-
Medical journals
- Career
Predicting Vitality Change in Older Breast Cancer Survivors after Primary Treatment – an Approach Based on Using Time-related Difference of Pro-inflammatory Marker C-reactive Protein
Authors: K. Skrivanova 1; L. Anderkova 1,2; D. Brancikova 3; Jiří Jarkovský 4; K. Benesova 4; N. Elfmarkova 1,2; T. Sverak 1,2; M. Bendová 5; H. Peterkova 1; J. Nedvěd 6; M. Protivánková 5; L. Minar 5; E. Holoubková 1; L. Dusek 4
Authors‘ workplace: Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Applied Neuroscience Research Group, CEITEC – Central European Institute of Technology, Masaryk University, Brno, Czech Republic 2; Department of Internal Medicine, Hematology and Oncology, University Hospital Brno and Masaryk University, School of Medicine, Brno, Czech Republic 3; Institute of Biostatistics and Analyses, Faculty of Medicine, Masaryk University, Brno, Czech Republic 4; Clinic of Obstetrics and Gynecology, University Hospital Brno and Masaryk University, School of Medicine, Brno, Czech Republic 5; Clinical psychology surgery of PhDr. Dagmar Konecna s. r. o, Zahreb na Morave, Czech Republic 6
Published in: Klin Onkol 2016; 29(1): 52-58
Category: Original Articles
doi: https://doi.org/10.14735/amko201652Overview
Backround:
We aimed to determine prognosis of vitality change and functional status of breast cancer survivors after primary oncological treatment using time-related differences of elevated levels of highly sensitive proinflammatory C-reactive protein (CRP).Patients and Methods:
The test group consisted of 46 elderly breast cancer survivors (median age was 65 years) who completed Vitality Scale of Short Form 36 (SF-36) after completing treatment and another retrospectively at diagnosis. Data on tumor-related factors, treatment, and outcomes were obtained retrospectively from medical records, and linear regression analysis was performed. CRP was followed at diagnosis and one year after primary treatment. Within the scope of this study, clinically important difference in the Vitality Scale was set at five points of change.Results:
Results showed a statistically significant relationship between CRP change and vitality component of SF-36 change (rs = – 0.350, p = 0.023) in which a decrease in CRP inversely correlated with the quality of life component. The overall change was 1.078 of the vitality scale score (approximately 1 point) for each 1 unit decrease of CRP (1 mg/ L). Association of CRP levels (before and after treatment, its difference between these time points) with age, number of comorbidities and stage of the disease was analyzed and no statistically significant relationship was found in our study.Conclusion:
Preliminary results suggested time-related differences in elevated CRP levels as a potentially suitable predictor for change in vitality status for long term, chronic condition for older breast cancer survivors. We suggest the interpretation schema including an understanding that CRP change of 5 mg/ L and more should be considered a potential risk factor for subsequent negative clinical outcomes.Key words:
breast cancer – vitality change prediction – linear regression model – functional status – C-reactive protein – risk factorIntroduction
Inflammation has been linked to a spectrum of conditions associated with ageing, including cardiovascular disease, osteoporosis, arthritis, type II diabetes, certain cancers, Alzheimer’s disease, frailty and functional decline, and periodontal disease [1]. Pro-inflam-matory cytokines, such as interleukin 6 (IL-6) are reliable predictors of quality of life (QOL), morbidity, and even mortality [2]. Inflammation is a key pathologic mechanism underlying heart disease, and elevated levels of biomarkers, such as C-reactive protein (CRP) and IL-6 are early indicators of clinical events, such as myocardial infarction or coronary death [3]. This issue is summarized in the review of Miller et al. [4]. Inflammation also plays an important role in carcinogenesis and tumor progression [5,6]. Now, it appears that its systemic manifestations can provide a valuable biomarker for prognosis and oncology treatment stratification [7]. Recently, the systemic inflammatory response, as evidenced by elevated circulating concentrations of CRP and/ or hypoalbuminaemia, has been shown to be independently associated with poorer survival in breast cancer patients [8 – 13]. A series of studies shed new light on how inflammation variables impact breast cancer prognosis prediction [14,15].
