-
Medical journals
- Career
CURRENT TREATMENT OPTIONS OF DUPUYTREN´S DISEASE
Authors: J. Kníže 1; J. Miletín 1; A. Nejedlý 1; M. Chorvát 1; K. Novotná 1; P. Tichá 1; A. Fibír 2; A. Sukop 1; A. Knížetová 3
Authors‘ workplace: Department of Plastic Surgery, rd Faculty of Medicine, Charles University, Hospital Královské Vinohrady, Prague, Czech Republic 1; Department of Plastic and Aesthetic Surgery and Burn Treatment, Hospital Hradec Králové, Hradec Králové, Czech Republic 2; Department of Paediatric and Trauma Surgery, 3rd Faculty of Medicine, Charles University, Thomayer Hospital, Prague, Czech Republic 3
Published in: ACTA CHIRURGIAE PLASTICAE, 59, 3-4, 2017, pp. 142-148
INTRODUCTION
One of the first to describe this disease was Felix Plater in 1614. The current opinion that Plater considered the pathological basis of disease to be a contracture of the flexor tendons is a misinterpretation of the original Latin text. Plater’s anatomical studies demonstrated that shortening of ligamentous palmar aponeurosis is the anatomical basis responsible for DD.1 During the eighteenth century, DD was also studied by Henry Cline (1777) and his student Astley Cooper, who reported in detail about DD in 1822 in his publication “A Treatise on Dislocations and Fractures of the Joints”.2 A Parisian surgeon, Baron Guillaume Dupuytren (1777–1835), is known as the father of treatment of the disease. He did not publish any comprehensive monograph, but since 1831 he lectured about DD for his colleagues and students and they kept his name associated with the disease through oral transcripts called Leçons orales and gave the disease its current name. It is known that Dupuytren himself did not like to write.3,4
DD occurs predominantly in males with a ratio varying from 2 : 1 to 10 : 1.5 The differences observed in male prevalence can be however attributed to the fact that women have more often milder forms without functionally significant flexion contractures and slower progression of DD. so some of the affected women may not be enrolled to the prevalence studies in operated patients.4 Most cases are observed in the 6th decade of life. The disease affects primarily the white race. The highest prevalence is reported in the Nordic countries and in the countries where civilization expansion from these countries occurred. Therefore, DD is sometimes called a Viking’s disease. However, by refining epidemiological studies on DD, it seems that this correlation is likely to be significantly lower. The worldwide prevalence of DD varies greatly from 0.6% to 31.6% (depending on the classification criteria; a large number of patients with mild stages of the disease do not visit doctors and the disease then appears more rare than it actually is). The origin of the disease is probably multifactorial with a significant genetic component. In patients with a positive family history is the manifestation of the disease in average 5 years earlier than in those without positive family history.6 Other major risk factors include civilization diseases, especially diabetes mellitus, abuse of alcohol, side effect of some antiepileptic drugs in patients with epilepsia and smoking. We cannot simply say that these diseases themselves are the cause of DD. It only appears to be more common in patients with DD than in the general population. The effect of heavy manual work itself has not been recently considered to be an aetiological factor. On the other hand, small non-penetrating injuries or long-term exposure to vibrations are considered to contribute on the development and progression of DD.7
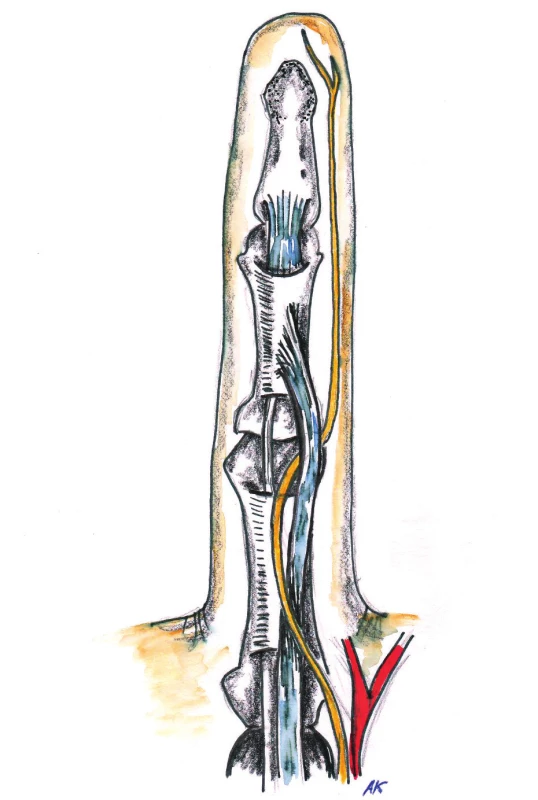
The palmar aponeurosis begins at the distal edge of the transverse carpal ligament and its projections continue into septa and skin of midpalmar space and on the fingers. In the mid-palmar space, it merges with the palmar fascia, then it inserts in the radial and ulnar aspect into thenar and hypothenar fascia. The tendon of palmaris longus muscle inserts into the palmar aponeurosis at the distal edge of the transverse carpal ligament and stretches the aponeurosis by exerting tension on it (this occurs mainly in cats). In the area above the tendons of the 2nd to the 5th finger, the palmar aponeurosis creates individual longitudinal bands, which are distributed distally in the palm into three portions – superficial, intermediate and deep fibers. The superficial fibers are attached into the skin near the MCP joints, the middle fibers are attached into the natatory ligaments (ligamentum metacarpale transversum superficiale) and finger fascia (they are called spiral fibers) and deep fibers into the area of the MCP joints and it goes deeply and attaches close to extensor aponeurosis. In the area of the fingers is the course of the fibers a little bit more complicated. The finger fascia is based on Grayson’s prevascular and Cleland’s retrovascular ligaments that extend into the lateral digital fascia, which continues as a portion of the fibers from the natatory ligaments and from the spiral fibers from the pretendious bands (Fig. 1, 2, 3).
