-
Medical journals
- Career
OLEOGEL-S10 TO ACCELERATE HEALING OF DONOR SITES: MONOCENTRIC RESULTS OF PHASE III CLINICAL TRIAL
Authors: Lipový B.a; B; Fiamoli M.a; Mager R.a; Jelínková Z.a; B; Jarkovský J.c; Chaloupková Z.a; Holoubek J.a; Suchánek I.a; Brychta P.a; B
Authors‘ workplace: bFaculty of Medicine, Masaryk University, Brno, Czech Republic ; cInstitute of Biostatistics and Analyses, Masaryk University, Brno, Czech Republic ; aDepartment of Burns and Reconstructive Surgery, University Hospital Brno, Czech Republic
Published in: ACTA CHIRURGIAE PLASTICAE, 59, 3-4, 2017, pp. 129-134
INTRODUCTION
During the recent years was observed substantial progress in the quality of care provided to burn patients. Such progress concerns antimicrobials and systemic therapy, but in particular wound management. For burn patients whose therapy includes split-thickness skin grafting, wound management concerns both burn and donor sites.1 Particularly in patients with critical burns, donor sites are often lacking and therefore any possibility to accelerate epithelialization and to minimize scarification is beneficial for both patients and personnel providing treatment.2
The time period for donor site healing is one of the most important factors responsible for duration of hospitalization. For patients with critical burns, this means shortening the time of donor site epithelialization, shortening the interval between two harvests from a single site, and earlier closure of large areas, from which necrotic skin has been removed.
If no complications occur, the meantime period for donor site healing is around 2–3 weeks. Infectious complications in particular interfere with the transition of healing phases and prolong healing. Healing with complications is also subject to increased risk of developing hypertrophic scars.3
MATERIAL AND METHODS
Study design and treatment
The project was officially entitled Open, Blindly Evaluated, Prospective, Controlled, Randomized, Multicenter Phase III Clinical Trial to Compare Intra-individually the Efficacy and Tolerance of Oleogel-S10 versus Standard of Care in Accelerating the Wound Healing of Split-Thickness Skin Graft Donor Sites. This phase monitored a total of 105 patients from various European centres.4 32 patients (30.5%) from these were included in prospective monitoring at the Department of Burns and Reconstructive Surgery, University Hospital Brno. This article focuses on the results of the treatment phase and 1 year of follow-up. This experiment was carried out with the approval of the Ethics committee of the University Hospital Brno provided on 27 June 2012. All patients provided their informed consent prior to enrolment to the study. The entire project was carried out while respecting the Principles of Good Clinical Practice (GCP).
The primary endpoint was intra-individual difference in time to wound closure (at least 95% epithelialization) between wound halves either treated with Oleogel-S10 and non-adhesive wound dressing or treated with non-adhesive wound dressing alone, based on blinded photo evaluation by three independent blinded experts.
Study protocol
Following split-thickness skin graft (STSG) collection (Zimmer® Air Dermatome) was the donor site photographed and a computer and the internet were used to split the site randomly into two halves. An STSG was harvested from the thigh or buttocks of each patient at uniform thickness of 0.20 mm. The first half was designated as verum and was treated with both Oleogel-S10 and a Mepilex non-adhesive wound dressing (Mölnlycke Health Care, Norcross, GA, USA), and the other half was treated following standard of care (SOC) with Mepilex only without any ointment. At each change of dressing was taken photographic documentation and the wound’s epithelialization extent was assessed. Swabs for microbiological monitoring were also collected. The wound was handled in this manner until it healed completely (at least 95% epithelialization). This was followed by monitoring at 3 and 12 months following STSG collection.
Assessment
Assessments of effectiveness (time to wound closure) and cosmetic effect were carried out for the two compared sites at the end of treatment (EOT), a 3-month follow-up (3m-FU), and a 12-month follow-up (12m-FU). To achieve optimal assessments, we used the Patient and Observer Scar Assessment Scale (POSAS). Laser speckle contrast analysis (LASCA) was carried out at all monitoring phases and the skin’s biomechanical properties were analysed after 12 months. Viscoelastic parameters were analysed. The R0–R9, F0, F1, and Q0–Q3 parameters were monitored. Table 1 shows the most important R, F and Q parameters and their meanings. For more objective results, only one physicians measured cutometric and perfusion parameters. The same physician was the observer in POSAS.