Fatigue is one of the most common adverse effects of cancer, and can persist for years after treatment completion in otherwise healthy survivors. Cancer-related fatigue (CRF) disrupts all aspects of QOL and might be a risk factor of reduced survival. Inflammation seems to play a key role in fatigue before, during, and after cancer treatment. This issue is summarized in the review of Bower et al. [16]. The relationship between inflammation and fatigue among cancer survivors showed reduced fatigue among patients with advanced lung cancer after supplementation with three polyunsaturated fatty acids (PUFAs) [17]. Alfano et al. [18] used vitality (VT) scale Short Form 36 (SF-36) to investigate relationships between inflammation and intake of both omega-3 and omega-6 PUFAs among breast cancer survivors. The results of this study link higher intake of omega-3 PUFAs, decreased inflammation, and decreased physical aspects of fatigue. Bower et al. [19] examined relationships between psychological states (anxiety, depression) and inflammatory proces-ses. Results of this cross-sectional study indicate a relationship between tumor necrosis factor (TNF) and the level of tiredness of breast cancer patients after chemotherapy. Courtier et al. [20] characterized fatigue in early-stage breast cancer patients during radiotherapy, and examined its association with IL-6. In a prospective study of breast cancer patients, persistent CRF was predicted by tumor size but not demographic, psychological, surgical, or hematologic parameters. CRF was associated with significant disability and health care utilization [21].
We examined status of vitality after primary treatment, which should be directly connected with cancer-related fatigue, with a focus on chronic conditions. To our knowledge, this is the first study to examine time-related changes in CRP levels as an independent predictor of VT change for chronic conditions in breast cancer survivors. We hypothesized that the differences of CRP levels at the time of diagnosis and 1 year after the completion of primary treatment were good long-term predictors of VT changes: decrease in CRP would be connected with increase of vitality. Potential physiologic mechanisms for prognostic effect were not investigated.
Patients and methods
Population assembly
For this study, 172 women diagnosed with breast cancer were enrolled at participating University Hospital Brno, Czech Republic, between May 2012 and May 2015. They represent a group of women recruited during four years from a larger cohort study examining the prognostic effect of a number of life-style related factors [22]. The eligible criteria for the patient group were breast cancer diagnosis, age 30 – 90, Czech language proficiency, and completion of primary oncological treatment. Women were excluded if they were unable to speak Czech. Women provided informed consent to participate in this study as approved by Ethics Committee at University Hospital Brno. The refusal rate was less than 1%.
Measurement
Women completed self-assessment questionnaires after breast cancer treatment. Initial semi-structured interviews were conducted by psychologists and were aimed at collecting socio-demographic data as well as promoting good personal contact with patients. Each interview took about 20 min. The study was designed to be retrospective, and patients were asked to fill in the questionnaires two times: first to answer the questions based on their current situation and second to answer the same questions retrospectively, i.e. what was their situation like prior to treatment. The interview were designed to collect socio-demographic characteristics and information of known breast cancer risk factors, such as age, BMI, number of children, smoking and alcohol abuse, age at the birth of first child, breast feeding, age at menarche and menopause, level of education, lifestyle, and marital status.
Vitality change measurement
Vitality was assessed using the VT subscale of Short Form-36 (SF-36) [23]. SF-36 is a survey constructed to study health-related QOL and consists of eight health concepts: physical functioning, role limitations caused by physical health problems, role limitations caused by emotional problems, social functioning, emotional well-being, vitality/ fatigue, pain, and general health perceptions. A Czech version of the SF-36 survey was used for the purpose of our research; this translation was validated, confirming that the Czech version of SF-36 is as effective as the original US version [24]. The SF-36 Vitality subscale is a valid and reliable four-item measure of vitality (energy)-fatigue supported by both observational and experimental data [25]. Moreover, the SF-36 vitality subscale is one of the most frequently used measures of energy (vitality) - -fatigue in cancer survivors [18,26]. The standardized scores range from 0 to 100, higher scores reflecting higher vitality, i.e. less severe fatigue [27].