1. Anatomy of palmar aponeurosis 1. Longitudinal pretendinous fibers 2. Proximal commisural ligament of 1<sup>st</sup> web space 3. Distal commisural ligament of 1<sup>st</sup> web space 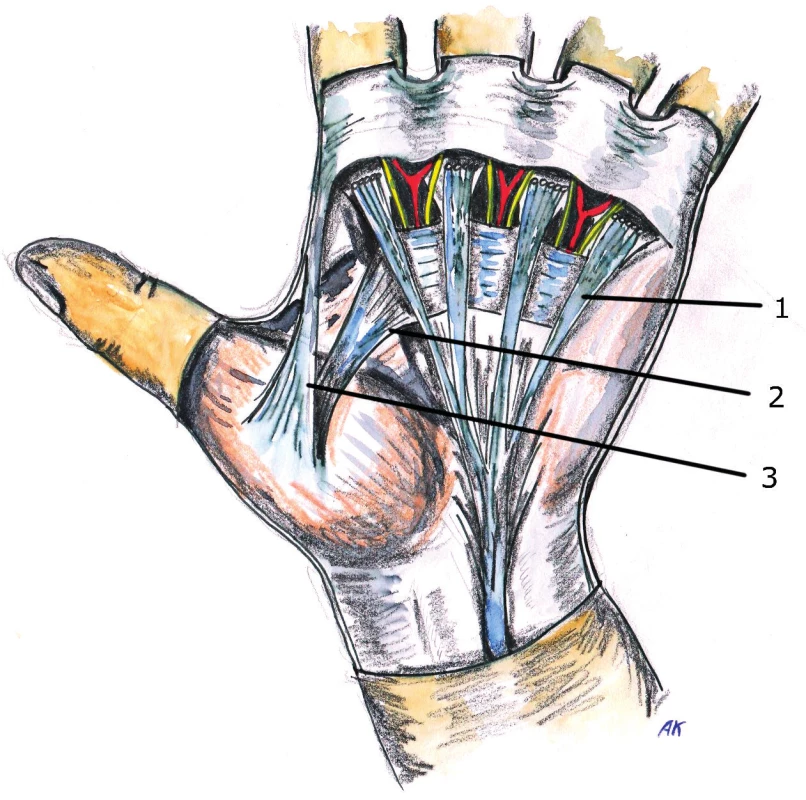
2. Anatomy of pretendinous fibers in the area of its transition to the fingers 1. Flexor tendons 2. Neurovascular bundle 3. Pretendinous fibres 4. Superficial fibers 5. Intermediary fibers 6. Deep fibers (an its course to dorsal extensor aponeurosis) 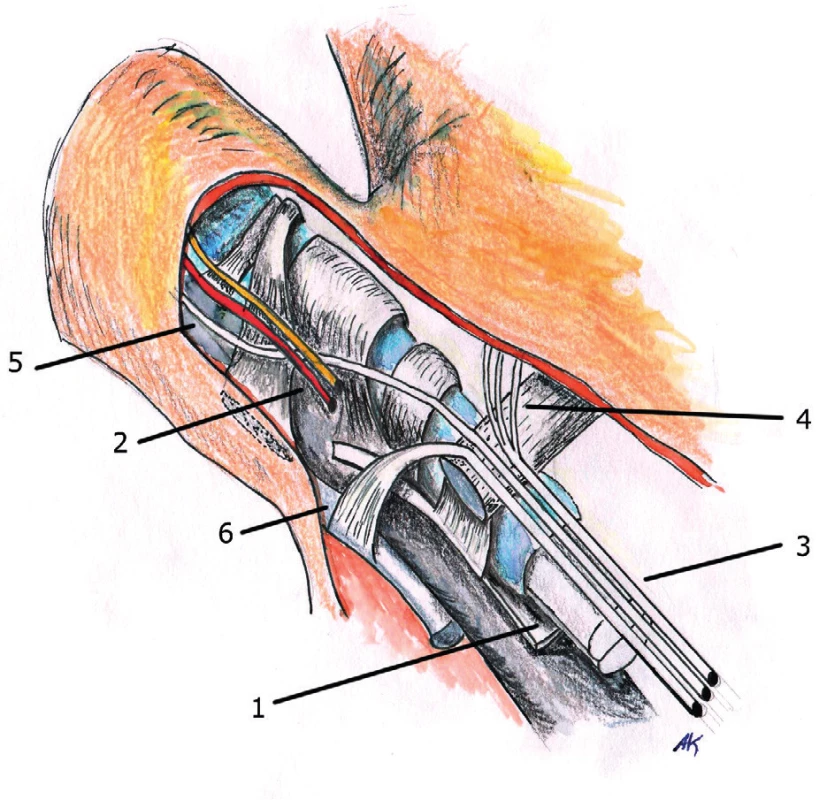
3. Anatomy of ligaments in the fingers 1. Extensor tendon 2. Dorsal vein 3. Cleland’s retrovascular ligament 4. Neurovascular bundle 5. Grayson’s prevascular ligament 
In case of pathologically affected aponeurosis the individual bands are transformed into thickened and rigid cords. The initial stages of the disease can be manifested by the formation of palmar skin pits, subcutaneous nodules and the contraction of the cords. In the area of the palmar fascia the pathologically modified cords cause a gradual contraction in MCP joints. If the fingers are also affected, there is a gradual pathological transformation of the individual parts of the digital fascia. In this area they gradually form three main structures that cause contracture of the PIP joint.8 The first one, the central cord, is a continuation of the pretendinous cord that is attached to the flexor sheath and periosteum of the middle phalanx. Furthermore, a lateral cord can be formed by transformation of the lateral digital sheath, which can also cause contracture of the DIP joint. A relatively complicated structure is the so-called McFarlan’s spiral cord. It is caused by the transformation of the spiral fibers, fibers of the natatory ligaments, parts of the Grayson’s ligaments and lateral digital sheath. It causes flexion contracture at the PIP joint and its course deviates the neurovascular (NV) bundle medially and superficially (Fig. 4).
