-
Medical journals
- Career
Aberrant axillary breast tissue with pseudoangiomatous stromal hyperplasia in a man
Authors: JF. Val-Bernal 1; C. Celeiro-Muñoz 2; E. Linares 2; E. Gallardo 3; E. García-Somacarrera 4
Published in: Čes.-slov. Patol., 54, 2018, No. 3, p. 143-146
Category: Original Articles
Overview
Diagnosing accessory breast tissue in a male patient is difficult when the condition is unilateral, and there is no areola or nipple. Pseudoangiomatous hyperplasia of the mammary stroma is an uncommon benign mesenchymal proliferation that may mimic low-grade angiosarcoma. We report herein an example of tumoriform pseudoangiomatous hyperplasia of the stroma arising in the accessory breast tissue of a 38-year-old man. The condition presented as a palpable tender axillary mass. Histopathologically, there were no changes of gynecomastia. Only two cases of pseudoangiomatous hyperplasia of the stroma have been previously reported in the accessory breast tissue of men showing unilateral or bilateral gynecomastia. Our case is the first report without associated gynecomastia. Radiologic imaging features are not sufficiently specific to enable a prospective diagnosis of pseudoangiomatous hyperplasia of the stroma. Microscopic examination of the lesion is indispensable in making a definitive diagnosis. Awareness of the condition can avoid difficulty in diagnosing it. Aberrant breast tissue with mass-forming pseudoangiomatous hyperplasia of the stroma, whilst rare, should be included among the benign proliferative mesenchymal lesions of the axilla.
Keywords:
aberrant breast tissue-accessory breast tissue-pseudoangiomatous stromal hyperplasia-gynecomastia-angiosarcoma-axilla
Accessory breast tissue (ABT) is a residual mammary tissue left over from embryologic development. The various forms of ABT can be classified simply as polymastia, polythelia and aberrant breast tissue (1). Polymastia corresponds to breast tissue containing glands with a duct system that communicates with overlying skin. Polythelia signifies the presence of accessory nipples or areolae. Aberrant breast tissue is the presence of disorganized secretory glandular tissue that is not related to the skin (1,2). ABT is most commonly observed in the axilla. However, it can be seen in the thoracoabdominal region of the milk line, and exceptionally in different ectopic sites outside the milk line such as the neck, face, chest, shoulder, arm, back, thigh, buttock, hip, and vulva (1,3-5).
ABT is more prevalent in women and can be bilateral. The incidence varies between 0.4 % and 6 % in women of various ethnic groups, with the highest prevalence being in the Japanese population (4). The condition is uncommon in male patients. The ratio of men to women is 1 : 5 (6).
We report herein a case of aberrant axillary breast tissue in a man presenting an axillary mass. The diagnosis was established after a histopathologic examination of the surgical specimen. The rare presence of the tumoriform of pseudoangiomatous stromal hyperplasia (PASH) was also found.
CASE REPORT
A 38-year-old man had noticed a right axillary mass that had developed over several years. The mass had increased in size and had been associated with discomfort and pain during palpation in the previous three months. The man’s past clinical history was irrelevant. A physical examination showed a lump in the right axilla measuring 3.5 x 1 cm that was firm in consistency, mobile, and painful on palpation. The skin over the swelling was normal. A provisional clinical diagnosis of axillary lymphadenopathy or lipoma was made.
Sonography revealed a poorly defined 3 x 2.5 cm, hypoechoic, oval subcutaneous area in the central region of the axilla. The echotexture was similar to the surrounding subcutaneous tissue with internal tubular hypoechoic structures and no vascular signal on the power Doppler ultrasound (Fig. 1A and B). The images suggested a low-flow vascular malformation. Magnetic resonance imaging (MRI) showed a clearly defined triangular-shaped isointense mass on the muscle on T1-weighted images that was slightly hyperintense on T2-weighted and DP-weighted images and became homogeneously enhanced after adjusting the contrast (Fig. 2A, B, and C). A malignant disease could not be excluded by the use of MRI, which prompted surgical removal.
