-
Medical journals
- Career
MAPK/ERK signal pathway alterations in patients with Langerhans Cell Histiocytosis
Authors: Novosad Olga 1; Skrypets Tanya 1; Pastushenko Yan 1; Titorenko Iryna 1; Martynchyk Arina 1; Skachkova Oksana 2; Inomistova Mariia 2; Gorbach Alex 2; Khranovska Nataliia 2; Kryachok Irina 1
Authors‘ workplace: Oncohematology Department, National Cancer Institute, Kiev, Ukraine 1; Experimental Oncology Department, National Cancer Institute, Kiev, Ukraine 2
Published in: Klin Onkol 2018; 31(2): 130-136
Category: Original Articles
doi: https://doi.org/10.14735/amko2018130Overview
Background:
Clinical outcomes of Langerhans cell histiocytosis (LCH) are highly variable. It has been suggested that mitogen-activated protein kinase (MAPK) /extracellular signal-regulated kinases (ERK) signaling pathway might be activated in LCH patients.Materials and Methods:
We investigated KRAS, BRAF and NRAS mutations in patients with LCH by qPCR.Results:
Eight adult patients with LCH were treated at the National Cancer Institute, Kiev, Ukraine. Five patients received chemo plus radiation therapy and three patients received only chemotherapy, resp. (p < 0.05). All patients received LCH–I study protocol, six cycles in average. A BRAF c.1799T > A, p. V600E mutation was detected in 25% (2/8) of cases – 1 patient had an early relapse in 6 months, and 1 patient – stable disease. We did not find any BRAF, KRAS or NRAS mutations in three patients with late relapses (in 15, 24 and 46 months). Notably, KRAS mutations were not revealed in any LCH samples. The NRAS c.182A > G, p. Q61R mutation was found in two cases – one patient had LCH transformed to Hodgkin’s lymphoma, one patient had a refractory disease. Time to relapse rate (TTR) in patients with and without BRAF V600E gene mutation was 13 vs. 28 months, resp. (p < 0.05). TTR was 31.3 vs. 6.41 months in patients with absence and presence of NRAS mutation, p < 0.05. Multivariate analysis showed the presence of NRAS Q61R mutation was associated with poor event-free survival in LCH patients with HR of 6.1 (95% CI 0.2–12.6; p = 0.008).Conclusion:
BRAF and NRAS mutations in LCH suggest a possibility of the disease being driven by the activation of the MAPK/ERK pathway. These oncogenic mutations provide new opportunities in understanding LCH pathogenesis and may be a potential target of therapy.Key words:
Langerhans cell histiocytosis – mutations – prognostic factors – relapse – survivalIntroduction
Langerhans cell histiocytosis (LCH) is a rare myeloproliferative disorder of unknown etiopathogenesis. Based on modern research, LCH is a disease of the immune system with an abnormal immune response, leading to the proliferation of Langerhans cells, eosinophilic infiltration, granuloma formation, fibrosis, osteolytic lesions, etc.
The age-adjusted incidence rate of LCH is 3–5 cases in children and 1–2 cases in adults per 1,000,000 per year, with the incidence rate higher in men than in women. The sex ratio (m: f) is 2 : 1.
Clinical symptoms in LCH patients are very different – from isolated bone lesions to multisystem disease, with outcomes ranging from spontaneous remission to progression during therapy. In addition, symptoms of LCH vary depending on the organ or system involved. Rapid progressive forms are often seen in children and usually not observed in adults [1].
Regardless to similarities in histological features between LCH lesions, clinical outcomes are highly variable and range from isolated skin or bone disease to highly aggressive subtypes with life-threatening multisystem lesions, which require intensive chemotherapy.
There are different types of LCH (stratification of LCH):
- multisystem LCH (MS-LCH) – with or without the involvement of ‘’risk organs”;
- single system LCH (SS-LCH) – unifocal or multifocal lesions;
- single system LCH (SS-LCH) – with “special site” lesions
There are no universal guidelines for the diagnosis and treatment of adult LCH patients. The largest number of patients was analyzed in a retrospective pooled analysis from several national registries [2,3]. In general, physicians should have an option to choose the course of chemotherapy treatment depending on the stratification of LCH and presence of genetic mutations.