Histopathologically the composition of a healthy and affected palmar aponeurosis is different. While the normal aponeurosis consists predominantly of collagen I (90%) and partially of collagen III (5%), the affected aponeurosis contains up to 40% of collagen III and numerous fibroblasts and myofibroblasts. One of the theories of the pathophysiological basis of the disease is based on the effect of oxygen radicals. It’s production is a reaction to local ischemia or high concentration of alcohol.9 Increased formation of oxygen radicals was indirectly demonstrated by higher concentrations of substances that catalyze their formation in pathological tissue.10 Environment with a high concentration of oxygen radicals further causes production of growth factors (BFGF, PDGF, TGF-β) that stimulate proliferation and subsequent contracture of myofibroblasts.
A simple classification according to Karfík is used historically. It classifies the clinical forms into 3 stages. This type of classification considers the extent and location of the pathological process: type 1 – palmar form (without finger contracture), type 2 – simple contracture, type 3 – complicated contracture. In the case of simple contracture, we can observe flexion contractures of MP and PIP joints, most often on the 4th and 5th fingers. The complicated type of contracture is manifested by the impairment of the whole ligamentous system of the palm itself . Contractures of several or all fingers, adduction contracture of the thumb and severe impairment of the aponeurosis and palmar skin are typical. The palm can have a cuplike appearance.3,8
