15 (13.3%) patients experienced immune-related serious adverse events grade III, including aspartate aminotransferase increase and grade II adrenal insufficiency, both of which were resolved after drug discontinuation [9].
In a phase II study of pembrolizumab, adverse events of any grade were counted. The safety profile of pembrolizumab in this study was notable because of a high percentage of irAEs. Among all patients, nine patients (27.3%) stated grade 3 or 4 irAEs, and eight (24.2%) discontinued pembrolizumab therapy. irAEs were more prevalent in patients with thymoma compared with patients with thymic carcinoma (71.4 vs. 15.4%, respectively). Furthermore, five out of nine patients (four with T and one with TC) experienced multiple autoimmune adverse events simultaneously, which were not frequently observed in other malignancies treated with pembrolizumab. Of note, severe immune-related myocarditis, which is a relatively rare autoimmune syndrome, even in TET, developed in three patients with thymoma, but all have fully recovered with high-dose corticosteroids and intravenous immunoglobulin [11].
The most common adverse events of any grade included dyspnea (11; 33.3%), chest wall pain (10; 30.3%), anorexia (7; 21.2%), and fatigue (7; 21.2%). Five (71.4%) of seven patients with thymoma and four (15.4%) of 26 patients with thymic carcinoma reported grade ≥ 3 immune-related adverse events, involving hepatitis (4; 12.1%), myocarditis (3; 9.1%), myasthenia gravis (2; 6.1%), thyroiditis (1; 3.0%), antineutrophil cytoplasmic antibody–associated rapidly progressive glomerulonephritis (1; 3.0%), colitis (1; 3.0%), and subacute myoclonus (1; 3.0%) [11]. Until now, no myocarditis was described in patients with thymic carcinoma treated with ICI.
ICI‐related myocarditis has a reported incidence of 0.04–1.14%, but when compared with irAEs, it has a significantly higher associated mortality of 25–50% [25]. The treatment of ICI‐associated myocarditis has largely been based on the use of glucocorticoids. Recommended pulse dose of methylprednisolone at 1 000 mg daily for 3 days was followed by 1 mg/kg daily of either oral or intravenous steroids [26]. The clinical practice guidelines by American Society for Clinical Oncology for irAEs suggest initiation at 1 mg/kg daily of either intravenous or oral steroids [30].
There have been case reports or small case series of successfully treated ICI‐related myocarditis with intravenous immunoglobulin, mycophenolate, infliximab, anti–thymocyte globulin, plasmapheresis, alemtuzumab and abatacept. The effectiveness of these agents in ICI‐related myocarditis is unclear, and they are generally reserved for those patients who have an inadequate response to glucocorticoids [25]. In our patient, despite the rapid administration of corticosteroids at an adequate dose of 1 mg/kg on the 3rd day after the onset of symptoms, no clinical improvement occurred. Biventricular heart failure progressed. As infliximab was contraindicated due to heart failure, we decided to initiate dual immunosuppression with mycophenolate. However, no improvement was observed and despite dual immunosuppressive therapy the course of myocarditis was fatal.
Conclusion
Myocarditis is the most serious form of the cardiovascular toxicity of ICI. It is necessary for both the oncologist and the cardiologist to have a high suspicion for the diagnosis of ICI‐related cardiotoxicity in patients with nonspecific symptoms even after the first dose of nivolumab. The exact mechanism of nivolumab cardiotoxicity is not fully understood. It remains unclear whether the combination of ICI with previous chemotherapy and radiotherapy could contribute to cardiac damage in our patient. Despite the low incidence of cardiotoxicity of ICI, potentially fatal cardiac adverse events following ICI treatment for cancer should be reported systematically.
Acknowledgment
We thank Dr. Lucia Masarova for helpful discussion.