The benign morphology of LCH proliferating cells and their characteristic inflammatory infiltrates suggest that LCH may be an inflammatory disorder [4]. Dysregulated expression of inflammatory cytokines, such as interleukin-17A, has been reported [5]. However, the pathologic Langerhans cells in LCH are clonal [6]. Although clonality is an important feature of neoplasia, recurrent genomic abnormalities will be required to demonstrate that LCH is a neoplasm; until now, none of them have been reported [7].
It is known that the mitogen-activated protein kinase (MAPK) pathway is constantly activated in LCH. Mutations of the downstream kinases BRAF and MAP2K1 mediate this activation in a subset of LCH lesions. The most common mis-sense mutation of BRAF (mainly V600E) contributes to the incidence of various types of cancer, including LCH. BRAF gene located on chromosome 7q34 encodes a cytoplasmic serine-threonine kinase. This mutated BRAF protein constitutively activates the MAPK signaling pathway, which results in increased cell proliferation, apoptosis resistance and tumor progression [8,9].
Specific inhibition of BRAF signaling is effective in blocking proliferation of melanoma cells that have additional genomic abnormalities. Recent identification of cancer-associated mutation BRAF V600E in LCH cases provided molecular evidence of the neoplastic nature of LCH [10]. Initially BRAF was discovered in other types of cancer; additionally, it was found in LCH. Based on such data, it is possible to suggest that melanoma treatment approaches can be used for LCH treatment taking in consideration the expression of this gene [11]. BRAF mutations are usually found in tumors that have wild-type KRAS and NRAS, KIT, and other driver mutations.
Initially, LCH was considered as a disorder of immune regulation. Activating mutations in the proto-oncogene BRAF-V600E were reported in approximately 50–60% of cases; mitogen-activated protein kinase (MEK) and extracellular signal-related pathway (ERK) phosphorylation was reported in 100% of these cases. These data allow to relate LCH to a dendritic cell neoplasm with a strong inflammatory component. Current international LCH trials are focused on further improving the outcome of patients with high-risk multisystem LCH, by decreasing the reactivation rate, optimizing early salvage regimens and preventing late complications.
Somatic mutations in ARAF and MAP2K1 were recently discovered; these mutations lead to activation of the RAS-RAF-MEK-ERK pathway in the setting of wild-type BRAF. It was also found that the activation of mutation in MAP2K1 is relatively insensitive to MEK inhibitors. This suggests that a more detailed understanding of this pathway in LCH may be necessary for the development of more efficient targeted therapies [12].
Materials and Methods
We performed the study from February 1, 2009 to March 31, 2017 on biopsy sam-ples received at the Department of Oncohematology of National Cancer Institute, Kiev, Ukraine. Altogether, 8 patients with LCH (6 males and 2 females; median age – 25, age range – 21–55) were included in this study. The diagnosis was based on clinical symptoms and immunohistochemistry results (CD1a+ or CD207+ and S-100+).
The study was approved by the institutional ethics committee review board at the National Cancer Institute of Ukraine.
Quantitative real-time PCR
All biomaterial was obtained before treatment. Fresh tumor samples for quantitative polymerase chain reaction (qPCR) analysis were stored in ’RNA-later’ (Ambion, USA) to stabilize nucleic acids.
DNA was extracted from formalin-fixed, paraffin-embedded tissues after histological control. Genomic DNA from tumor samples was isolated using NucleoSpin Tissue Kit (Macherey-Nagel, Germany). Primers and probes were specially engineered to determine KRAS tumor genotype (7 mutations located within codons 12 (6) and 13 (1)) by the multiplexed qPCR. Sequences of primers were experimentally selected with Primer Express Software v3.0 (Applied Biosystems, USA) and synthesized by Applied Biosystems, USA. All primer and probe sequences are listed in Tab. 1.