Another known, simple and used classification is the Iselin system. He has divided DD into 4 stages according to the location of the disease; see table (Table 1). Better knowledge of the stage of contracture is provided by the classification according to Mikkelson. The various stages of the disease are assigned a degree of contracture (Table 2). Complex evaluation of DD is provided by Tubiana classification (Table 3). It considers the localization of the disease (including both two and three phalanx fingers), the degree of contracture and the impairment of the webspaces.8 Generally, the best classification is such one that provides instructions for treatment of DD. From this perspective, we can be satisfied particularly with Tubiana classification and with Mikkelson classification.
1. Stages of DD according to Iselin 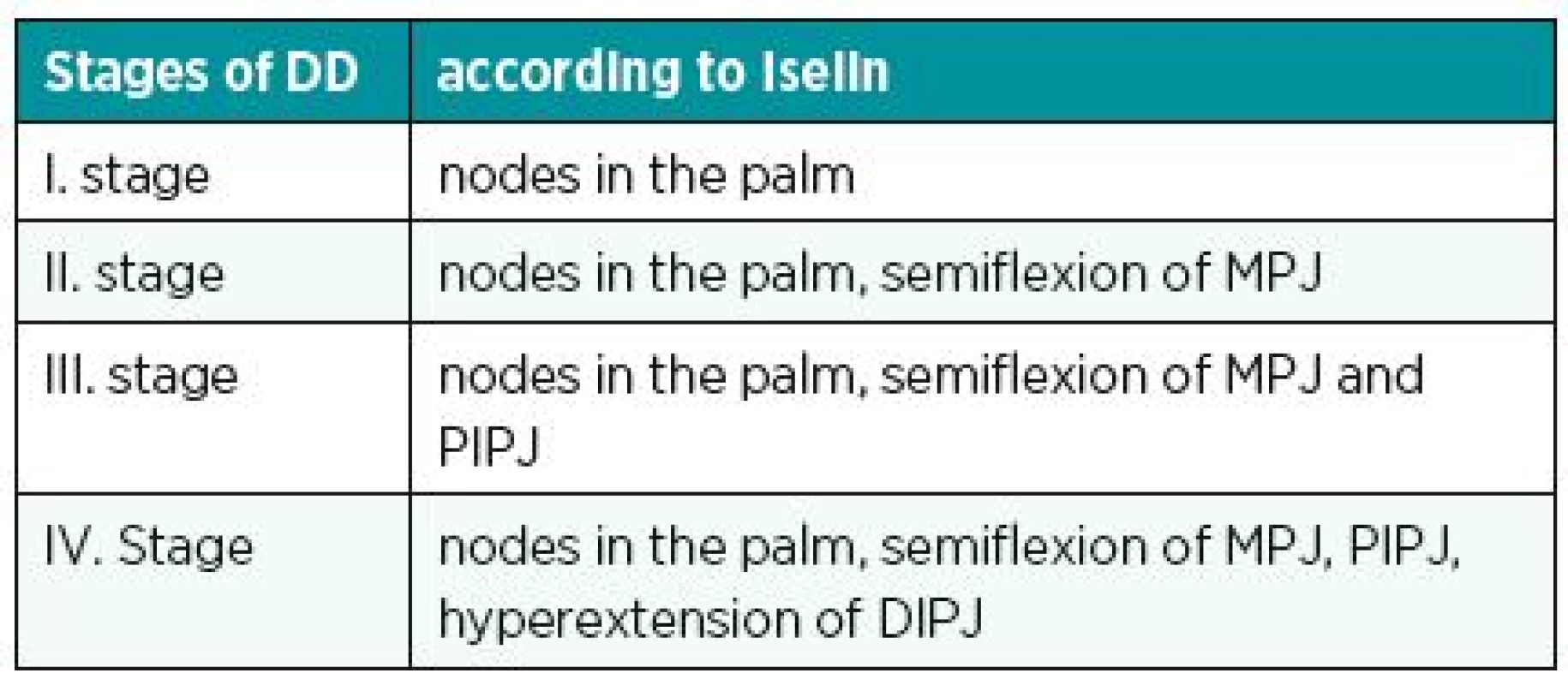
2. Stages of DD according to Mikkelson 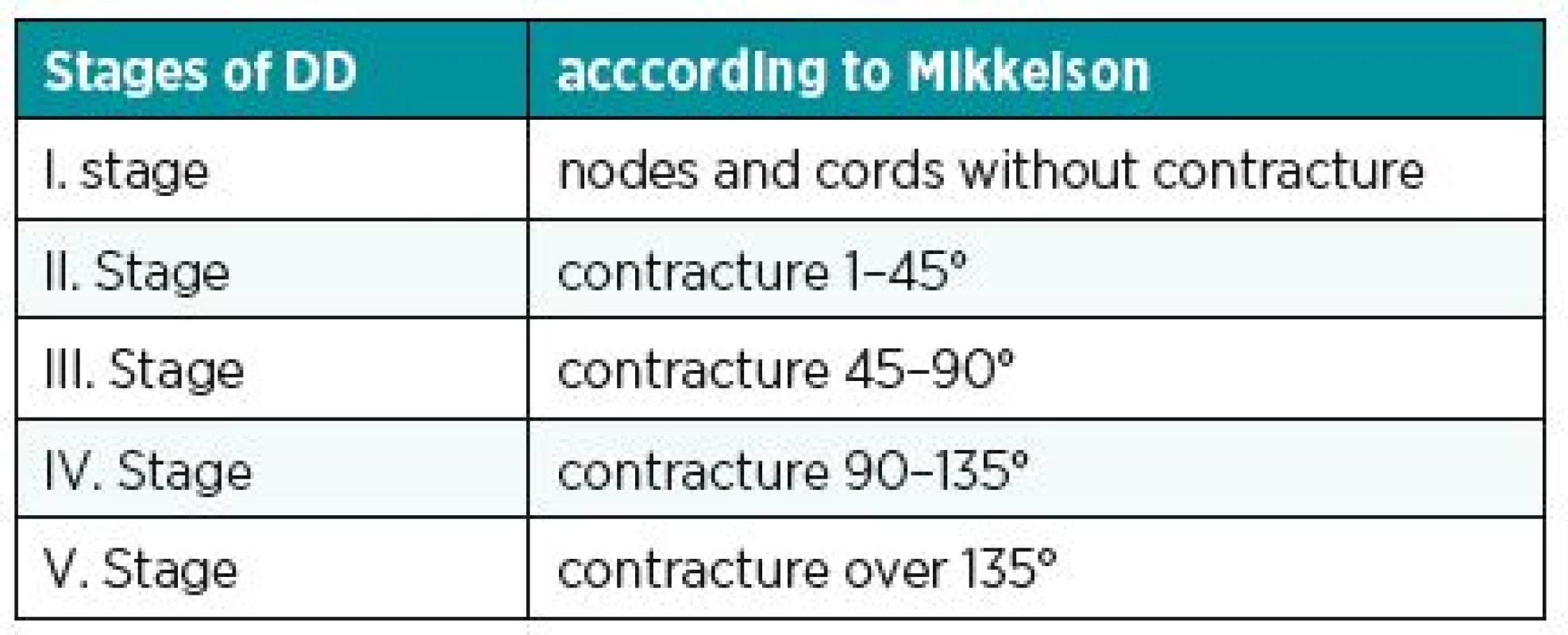
3. Classification of DD according to Tubiana 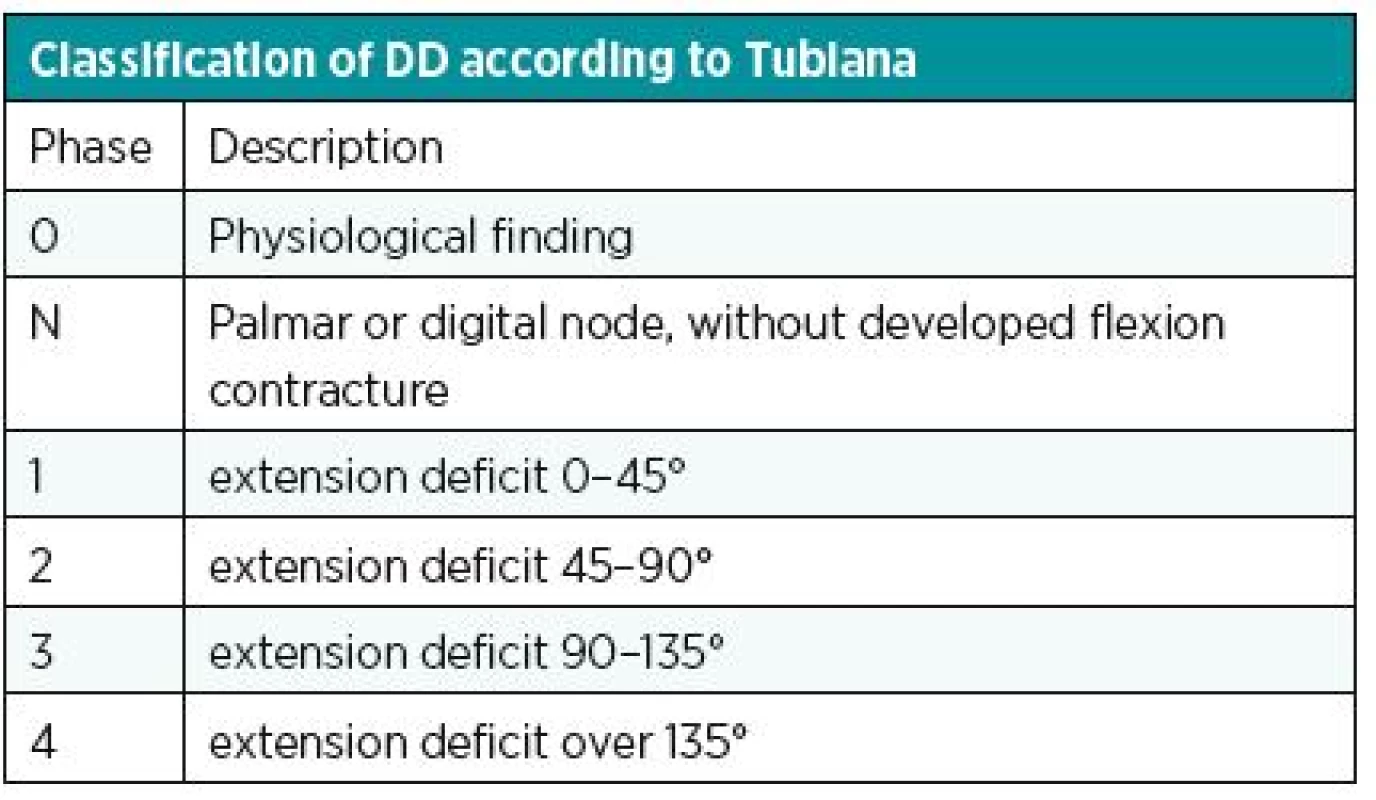
TREATMENT OF DUPUYTREN´S DISEASE
In patients with functional disability of the hand due to DD the fastest and most radical removal of the affected tissue is not the main objective – unlike in cancer patients. The most important for the correct treatment of DD is currently, in addition to proper timing, also the choice of the most optimal steps that ensures the maximal function of the affected hand with minimal morbidity and minimal risk for the patient. Radical and apparently the most effective therapy of DD is the classical surgical treatment that, however, causes considerable morbidity.8 The decision about the suitable surgical technique should be based on the local finding as well as other factors such as social history, occupational history, age and mental status of the patient.