Prof. Michal Mego, MD, PhD
2nd Department of Oncology
Comenius University
Faculty of Medicine
National Cancer Institute
Klenova 1
833 10 Bratislava
Slovak Republic
e-mail: misomego@gmail.com
Submitted/Přijato: 9. 2. 2022
Accepted/Přijato: 13. 4. 2022For Fig. 1 and 2, see the online version of the article at https:/ / www.linkos.cz/ casopis-klinicka-onkologie/ .
Sources
1. Agrawal B. New therapeutic targets for cancer: the interplay between immune and metabolic checkpoints and gut microbiota. Clin Transl Med 2019; 8 (1): 23. doi: 10.1186/s40169-019-0241-x.
2. Bayat Mokhtari R, Homayouni TS, Baluch N et al. Combination therapy in combating cancer. Oncotarget 2017; 8 (23): 38022–38043. doi: 10.18632/oncotarget.16723.
3. Yeung SJ, Qdaisat A, Chaftari P et al. Diagnosis and management of immune-related adverse effects of immune checkpoint therapy in the emergency department. J Am Coll Emerg Physicians Open 2020; 1 (6): 1637–1659. doi: 10.1002/emp2.12209.
4. Hsu HC, Huang EY, Wang CJ et al. Postoperative radiotherapy in thymic carcinoma: treatment results and prognostic factors. Int J Radiat Oncol Biol Phys 2002; 52 (3): 801–805. doi: 10.1002/emp2.12209.
5. Berghmans T, Durieux V, Holbrechts S et al. Systemic treatments for thymoma and thymic carcinoma: a systematic review. Lung Cancer 2018; 126 : 25–31. doi: 10.1016/j.lungcan.2018.10.018.
6. Luo Y, Li JL, Yang L et al. Chemotherapy with gemcitabine plus cisplatin in patients with advanced thymic squamous cell carcinoma: evaluation of efficacy and toxicity. Thorac Cancer 2016; 7 (2): 167–172. doi: 10.1111/1759-7714.12300.
7. Zander T, Aebi S, Rast AC et al. Response to pembrolizumab in a patient with relapsing thymoma. J Thorac Oncol 2016; 11 (12): e147–e149. doi: 10.1016/j.jtho.2016.07.018.
8. Yang PC, Guo JC, Hsieh MS et al. Response to nivolumab as salvage therapy in a patient with thymic carcinoma. J Thorac Oncol 2018; 13 (3): e36–e39. doi: 10.1016/j.jtho.2017.10.022.
9. Katsuya Y, Horinouchi H, Seto T et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer 2019; 113 : 78–86. doi: 10.1016/j.ejca.2019.03.012.
10. Giaccone G, Kim C, Thompson J et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single--centre, phase 2 study. Lancet Oncol 2018; 19 (3): 347–355. doi: 10.1016/S1470-2045 (18) 30062-7.
11. Cho J, Kim HS, Mi Ku B et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol 2019; 37 (24): 2162–2170. doi: 10.1200/JCO.2017.77.3184.
12. Zhao C, Rajan A. Immune checkpoint inhibitors for treatment of thymic epithelial tumors: how to maximize benefit and optimize risk? Mediastinum 2019; 3 : 35. doi: 10.21037/med.2019.08.02.
13. Rajan A, Heery CR, Thomas A et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (Avelumab) treatment in advanced thymoma. J Immunother Cancer 2019; 7 (1): 269. doi: 10.1186/s40425-019-0723-9.
14. Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw 2020; 20 (1): e9. doi: 10.4110/in.2020.20.e9.
15. Matsuo K, Ishiguro T, Najama T et al. Nivolumab-induced myocarditis successfully treated with corticosteroid therapy: a case report and review of the literature. Intern Med 2019; 58 (16): 2367–2372. doi: 10.2169/internalmedicine.2596-18.
16. Moslehi JJ, Salem JE, Sosman JA et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018; 391 (10124): 933. doi: 10.1016/S0140-6736 (18) 30533-6.