1. Sonography study. Axial (A) and longitudinal (B) sonograms of the lesion. Notice the anechoic tubular structures (*) and the poorly defined margins of the mass (white arrows). 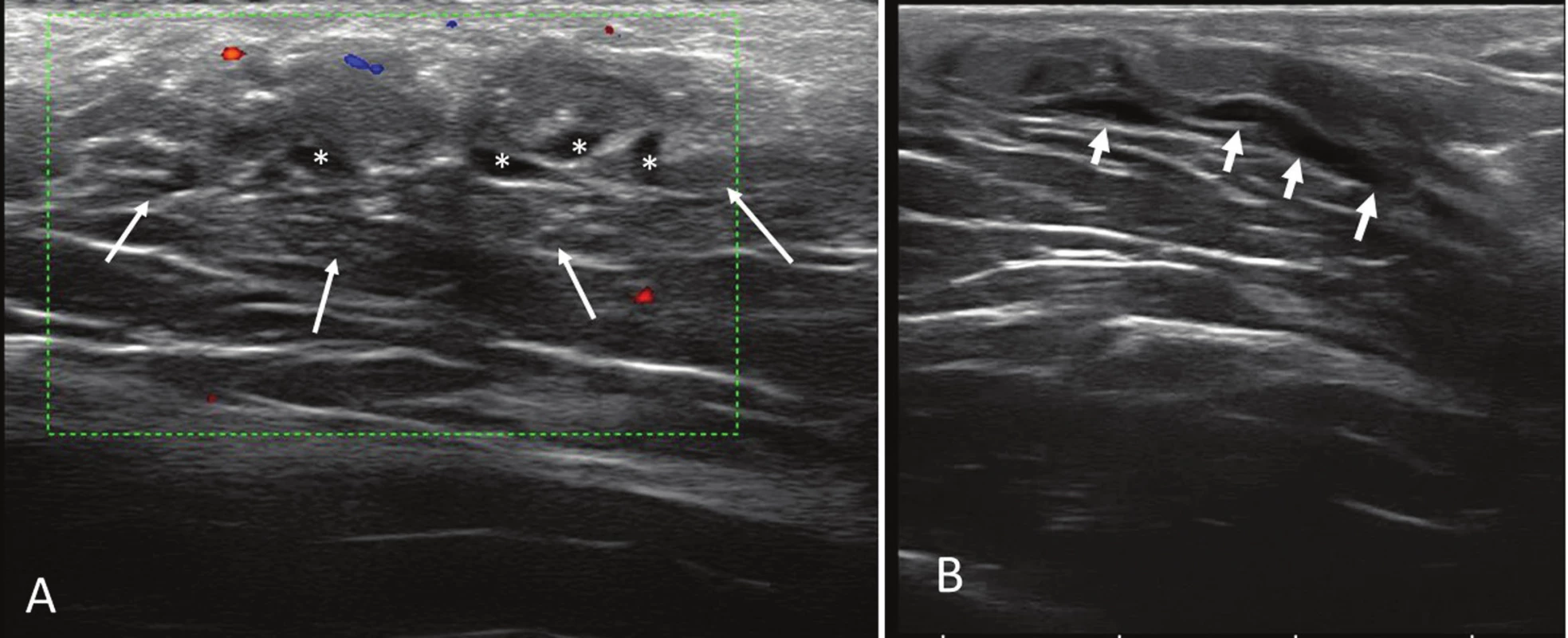
2. Magnetic resonance imaging (MRI). Axial T1-weighed (A), DP-weighed fat-suppressed (B), and T1-weighed fat suppressed gadolinium enhanced (C). Images demonstrate a superficial triangular shaped mass (white arrows), isointense to muscle on T1 with homogeneous contrast enhancement 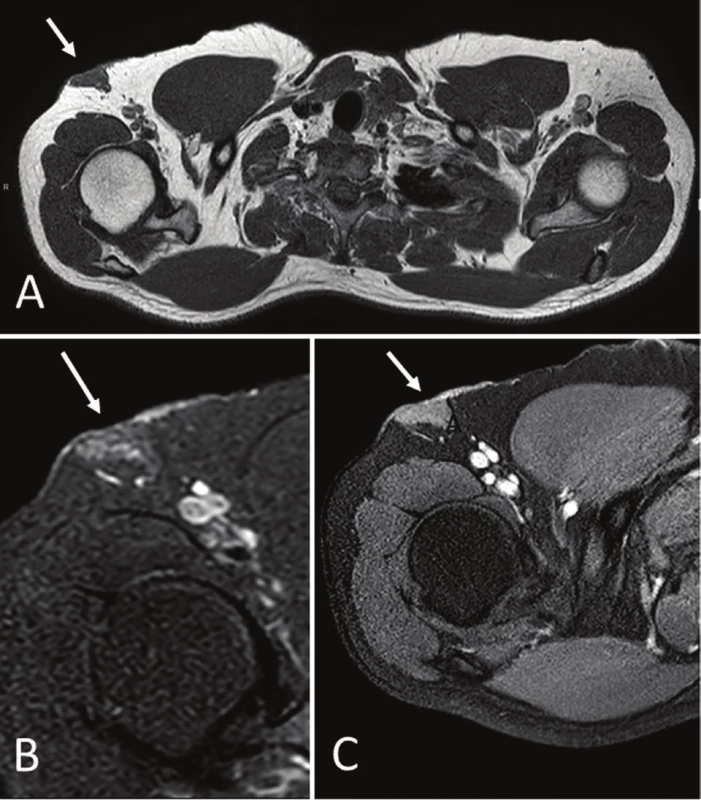
The specimen consisted of an elliptic area of skin measuring 3 x 0.6 cm, underlying which was a firm, homogeneous 4 x 4 x 1.2 cm whitish yellow solid tissue. It was grayish white in cross section. The histopathologic study showed skin rich in apocrine glands. In the hypodermis, abundant, well-delimited, not encapsulated mammary glandular parenchyma was present. This tissue showed scattered dilatation of ducts associated with slight periductal lymphocyte infiltration and fibrosis (Fig. 3A). Floccular secretory material and foamy histiocytes were occasionally present in the ductal lumina. Apocrine differentiation was observed in some ducts. There were no acinar structures. Extensive interductal areas consisting of empty, interanastomosing, slit-like elongated spaces lined by a discontinuous layer of spindly flat cells were prominent (Fig. 3B). These areas involved 90 % of the