Conservative treatment
Conservative treatment of DD includes or in the past included radiotherapy, splinting, massages, topical use of vitamin E ointments, steroid injections, ultrasound, shockwave and laser therapy. The relatively new treatment option, which has changed the DD treatment algorithm in recent years, is the use of Clostridium histolyticum enzyme (enzymatic fasciotomy). It is not classified as a conservative treatment but rather as a separate group of so-called mini-invasive procedures. Conservative treatment has its place as a surgical therapy or a method to slow the progression in early stages of the disease. Physical methods (ultrasound, shock wave, laser, ...) usually lead to softening of the nodes, but in principle they do not affect contracting cords. A good effect is known in the case of long-term use of splinting.3,7,8,11 However, this is often more restrictive for the patient than the contracture itself. A more effective conservative treatment is the application of steroid injections. They influence the formation of fibrous tissue, whereas they do not affect matured cords, and therefore they are used to treat the nodular form of DD only. The most serious local complication of this treatment is the possibility of spontaneous rupture of the flexor tendon. The exact percentage risk is not known. In addition, up to 50% of the patients usually report only transient atrophy and depigmentation of the skin at the injection site.12 The efficiency of various conservative procedures is controversial and with little clinical benefit to the patient, conservative treatment is not commonly indicated as first treatment.
Mini-invasive treatment
A relatively new group of therapeutic procedures includes needle aponeurotomy and treatment with Clostridium histolyticum collagenase. Both of these procedures cannot be included simply among surgical procedures or conservative procedures.
Aponeurotomy (some authors also use the synonym fasciotomy) means simple interruption of a contracture cord with immediate release of flexion contracture, followed by patient’s rehabilitation. This was the basic technique used by Baron Dupuytren in the 19th century. Aponeurotomy can be performed with small scalpel incisions, but more often it is performed as mini-invasive needle technique (percutaneous needle fasciotomy). Recommended technique includes technique with swinging movements or multiple puncture technique in one spot with a simultaneous passive tension applied with extension of the finger. This interruption is performed several times over the entire contracture cord, and with subsequent manipulation may be achieved full extension of the finger according to the degree and character of the disease. Needle aponeurotomy may be supplemented with lipografting, which, according to the authors, allows shorter recovery and less scarring of the skin by adding fat to the atrophic subcutaneous tissue.13 But there is not more extensive experience from more independent workplaces in case of this method.
The application of Clostridium histolyticum collagenase (CCH) is also a mini-invasive technique that has already gained its place in addition to the classical aponeurotomy. Clostridium histolyticum collagenase is used under the tradename Xiapex (Auxilium, USA). CCH contains 2 different types of collagenases: collagenase AUX-I (class I) that splits the terminal portions of collagen chains and AUX-II collagenase (class II) that affects the middle parts of the chain. Collagenase should specifically split collagen I and III, especially pathological cords. However, there are other connective tissues of the hand with a similar structure, including the tendons and joint ligaments, which results in a risk of rupture of the flexor tendon as a complication after the procedure. However, this complication is rare when the application guidelines are followed (2 ruptures per 1000 injections are reported in the literature).14 Other important structures such as vessels and nerves contain predominantly collagen type IV in the walls, which should not be affected by CCH. CCH should always be applied safely to the cord. It is then followed by 1 day of resting or limb fixation with a splint. The next day, the doctor performs manipulation under anesthesia, which breaks the collagenase-infiltrated cords to straighten the finger. Extensions can also be improved by continuous rehabilitation or by stretching or splinting. Regarding the long-term results of CCH treatment, there are currently no sufficient long-term studies available. The results of comparative studies show that the estimated recurrence rate ranges from 10 to 31% over a period of 120 days to 4 years, which is less than in percutaneous needle aponeurotomy (50-58% over 3 to 5 years). In 2010, preliminary results of recurrence in patients enrolled in a phase II trial were published. Follow-up was 8 years. From the original 23 patients, only 8 were followed and examined, so the results cannot be generalized. Results show that CCH treatment can achieve full joint extension in most patients and clostridium collagenase is more effective in treating metacarpophalangeal (MCP) than proximal interphalangeal (PIP) joints. From the 8 patients who were finally checked, 6 had signs of recurrence of the disease, although less than in the previous treatment. None of them underwent surgery, and satisfaction with CCH treatment was high in these patients.15