17. Chen Q, Huang DS, Zhang LW et al. Fatal myocarditis and rhabdomyolysis induced by nivolumab during the treatment of type B3 thymoma. Clin Toxicol (Phila) 2018; 56 (7): 667–671. doi: 10.1080/15563650.2017.1401079.
18. Wang DY, Okoye GD, Neilan TG et al. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep 2017; 19 (3): 21. doi: 10.1007/s11886-017-0835-0.
19. Frigeri M, Meyer P, Banfi C et al. Immune checkpoint inhibitor-associated myocarditis: a new challenge for cardiologists. Can J Cardiol 2018; 34 (1): 92e1–92e3. doi: 10.1016/j.cjca.2017.09.025.
20. Agostinetto E, Eiger D, Lambertini M et al. Cardiotoxicity of immune checkpoint inhibitors: a systematic review and meta-analysis of randomised clinical trials. Eur J Cancer 2021; 148 : 76–91. doi: 10.1016/j.ejca.2021.01.043.
21. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378 (2): 158–168. doi: 10.1056/NEJMra1703481.
22. Muller OJ, Spehlmann ME, Frey N. Cardio-toxicity of checkpoint inhibitors. J Thorac Dis 2018; 10 (Suppl 35): S4400–S4404. doi: 10.21037/jtd.2018.12.78.
23. Ball S, Ghosh RK, Wongsaengsak S et al. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol 2019; 74 (13): 1714–1727. doi: 10.1016/j.jacc.2019.07.079.
24. Anscher MS, Arora S, Weinstock C et al. Impact of radiotherapy on risk of adverse events in patients receiving immunotherapy: a U.S. food and drug administration pooled analysis. J Clin Oncol 2020; 38 (Suppl 15): 3018–3018. doi: 10.1200/JCO.2020.38.15_suppl.3018.
25. Palaskas N, Lopez-Mattei J, Durand JB et al. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc 2020; 9 (2): e013757. doi: 10.1161/JAHA.119.013757.
26. Mahmood SS, Fradley MG, Cohen JV et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018; 71 (16): 1755–1764. doi: 10.1016/j.jacc.2018.02.037.
27. Escudier M, Cautela J, Malissen N et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 2017; 136 (21): 2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571.
28. Shelly S, Agmon-Levin N, Altman A et al. Thymoma and autoimmunity. Cell Mol Immunol 2011; 8 (3): 199–202. doi: 10.1038/cmi.2010.74.
29. Suzuki S, Ishikawa N, Konoeda F et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 2017; 89 (11): 1127–1134. doi: 10.1212/WNL.0000000000004359.
30. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36 (17): 1714–1768. doi: 10.1200/JCO.2017.77.6385.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2022 Issue 6-
All articles in this issue
- Editorial
- Fecal microbiota transplantation – new possibility to influence the results of therapy of cancer patients
- Prognostic and predictive factors of brain meningiomas
- Informace z České onkologické společnosti
- Cardiovascular complications among hematopoietic cell transplantation survivors – the role of cardiomarkers
- Aktuality z odborného tisku
- Souhra klinické onkologie, radiační onkologie a chirurgie v léčbě pacientů s nádory GIT
- Tebentafusp
- Comparison of the efficiency of peripheral blood stem cell apheresis on the blood cell separators
- Reconstruction and analysis of circRNA-miRNA-mRNA network in the pathology of lung cancer
- Advantages and limitations of 3D organoids and ex vivo tumor tissue culture in personalized medicine for prostate cancer
- Benign lymphoid hyperplasia mimicking oligometastasis from non-small cell lung cancer after stereotactic ablative radiotherapy
- Fatal myocarditis after the first dose of nivolumab
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Prognostic and predictive factors of brain meningiomas
- Advantages and limitations of 3D organoids and ex vivo tumor tissue culture in personalized medicine for prostate cancer
- Fecal microbiota transplantation – new possibility to influence the results of therapy of cancer patients
- Fatal myocarditis after the first dose of nivolumab
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career