1. Primer and probe sequences for analysis of qPCR mutations. 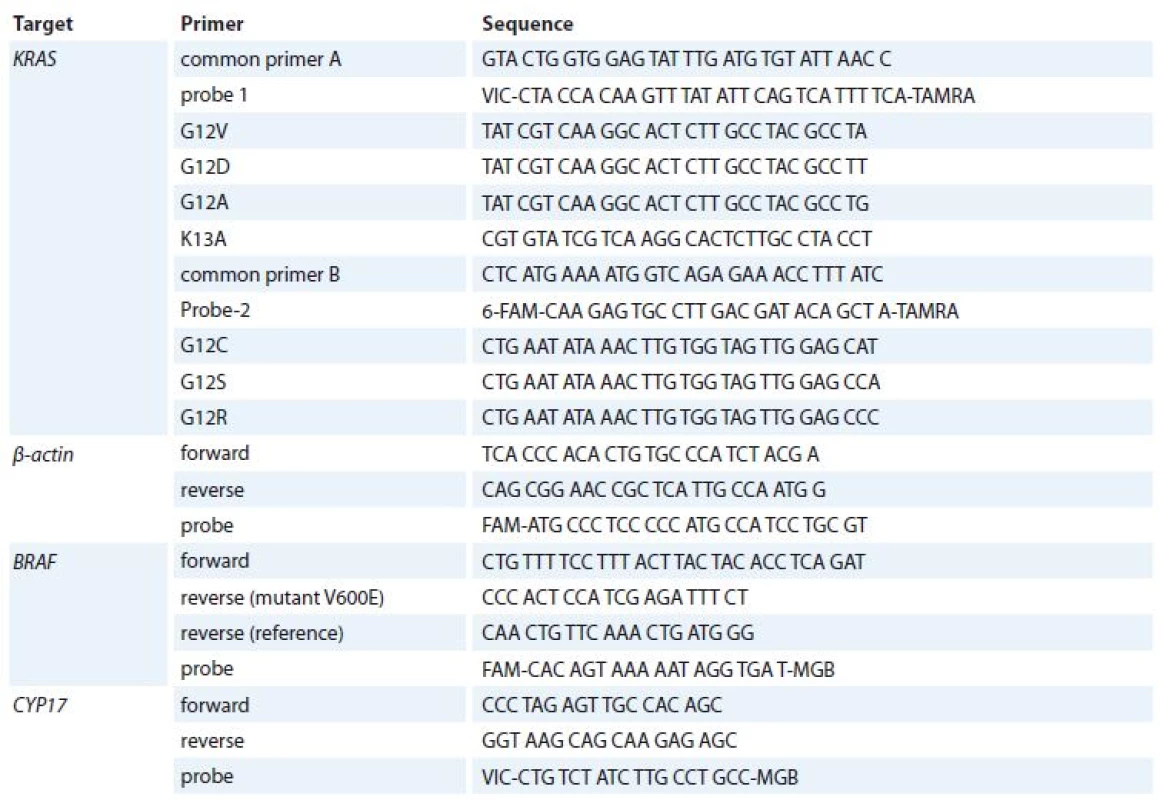
qPCR – quantitative polymerase chain reaction For KRAS mutation analysis, primers and TaqMan probes were used at 20 μM and 5 μM concentrations, resp. TaqMan Universal PCR Master Mix (Applied Biosystems, USA). Common primer A, mutation-specific primer and probe 1 were used for KRAS G12V, G12D, G12A, K13A mutation analysis, common primer B, mutation-specific primer and probe 2 were used for KRAS G12C, G1S, G12R mutation analysis; 5–10 ng of DNA were used to prepare the reaction mixture in a total volume of 25 μl. A total of 55 cycles of qPCR (95°C – 42 seconds, 60°C – 42 sec and 72°C – 52 sec) were run on 7,300/7,500 Real-Time PCR Systems (Applied Biosystems, USA). This assay uses six primers, two common primers, two TaqMan probes for seven different KRAS mutations. qPCR for an inner control β-actin was performed for each sample.