mammary stroma. The cells had thin, elongated nuclei with pointed ends (Fig. 3C), and some single cells formed delicate bridges between the walls of a space. Adjacent spaces were separated by dense collagen bands (Fig. 3D), and small groups of adipocytes were scattered in the stroma. There were no mitoses, cellular atypia, giant cells, or necrotic areas. Gynecomastia-like changes were not observed.
3. Histopathology of the axillary breast tissue. (A) Aberrant axillary breast tissue including ectatic mammary ducts, absence of acinar tissue and increase of the fibrous tissue (H&E, original magnification x100). (B) Interlobular stroma showing elongated slit-like spaces lined by flattened spindle cells (H&E, original magnification x100). (C) The spaces are empty, devoid of red blood cells (H&E, original magnification x200). (D) Keloid-like fibrosis containing slit-like spaces that simulate vascular channels. No cytologic atypia or mitotic activity was seen in the spindle cells (H&E, original magnification x400). 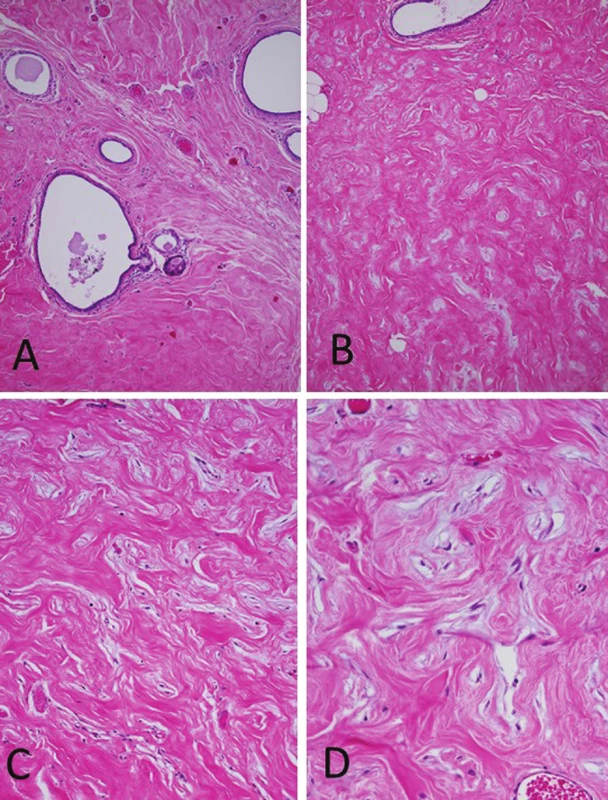
The immunohistochemical study revealed positivity for vimentin and CD34 (Fig. 4) in the flat spindly cells. These cells were not reactive for CD31, smooth muscle actin, desmin, bcl2, calponin, estrogen receptor, or progesterone receptor. The diagnosis was aberrant axillary breast tissue associated with the tumoriform form of PASH involving 90 % of the stroma.