In the study by Peimer et al. (2015), a 5-year recurrence was published after the use of collagenase. The recurrence was defined as an extension deficit of 20 and more degrees in the joint, where the extension deficit achieved by therapy was 0-5 degrees. The total recurrence reported was 47% (for MCP joints 39% and for PIP joints 66%).16 If we define the recurrence as extension deficit 30 or more degrees, we assume that the resulting recurrence is smaller. Recurrence after fasciectomy varies depending on the criteria for defining recurrences and it is in average around 39%. 17
Surgical therapy
“The development of experience with surgical therapy follows the development of insight into the basis, the anatomical extent of the disease. From the interruption of the cords, it was correctly concluded that the most effective therapy is removal of affected aponeurosis. Experience with recurrences has shown the possibility that the disease develops at any area of palmar aponeurosis and resulted in logical removal of the entire palmar aponeurosis in the palm.” 3 This idea of prof. Karfík fully reflects the previous efforts to achieve maximal radicality in removal of pathological palmar aponeurosis. However, when selecting a suitable surgical procedure, it is necessary to consider the clinical findings and overall condition, but also the social background and the patient’s needs, considering the expected total duration of treatment and the risk of relapse or recurrence. Radical operations have their indications, but there is no need to perform extensive surgeries with complete removal of unaffected aponeurosis. Secondary morbidity after such an extensive operation is considered to be a greater problem than any other future surgery on other parts of the hand that were not previously affected. General indications of surgical therapy are regular, for example, in a painful palmar form. The most common indication is a contracture in the MCP or PIP joints (30° in MCP or any degree in PIP joint) limiting the patient in everyday life. The commonly used standard for indication of surgical treatment is also the so-called positive “table top test”, in which the patient with clinically significant contracture of the finger can not place the palm on a flat tabletop surface.
Together with the choice of the right surgical procedure, we also have to choose the optimal skin incisions so that we could remove maximum of the affected tissue without the need for extensive dissection and at the same time could sufficiently visualize the structures to be protected, such as the tendons, blood vessels and nerves. The most common incisions are shown in Fig. 5, 6, 7. The open surgical procedures for treatment of DD include limited, segmental and radical aponeurectomy or dermofasciectomy.
5. Incisions according to Meyerding 
6. Incisions according to Burian 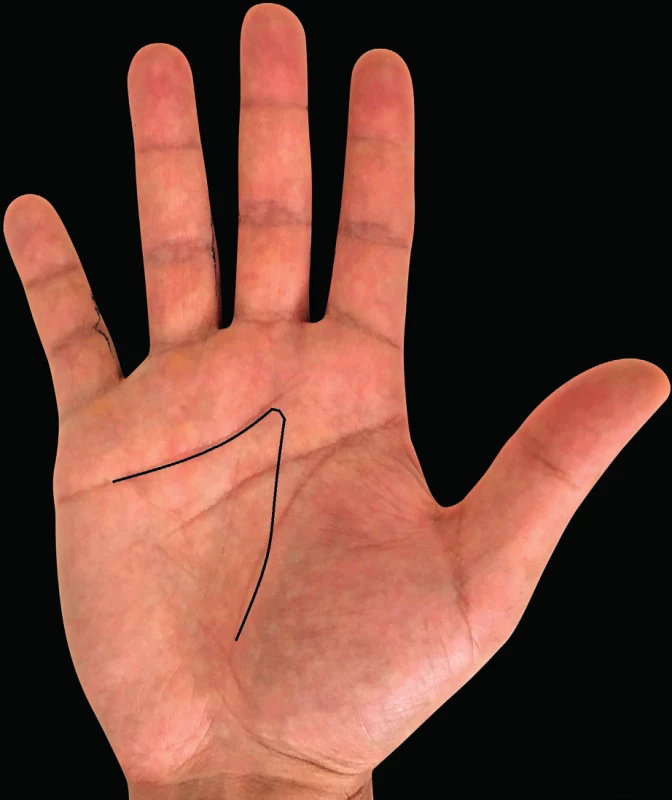
7. Incisions according to Hurst 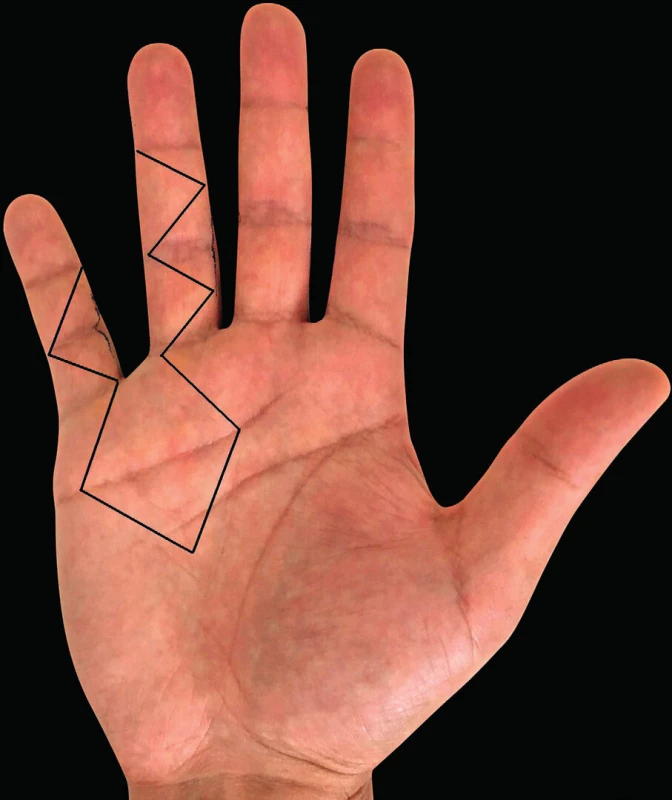
Limited aponeurectomy (also limited fasciectomy) is currently the most commonly used surgical technique for treatment of DD. This is a procedure with low morbidity. The principle is removal of only pathologically altered tissue in a necessary extent. Any possible skin defects are usually covered by local skin plasties (Z plasty, V-Y plasty), or local skin flaps. Limited aponeurectomy has a significantly lower recurrence rate (20.9% vs. 84.9%) compared with needle aponeurotomy in a 5-year comparison, which is associated with greater patient satisfaction over the long term.18,19,20 In 1964, McCash published the so-called open palm technique, in which the defects were left for secondary healing.21 The use of open palm technique has a higher recurrence rate (32%) and a greater limitation of the range of motion in long-term evaluation.22
Segmental aponeurectomy (also called segmental fasciectomy) is a procedure that removes approximately 1cm segments of affected aponeurosis without mobilizing or undermining the skin. Part of the affected tissue is left in situ.