Tumor-related measures and variables
Data on tumor-related factors and treatment were obtained retrospectively from medical records. Case history data contained information on the presence of comorbidities, including depression. Disease and treatment data contained information about the stage of disease (I – IV), type of treatment (surgery, chemotherapy, radiotherapy, biological therapy, and hormone therapy), progression, relapse, and treatment response (complete, partial, stable, and progressive). CRP was measured by surgeons over two separate time periods (at diagnosis and one year after primary treatment completion), but the second time point measure was conducted primarily in women with postoperative inflammation.
CRP was chosen as an inflammatory marker because it responds to physical activity and weight changes [28], is more stable than cytokines [18], and is being investigated in The Health, Eating, Activity, and Lifestyle Study (HEAL) as a mechanism that links lifestyle factors to survival [29]. CRP was measured by a high-sensitivity assay using Cobas Integra 400 plus with an interassay variation of 0 – 5 mg/ L.
Statistical analyses
Standard descriptive statistics were applied in the analysis; absolute and relative frequencies for categorical variables and mean with standard deviation or median with minimum-maximum range for continuous variables and scores. Statistical significance of differences among groups of patients was tested using the Fisher exact test for categorical variables, and the Mann-Whitney U test or the Kruskal Wallis test for continuous variables and scores. Statistical significance of time-related changes of continuous variables and scores was tested using the Wilcoxon paired signed-rank test. The significance of correlation between continuous variables was tested by the Spearman rank correlation coefficient. Quantification of the relationship between change in CRP and QOL as a dependent variable was estimated using a linear regression model. Statistical analysis was computed using SPSS 22.0.0.1 (IBM Corporation, 2014).
Results
Characteristics of the study population
The median age was 65 years, median BMI 28, 91% of women had children and 83% were postmenopausal at the time. The primary stage of the disease was 0 – I in 26%, II – III in 59% and IV in 15% of patients; surgery treatment was applied in 94%, chemotherapy in 67%, radiotherapy in 78%, biological and hormonal therapy in 22% and 80% of patients, resp. The median CRP at diagnosis was 5.0 mg/ L, after treatment 3.2 mg/ L; nevertheless, the change in CRP was not statistically significant (Wilcoxon paired test; p = 0.544) and patients’ change in CRP was both positive and negative (Graph 1). Detailed description of patients’ characteristics is presented in Tab. 1. Follow-up questionnaires were completed by 107 women. Completed medical records with both CRP time-related information were collected from 109 women. Sixty-six women completed both forms of SF-36 questionnaires, and their CRP information was available. Four patients were excluded for missing information about the length of follow-up, and 16 women were excluded for inconsistent length of CRP follow-up. Ultimately, the dataset consisted of 46 women with available data on CRP and SF-36 at diagnosis and one year after primary treatment completion; the median of follow-up time was 45 months.
1. Basic characteristics. 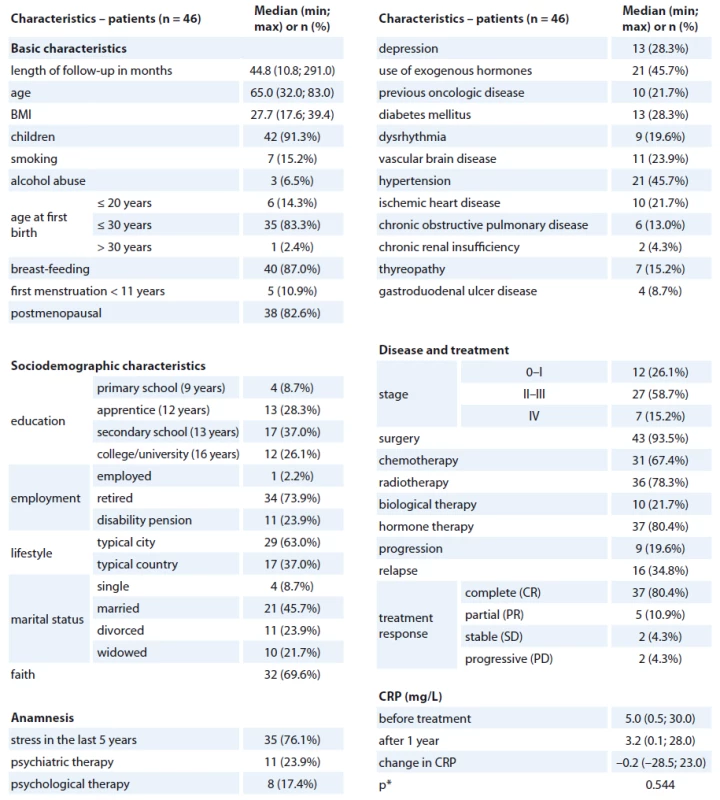
*p value of paired Wilcoxon test for the change in CRP level 1. The level of CRP (mg/L) before primary treatment and after one year. 