4. CD34 stain decorates cells lining the pseudovascular spaces as well as single spindle stromal cells. Delicate bridges between the cells lining spaces can be observed (original magnification x200). 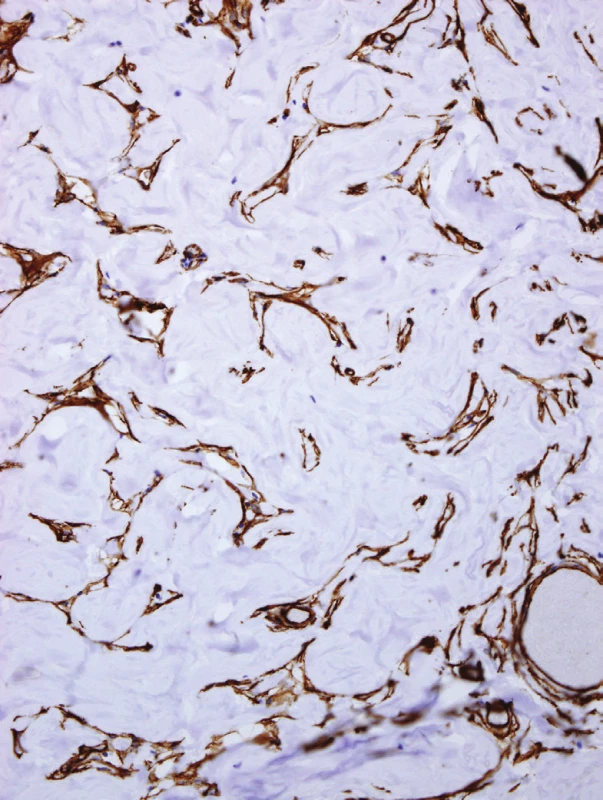
The postoperative course was uneventful. The patient left the hospital on the third postoperative day, and no evidence of recurrence of the axillary mass was noted after three months of follow-up.
DISCUSSION
According to embryologic theory, ABT represents the failure to regress of primordial breast tissue in the milk line, or the absence of involution of migrating nests of primordial breast cells outside the milk line in a completely random manner (2). ABT is traditionally classified into eight classes according to the number of elements present and their position (7). Class 4 of the classification includes glandular tissue only and corresponds to aberrant breast tissue. This is the most common clinical manifestation of accessory breast tissue (2). The condition has been reported as being bilateral in 30.3 % of cases (1,8) and is usually sporadic, but familial cases are also reported.
Aberrant breast tissue in the axilla should be distinguished from Spence’s axillary tail. Aberrant breast tissue in the axilla is separate from the breast, unlike the axillary tail of Spence, which is a direct extension toward the axilla from the main breast tissue.
Mammary tissue is influenced by endocrine hormones. Thus, the most common presentation of axillary breast tissue in women is exacerbation during puberty, pregnancy, and/or the lactation period (1). In men, the condition may cause discomfort, pain, or be asymptomatic. Diagnosing ABT in a male patient is difficult when the condition is unilateral, and there is no areola or nipple. The most common presumptive diagnoses reported include lymphadenopathy, lipoma, hidradenitis, sebaceous cyst, vascular malformation, and malignancy (1,9).
ABT can develop all the diseases that may affect the normal pectoral breast, such as mastitis, milk fistula, abscesses, cysts, fibroadenoma, phyllodes tumors, and carcinomas (1). On rare occasions, PASH may involve the axillary tail of the breast (10). Very uncommonly, aberrant breast tissue is associated with PASH in the axilla of women (11-15). One of these cases showed simultaneous involvement of the ABT in the axilla and vulva (12).
PASH has been reported in approximately 24 % of cases of gynecomastia (16). On the other hand, only two cases of PASH in axillary gynecomastia have previously been described in men. Seidman et al (17) reported the case of a 39-year-old man who presented a rapid growth of PASH in axillary gynecomastia one year after a renal transplant. Thus, a tumoriform PASH occurred in the setting of immunosuppression. Vega et al (18) described a 44-year-old man with bilateral axillary gynecomastia associated with PASH. We believe that both reports are cases of aberrant axillary breast tissue with mass-forming PASH. Ferreira et al (10) observed that there is a significant association between PASH and the presence of gynecomastia-like changes in the pectoral breast tissue of women. This association was present in 65.4 % of their patients.
