-
Medical journals
- Career
Recent Trends in Survival of Testicular Cancer Patients – Nation-wide Population Based Study
Authors: Ondrus Dalibor 1; Ondrusova Martina 2; Suchansky Martin 2
Authors‘ workplace: 1st Department of Oncology, Comenius University Faculty of Medicine and St. Elisabeth Cancer Institute in Bratislava, Slovak Republic 1; Pharm-In Ltd., Bratislava, Slovak Republic 2
Published in: Klin Onkol 2018; 31(2): 137-142
Category: Original Articles
doi: https://doi.org/10.14735/amko2018137Overview
Introduction and Aim:
Survival of germ cell testicular cancer (TC) patients is better than for other malignancies and has not yet been exactly studied in the Slovak Republic. The aim of the study, based on the analyses of epidemiological data over time, was to present 5-year survival trends for germ cell TC patients.Patients and Methods:
Survival is assessed within the framework of a nation-wide retroprospective study among TC patients newly diagnosed between 1993–2007 (divided to three 5-year periods according the time of diagnosis – 1993–1997, 1998–2002 and 2003–2007). Standardized 5-year survival rates were calculated and compared between the periods using a widely accepted methodology. TC patients were divided into two groups (seminomas and non-seminomas histopathologically) and to two groups according the age at diagnosis (< 40 vs. ≥ 40 years). The demographic characteristics of TC patients were analyzed using descriptive statistics. Statistical analysis was carried out using Microsoft Excel 2013, statistical software STATISTICA and Joinpoint Regression Programe, Version 4.3.1.0.Results:
Five-year survival of TC patients (n = 2.748) diagnosed from 1993 to 2007 was 92.21%. TC patients diagnosed between 1993 and 1997 (n = 810) reached 5-year survival at 91.23%, between years 1998 and 2002 (n = 916) at 92.14% and between years 2003 and 2007 (n = 1.022) at 93.05%. There was not a statistically significant difference in survival among these three 5-year periods. Significant difference in 5-year survival was observed between seminomas and non-seminomas in each 5-year period. Compared with younger patients (age < 40 years), there was a significantly worse survival for TC patients (age ≥ 40 years) in all groups.Conclusion:
Moderate improvement in survival for TC patients in the Slovak Republic is probably influenced by diagnostic and therapeutic progress, including multidisciplinary care and patient’s concentration in specialized centers. The long-term follow-up of TC survivors can also help to prevent late side effects of the treatment modalities and to detect second malignancies.Key words:
testicular cancer – seminoma – non-seminoma – age at diagnosis – survivalIntroduction
Testicular cancer (TC) offers challenges due to its unique descriptive epidemiology and unknown etiology. Despite its relatively rare occurrence and high curability, it is the most commonly diagnosed malignancy among males aged 15–44 in developed countries. Moreover, its incidence has been increasing to epidemic proportions while mortality is decreasing in many countries [1,2]. Information on survival of cancer patients is an important indicator of cancer control. Survival information is needed for estimating how many cancer survivors are alive at any time in order to plan health services [3]. Survival for TC patients is better than for all other malignant diseases (excluding non-melanoma skin cancers), but significant differences worldwide have been documented [4].
With modern therapeutic approaches, 5-year survival after diagnosis of TC exceed 90% in many European countries [5].
The survival for TC patients in the Slovak Republic has not yet been particularly and exactly studied. Therefore, this nation-wide study, based on the analyses of epidemiological data over time presents 5-year survivals. In addition, this paper discusses possible factors accounting for these trends and compares national data to the international context.
Patients and Methods
Study design and data collection
Survival is assessed within the framework of a nation-wide retro-prospective study among patients with germ cell TC diagnosed between 1993–2007 (divided to three periods of diagnosis 1993–1997, 1998–2002 and 2003–2007). There were analyzed data from the medical records of patients with newly diagnosed germ cell TC, where the histology of the removed testis was primarily evaluated, consulted or revised by the only pathologist, specialist in the morphology of TC in the Slovak Republic. Patients with non-germ cell TC and with spermatocytic seminoma were not included in this study. TC patients were divided into seminomas and non-seminomas histologically and to two groups according the age at diagnosis (< 40 vs. ≥ 40 years). The analyzed database of germ cell TC comprised 2.748 of all 2.978 TC cases. The newly diagnosed cases in the database are fully representative as a national source of incidence data of TC in the Slovak Republic [6,7]. The database contains personal data on patients, data describing cancer and other diagnostic and histopathologic findings, basic data on patient‘s treatment, as well as data on post-treatment follow-up. The starting point of the evaluation of survival time was defined as the date of the first diagnosis (morphological verification of germ cell TC); the closing date was defined as the date of the end of follow-up (December 15, 2017) or the date of patient‘s death.