Prognostic association of CRP, and CRP time-related difference
The association of the CRP levels (before and after primary treatment, its difference between these time points) with age, number of comorbidities and stage of the disease was analyzed and no statistically significant relationship was found (Tab. 2). Prognostic associations for the SF-36 questionnaire are listed in Tab. 3. The relationship between the change in CRP and the change of QOL components of SF-36 (at baseline and follow-up) is shown; the only statistically significant relationship was found for CRP change and Vitality scale component of SF-36 change (rs = – 0.350, p = 0.023), in which a decrease in CRP was related to an increase in QOL component (Graph 2). The change was 1.078 of QOL score (1 point) for each 1 unit decrease of CRP (1 mg/ L) (Graph 2). Evidence of significant prognostic association of a CRP time-related difference (at diagnosis and one year after treatment) was observed. The associations that were significant at a p < 0.05 included Vitality (SF-36) change in Vitality Scale. The overall p value was 0.004, suggesting that a decrease in CRP was statistically significantly related to an increase in vitality, consistent with our hypothesis.
2. The relationship between CRP level (mg/L) and basic characteristics. 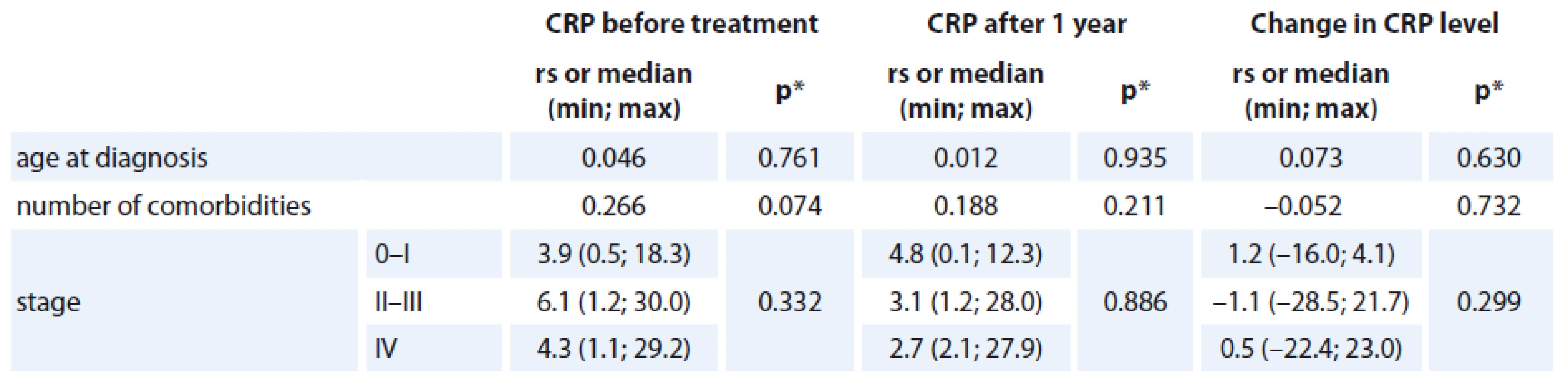
rs = Spearman‘s correlation coefficient *p value of the significance of Spearman‘s correlation coefficient or p value of Kruskal-Wallis test 2. The correlation between the change in CRP level (mg/L) and the change in VT score. 