1. Examined R parameters for patients in the set 
POSAS is currently one of the most frequently used assessment scales.5 Due to its universality and high objectivity it is used for many scars arising from various types of wounds. This scale is divided into two parts. The observer scale comprises six indicators (vascularity, pigmentation, thickness, relief, pliability, and surface area). Patients respond to seven questions concerning such parameters as pain, the irregularity of the scar, and colour differences. Each indicator and question is scored on a scale from1 to 10, with 1 representing the best result and 10 the worst.
LASCA (laser speckle contrast analysis) is a very sensitive method used to monitor differences in blood perfusion.6 It is a non-invasive method where by a laser beam is used to detect blood perfusion in the microcirculation of the skin and subcutaneous tissue. It measures blood flow at an average depth of 0.5–1.0 mm. Data was measured using the PIMsoft (Perimed, Sweden) software analysis tool. This method is primarily useful for early assessment of burn site’s depth, although it can also assess very precisely the quality of perfusion surrounding and wound already healed, and thereby reveal sites at risk for hypertrophic scars. LASCA was carried out three times: at EOT, 3m-FU, and 12m-FU.
The Cutometer® MPA 580 (Courage+Khazaka electronic GmbH, Cologne, Germany) is an instrument used to measure skin’s viscoelastic properties.7,8 The device creates negative pressure, which is variably configurable between 2 and 50 kPa, and operates based on skin suction. During measurement, the selected skin site is drawn in to the probe’s aperture. The depth the skin penetrates into the aperture is measured through a contactless optical system. The skin’s biomechanical properties were measured at 12m-FU. A probe with a 2mm aperture was used, and constant negative pressure of 45 kPa was applied for all measurements. On-time was 2s and was followed by off-time (relaxation time) of 2s. The measurement was repeated three times. For each patient, two points on the verum half and two points on the control half were selected. The measurement was repeated four times at each point, i.e. 48 measurements were performed for each patient. Resulting values were then averaged for each point. The acquired parameters could then be interpreted as several indicators, such as skin extensibility (Ue), delayed distension (Uv), final deformation (Uf), immediate retraction (Ur), total recovery (Ua), and residual deformation at the end of measuring cycle (R).
Statistical analysis
Means with standard deviations were adopted as descriptive statistics for the evaluated parameters as well as for their differences in time, between treatments, and between observers. The statistical significance of differences was analysed using the Wilcoxon paired test. Time to healing was visualized using Kaplan–Meier analysis and described by median survival and its 95% confidence interval; the statistical significance of the difference between treatment curves was tested by means of the Cox proportional hazards model with random effects. Statistical analysis was conducted using SPSS 22.0.0.1 (IBM, Armonk, NY, USA).
RESULTS
A total of 32 patients were included from the Department of Burns and Reconstructive Surgery, University Hospital Brno. There were 25 men and 7 women and mean patient age in the set was 41.8 years (SD, ±11.66). A total of 31 patients had been hospitalized with burns, and only 1 patient was hospitalized for closure of a wound following free-flap surgery. The mean extent of patients’ donor sites in the study was 56.77 cm2 (SD, ±20.39). Two patients did not come for follow-ups, consequently 30 patients went through the entire protocol.
Donor site healing time
On average, donor sites treated with Oleogel-S10 healed completely in 10.03 days and control sites in 11.09 days. Median healing time for experimental sites was 7 days, and for comparator sites it was 8 days. Median healing time (95% confidence interval), verum 7 days (7–8), comparator 8 days (7–10). P-value based on the Cox model with random effects: 0.003. Fig. 1 displays the median healing time of donor sites for patients in the set. Fig. 2 presents real photographic documentation within donor site wound healing.