We report herein a case of aberrant axillary breast tissue in a man. The histopathology of the aberrant breast tissue included mammary duct ectasia, the absence of acinar tissue, and an increase in fibrous tissue. The microscopic study showed no changes of gynecomastia. The aberrant tissue was complicated by an area of PASH involving 90 % of the glandular stroma. The enlargement of the axillary tissue was predominantly due to the excessive proliferation of the tumorous PASH, and the lesion required excision to rule out a malignant tumor. The cause of PASH remains unknown. The patient was not undergoing immunosuppressive treatment. Most authors believe that PASH represents a proliferative response of myofibroblasts, probably to hormonal stimuli (19). In our case, there was no evidence of hormonal imbalance.
Imaging features are not sufficiently specific to enable a prospective diagnosis of PASH (20). The appearance of PASH on MRI has been described in very few cases. A malignant tumor could not be excluded in our patient or in the case reported by Alikhassi et al (13). Histopathologic examination of the lesion is necessary to make a definitive diagnosis and rule out malignancy (21).
Differential diagnosis of PASH includes angioma and low-grade (differentiated) angiosarcoma. Features of PASH such as empty spaces devoid of red cells, a discontinuous layer of cells bordering the lumens, the absence of cellular atypia, the absence or rarity of mitoses, and negativity for CD31 are useful criteria in differential diagnosis.
Excision with adequate margins is the recommended treatment for mass-forming PASH. The prognosis is excellent, but recurrence if the lesion is not completely excised ranges from 0 % to 22 % (16). An exceptional case of histological malignant transformation of PASH (a type of myofibroblastic sarcoma) has been reported (22).
In conclusion, we present what we consider to be the third described example of tumoriform PASH occurring in the aberrant axillary breast of a man. The condition presented as a palpable, unilateral, tender mass in a 38-year-old man with no signs of gynecomastia or immunosuppression. To the best of our knowledge, our case is the first report of aberrant axillary breast tissue with PASH in a man without gynecomastia changes. A histopathological study is essential for making the diagnosis, and the main differential diagnosis is low-grade angiosarcoma. Awareness of the condition can avoid difficulty in making a diagnosis. In sum, aberrant breast tissue with mass-forming PASH, whilst rare, should be included among the benign proliferative mesenchymal lesions of the axilla.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
FUNDING
No external funding for this work.
Correspondence address:
José-Fernando Val-Bernal, MD, PhD
Unidad de Patología, Departamento de Ciencias Médicas y Quirúrgicas,
Universidad de Cantabria, Avda. Cardenal Herrera Oria s/n,
39011 Santander, Spain
tel.: +34 942 203492; extension 73232
e-mail: fernando.val@unican.es
Sources
1. Arora BK, Arora R, Aora A. Axillary accessory breast: presentation and treatment. Int Surg J 2016; 3(4): 2050-2053.
2. DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. AJR Am J Roentgenol 2014; 202(5): 1157-1162.
3. Camisa C. Accessory breast on the posterior thigh of a man. J Am Acad Dermatol 1980; 3(5): 467-469.
4. Grossl NA. Supernumerary breast tissue: historical perspectives and clinical features. South Med J 2000; 93(1): 29-32.
5. Dordevic M, Jovanovic B, Mitrovic S, Dordevic G. Ectopic mammary tissue in vulva. Vojnosanit Pregl 2008; 65(5): 407-409.
6. Xia W, Cheng J, Zhang H, Lu Y. A male patient with Kallman syndrome and accessory breasts. Radiol Infect Dis 2015; 2(3): 141-145.
7. Schultz M, Vatsayan A. Accessory breast tissue. Women´s Health 2013; 15(2): 64-67.
8. Aydogan F, Baghaki S, Celik V, et al. Surgical treatment of axillary accessory breasts. Am Surg 2010; 76(3): 270-272
9. Vidyasagar R, Sudarshan P, Sing NR, Shivaram S. A rare presentation of an ectopic breast tissue in axilla. Our Dermatol Online 2015; 6(4): 450-452.
10. Ferreira M, Albarracin CT, Resetkova E. Pseudoangiomatous stromal hyperplasia tumor: a clinical, radiologic and pathologic study of 26 cases. Mod Pathol 2008; 21(2): 201-207.