Statistical analysis
Standardized 5-year relative survival rates were calculated on the basis of a widely accepted methodology, using computer program package Microsoft Excel 2013, STATISTICA 12 (data analysis software system) (Stat Soft, Inc.) [8] and Joinpoint Regression Program, Version 4.3.1.0. Recently proposed period analysis was applied to calculate survival in the latest period with accessible data [9].
The demographic characteristics of all patients were analyzed using descriptive statistics. Survival curves were generated using the method of Kaplan and Meier. The multiple-sample test implemented in Survival Analysis is an extension (or generalization) of Gehan‘s generalized Wilcoxon test, Peto and Peto‘s generalized Wilcoxon test, and the log-rank test. By this method, a score is first assigned to each survival time using Mantel‘s procedure. Next, a Chi-square value is computed based on the sums (for each group) of this score. If only two groups are specified, then this test is equivalent to Gehan‘s generalized Wilcoxon test, and the computations will default to that test in this case. All statistical tests were two-sided, and statistical significance was set at a p < 0.05. Mann Whitney U Test was used for comparing median age of two main histological types of TC (seminoma vs. non-seminoma).
Results
The median age of all 2.748 germ cell TC patients in the period of 1993–2007 at the time of diagnosis was 31.97 years (95% CI 32.47–33.21; interquartile range (IQR) 26.13–38.80; standard deviation (SD) 9.83). Average annual percentage change (AAPC) of the median age at the time of diagnosis was not significantly different (increase from 31.38 years (1993) to 32.79 years (2007)) (p > 0.05), it was + 0.08% (difference between 1993 and 2007 was + 4.48%), SD increased from 9.37 (1993) to 10.42 (2007).
The median age of 1.213 patients with pure seminoma testis was 35.83 years (95% CI 36.34–37.36; IQR 30.37–42.26; SD 9.08). The median age of 1.535 patients with non-seminoma testis was 28.46 years (95% CI 29.22–30.15; IQR 23.45–34.79; SD 9.22). Significant difference was observed between the age of patients with pure seminoma testis vs. non-seminoma testis (p < 0.001) (Tab. 1).
1. Age difference of patients at the TC diagnosis according to the histological types (seminoma, non-seminoma). 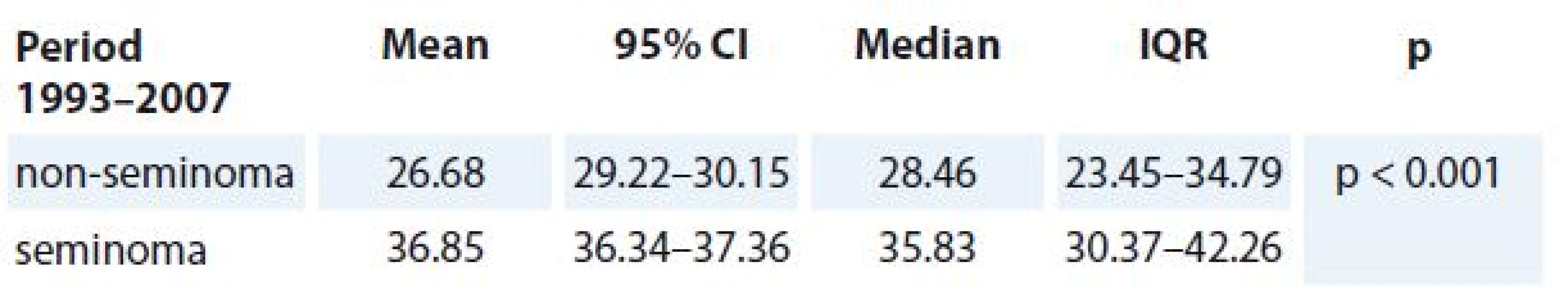
TC – testicular cancer, IQR – interquartile range Five-year overall survival (OS) for all 2.748 germ cell TC patients diagnosed in the period of 1993–2007 reached 92.21% (95% CI 91.21–93.22). Five-year survival of TC patients diagnosed between years 1993–1997 (n = 810) reached 91.23% (95% CI 89.28–93.19), between years 1998–2002 (n = 916) it reached 92.14% (95% CI 90.39–93.89) and between years 2003–2007 (n = 1.022) reached 93.05% (95% CI 91.49–94.61). The difference in survival between 5-year periods was not statistically significant (p > 0.05) (Tab. 2).