1. Median healing time (95% confidence interval). Verum 7 days (7–8), comparator 8 days (7–10). P-value based on the Cox model with random effects: 0.003 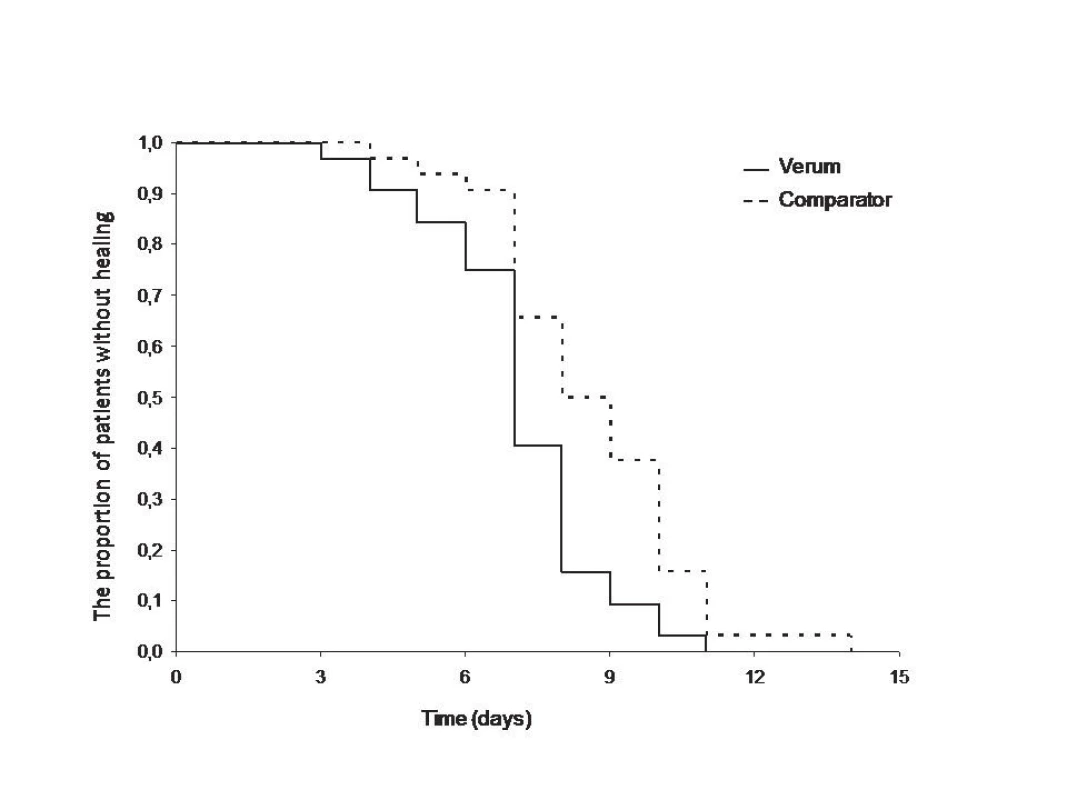
2. Donor site wound healing (Pr.– proximal part with application of Oleogel-S10). A – Immediately after surgical procedure, B – 2<sup>nd</sup> day post procedure, C – 7<sup>th</sup> day post procedure, D – 9<sup>th</sup> day post procedure – complete wound closure (end of treatment) 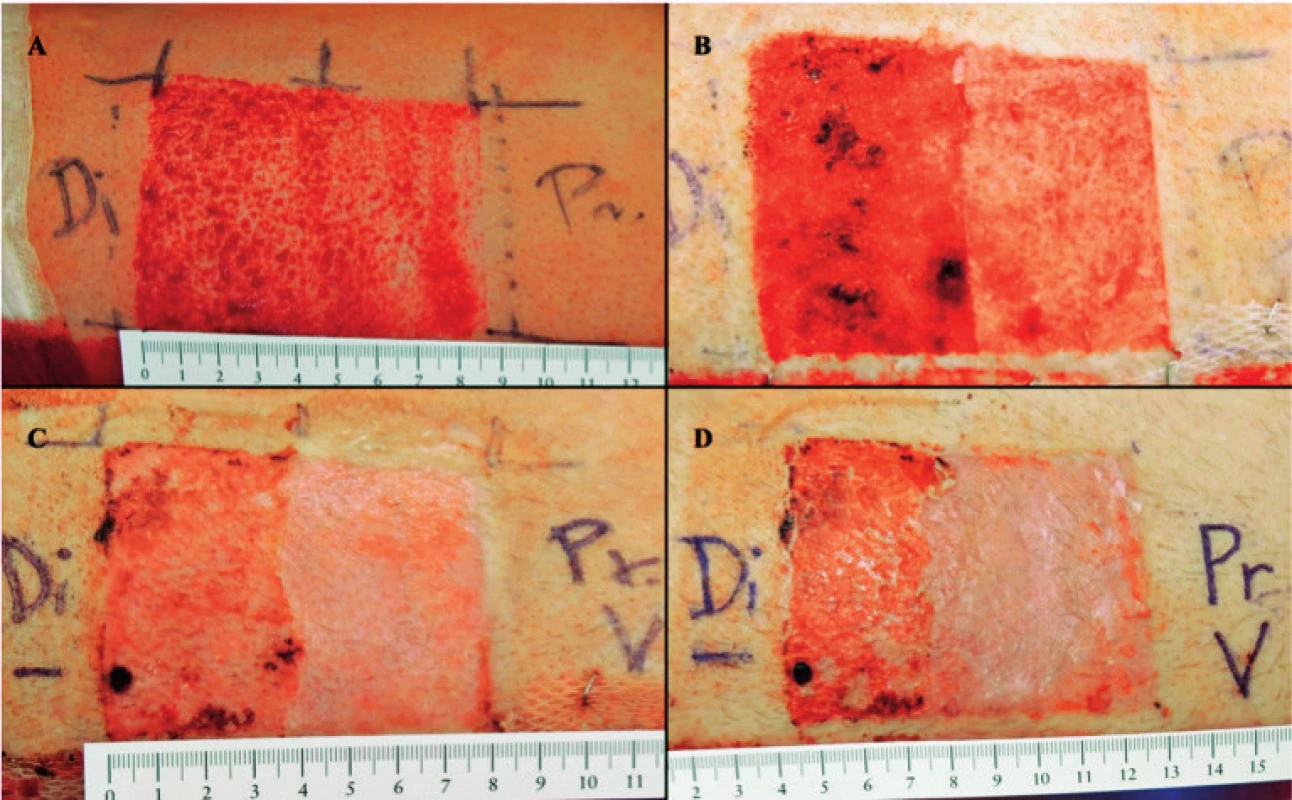
The scar assessment included repeated use of POSAS (at EOT, 3m-FU, 12m-FU). On the patient scale, the verum half recorded decreasing means of 12.1 points at EOT, 9.0 points at 3m-FU, and 7.1 points at 12m-FU with significantly lower values over time (see Table 1). Values for the control half at corresponding time points were significantly higher (see