For BRAF mutation analysis qPCR reference PCR was performed in a 25 mkl reaction volume with 1 mkl TaqMan Universal Master Mix (Applied Biosystems, USA), 900 nmol/L of each BRAF mutation-unspecific primer, 100 nmol/L of the BRAF probe, 112.5 nmol/L of each internal control primer, 25 nmol/L of the internal control probe, and 5–10 ng of DNA of varying concentrations. Allele-specific PCRs were performed according to the same protocol but using a concentration of 450 nmol/L of allele-specific primer [13]. All real-time PCRs were performed on 7,300/7,500 Real-Time PCR Systems (Applied Biosystems, USA) under the following thermocycling conditions – 95°C for 10 min, followed by 50 cycles of 90°C for 15 sec and 60°C for 1 min. Cycle threshold (Ct) values were recorded for reference PCR and for each allele-specific PCR, and corresponding Ct values (ie, allele-specific Ct minus reference Ct) were calculated.
We also studied KRAS minor mutations G13C, Q61R, Q61H, A146T and NRAS mutations G12V, G12D, G12C, G12S, G13V, G13R, Q61K, Q61L, Q61R, Q61HC, Q61HT in tumor samples with TaqMan Mutation Detection Assays (Cat. #4465804, Applied Biosystems, USA) and a mutation-unspecific region was used as a reference amplicon (Cat. #4465807, Applied Biosystems, USA).
Statistical analysis
A Spearman correlation coefficient (r) test and Cox test were used to determine the relationship between two continuous measurements. We used Chi-square tests for comparison of the time to relapse (TTR); p < 0.05 was considered to indicate a statistically significant difference. Survival curves were generated by the Kaplan-Meier method. An overall survival (OS) was calculated from the date of pathological diagnosis to the death or to last follow-up. An event-free survival (EFS) was calculated from the first day of treatment to relapse, progression or death, or to last date of follow-up. All the analyses were carried out using Statistica 10 and MedClac Version 12.6.1.0.
Results
Single-system disease (SS-LCH) and multi-system LCH (MS-LCH) had 25% (2/8) and 75% (6/8) of patients, resp. (p < 0.05).
In two cases, SS-LCH patients had bone involvement and one patient had pulmonary system involvement. The patient with primary LCH of lungs had a long-term history of smoking (> 10 years).
Two patients had MS-LCH with the involvement of “risk organs” such as central nervous system and bone marrow, there was only one patient with the involvement of “special sites”. In addition, we diagnosed two cases of MS-LCH without the involvement of “risk organs”. In our study, the patients older than 32 (50 and 55 years old) had MS-LCH with bone involvement, while patients younger than 24 (22 years old) had their pulmonary system involvement at diagnosis. A total of 62.5% patients received chemotherapy in combination with radiation therapy, and only 1/4 of patients (37.5%) received only chemotherapy, (p < 0.05). All patients received chemotherapy according to LCH-I protocol, six cycles on average (range 2–8).
The evaluation of response was done after the second or third cycle of chemotherapy. The response to treatment was achieved in 87.5% (7/8) of cases (Tab. 2.).
The maximum follow-up period in this group of patients was 96.72 months (median 44.92 months). A total of 87.5% (7/8) patients is still being followed up. During the follow-up period, there was one death registered due to the progression in LCH with pulmonary involvement.
There were 2/8 patients with early relapses (in 6 months), 2/8 patients with late relapses and 1/8 patient with refractory disease. In addition, one patient had transformed LCH into Hodgkin lymphoma, which was confirmed by immunohistochemical study.
Three-year EFS was 28.3%. It was impossible to calculate the OS due to the loss to follow-up of one patient. The analysis of data for presence of MAPK/ERK pathway gene mutations did not show any BRAF/KRAS/NRAS mutations in 50% (4/8) of patients.