3. The correlation between change in CRP level (mg/L) and changes in QOL subscales (n = 46). 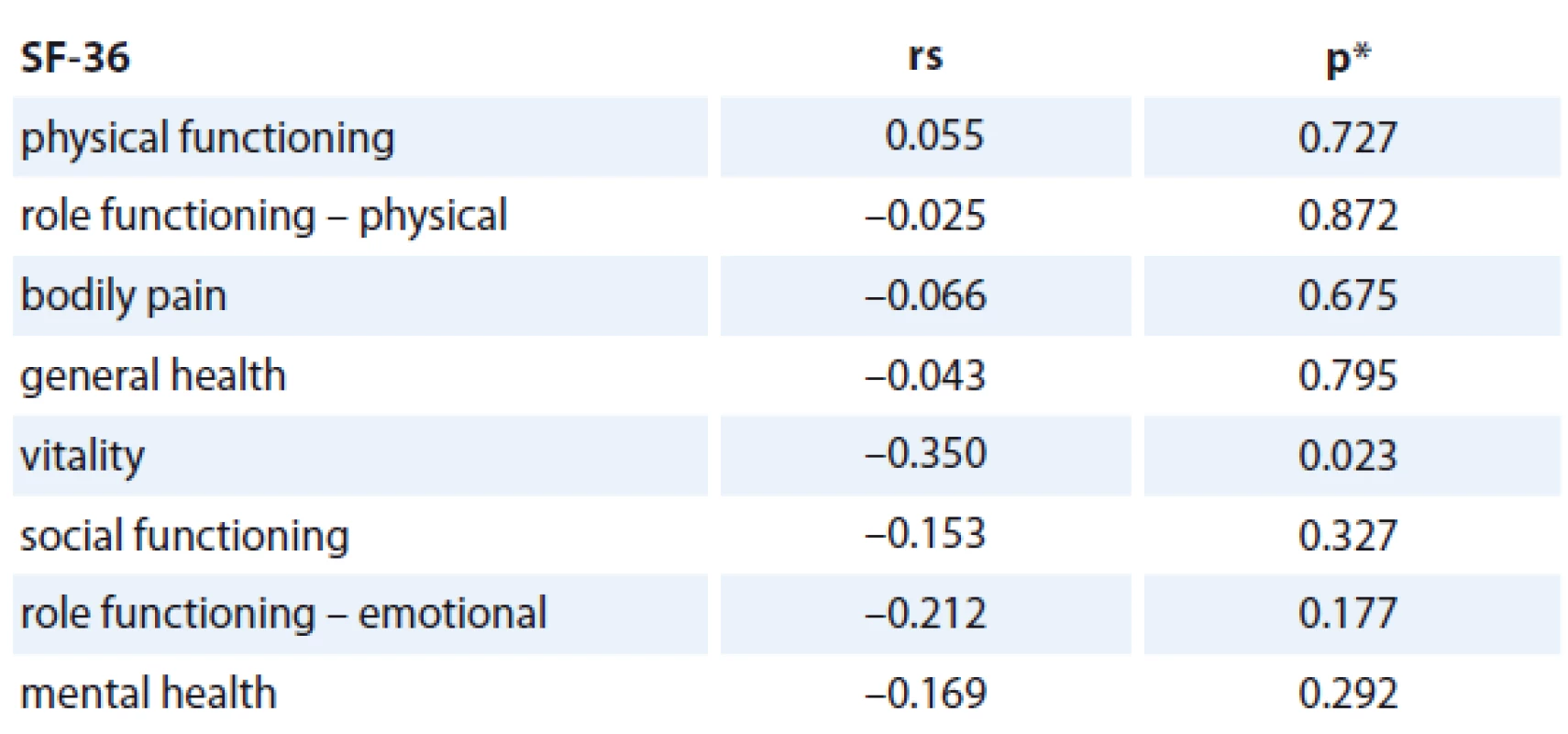
rs = Spearman‘s correlation coefficient *p value of the significance of Spearman‘s correlation coefficient Interpretation of our results
Results of our prognostic analyses show that CRP time-related change was asso-ciated with a change in Vitality status after treatment measured by SF-36 (change before and after treatment). We suggest the interpretation frame described by Bjorner et al. [30], where 5 points in the Vitality scale is connected with a significant decrease in functional status in older adults. We suggest a cut--off point of five units of CRP change – increase (mg/ L) as a risk factor. The change was calculated to be 1.078 of QOL score (1 point) for each 1-unit decrease of CRP (1 mg/ L) for a significant decrease in vitality in breast cancer patients after treatment completion, which could lead to a clinically observed decrease in functional status. In our research, six women (three retired, three on disability pension) fell within this risk group of patients (change in CRP ≥ 5 mg/ L) at risk of a decline in functional status after primary treatment completion. Limitation of this statistical analysis is a relatively low sample size resulting in low statistical power of tests and problematic testing of potential confounders in stratified analysis or the multivariate model. In addition to analysis of vitality as a continuous variable, we adopted a binary vitality endpoint as well. The advantage of binary endpoints is their straightforward interpretation in clinical practice for risk stratification of patients using defined cut-offs.