Table 1). On the observer scale, values from the verum half were better (lower), and significant differences favouring the verum half were found for all monitoring times. Table 2 presents the acquired values.
2. POSAS – comparing effects of verum and control at various times on patient and observer scales 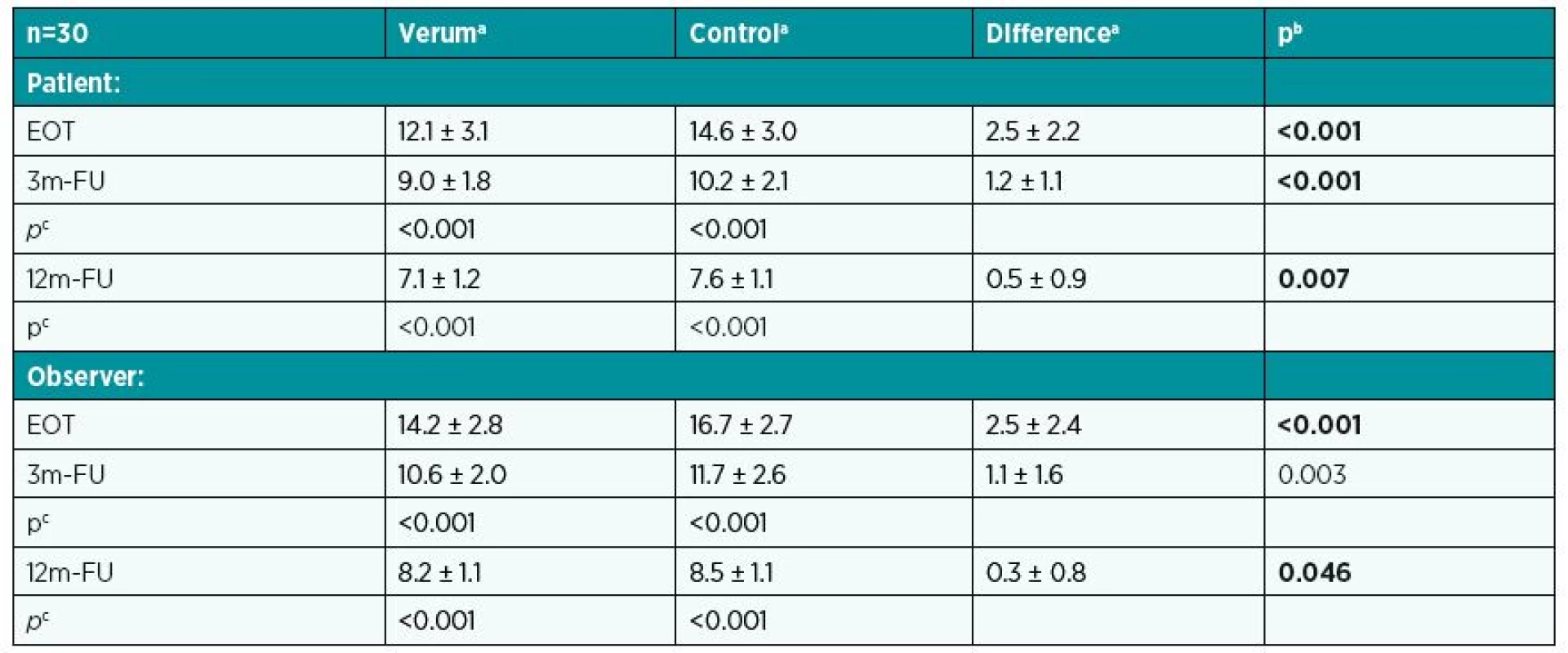
aMean and standard deviation. bp-value for Wilcoxon paired test. cp-value for Wilcoxon paired test to compare values at EOT with those at 3m-FU and 12m-FU. LASCA was carried out in all three phases and the values acquired were expressed as perfusion units (PU). While experimental sites reached on average 115 PU at EOT, the average was 69.8 PU at 3m-FU and 50.2 PU at 12m-FU. Control sites displayed significant higher values of vascularity (122.2 PU at EOT, 73.9 PU at the 3m-FU, and 52.2 PU at the 12m-FU). In all three