We did not find any BRAF, KRAS or NRAS mutations in three patients with late relapses (in 15, 24 and 46 months). Notably, KRAS mutations were not revealed in any LCH samples.
A BRAF c.1799T>A, p. V600E mutation was present only in 2 out of 8 evaluated cases (25%):
- one patient with early relapse (in 6 months after the treatment);
- one patient with stable disease (Graph. 1 and 2)
1. Case 1 SS-LCH with the involvement of bone system, identified by qPCR, no BRAF V600E mutation found. 
SS-LCH – single sytem Langerhans cell histocystis, qPCR – quantitative polymerase chain reaction 2. Case 7 MS-LSH with the involvement of “special sites”, identified by qPCR, with a BRAF V600E mutation. 
MS-LCH – multisystem Langerhans cell histocystis, qPCR – quantitative polymerase chain reaction We also identified two patients with NRAS c. 182A>G, p. Q61R mutation:
- one patient had LCH transformed to Hodgkin’s lymphoma during 12 months after treatment LCH;
- one patient had a refractory disease (Graph 3 and 4.)
3. Case 2 MS-LCH, with the involvement of risk organ, identified by qPCR, no NRAS Q61R mutation found. 
MS-LCH – multisystem Langerhans cell histocystis, qPCR – quantitative polymerase chain reaction 4. Case 6 MS-LCH, without the involvement of risk organ, identifi ed by qPCR, with a NRAS Q61R mutation. 
MS-LCH – multisystem Langerhans cell histocystis, qPCR – quantitative polymerase chain reaction Due to a small number of patients in the research group, we decided to introduce TTR in months in order to be able to determine the impact of MAPK/EPK pathway mutation on relapse occurrence (Tab. 3).
3. TTR based on disease stratifi cation and BRAF/KRAS/NRAS gene mutations. 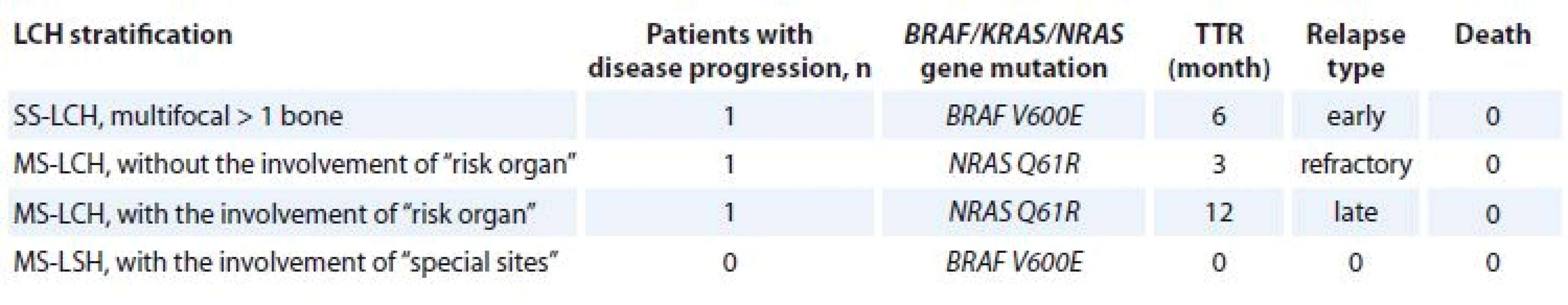
TTR – time to relaps, LCH – Langerhans cell histiocytosis, MS-LCH – multisystem LCH, SS-LCH – single system LCH We also analyzed correlation of disease response to the therapy, OS and EFS with age, sex, LCH stratification, number of cycles, response totherapy, radiation therapy, presence of gene mutations BRAF/KRAS/NRAS.
Unfortunately, due to a small number of patients, we did not identify a statistically significant difference of EFS in patients with or without BRAF V600E gene mutation. However, the comparison of patients with TTR with and without BRAF V600E gene mutation was significant, 13 vs. 28 months, resp.; p < 0.05.