2. Five-year survival for TC patients by year periods and histological type. 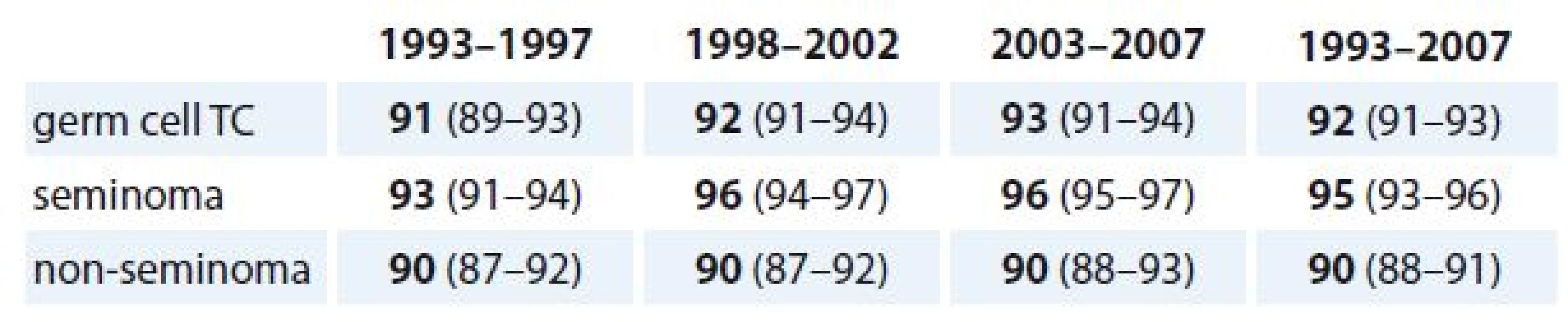
TC – testicular cancer Data are % relative survival (95% CI). Compared with younger patients (age < 40 years), higher diagnostic age (≥ 40 years) was associated with declined 5-year survival for TC patients (93.70 vs. 86.78%) in the time period of 1993–2007 (Tab. 3, Graph 1).
3. Five-year survival for TC patients by age groups and year periods. 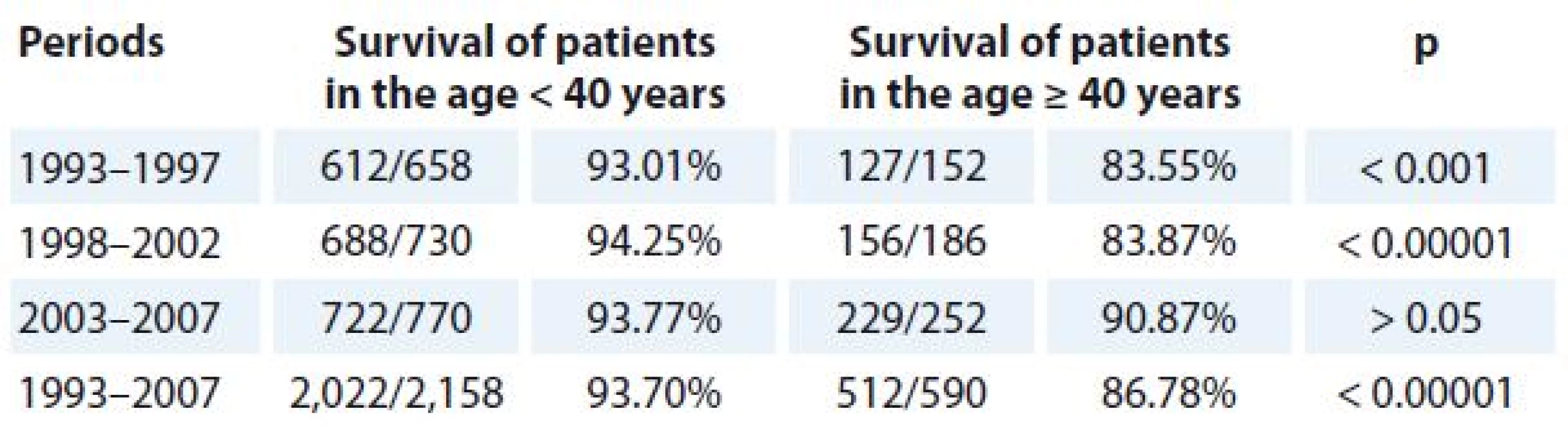
TC – testicular cancer 1. Five-year survival for TC patients by age groups. 