Radical aponeurectomy (also radical fasciectomy), i.e. complete removal of the whole aponeurosis, was based on the principle that DD is an analogue to a tumor. This method, however, is associated with a high postoperative morbidity. A lower risk of recurrence with this method has not been demonstrated.7
Dermofasciectomy is a method in which the affected aponeurosis, including the affected skin, is removed. The resulting defects are mostly covered by full thickness skin grafts. The advantage of this procedure is reduced risk of recurrence by radical removal of the cord, including pathologically changed skin, which may contain myofibroblasts responsible for early recurrence. The main disadvantage is a longer post-operative immobilization with regards to the healing of the skin graft. This method is indicated especially in case of relapses or in young patients with an aggressive form of the disease.
If we want to evaluate the recurrence rate after various treatment options, we must be aware of one important thing – whether it is possible to satisfactorily define recurrence as itself and how. The limits of recurrence have often been defined in the literature differently and it was not possible to make comparisons in various studies. Significantly different definitions for recurrence limits made almost impossible the effectiveness of individual treatment options to compare. Until recently, there has been some unification of the criteria that the condition is defined as recurrence. The recurrence is now considered as condition where the contracture of the joint (or the extension deficit) returned to 20 to 30 degrees. It is in the case of previously treated joint to full extension or a maximum of 5 degree limitation of extension.16 Most current studies are based on this definition. This definition often does not correspond to the patient’s own disability, and may therefore be more academic than practical or functional. For one patient, a 20-degree extension deficit may be functionally significant, for another, this may be no problem and an extension deficit of 50 to 60 degrees may only be considered significant. Therefore, the limit of recurrence cannot be specified precisely in relation to the functional results of the therapy, which is generally important in hand surgery. This discrepancy between the recurrence defined in literature and the “functionally” significant recurrence together with the considerable heterogeneity of the disease make it difficult to compare data published in literature and to compare the effectiveness of the individual treatment options. On the other hand, we have no other choice than to use partially academic criteria for clinical trials in DD, but we should always consider the functional aspect and not to forget the disability noticed by the patient.
DISCUSSION
Although new mini-invasive techniques have begun to be promoted in recent years in DD treatment, classical open surgical therapy remains the gold standard of DD treatment. In most cases, limited aponeurectomy (possibly extended with removal of cords in individual fingers) appears to be the most effective technique in the case of primary disease. The disadvantage of open surgery is both longer recovery and also the risk of complications during healing or the risk of neurovascular structures damage. It can also be said that the results of surgical therapy are dependent in some extent on the surgeon’s experience and skills.
Disadvantages of open surgical procedures are eliminated by mini-invasive techniques, however lower effectiveness and higher risk of recurrence must be expected. They have a good effect especially in the case of elderly patients or patients who cannot undergo surgery for any medical or social reason. Mini-invasive operations can also be useful for advanced contractures of multiple fingers. In these cases, the mini-invasive procedure can precede a few weeks the radical procedure, thereby improving operational comfort during radical surgery and accelerating post-operative rehabilitation. The use of CCH provides comparable results to open surgical therapy, but we need to wait for a definitive assessment of the effectiveness of CCH treatment. We cannot also forget the high economic burden of CCH treatment.
Previously published results of expert studies investigating the efficiency of various DD treatment regimens are still insufficient to determine, which treatment methods are “better” or “worse”.23 Given the significant heterogeneity of disease manifestations, inconsistent criteria for the recurrence, both academic and functional, dependence of the treatment effect on the personal experience and surgeon’s skills (especially in fasciectomy) and other factors, it is possible to say that a randomized and blinded study to clearly answer these questions can not be done now or in the future. The situation is completely different for example to drug studies that can be randomized and double-blinded. Thus, the “heretical” question can be asked whether we will ever have enough information and experience in the future to be able to accurately assess the effectiveness of these treatments.
We also have to remember that the optimal goal of DD therapy is to remove the contracting tissue with least possible intervention on the soft tissues of the hand, and therefore, with the least risk of complications. After every open surgery, there is more or less scar tissue produced, which limits the movements of the finger, and, if further reoperation in case of recurrence is necessary, increases the risk of perioperative damage to the vessels, nerves or skin cover. Every other open surgery at the site where it was previously operated also means a worse prognosis of healing. This does not have to be the case for post-CCH surgery, when collagenase has been shown to provide safe future fasciectomy.24 However, we should still await long-term CCH treatment results, including this aspect. Only after gaining further experience, we will be able to tell more precisely for what disease stage or for which groups of patients this treatment may have the greatest benefit.
CONCLUSION
Dupuytren’s disease is clinically heterogeneous and has a significant impact on the function of the hand. We now have a range of therapeutic procedures available for DD, ranging from conservative, mini-invasive to open surgical methods. The method of treatment should be chosen not only on the basis of the clinical manifestations of the disease but also with regards to other medical, functional and social aspects of the disease. Dupuytren’s disease should be treated at specialized hand surgery departments that are able to offer a whole range of treatments, from nonsurgical to mini-invasive or surgical.