In addition, TTR was 31.3 vs. 6.41 months in patients with the absence or presence of NRAS mutation, p < 0.05. Multivariate analysis confirmed that the presence of NRAS Q61R mutation has a significant association with shorter EFS in LCH patients with HR of 6.1 (95% CI 0.2–12.6; p = 0.008).
Discussion
LCH is a rare disease characterized by clonal proliferation of Langerhans cells [14]. Granuloma-like lesions of LCH have heterogeneous cellular composition. The etiology of Langerhans cell histiocytosis is largely unknown [15,16]. Recent studies have suggested that LCH is related to the clonal neoplastic proliferation of myeloid-derived precursor cells with a high frequency of somatic oncogenic BRAF V600E mutations in 25–60 % of LCH patients [17–20]. Since the follow-up time in our study for some patients was short, the relationship of BRAF V600E mutation to survival has not been sufficiently analyzed. Smoking is an important factor in primary LCH of lungs [20]; one patient with pulmonary disease had a long-term history of smoking in our study, which may be related to this form of LCH.
Symptoms, such as lytic bone lesions, exophthalmos, polyuria, hepatosplenomegaly, lymphadenopathy, skin rash, and hematological compromise, are most common in patients with LCH [21]. During our study, we most commonly came across such symptoms as lytic bone lesions (62.5%), lymphadenopathy (50%), polyuria (25%).
The overall 5-year survival rate of LCH patients is 88% [22]. Patients with unifocal LCH have an excellent prognosis and a high long-term survival rate (99%), may spontaneously recover or require minimal treatment [22]. In our cohort, one patient died because of pulmonary disease. The other seven patients are still alive, and further follow-up should be performed. There were no spontaneous recovery cases in our study.
The BRAF V600E mutation has been reported in 39–57% of Langerhans cell histiocytosis cases [17,19]. Although the pathophysiology of LCH remains unclear, activation of the MAPK/ERK signal pathway appears to play a significant role [23]. According to our data, BRAF gene mutation has been registered in only 25% of patients. The comparison of patients with TTR with and without BRAF V600E gene mutation was significant, 13 vs. 28 months, resp.; p < 0.05. Hypothetically, we can assume that the data analysis of a larger patient’s cohort would be able to confirm the relation of the response to treatment with or without the expression of BRAF V600E gene mutation. BRAF gene can be used as a prognostic marker for the assessment of patient’s response to treatment, receiving a standard chemotherapy for LCH as well as for patients participating in clinical trials.
The fact that some of LCH patients do not carry BRAF gene mutations prompts a possibility of other mutations that are partially or entirely related to ERK pathway and require further research. We did not find any BRAF, KRAS or NRAS mutations in three patients with late relapses.
Approximately 30% of LCH tumors have MAP2K1 mutations. Somatic MAP2K1 mutations have been identified in approximately 50% of BRAF– LCH samples [24]. In our study, there were also two BRAF patients who had a NRASQ61K/R somatic mutation. In addition, there are studies showing that the presence of concurrent BRAF V600E or NRASQ61K/R mutations was strongly associated with patient outcome [25]. Multivariate analysis in our cohort showed that presence NRASQ61K/R gene mutation has a significant association with poor clinical outcome in patients with LCH. Moreover, somatic mutations affecting other BRAF residues, as well as the ARAF gene, have recently been described [18,23].
The determination of the genetic mutation in LCH lesions has important implications for specific treatment. BRAF inhibitors, such as vemurafenib, have been successfully used to treat LCH patients with the V600E mutation [26]. The test for this mutation may play an important role for an individualized treatment. Additionally, a recent report on the successful use of BRAF protein inhibitor on refractory hairy cell leukemia [27] emphasized that LCH can potentially be treated with targeted therapy.