TC – testicular cancer Five-year survival for patients with non-seminoma testis from the cohort of years 1993–1997 (n = 438) reached 89.50% (95% CI 86.62–92.38), from the cohort of years 1998–2002 (n = 538) reached 89.41% (95% CI 86.80–92.01) and from the cohort of years 2003–2007 (n = 559) reached 90.16% (95% CI 87.68–92.64). The difference in survival between these cohorts was not statistically significant (p > 0.05) (Graph 2).
2. Five-year survival for TC patients according to year periods. 
TC – testicular cancer Five-year survival for patients with seminoma testis from the 1993–1997 cohort (n = 372) reached 93.28% (95% CI 90.72–95.84), from the 1998–2002 cohort (n = 363) it reached 96.03% (95% CI 94.05–98.01) and for the 2003–2007 cohort (n = 463) it reached 96.54% (95% CI 94.87–98.21). The difference in survival between these cohorts was not statistically significant (p > 0.05) (Graph 3).
3. Five-year survival for patients with seminoma. 
TC – testicular cancer Five-year survival was in these three cohorts higher for seminoma testis (93.28, 96.03 and 96.54%, resp.) than for non-seminoma testis (89.50, 89.41 and 90.16%, resp.). There was a statistically significant difference in survival between histological types in years 1993–2007 (p < 0.05) (Graph 4).
4. Five-year survival for patients with non-seminoma. 
TC – testicular cancer Discussion
Survival of patients having (not only) TC depends on multiple factors, such as the effectiveness of treatment, diagnostic practice, follow-up of the patients, methods of data collection and analysis, characteristics of patients (such as age, comorbidity) and cancer (such as clinical stage, histologic type). The increase in survival and the decrease in mortality are attributed to the introduction of cisplatin-containing chemotherapy, which has proven to be the most effective treatment for non-seminomas [10]. Improved survival is also the result of the use of more effective imaging techniques, the introduction of appropriate serum tumor markers (STM), which allow for careful follow-up, and modification of surgical techniques [11]. Another explanation of the improved survival is a shift toward seminomas, which have a better prognosis than non-seminomas. Prognosis is also influenced by stage and age at diagnosis, with younger patients exhibiting better survival than older patients [12,13].
Aareleid et al. [11] described the results of EUROCARE-2 study, where 5-year relative survival for TC diagnosed in 1985–1989 was between 90–95%, which were observed in most participating countries of Northern, Western, Central and Southern Europe, and the United Kingdom. Five-year survival rate under 90% was seen in France (87%), Slovenia (88%), Poland (83%), Slovakia (82%) and Estonia (51%). Rates in Poland, Slovakia and Estonia were significantly lower than the summary rate for Europe (p < 0.05). From 1978–1980 to 1987–1989, the 5-year relative survival rate for Europe increased from 79 to 93% (p < 0.05)
According to EUROCARE-3 [14], the European 5-year relative survival for TC diagnosed in 1990–1994 was 93%. Survival differences across Europe were striking, with Estonia standing out for its exceptionally low age-standardized survival (71% at 5 years). Poland, Slovakia, Spain and Wales had survival just under the European mean (82–89%), while other countries had 5-year survival > 93%. Survival for TC decreases noticeably with advancing age. For patients aged 15–44 (age of the greatest incidence), 5-year relative survival was 94%, whereas for patients aged 65–74, 5-year survival was only 40%. The main explanation for the differences in survival for TC across Europe is probably poor access to care of sufficient quality in low survival areas, resulting also in advanced stage at diagnosis. Survival for TC increased in most western European countries over the entire EUROCARE study period – from 1983–1985 to 1992–1994 – European 5-year relative survival improved from 89 to 95%. While major improvements in chemotherapy or radiotherapy for TC have not been seen since cisplatin therapy was introduced at the end of the 70s, the use of STM has made treatment monitoring and follow-up more precise.