Discussion
We believe that changes in vitality status are associated with changes of CRP levels at diagnosis and one year after treatment completion in older breast cancer survivors. As discussed earlier, we have not examined any physiological mechanisms in our research, nor have we measured dynamics of change of vitality as a psychological, subjectively experienced attribute and its potential somatic, biologic correlate CRP. Nowakovski et al. [31] and Cho et al. [32] identified CRP levels as a significant predictor of QOL/ fatigue with chronic inflammation in older adults. Their studies review this issue in the biopsychosocial context and suggest CRP measures as a potentially convenient indicator for the QOL status/ fatigue in such an age group. Chronic inflam-mation predicts significantly lower odds of reporting high QOL on both emotional and relational measures. Social structural factors do not confound these associations [31].
We chose to measure CRP at diagnosis and after treatment for its potential to assess long-term effect of cancer and its treatment. Acute post-operative CRP which is influenced by surgical trauma was not added to the linear regression model as a vitality predictor. The association of the CRP levels (before and after treatment, its difference between these time points) with age, number of comorbidities and stage of the disease was analyzed, and no statistically significant relationship was found in our study. CRP at diagnosis has been shown to be independently associated with poorer survival in breast cancer patients [13]. Postoperative CRP was dependent on age, comorbidity and stage of disease [33].
The interpretation schema includes results of linear regression analysis (the change was 1.078 of QOL score (1 point) for each 1-unit decrease of CRP (1 mg/ L)). Bjorner et al. [30] recommend 5 points as a minimally important difference (MID) for analyses of groups with VT scores below average for risk of negative outcome (decline in functional status). Analyses were performed on data from the Medical Outcomes Study (n = 3,445). The first analyses regressed VT scores (on a scale of 0 – 100) on chronic conditions that cause fatigue in order to determine the impact of each condition on VT. The second set of analyses examined the relationship between baseline VT scores and other outcomes at baseline, 1-year, and 7-year follow-ups. VT decrements of 5 – 10 points were seen for diseases known to cause fatigue. Furthermore, differences of 5 – 10 points in the VT score were associated with significant increased risk of negative outcomes. They recommend MID of 5 points for analyses of groups with VT scores that are below average. For follow-up of individual patients, they recommend a 10-point difference as important [30]. As mentioned above, at least a 5-point cut-off was recommended for diseases causing fatigue, which includes cancer. We suggest a cut-off point of 5 units of CRP change – increase (mg/ L) as a risk factor for a significant decrease in vitality in breast cancer patients after treatment completion, which could lead to a clinically observed decrease in functional status. Functional status of older cancer patients is an important clinical outcome and was previously examined [34].
According to our time-dynamic, focused schema, change in CRP levels over time could represent a more suitable long-term predictor of possible negative clinical outcomes connected with vitality changes in breast cancer survivors than CRP levels obtained as a stationary variable (at diagnose or other time during the treatment). These suggested paradigms reflect an individual’s capability to deal with disease and its treatment, connected with a subjective evaluation of vita-lity change/ fatigue. We see this as essential for understanding individual experiences of cancer survivors with disease and its treatment in relation to cancer-related fatigue/ vitality. This is the main strength of our study.
Limitations
Our observations should not be generalized to other aspects of QOL in women with breast cancer and should not be generalized to other cancer types without replication. The patients we studied represent a relatively heterogeneous group of patients (healthy survivor versus relapsed); median age of these patients was 65. Retrospective design of QOL-related part of the study represents another limiting factor of the study. Due to a small sample size, the study has a low statistical power, and reported results of our study might be considered only preliminary; further investigation is needed in order to confirm them. Moreover, CRP data were obtained from medical records. If performed, these analyses should be viewed as a means of generating a hypothesis.