Conclusion
In summary, the presence or absence of one of MAPK/EPK pathway mutations, such as BRAF, NRAS or KRAS in a tumor, does not confirm or rule out a diagnosis of LCH. The results of these tests should be correlated with clinical findings and histopathologic features. Unfortunately, we did not find a strong significant impact of BRAFV600E gene mutation on clinical outcomes in patients with LCH in our study. However, our results provide evidence that NRASQ61K/R gene mutation can predict clinical outcome in patients with LCH and seem to be promising for the future studies.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Submitted: 29. 6. 2017
Accepted: 29. 12. 2017
Olga Novosad, MD, PhD
Oncohematology Department National Cancer Institute
Lomonosova str. 33/43
Kiev-03022 Ukraine
e-mail: novosad.o.ua@gmail.com
Sources
1. Lee JS, Ko GH, Kim HC et al. Langerhans cell sarcoma arising from Langerhans cell histiocytosis: a case report. J Korean Med Sci 2006; 21 (3): 577–580. doi: 10.3346/jkms.2006.21.3.577.
2. Arico M, Girschikofsky M, Généreau T et al. Langerhans cell histiocytosis in adults. report from the international registry of the histiocyte society. Eur J Cancer 2003; 39 (16): 2341–2348.
3. Kriachok IA, Skrypets TV, Novosad OI et al. Therapeutic approaches to the treatment of adult patients with Langerhans cell histiocytosis. J Clin Oncol Ukraine 2016; 1 (21): 58–62.
4. Broadbent V, Pritchard J, Davies EG et al. Spontaneous remission of multi-system histiocytosis X. Lancet 1984; 1 (8371): 253–254.
5. Coury F, Annels N, Rivollier A et al. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med 2008; 14 (1): 81–87. doi: 10.1038/nm1694.
6. Willman CL, Busque L, Griffith BB et al. Langerhan‘s-cell histiocytosis (histiocytosis X): a clonal proliferative disease. N Engl J Med 1994; 331 (3): 154–160. doi: 10.1056/NEJM199407213310303.
7. da Costa CE, Szuhai K, van Eijk R et al. No genomic aberrations in Langerhans cell histiocytosis as assessed by diverse molecular technologies. Genes Chromosomes Cancer 2009; 48 (3): 239–249. doi: 10.1002/gcc.20634.
8. Cantwell-Dorris ER, O’Leary JJ, Sheils OM. BRAFV600E: implications for carcinogenesis and molecular therapy. Mol Cancer Ther 2011; 10 (3): 385–394. doi: 10.1158/1535-7163.MCT-10-0799.
9. Davies H, Bignell GR, Cox C et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417 (6892): 949–954. doi: 10.1038/nature00766.
10. Novosad O, Kryachok I, Khranovska N et al. The MAPK/ERK pathway activation in patients with Langerhans Cell Histiocytosis. abstr. BMS-P-3. In: 18th Meeting of the European Association for Haematopathology – EAHP. Basel, 3.–8. September 2016.
11. Badalian-Very G, Vergilio JA, Degar BA. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 2010; 116 (11): 1919–1923. doi: 10.1182/blood-2010-04-279 083.
12. Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program 2015; 2015 : 565–570. doi: 10.1182/asheducation-2015.1.565.
13. Lang AL, Drexel H, Geller-Rhomberg S et al. Optimized allele-specific real-time PCR assays for the detection of common mutations in KRAS and BRAF. J Mol Diagn 2011; 13 (1): 23–28. doi: 10.1016/j.jmoldx.2010.11.007.
14. Allen CE, Li L, Peters TL et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol 2010; 184 (8): 4557–4567. doi: 10.4049/jimmunol.0902336.
15. Jaffe R, Weiss LM, Facchetti F. Tumours derived from Langerhans cells. In: Swerdlow SH, Campo E, Harris NL (eds). WHO classification of tumours the haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008 : 358–360.
16. Paulus W, Perry A. Histiocytic tumours. In: Louis DN, Ohgaki H, Wiestler OD (eds). WHO classification of tumours the central nervous system. Lyon: IARC Press; 2007 : 193–196.