According to EUROCARE-4 study in the period of 1995–1999, European 5-year standardized relative survival for TC was 90% with generally small variation between countries [5].
Matsuda et al. [15] suggested an improvement in TC 5-year survival also in Japan from 89.6% (1993–1996) to 92% (1997–1999).
Results of EUROCARE-5 population-based study [16] showed that the European mean age-standardized 5-year relative survival for TC (in patients aged 15–99 years) recorded in 2000–2007 was 88.6%. Age standardized 5-year relative survival was 92.8% for TC patients from Northern Europe, 91.8% for those from Ireland/UK and from Central Europe, 89.1% for patients from Southern and 80.1% for patients from Eastern Europe. Age standardized 5-year relative survival was highest for patients from Sweden (94.6%) and The Netherlands (93.5%) and lowest for those from Bulgaria (71.7%) and Lithuania (67.1%). According to EUROCARE-5, age standardized relative survival for TC patients from the Czech Republic was 84.1% and from the Slovak Republic 90.7%.
Our present study (1993–2007) showed better 5-year OS of germ cell TC patients – 92.21% compared with European mean [16]. Improvement of 5-year survival is shown between 1993 and 1997 (91.23%), 1998–2002 (92.14%) and 2002–2007 (93.05%). It shows a clearly upward trend, and thus its total value in the Slovak Republic ranks it among the developed countries in Europe.
However, in some Baltic countries, the results were poorer, e. g. in Lithuania, it was only 71.2% in the same time-period [17]. Even despite the significant improvement in survival recorded in Estonia, the values of the 5-year relative survival in 2000–2004 are at a relatively low level, 74.5% [18].
Worst trends of survival of TC patients in several countries of Europe (for example even in southern Portugal) may be dependent especially on TC diagnosis delay [19].
According to a Nordic study [20], the relative 5-year survival of TC patients in 1999–2003 is ranging from 88% in Finland to 94% in Sweden.
The recent Czech analysis showed a statistically significant improvement in the stageadjusted relative survival of all TC patients between 2000–2004 (92.4%) and 2005–2008 (94.4%) [21]. Moreover, age standardized 5-year relative survival between time periods 2001–2005 (91.2%) and 2006–2010 (92.7%) improved as well [22].
According to published results of the framework of the EUNICE Survival Working Group [23], projections for 2005–2009 suggest that 5-year relative survival will reach 90% in all cancer registries in Europe except Estonia, where survival is projected to be 82%.
In the USA, the 5-year relative survival rate in whites, for all stages, was 95.8% for patients diagnosed 1992–1999, in blacks it was 86.9% [24].
Verhoeven et al. [25] observed improvement in relative survival rate in the Netherlands. TC 5-year survival improved from 95% in 1989–1993 to 98% in 2004–2009.
Potential explanations for the age dependency of prognosis are reduced treatment intensity combined with increased therapy – related toxicity among older men [3,26]. In Germany, worse age specific 5-year relative survival for older patients with non-seminomas was observed (15–24 years 97.1%, 45+ years 86.6%) [3].
Compared with younger patients (age < 40 years) diagnostic age (≥ 40 years) was associated with declined 5-year OS for TC in our study (93.7 vs. 86.8%) between 1993 and 2007.
Conclusion
The main factors contributing to survival improvement of TC patients are – interdisciplinary management involving staging (advances in diagnostic imaging), early treatment, attentive follow-up and use of salvage treatments with concentration of TC patients in specialized centers [16]. An increasing concern is the observation that, life threatening conditions such as second malignancy and cardiovascular disease, occur more frequently in TC patients than in the general population. Long-term monitoring of TC survivors is necessary for possibility of the development of late side effects of the treatment modalities [27,28].