Conclusion
In conclusion, we have found indications that changes in vitality status have an important association with CRP level changes between diagnosis and one year after primary treatment completion in older breast cancer survivors. We suggest a change of CRP 5 mg/ L and more as a potential risk factor for later negative clinical outcomes.
Acknowledgments
We deeply thank our participants for their commitment to our research project.
This study was supported by The Czech Science Foundation as the Project P407/12/0607.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
RNDr. Jiri Jarkovsky, PhD.
Institute of Biostatistics and Analyses
Faculty of Medicine
Masaryk University
Kamenice 3, 625 00 Brno
Czech Republic
e-mail: jarkovsky@iba.muni.cz
Submitted: 31. 8. 2015
Accepted: 7. 12. 2015
Sources
1. Kiecolt-Glaser JK, McGuire L, Robles TF et al. Emotions, morbidity and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol 2002; 53 : 83 – 107.
2. Kiecolt-Glaser JK, Preacher KJ, Mac Callum RC et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A 2003; 100(15): 9090 – 9095.
3. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 2005; 111(25): 3481 – 3488.
4. Miller G, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annu Rev Psychol 2009; 60 : 501 – 524. doi: 10.1146/ annurev.psych.60.110707.163551.
5. Colotta F, Allavena P, Sica A et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenisis 2009; 30(7): 1073 – 1081. doi: 10.1093/ carcin/ bgp127.
6. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1): 57 – 70.
7. Stotz M, Gerger A, Eisner F et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 2013; 109(2): 416 – 421. doi: 10.1038/ bjc.2013.332.
8. Albuquerque KV, Price MR, Badley RA et al. Pre-treatment serum levels of tumour markers in metastatic breast cancer: a prospective assessment of their role in predicting response to therapy and survival. Eur J Surg Oncol 1995; 21(5): 504 – 509.
9. Zhang G, Adachi I. Serum interleukin-6 levels correlate to tumour progression and prognosis in metastatic breast carcinoma. Anticancer Res 1999; 19(2B): 1427 – 1432.
10. Al Murri AM, Bartlett JM, Canney PA et al. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer 2006; 94(2): 227 – 230.
11. Al Murri AM, Wilson C, Lannigan A et al. Evaluation of the relationship between the systemic inflammatory response and cancer specific survival in patients with primary operable breast cancer. Br J Cancer 2007; 96(6): 891 – 895.
12. Al Murri AM, Hilmy M, Bell J et al. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br J Cancer 2008; 99(7): 1013 – 1019. doi: 10.1038/ sj.bjc.6604667.
13. Allin KH, Nordestgaard GB, Flyger H et al. Elevated pre-treatment levels of C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res 2011; 13(3): R55. doi: 10.1186/ bcr2891.
14. Gnant M, Pfeiler G, Stöger H et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: an analysis of the randomized ABCSG-6a trial. Br J Cancer 2013; 109 : 589 – 596. doi: 10.1038/ bjc.2013.367.
15. Syed BM, Green AR, Paish EC et al. Biology of primary breast cancer in older women treated by surgery: with correlation with long-term clinical outcome and comparison with their younger counterparts. Br J Cancer 2013; 108(5): 1042 – 1051. doi: 10.1038/ bjc.2012.601.
16. Bower JE. Cancer-related fatigue-mechanism, risk factors, and treatments. Nat Rev Clin Oncol 2014; 11(10): 597 – 609. doi: 10.1038/ nrclinonc.2014.127.
17. Cerchietti LC, Navigante AH, Castro MA. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr Cancer 2007; 59(1): 14 – 20.
18. Alfano CM, Imayama I, Neuhouser LM et al. Fatigue, inflammation, and ω-3 and ω-6 fatty acid intake among breast cancer survivors. J Clin Oncol 2012; 30(12): 1280 – 1287. doi: 10.1200/ JCO.2011.36.4109.