17. Badalian-Very G, Vergilio JA, Degar BA et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood 2010; 116 (11): 1919–1923. doi: 10.1182/blood-2010-04-279083.
18. Satoh T, Smith A, Sarde A et al. B-RAF mutant alleles associated with Langerhans cell histiocytosis, a granulomatous pediatric disease. PLoS One 2012; 7 (4): e33891. doi: 10.1371/journal.pone.0033891.
19. Sahm F, Capper D, Preusser M et al. BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood 2012; 120 (12): e28–e34. doi: 10.1182/blood-2012-06-429597.
20. Roden AC, Hu X, Kip S et al. BRAF V600E expression in Langerhans cell histiocytosis: clinical and immunohistochemical study on 25 pulmonary and 54 extrapulmonary cases. Am J Surg Pathol 2014; 38 (4): 548–551. doi: 10.1097/PAS.0000000000000129.
21. Badalian-Very G, Vergilio JA, Fleming M et al. Pathogenesis of Langerhans cell histiocytosis. Annu Rev Pathol 2013; 8 : 1–20. doi: 10.1146/annurev-pathol-020712-163959.
22. Grana N. Langerhans cell histiocytosis. Cancer Control 2014; 21 (4): 328–334. doi: 10.1177/107327481402100409.
23. Nelson DS, van Halteren A, Quispel WT et al. MAP2K1 and MAP3K1 mutations in Langerhans cell histiocytosis. Genes Chromosomes Cancer 2015; 54 (6): 361–368. doi: 10.1002/gcc.22247.
24. Brown, NA, Furtado LV, Betz BL et al. High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood 2014; 124 (10): 1655–1658. doi: 10.1182/blood-2014-05-577361.
25. Mourah S, How-Kit A, Meignin V et al. Recurrent NRAS mutations in pulmonary Langerhans cell histiocytosis. Eur Respir J 2016; 47 (6): 1785–1796. doi: 10.1183/13993003.01677-2015.
26. Haroche J, Cohen-Aubart F, Emile JF et al. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood 2013; 121 (9): 1495–1500. doi: 10.1182/blood-2012-07-446286.
27. Dietrich S, Glimm H, Andrulis M et al. BRAF inhibition in refractory hairy-cell leukemia. N Engl J Med 2012; 366 (21): 2038–2040. doi: 10.1056/NEJMc1202124.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2018 Issue 2-
All articles in this issue
- Human Papillomavirus – Role in Cervical Carcinogenesis and Methods of Detection
- Anogenital HPV Infection as the Potential Risk Factor for Oropharyngeal Carcinoma
- Treatment of Metastatic Renal Cell Carcinoma
- New Approaches for Chemosensitivity Testing in Malignant Diseases
- Quality of Life After High-dose Brachytherapy in Patients with Early Oral Carcinoma
- MAPK/ERK signal pathway alterations in patients with Langerhans Cell Histiocytosis
- Recent Trends in Survival of Testicular Cancer Patients – Nation-wide Population Based Study
- Cutaneous and Subcutaneous Metastases of Adenocarcinoma as a Dominant Clinical Manifestation of Malignancy of Unknown Origin – a Case Report
- Diagnostic, Prognostic and Predictive Immunohistochemistry in Malignant Melanoma of the Skin
- Long Non-coding RNAs as Regulators of the Mitogen-activated Protein Kinase (MAPK) Pathway in Cancer
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Cutaneous and Subcutaneous Metastases of Adenocarcinoma as a Dominant Clinical Manifestation of Malignancy of Unknown Origin – a Case Report
- MAPK/ERK signal pathway alterations in patients with Langerhans Cell Histiocytosis
- Long Non-coding RNAs as Regulators of the Mitogen-activated Protein Kinase (MAPK) Pathway in Cancer
- Human Papillomavirus – Role in Cervical Carcinogenesis and Methods of Detection
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career