phases, results for experimental sites were significantly better than control sites. Table 3 presents all the mean values from LASCA for patients in the set.
3. LASCA – comparing effects of verum and control at various times (perfusion unit) 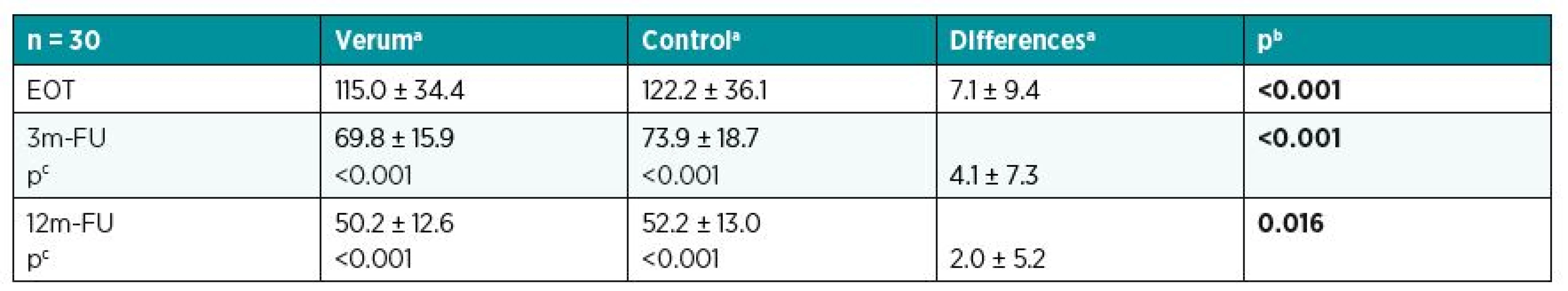
aMean and standard deviation. bp-value for Wilcoxon paired test. cp-value for Wilcoxon paired test to compare values at EOT with those at 3m-FU and 12m-FU. Significant differences were found in the values for R0, R1, R3, R4, R5, R6, R8, F0, F1, and Q0, with sites treated with Oleogel-S10 displaying more favourable values. The mean R0 value for sites treated with Oleogel-S10 was 0.079 while the mean for control sites was only 0.063 (p<0.001). R8 (or Ua) had similar results with a mean of 0.074 for treated sites and 0.059 for control sites (p<0.001). Table 4 states all measured values.