19. Bower JE, Ganz PA, Irwin MR et al. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol 2012; 29(26): 3517 – 3522. doi: 10.1200/ JCO.2011.36.1154.
20. Courtier N, Gambling T, Enright S et al. Psychological and immunological characteristic of fatigued women undergoing radiotherapy for early-stage breast cancer. Support Care Cancer 2013; 21(1): 173 – 181. doi: 10.1007/ s00520-012-1508-6.
21. Goldstein D, Bennett BK, Webber K et al. Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol 2012; 30(15): 1805 – 1812. doi: 10.1200/ JCO.2011.34.6148.
22. Skřivanová K, Brančíková D, Bendová M et al. Evaluating disease and patient characteristics which predict (retrospectively) changes in quality of life after breast cancer diagnosis and treatment. Psycho-Oncology 2014; 23 (Suppl 3): 396 – 397. doi:10.1111/ j.1099-1611.2014.3697.
23. Ware JE. Sherbourne: the mos 36-item short-form health survey (SF-36) 1. Conceptual-framework and item selection. Med Care 1992; 30(6): 473 – 483.
24. Sobotik Z. Our experience with the use of the preliminary Czech version of the US questionnaire on health (SF-36). Zdravotnictvi v Ceske Republice 1998; 1 : 50 – 54.
25. O’Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res 2004; 57(5): 435 – 441.
26. Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: a controlled comparison. J Behav Med 1998; 21(1): 1 – 18.
27. Ware JE. SF-36 Health Survey: Manual and Interpretation Guide. Boston: the Health Institute, New England Medical Center: 1993.
28. Campbell PT, Campbell KL, Wener MH et al. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med Sci Sports Exerc 2009; 41(8): 1533 – 1539.
29. Meeske K, Wilder-Smith A, Alfano CM et al. Fatigue in breast cancer survivors two to five years post-diag-nosis: a heal study report. Qual Life Res 2007; 16(6): 947 – 960.
30. Bjorner JB, Wallenstein GV, Martin MC et al. Interpreting score differencies in the SF-36 Vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Res Med Opin 2007; 23(4): 731 – 739.
31. Nowakovski AC. Chronic inflammation and quality of life in older adults: a cross-sectional study using biomarkers to predict emotional and relational outcomes. Health Qual Life Outcomes 2014; 12 : 141. doi: 10.1186/ s12955-014-0141-0.
32. Cho HJ, Kivimaki M, Bower JE et al. Association of C-reactive protein and Interleukin 6 with new onset fatigue in the Whitehall II prospective cohort study. Psychol Med 2013; 43(8): 1773 – 1783. doi: 10.1017/ S0033291712002437.
33. Tibau A, Ennis M, Goodwin P. Post-surgical highly sensitive C-reactive protein and prognosis in early-stage breast cancer. Breast Cancer Res Treat 2013; 141(3): 485 – 489. doi: 10.1007/ s10549-013-2694-8.
34. Extermann M, Overcash J, Lyman GH et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 1998; 16(4): 1582 – 1587.
Labels
Paediatric clinical oncology Surgery Clinical oncology
Article was published inClinical Oncology

2016 Issue 1-
All articles in this issue
- Genomic Tests as Predictors of Breast Cancer Patients’ Prognosis
- Potential of Long Non- coding RNA Molecules in Diagnosis of Tumors
- Survival Analysis Three-year Follow-up of Pacients with Head and Neck Cancer
- Successful Therapy of Czech Patients with ROS1 Translocation by Crizotinib
- The Role of Heat Shock Proteins in Leukemia
- Predicting Vitality Change in Older Breast Cancer Survivors after Primary Treatment – an Approach Based on Using Time-related Difference of Pro-inflammatory Marker C-reactive Protein
- Successful Associating Liver Partition and Portal Vein Ligation after Unsuccessful Double TACE Procedure Complicated with Sepsis and Pancreatitis
- Clinical Oncology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Survival Analysis Three-year Follow-up of Pacients with Head and Neck Cancer
- Genomic Tests as Predictors of Breast Cancer Patients’ Prognosis
- The Role of Heat Shock Proteins in Leukemia
- Potential of Long Non- coding RNA Molecules in Diagnosis of Tumors
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