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Submitted: 20. 12. 2017
Accepted: 23. 1. 2018
prof. Dalibor Ondrus, MD, DrSc.
1st Department of Oncology Comenius University Faculty of Medicine
St. Elisabeth Cancer Institute in Bratislava
Heydukova 10
812 50 Bratislava Slovak Republic
e-mail: dalibor.ondrus@ousa.sk
Sources
1. Purdue MP, Devessa SS, Sigurdson AJ et al. International patterns and trends in testis cancer incidence. Int J Cancer 2005; 115 (5): 822–827. doi: 10.1002/ijc.20 931.
2. Tamini R, Adami HO. Testicular cancer. In: Adami HO, Hunter D, Trichopoulos D (eds). Textbook of cancer epidemiology. New York: Oxford University Press Inc; 2002 : 429–445.
3. Stang A, Jansen L, Trabert B et al. Survival after a diagnosis of testicular germ cell cancers in Germany and the United States, 2002–2006: A high resolution study by histology and age. Cancer Epidemiol 2013; 37 (4): 492–497. doi: 10.1016/j.canep.2013.03.017.
4. Berrino F, De Angelis R, Sant M et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: results of the EUROCARE-4 study. Lancet Oncol 2007; 8 (9): 773–783. doi: 10.1016/S1470-2045 (07) 70245-0.
5. Sant M, Allemani C, Santaquilani M et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer 2009; 45 (6): 931–991. doi: 10.1016/j.ejca.2008.11.018.
6. Ondrus D, Ondrusova M, Dusek L. Recent patterns in testicular cancer incidence, mortality and survival in the Slovak Republic with reference to international comparison. Cancer Invest 2012; 30 (8): 545–551. doi: 10.3109/07357907.2012.700984.
7. Safaei-Diba C, Plesko I, Hlava P (eds). Cancer incidence in the Slovak Republic 2007. Bratislava: National Cancer Registry of Slovakia, National Health Information Center; 2012. 135.
8. Hakulinen T, Abeywickrama KH. A computer program package for relative survival analysis. Comp Progr Biomed 1985; 19 (2–3): 197–207.
9. Brenner H, Gefeller O, Hakulinen T. Period analysis for up-to-date cancer survival data: Theory, empirical evaluation, computational realization and applications. Eur J Cancer 2004; 40 (3): 326–335.
10. Verhoeven R, Houterman S, Kiemeney B et al. Testicular cancer: marked birth cohort effects on incidence and a decline in mortality in southern Netherlands since 1970. Int J Cancer 2008; 122 (3): 639–642. doi: 10.1002/ijc.23061.
11. Aareleid T, Sant M, Hédelin G. Improved survival for patients with testicular cancer in Europe since 1978. Eur J Cancer 1998; 34 (14 Spec. No): 2236–2240.
12. Sant M, Aareleid T, Artioli ME et al. Ten-year survival and risk of relapse for testicular cancer: a EUROCARE high resolution study. Eur J Cancer 2007; 43 (3): 585–592. doi: 10.1016/j.ejca.2006.11.006.
13. Karim-Kos HE, De Vries E, Soerjomataram I et al. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer 2008; 44 (10): 1345–1389. doi: 10.1016/j.ejca.2007.12.015.
14. Sant M, Aareleid T, Berrino F et al. EUROCARE-3: Survival of cancer patients diagnosed 1990-94 – results and commentary. Ann Oncol 2003; 13 (Suppl 5): v61–v118.
15. Matsuda T, Ajiki W, Marugame T et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: A chronological and international comparative study. Jpn J Clin Oncol 2011; 41 (1): 40–51. doi: 10.1093/jjco/hyq167.
16. Trama A, Foschi R, Larrañaga N et al. Survival of male genital cancers (prostate, testis and penis) in Europe 1999–2007. Results from the EUROCARE-5 study. Eur J Cancer 2015; 51 (15): 2206–2216. doi: 10.1016/j.ejca.2015.07.027.
17. Juška A, Ulys A, Kaireviče L et al. Survival of patients with testicular cancer in Lithuania during 1999–2002. Medicina 2011; 47 (1): 52–56.
18. Aareleid T, Gondos A, Brenner H et al. Testicular cancer survival in Estonia; improving but still relatively low. Acta Oncol 2011; 50 (1): 99–105. doi: 10.3109/0284186X.2010.480981.