4. Viscoelastic parameters with Oleogel – verum vs. control 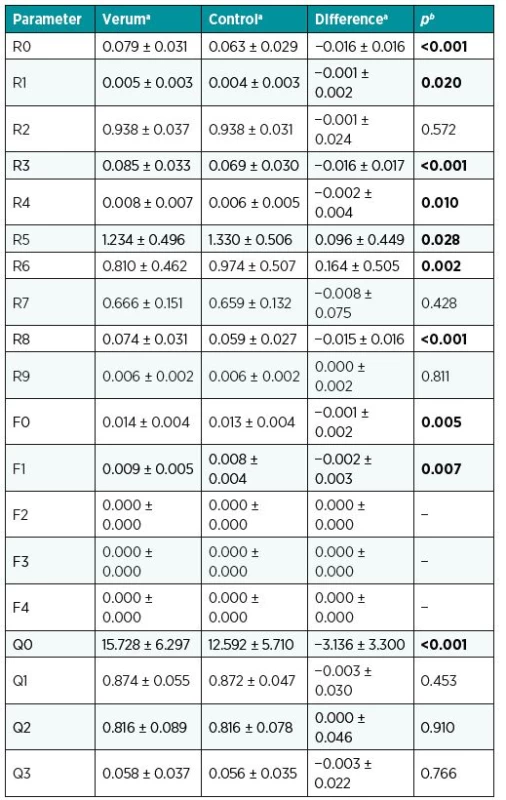
aMean and standard deviation. bp-value for Wilcoxon paired test. DISCUSSION
The search for high-quality wound dressings and topical products has led to a great number of different dressings, which are used today in wound management of wounds including donor site wounds. The optimal dressing or topical product should ensure a proper environment for supporting epithelialization, prevent multiplication of potentially pathogenic microorganisms and thereby the development of infectious complications at the donor site area, to minimize patient’s pain and discomfort during changes of dressing, to ensure a favourable final cosmetic effect, and, last but not least, be easy to handle and not too expensive.9
Oleogel-S10, the ointment used in this study, is a new product with the clear ambition to come closer to the ideal for a wound topical product.10 It is a betulin-rich triterpene dry extract from birch bark. The potential of triterpenes to accelerate wound healing has been frequently mentioned recently.11 Triterpenes, such as betulin, lupeol, and betulinic acid, represent a promising group of natural substances with interesting potential to support keratinocyte division and may thus accelerate wound healing and support epithelialization.12 The extract is acquired from birch cork (the outer layer of bark). Oleogel-S10 contains sunflower oil and birch bark extract (10%). Birch bark contains in particular lupeol (0.4–4%), erythrodiol (0.5–2%), betulin (60–95%), betulinic acid (0.5–6%), and many other substances.13 Oleogel-S10 has been designated by the European Commission as an orphan medicinal product for the treatment of the hereditary skin disorder Epidermolysis bullosa. Another area for its use is as part of therapy for actinic keratosis.14,15 Oleogel-S10 is currently being studied very intensively in terms of its effect not only on healing donor sites but also on healing burns themselves.12 One of the important differences between these two types of skin wounds is in their homogeneity. No burn has ever the same depth across its entire extent and so various healing times can be expected for different parts of a wound. In contrast, donor sites are far more homogenous, and this simplifies randomization as well as monitoring of the course of healing because there is no effect of different wound depths.
Our results clearly indicate accelerated healing at donor sites treated with Oleogel-S10 in comparison to control sites. Although the difference between the groups was relatively small, it may have relevant impact for difficult to heal wounds. In general, donor sites are standardised wounds without disturbing factors. Similar to our study, other studies have also reported only small differences in time to heal. The small difference in healing related to a significant improvement in scar quality. With the use of objective methods such as LASCA and the Cutometer MPA580 we also demonstrated that the wound half treated with Oleogel-S10 has shown significantly better rheological and functional (viscoelastic) results. The Cutometer provides one of the most widely used methods for objectively determining viscoelastic skin parameters.8 In our patient set, we always evaluated two defined points for each site half. Individual measurements were conducted 12 times in total at each point, and the resulting values were averaged. With the use of the Cutometer, the vertical deformation of skin is measured as the skin is drawn into the probe. Significant differences were found in the values for R0, R1, R3, R4, R5, R6, R8, F0, F1, and Q0, with sites treated with Oleogel-S10 displaying more favourable values. The most important parameter, R0, corresponds to the first maximal amplitude or final deformation. This parameter represents the passive behaviour of skin to force (firmness). R0 on the half treated with Oleogel-S10 was 0.079 ± 0.031 and on the control half 0.063 ± 0.029 (p< 0.001). The R0 results unambiguously indicate that experimental sites had greater ability to achieve maximum amplitude. Another important R parameter is R8 (Ua), redeformation ability. For examined sites, R8 was 0.074 ± 0.031 and for control sites 0.059 ± 0.027 (p< 0.001). These values indicate that the redeformation ability (i.e. the ability to return to the original state) was greater for the wound half treated with Oleogel-S10.