Corresponding author:
Jakub Kníže, M.D.
Department of Plastic Surgery, 3rd Faculty of Medicine, Charles University,
Hospital Královské Vinohrady
Šrobárova 50, 100 34 Prague 10
Czech Republic
E-mail: jakubknize@gmail.com
Sources
1. Belusa L, Selzer AM, Partecke BD. Description of Dupuytren disease by the Basel physician and anatomist Felix Plater in 1614. Handchir Mikrochir Plast Chir. 1995 Sep;27(5):272-5.
2. Thurston A. Dupuytren’s disease or Cooper’s contracture?: Kenneth Fitzpatrick Russell Memorial Lecture. ANZ J Surg. 2003 Jul;73(7):529-35.
3. Karfík V. Duputrenova kontraktura. Praha: Spolek lékařů českých, c1949. 133p.
4. Warwick, D. Dupuytren’s disease FESSH instructional course 2015. CG Edizioni Medico Scientifiche, Torino (2015).
5. Green DP, et al. Green’s operative hand surgery. 6th ed. Philadelphia: Saunders/Elsevier, 2011. Chapter Dupuytren’s contracture; p141-158.
6. Becker K, et al. The importance of genetic susceptibility in Dupuytren’s disease. Clin Genet. 2015 May;87(5):483-7.
7. Pilny J, et al. Chirurgie ruky. Praha: Grada, c2011. p327-341.
8. Krejča M. Dupuytrenova nemoc. Praha: Grada, c2003. 124p.
9. Hueston JT, Murrell GA. Cell-controlling factors in Dupuytren’s contracture. Ann Chir Main Memb Super. 1990;9(2):135-7.
10. Murrell GA. An insight into Dupuytren’s contracture. Annals of The Royal College of Surgeons of England. 1992;74(3):156-161.
11. Thomson GR. Treatment of Dupuytren’s contracture with vitamin E. Br Med J. 1949 Dec 17;2(4641):1382.
12. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000 Nov;25(6):1157-62.
13. Hovius SE, Kan HJ, Smit X, Selles RW, Cardoso E, Khouri RK. Extensive percutaneous aponeurotomy and lipografting: A new treatment for Dupuytren disease. Plast Reconstr Surg. 2011 Jul;128(1):221-8.
14. Zhang AY, Curtin CM, Hentz VR. Flexor tendon rupture after collagenase injection for Dupuytren contracture: case report. J Hand Surg Am. 2011 Aug;36(8):1323-5.
15. Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg Am. 2010 Apr;35(4):534-9, 539.e1.
16. Peimer CA, Blazar P, Coleman S, Kaplan FT, Smith T, Lindau T. Dupuytren Contracture Recurrence Following Treatment With Collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-Year Data. J Hand Surg Am. 2015 Aug;40(8):1597-605.
17. Crean SM, Gerber RA, Le Graverand MP, Boyd DM, Cappelleri JC. The efficacy and safety of fasciectomy and fasciotomy for Dupuytren’s contracture in European patients: a structured review of published studies. J Hand Surg Eur Vol. 2011 Jun;36(5):396-407.
18. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012 Feb;129(2):469-77.
19. van Rijssen AL, Gerbrandy FS, Ter Linden H, Klip H, Werker PM. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren’s disease: a 6-week follow-up study. J Hand Surg Am. 2006 May-Jun;31(5):717-25.
20. Van Rijssen AL, Werker PMN. Percutaneous needle fasciotomy in Dupuytren’s disease. J Hand Surg Br. 2006 Oct;31(5):498-501.
21. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964 Jul;17 : 271-80.
22. Schneider LH, Hankin FM, Eisenberg T. Surgery of Dupuytren’s disease: a review of the open palm method. J Hand Surg Am. 1986 Jan;11(1):23-7.
23. Rodrigues JN, Becker GW, Ball C, Zhang W, Giele H, Hobby J, Pratt AL, Davis T. Surgery for Dupuytren’s contracture of the fingers. Cochrane Database Syst Rev. 2015 Dec 9;(12):CD010143.
24. Hay DC, Louie DL, Earp BE, Kaplan FT, Akelman E, Blazar PE. Surgical findings in the treatment of Dupuytren’s disease after initial treatment with clostridial collagenase (Xiaflex). J Hand Surg Eur Vol. 2014 Jun;39(5):463-5.
Labels
Plastic surgery Orthopaedics Burns medicine Traumatology
Article was published inActa chirurgiae plasticae

2017 Issue 3-4-
All articles in this issue
- Editorial
- PATIENT SATISFACTION AFTER BREAST RECONSTRUCTION: IMPLANTS VS. AUTOLOGOUS TISSUES
- OLEOGEL-S10 TO ACCELERATE HEALING OF DONOR SITES: MONOCENTRIC RESULTS OF PHASE III CLINICAL TRIAL
- THE NASOLABIAL FLAP: THE MOST VERSATILE METHOD IN FACIAL RECONSTRUCTION
- CURRENT TREATMENT OPTIONS OF DUPUYTREN´S DISEASE
- SURGICAL TREATMENT OF MELANOMA
- POSTOPERATIVE FIXATION AFTER REPOSITIONING OF PREMAXILLA IN A PATIENT WITH BILATERAL CLEFT LIP AND PALATE – CASE REPORT
- Acta chirurgiae plasticae
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- THE NASOLABIAL FLAP: THE MOST VERSATILE METHOD IN FACIAL RECONSTRUCTION
- CURRENT TREATMENT OPTIONS OF DUPUYTREN´S DISEASE
- PATIENT SATISFACTION AFTER BREAST RECONSTRUCTION: IMPLANTS VS. AUTOLOGOUS TISSUES
- OLEOGEL-S10 TO ACCELERATE HEALING OF DONOR SITES: MONOCENTRIC RESULTS OF PHASE III CLINICAL TRIAL
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career