19. Passos-Coelho JL, Castro Ribeiro M, Santos E et al. Suboptimal survival of male germ-cell tumors in southern Portugal – a population-based retrospective study for cases diagnosed in 1999 and 2000. Ann Oncol 2011; 22 (5): 1215–1220. doi: 10.1093/annonc/mdq551.
20. Bray F, Klint A, Gislum M et al. Trends in survival of patients with male genital cancers in the Nordic countries 1964–2003 followed up until the end of 2006. Acta Oncol 2010; 49 (5): 644–654. doi: 10.3109/02841860903575 315.
21. Pavlik T, Majek O, Büchler T et al. Trends in stage-specific population-based survival of cancer patients in the Czech Republic in the period 2000–2008. Cancer Epidemiol 2014; 38 (1): 28–34. doi: 10.1016/j.canep.2013.11.002.
22. Uher M, Pavlik T, Majek O et al. On the importance of standardization in the assessment of population-based cancer patient survival in the Czech Republic – methodology and results from the Czech National Cancer Registry. Klin Onkol 2014; 27 (2): 127–135. doi: 10.14735/amko2014127.
23. Gondos A, Bray F, Hakulinen T et al. Trends in cancer survival in 11 European populations from 1990 to 2009: a model-based analysis. Ann Oncol 2009; 20 (3): 564–573. doi: 10.1093/annonc/mdn639.
24. Biggs ML, Schwartz SM. Differences in testis cancer survival by race and ethnicity: a population-based study, 1973–1999 (United States). Cancer Causes Control 2004; 15 (5): 437–444. doi: 10.1023/B: CACO.00000 36443.95995.40.
25. Verhoeven RH, Karim-Kos HE, Coebergh JW et al. Markedly increased incidence and improved survival of testicular cancer in the Netherlands. Acta Oncol 2014; 53 (3): 342–350. doi: 10.3109/0284186X.2013.819992.
26. Fossa, SD, Cvancarova M, Chen L et al. Adverse prognostic factors for testicular cancer –specific survival: A population-based study of 27,948 patients. J Clin Oncol 2011; 29 (8): 963–970. doi: 10.1200/JCO.2010.32.3204.
27. Ondrus D, Ondrusova M, Friedova L. Second malignancies in long-term testicular cancer survivors. Int Urol Nephrol 2014; 46 (4): 749–756. doi: 10.1007/s11255-013-0554-4.
28. Kvammer Ø, Myklebust TÅ, Solberg A et al. Long-term relative survival after diagnosis of testicular germ cell tumor. Cancer Epidemiol Biomarkers Prev 2016; 25 (5): 773–779. doi: 10.1158/1055-9965.EPI-15-1153.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2018 Issue 2-
All articles in this issue
- Human Papillomavirus – Role in Cervical Carcinogenesis and Methods of Detection
- Anogenital HPV Infection as the Potential Risk Factor for Oropharyngeal Carcinoma
- Treatment of Metastatic Renal Cell Carcinoma
- New Approaches for Chemosensitivity Testing in Malignant Diseases
- Quality of Life After High-dose Brachytherapy in Patients with Early Oral Carcinoma
- MAPK/ERK signal pathway alterations in patients with Langerhans Cell Histiocytosis
- Recent Trends in Survival of Testicular Cancer Patients – Nation-wide Population Based Study
- Cutaneous and Subcutaneous Metastases of Adenocarcinoma as a Dominant Clinical Manifestation of Malignancy of Unknown Origin – a Case Report
- Diagnostic, Prognostic and Predictive Immunohistochemistry in Malignant Melanoma of the Skin
- Long Non-coding RNAs as Regulators of the Mitogen-activated Protein Kinase (MAPK) Pathway in Cancer
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Cutaneous and Subcutaneous Metastases of Adenocarcinoma as a Dominant Clinical Manifestation of Malignancy of Unknown Origin – a Case Report
- MAPK/ERK signal pathway alterations in patients with Langerhans Cell Histiocytosis
- Long Non-coding RNAs as Regulators of the Mitogen-activated Protein Kinase (MAPK) Pathway in Cancer
- Human Papillomavirus – Role in Cervical Carcinogenesis and Methods of Detection
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