POSAS is currently one of the most universal scales for assessing scars’ character and changes over time.16 In comparison to other scar scales it has a linear scale and allows proper calculation.17 The assessment includes two scales, the patient’s and the observer’s scale. We used POSAS for assessment three times during the study, first at wound healing (i.e. at EOT) and then at 3m-FU and 12m-FU. As expected the acquired results indicate that over the monitored period values improved not only at experimental sites but also at control sites. This underscores the necessity for any anti-scarring strategy to include a control group. Values from within-subject comparison were significantly higher at all monitoring times from the half treated with Oleogel-S10, clearly indicating the benefit of the drug. The acquired results correlate very closely with objective measurements and data acquired from LASCA and the Cutometer MPA 580.
In general, the ointment was well tolerated and no allergic reaction was recorded. Across the entire clinical study, only one adverse event was recorded. This event was the development of infectious complications, which had originated in the control half, but the potentially pathogenic microorganism spread over 5 days to the already epithelialized site treated with Oleogel-S10. This complication required antibiotic therapy. The infectious agent was identified as Staphylococcus aureus. It can nevertheless be assumed that support for keratinocyte differentiation alone would have had an antimicrobial effect. Moreover, studies have unambiguously confirmed that certain triterpenes inhibit the growth of potentially pathogenic micro-organisms.18 Awolola et al. had examined the antibacterial effects of three triterpenes and three flavonoids against a number of bacteria – S. aureus and Escherichia coli.19 In particular, the activity of lupeol acetate against S. aureus strains was observed to be very promising. Antibacterial as well as antifungal effects had been determined by Mutai et al. in triterpenes acquired from extracts from the stem bark of Acacia mellifera.20 That study again tested against S. aureus strains, as well as Microsporum gypseum and Trichophyton mentagrophytes.
CONCLUSION
The acquired results clearly demonstrate the effectiveness of using Oleogel-S10 to accelerate donor site epithelialization. This acceleration is accompanied not only by reduced wound healing time but also the creation of much higher-quality skin with viscoelastic parameters comparable to those of intact skin. We must also conclude that this product was completely safe across the entire clinical study, and despite the single infectious complication, no undesirable side effects were recorded.
Other clinical trials using Oleogel-S10 in patients with acute non-thermal skin loss (epidermolysis bullosa, toxic epidermal necrolysis, etc.) are now at different stages of progression.
Corresponding author:
Břetislav Lipový, M.D., PhD.
Department of Burns and Reconstructive Surgery
University Hospital Brno
Jihlavská 20, 625 00 Brno
Czech Republic
E-mail: bretalipovy@gmail.com
Sources
1. McBride CA, Kimble RM, Stockton K. Three donor site dressings in pediatric split-thickness skin grafts: study protocol for a randomised controlled trial. Trials. 2015 Feb 8;16 : 43.
2. Caliot J, Bodin F, Chiriac S, Correia N, Poli-Mérol ML, François-Fiquet C. Split-thickness skin graft donor site: which dressing use? [Article in French]. Ann Chir Plast Esthet. 2015 Apr;60(2):140-7.
3. Stella M, Castagnoli C, Gangemi EN. Postburn scars: an update. Int J Low Extrem Wounds. 2008 Sep;7(3):176-81.
4. Barret JP, Podmelle F, Lipový B et al.; BSH-12 and BSG-12 study groups. Accelerated re-epithelialization of partial-thickness skin wounds by a topical betulin gel: Results of a randomized phase III clinical trials program. Burns. 2017 Sep;43(6):1284-1294.
5. Draaijers LJ, Tempelman FR, Botman YA et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004 Jun;113(7):1960-5; discussion 1966-7.
6. Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001 Nov;22(4):R35-66.
7. Sin P, Stupka I, Brychta P. Evaluation and comparison of composite and split-thickness skin grafts using cutometer mpa 580. Ann Burns Fire Disasters. 2010 Dec 31;23(4):208-13.