11. Lee JS, Oh HS, Min KW. Mammary pseudoangiomatous stromal hyperplasia presenting as an axillary mass. Breast 2005; 14(1): 61-64.
12. Jordan AC, Jaffer S, Mercer SE. Massive nodular pseudoangiomatous stromal hyperplasia (PASH) of the breast arising simultaneously in the axilla and vulva. Int J Surg Pathol 2011; 19(1): 113-116.
13. Alikhassi A, Ensani F, Omranipour R, Abdollahi A. Bilateral simultaneous pseudoangiomatous stromal hyperplasia of the breast and axillae: imaging findings with pathological and clinical correlation. Case Rep Radiol 2016; 2016 : 9084820.
14. Shimpi TR, Baska Reynolds V, Shikare S, et al. Synchronous large tumoral pseudoangiomatous stromal hyperplasia (PASH) in the breast and axilla with subsequent carcinoma in the contralateral breast: routine and strain imaging with histopathological correlation. BJR Case Rep 2015; 1(3): 20150017.
15. Nabeyama K, Wada Y, Saito N, Miyakazi R. A case of bilateral axillary accessory breasts and mammary pseudoangiomatous stromal hyperplasia (PASH). Nihon Rinsho Geka Gakkai Zasshi 2008; 69(7): 1595-1600.
16. Milanezi MFG, Saggioro FP, Zanati SG, Bazan R, Schmitt FC. Pseudoangiomatous hyperplasia of mammary stroma associated with gynaecomastia. J Clin Pathol 1998; 51(3): 204-206.
17. Seidman JD, Borkowski A, Aisner SC, Sun CC. Rapid growth of pseudoangiomatous hyperplasia of mammary stroma in axillary gynecomastia in an immunosuppressed patient. Arch Pathol Lab Med 1993; 117(7): 736-738.
18. Vega RM, Pechman D, Ergonul B, Gomez C, Moller MG. Bilateral pseudoangiomatous stromal hyperplasia tumors in axillary male gynecomastia: report of a case. Surg Today 2015; 45(1): 105-109.
19. Bowman E, Oprea G, Okoli J, et al. Pseudoangiomatous stromal hyperplasia (PASH) of the breast: a series of 24 patients. Breast J 2012; 18(3): 242-247.
20. Jones KN, Glazebrook KN, Reynolds C. Pseudoangiomatous stromal hyperplasia: imaging findings with pathologic and clinical correlation. AJR Am J Roentgenol 2010; 195(4): 1036-1042.
21. Virk RK, Khan A. Pseudoangiomatous stromal hyperplasia. An overview. Arch Pathol Lab Med 2010; 134(7): 1070-1074.
22. Nassar H, Elieff MP, Kronz JD, Argani P. Pseudoangiomatous stromal hyperplasia (PASH) of the breast with foci of morphologic malignancy: a case of PASH with malignant transformation. Int J Surg Pathol 2010; 18(6): 564-569.
Labels
Anatomical pathology Forensic medical examiner Toxicology
Article was published inCzecho-Slovak Pathology

2018 Issue 3-
All articles in this issue
- Intraoperative consultation in gynecologic pathology
- Frozen section of lung, pleura and mediastinum specimen: Retrospective analysis of 5-years practical experiences and review of the literature
- Molecular methods for detection of prognostic and predictive markers in diagnosis of adenoid cystic carcinoma of the salivary gland origin
- Clinicopathological analysis of programmed death-ligand 1 testing in tumor cells of 325 patients with non-small cell lung cancer: Its predictive and potential prognostic value
- Aberrant axillary breast tissue with pseudoangiomatous stromal hyperplasia in a man
- JOSE VEROCAY - “Prague’s pathologist”. The history of a Latin-American doctor
- Pathology will stay as a cornerstone of personalized medicine
- Czecho-Slovak Pathology
- Journal archive
- Current issue
- Online only
- About the journal
Most read in this issue- Intraoperative consultation in gynecologic pathology
- Aberrant axillary breast tissue with pseudoangiomatous stromal hyperplasia in a man
- Clinicopathological analysis of programmed death-ligand 1 testing in tumor cells of 325 patients with non-small cell lung cancer: Its predictive and potential prognostic value
- Molecular methods for detection of prognostic and predictive markers in diagnosis of adenoid cystic carcinoma of the salivary gland origin
Login#ADS_BOTTOM_SCRIPTS#Forgotten passwordEnter the email address that you registered with. We will send you instructions on how to set a new password.
- Career