8. Bonaparte JP, Chung J. The effect of probe placement on inter-trial variability when using the Cutometer MPA 580. J Med Eng Technol. 2014 Mar;38(2):85-9.
9. Karlsson M, Lindgren M, Jarnhed-Andersson I, Tarpila E. Dressing the split-thickness skin graft donor site: a randomized clinical trial. Adv Skin Wound Care. 2014 Jan;27(1):20-5.
10. Metelmann HR, Brandner JM, Schumann H et al. Accelerated reepithelialization by triterpenes: proof of concept in the healing of surgical skin lesions. Skin Pharmacol Physiol. 2015;28(1):1-11.
11. Agra LC, Ferro JN, Barbosa FT, Barreto E. Triterpenes with healing activity: A systematic review. J Dermatolog Treat. 2015 Oct;26(5):465-70.
12. Woelfle U, Laszczyk MN, Kraus M et al. Triterpenes promote keratinocyte differentiation in vitro, ex vivo and in vivo: a role for the transient receptor potential canonical (subtype) 6. J Invest Dermatol. 2010 Jan;130(1):113-23.
13. Metelmann HR, Brandner J, Schumann H, Bross F, Hoffmann M, Podmelle F. Accelerating the aesthetic benefit of wound healing by triterpene. J Craniomaxillofac Surg. 2012 Jul;40(5):e150-4.
14. Pflugfelder A, Andonov E, Weide B et al. Lack of activity of betulin-based Oleogel-S10 in the treatment of actinic keratoses: a randomized, multicentre, placebo-controlled double-blind phase II trial. Br J Dermatol. 2015 Apr;172(4):926-32.
15. Huyke C, Reuter J, Rödig M et al.Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J Dtsch Dermatol Ges. 2009 Feb;7(2):128-33.
16. van der Wal MB, Tuinebreijer WE, Lundgren-Nilsson Å, Middelkoop E, van Zuijlen PP. Differential item functioning in the Observer Scale of the POSAS for different scar types. Qual Life Res. 2014 Sep;23(7):2037-45.
17. Tyack Z, Simons M, Spinks A, Wasiak J. A systematic review of the quality of burn scar rating scales for clinical and research use. Burns. 2012 Feb;38(1):6-18.
18. Jesus JA, Lago JH, Laurenti MD, Yamamoto ES, Passero LF. Antimicrobial activity of oleanolic and ursolic acids: an update. Evid Based Complement Alternat Med. 2015;2015 : 620472.
19. Awolola GV, Koorbanally NA, Chenia H, Shode FO, Baijnath H. Antibacterial and anti-biofilm activity of flavonoids and triterpenes isolated from the extracts of Ficussansibarica Warb. subsp. sansibarica (Moraceae) extracts. Afr J Tradit Complement Altern Med. 2014 Apr 3;11(3):124-31.
20. Mutai C, Bii C, Vagias C, Abatis D, Roussis V. Antimicrobial activity of Acacia mellifera extracts and lupane triterpenes. J Ethnopharmacol. 2009 May 4;123(1):143-8.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2017 Issue 3-4-
All articles in this issue
- Editorial
- PATIENT SATISFACTION AFTER BREAST RECONSTRUCTION: IMPLANTS VS. AUTOLOGOUS TISSUES
- OLEOGEL-S10 TO ACCELERATE HEALING OF DONOR SITES: MONOCENTRIC RESULTS OF PHASE III CLINICAL TRIAL
- THE NASOLABIAL FLAP: THE MOST VERSATILE METHOD IN FACIAL RECONSTRUCTION
- CURRENT TREATMENT OPTIONS OF DUPUYTREN´S DISEASE
- SURGICAL TREATMENT OF MELANOMA
- POSTOPERATIVE FIXATION AFTER REPOSITIONING OF PREMAXILLA IN A PATIENT WITH BILATERAL CLEFT LIP AND PALATE – CASE REPORT
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- THE NASOLABIAL FLAP: THE MOST VERSATILE METHOD IN FACIAL RECONSTRUCTION
- CURRENT TREATMENT OPTIONS OF DUPUYTREN´S DISEASE
- PATIENT SATISFACTION AFTER BREAST RECONSTRUCTION: IMPLANTS VS. AUTOLOGOUS TISSUES
- OLEOGEL-S10 TO ACCELERATE HEALING OF DONOR SITES: MONOCENTRIC RESULTS OF PHASE III CLINICAL TRIAL
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

